Abstract
The aim of this study was to investigate pharmaceutical potentialities of a polymeric microparticulate drug delivery system for modulating the drug profile of poorly water-soluble quercetin. In this research work two cost effective polymers sodium alginate and chitosan were used for entrapping the model drug quercetin through ionic cross linking method. In vitro drug release, swelling index, drug entrapment efficiency, Fourier Transforms Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), X-ray Diffraction (XRD) and Differential Scanning Calorimetric (DSC) studies were also done for physicochemical characterization of the formulations. Swelling index and drug release study were done at a pH of 1.2, 6.8 and 7.4 to evaluate the GI mimetic action which entails that the swelling and release of the all the Formulation1 (F1), Formulation2 (F2) and Formulation3 (F3) at pH 1.2 were minimal confirming the prevention of drug release in the acidic environment of stomach. Comparatively more sustained release was seen from the formulations F2 & F3 at pH 6.8 and pH 7.4 after 7 h of drug release profiling. Drug entrapment efficiency of the formulations shows in F1 (D:C:A = 2:5:30) was approximately 70% whereas the increase in chitosan concentration in F2 (D:C:A = 2:10:30) has shown an entrapment efficiency of 81%. But the comparative further increase of chitosan concentration in F3 (D:C:A = 2:15:30) has shown a entrapment of 80% which is not having any remarkable difference from F2. The FTIR analysis of drug, polymers and the formulations indicated the compatibility of the drug with the polymers. The smoothness of microspheres in F2 & F3 was confirmed by Scanning Electron Microscopy (SEM). However F1 microsphere has shown more irregular shape comparatively. The DSC studies indicated the absence of drug-polymer interaction in the microspheres. Our XRD studies have revealed that when pure drug exhibits crystalline structure with less dissolution profile, formulated microparticles can help us to obtain amorphous form of the same drug that is likely to have more dissolution property. The findings of the study suggest that the microsphere formulations were a promising carrier for quercetin delivery and can be considered as a favorable oral controlled release dosage form for hydrophobic drug quercetin.
Keywords: Alginate, Chitosan, Quercetin, Microparticles, Characterization
1. Introduction
Researchers have treasured the potential benefits of micro technology in providing vast improvements in drug delivery. The method by which a drug is delivered can have a noteworthy effect on its efficacy. One of the most attractive areas of research in drug delivery today is the design of micro-particulate systems that are able to deliver drugs to the right place, at appropriate times and at the right dosage. Recently, the idea of using microparticles made from natural biodegradable polymers to deliver drugs has provoked great interests. Among them, keeping the cost effectiveness in mind two polymers have been used alginate and chitosan which are very promising and have been widely subjugated in pharmaceutical industry for controlling drug release as revealed from the previous article of Hamidi et al. (2008).
Gombotz and Wee (1998), Smidsrod and Skjakbraek (1990) and Murata et al. (2007) have reported that alginate can be used as mucoadhesive, biodegradable and has prospective for numerous pharmaceutical and biomedical applications such as drug delivery system and cell encapsulation. Chitosan, a linear polysaccharide consisting of glucosamine and N-acetyl glucosamine units, is biocompatible, biodegradable, and nontoxic in the application of peroral delivery of drugs as reported by Kotze et al. (1999) and Shi et al. (2008).
The drug Quercetin has been extensively investigated for its antioxidant, antitumor, hepatoprotective activity by the previous researchers like Inal and Kahraman, 2000, Hertog et al., 1992, Kanadaswami et al., 2005. But at the same time it has been noticed in spite of having so many therapeutic effect oral administration of quercetin has been limited by its poor bioavailability which can be evidenced from the work of Davis et al. (2000). Several attempts were made for increasing the bioavailability of the quercetin by different researchers including the complexation with cyclodextrin and liposome as seen in the work of Jessy and Sneha (2012). Nevertheless, the use of cyclodextrin is associated with a risk of nephrotoxicity and employing liposome might incur stability problems during storage as supported by the work of Frijlink et al. (1990). However no attempts have been made for increasing the oral bioavailability of quercetin by incorporating the drug in the matrix of biopolymers like chitosan and sodium alginate which on the other hand promises the drug delivery to a higher intestinal pH in which the drug is soluble enough.
It is therefore clear that a safe, stable, and efficient delivery method in increasing the solubility of quercetin is warranted. In the present research work quercetin microparticulate system has been prepared by a simple ionic cross linking technology with sodium alginate and chitosan as carriers and also the effect of the chitosan concentration on drug release profile has been evaluated. Physicochemical characterization of the formulations has been done by in vitro drug swelling index study, Scanning Electron Microscopy (SEM), differential scanning calorimetry (DSC), powder X-ray Diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR).
2. Experimental
2.1. Materials
Chitosan, 75–85% deacetylated, intermediate viscosity (Brookfield, 1% solution in acetic acid) 20–300 cP, sodium alginate and quercetin were purchased from Sigma Aldrich, India. The other entire reagent used was of analytical grade.
2.2. Preparation of quercetin microparticles
The formulations were made according to the described method of the previous researcher Wittaya-areekul et al. (2006). Briefly all the chitosan solutions (0.5%, 1% and 1.5%w/v) were prepared by dissolving chitosan in a solution of 1% acetic acid followed by the addition of 4% calcium chloride. The whole solution was homogenized at 500 r.p.m for 1 h. The sodium alginate solution at a concentration of 3% (w/v) was prepared. After complete mixing of sodium alginate quercetin was added in the concentration of 0.2% (w/v). The sodium alginate-drug solution was then dropped to the chitosan–calcium chloride solution through a 0.45 mm syringe needle at a dropping rate of 1.0 ml/min. The microparticles were allowed to harden for 2 h before washing them and were then dried at room temperature.
2.3. Microparticles characterization
2.3.1. Determination of Drug Entrapment Efficiency (DEE)
The quercetin content was determined by dissolving 100 mg of microparticles in phosphate buffer of pH 7.4 under sonication (Front line sonicator FS600) for 30 min until the microparticles were completely dissolved. After that the samples were filtered and analyzed spectrophotometrically (Thermo Spectronic, UK) at 370 nm.
The encapsulation efficiency was calculated using the following formula.
2.3.2. In vitro release studies
Drug release profiles from alginate/chitosan microparticles containing quercetin with an accurately weighed amount of microparticles were obtained using USP dissolution apparatus II (Erweka, Germany). The study conditions were set at a stirring speed of 75 r.p.m, dissolution medium volume of 500 ml at 37 °C. The dissolution mediums were simultaneously 0.1 N HCl buffer pH 1.2, phosphate buffer pH 6.8 and pH 7.4 as a representative of the gastric and intestinal fluids respectively. The samples were filtered and analyzed spectrophotometrically at 370 nm. The results measured in triplicate are expressed as a percentage of the drug released.
2.3.3. Swelling index study
Swelling study for microsphere was conducted by immersing the microsphere in 50 ml of three respective medias namely, simulated gastric fluid pH 1.2 and simulated intestinal fluid phosphate buffer pH 6.8 and pH 7.4. Increase in weight of the microsphere was determined at preset time interval until a constant weight was observed by using Mettler Toledo single pan electronic balance.
Swelling index was then calculated as:
2.3.4. Imaging through high resolution digital scanning microscope
The images of the microspheres were taken using high resolution scanning microscope (RMM SCOPE, India). Randomly chosen microparticles were taken to measure their individual shape and morphology. Microparticles were visualized under 30× magnification.
2.3.5. Scanning electron microscopy
The surface morphology of the microparticles was examined using scanning electron microscopy (Jeol Datum Ltd., G5/IL/42/08. Tokyo, Japan). Samples were deposited on stub using one side of a double-sided adhesive dried carbon tape (NEM Tape, Nisshin Em. Co. Ltd., Tokyo, Japan). The working distance 25 mm was maintained and acceleration voltage used was 17 kV with secondary electro image (SEI) as a detector.
2.3.6. Drug excipients interaction study by Fourier Transforms Infrared Spectroscopy
Fourier Transform Infrared Spectrum of pure quercetin, chitosan, sodium alginate, alginate–chitosan physical mixture and drug loaded microsphere were recorded in range of 4000–400 cm−1 using FT-IR (Perkin Elmer) from KBr pellet to find out the drug excipient compatibility study.
2.3.7. Differential scanning calorimetry
A DSC thermogram of pure drug and drug loaded formulated microparticle was performed to determine the thermal properties of the drug and that of formulations. The thermogram was covered by heating from 32.00 °C to 400.00 °C at 12 °C/min. The vessel is constantly purged with nitrogen at a rate of 20.0 mL/min by using PerkinElmer (SINGAPORE) instruments, (DTA, Osaka, Japan).
2.3.8. X-ray diffraction Analysis
This study is useful to investigate crystallinity of the drug in cross-linked microspheres. It was performed with X-ray diffractometer (ULTIMA-III, Rigaku, Japan), using nickel-filtered Cu Kα radiation (a voltage of 40 kV and a current of 30 mA) for pure drug and formulated microparticles.
3. Results & discussion
3.1. Drug entrapment efficiency study results
All the three microparticulate formulations were evaluated for drug entrapment efficiency and the results were illustrated in Table 1 which shows that the entrapment efficiency was affected by the chitosan concentration which reflects that with the increase in polymer ratio the drug entrapment in the polymer matrix get increased. F1 is having the minimum drug entrapment efficiency of approximately 70% whereas the entrapment efficiency of F2 with Drug:Chitosan:Alginate ratio of 2:10:30 shows an approximate entrapment of 80%. However, no significant differences were found in the quercetin entrapment efficiency between the microparticles of F2 and F3 prepared with varied chitosan to sodium alginate concentration. This could be revealed as the increase in polymer concentration has significant effect on the drug entrapment efficiency.
Table 1.
Drug entrapment efficiency (DEE), release profile after 7 h and swelling index of all the 3 formulationsa.
| FMSb | Ratio of D:C:Ac | DEE% | % Drug release profile after 7 h at pH |
Swelling index |
||||
|---|---|---|---|---|---|---|---|---|
| 1.2 | 6.8 | 7.4 | 1.2 | 6.8 | 7.4 | |||
| F1 | 2:5:30 | 70 ± 3.4 | 25.79 ± 2.8 | 79.45 ± 2.0 | 87.2 ± 1.9 | 195.6 ± 4.5 | 715.2 ± 4.1 | 850.5 ± 5.1 |
| F2 | 2:10:30 | 81.59 ± 4.8 | 20.78 ± 2.5 | 65.29 ± 2.9 | 78.45 ± 2.0 | 170.5 ± 4.7 | 653.9 ± 4.5 | 756.2 ± 5.0 |
| F3 | 2:15:30 | 80.33 ± 2.9 | 18.57 ± 1.8 | 63.45 ± 3.5 | 72.15 ± 2.7 | 150.5 ± 4.6 | 620.1 ± 4.6 | 715.4 ± 4.85 |
Each observation is the mean ± S.D (n = 3).
FMS – Formulation code.
D:C:A – Drug:Chitosan:Na-alginate.
3.2. In vitro drug release study results
Figure 1, Figure 2, Figure 3 are showing the in vitro drug release from F1, F2 and F3 respectively which was carried out for 7 h in 500 mL of three different physiological pH buffer solution (namely pH 7.4, pH 6.8 and pH 1.2). Formulation F1 has shown minimum amount of drug release at pH 1.2 which is 25%. But a sudden release rate was observed at pH 6.8 and pH 7.4 which were respectively 79% and 87%. The sudden release could be due to less polymer concentration of F1. On the other hand F2 and F3 have a minimum release of 20% and 18% respectively at stomach pH environment of pH 1.2. A steady drug release of 65% & 78% from F2 and 63% & 72% from F3 has been noted at pH 6.8 and pH 7.4. The results of the dissolution study indicated that the amount of drug release appreciably decreased and became steady with an increase in the concentration of chitosan in the matrix. It can be ascribed to increase in the densities of the polymer matrix ensuing in larger microspheres and this in turn increases the diffusional path length, which the drug molecules have to traverse as supported by the preceding work of Sultana et al. (2009) in preparation of diltiazem microspheres. Drug release study also entails that the release from all the Formulation1 (F1), Formulation2 (F2) and Formulation3 (F3) at pH 1.2 was minimal confirming the prevention of drug release in the acidic environment of stomach.
Figure 1.
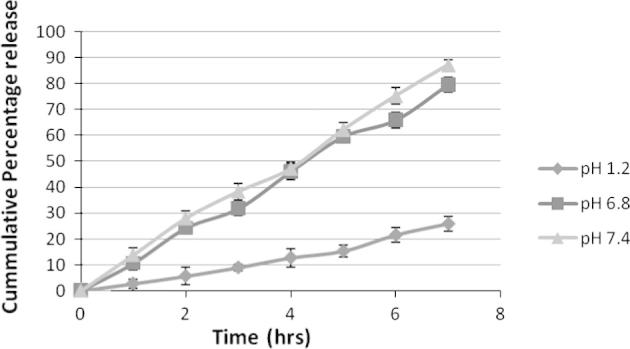
Drug release study from F1 at different physiological pH. Each observation is the mean ± SD (n = 3).
Figure 2.
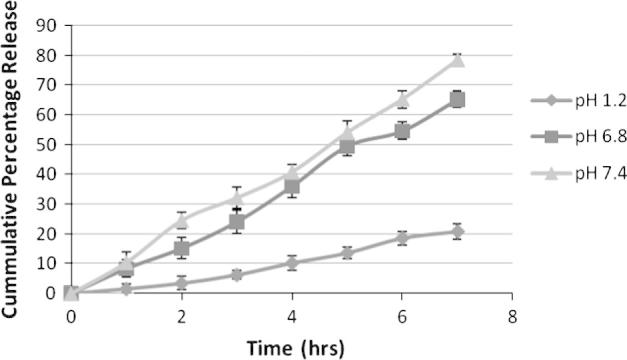
Drug release study from F2 at different physiological pH. Each observation is the mean ± SD (n = 3).
Figure 3.
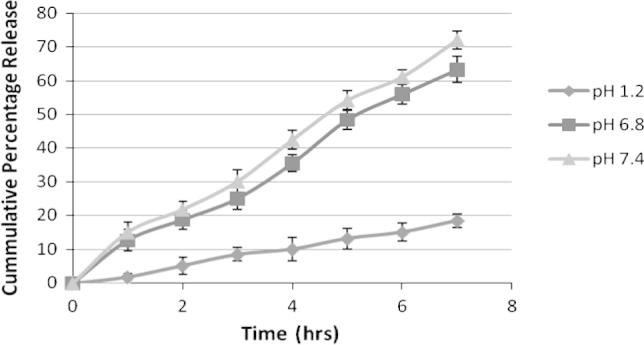
Drug release study from F3 at different physiological pH. Each observation is the mean ± SD (n = 3).
3.2.1. Swelling index study results
The swelling behavior of the microspheres shown in Figure 4, Figure 5, Figure 6 was studied by measuring the water uptake at definite time intervals in pH 1.2, pH 6.8 and pH 7.4. In pH 1.2, the ratio of water uptake by the microspheres of all three formulations was low and independent of time. However gradual increase in chitosan concentration in F1, F2 and F3 controlled the swelling of microsphere at all the three buffers. The maximum water uptake for all three formulations was seen for 7 h. More or less up to this stage microspheres behave as matrix for controlled release of incorporated drug, after which erosion and break down of microspheres occurred.
Figure 4.
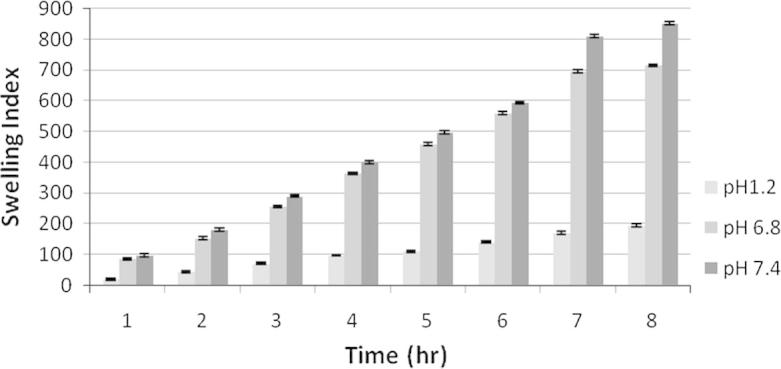
Swelling index study of F1 at different physiological pH. Each observation is the mean ± SD (n = 3).
Figure 5.
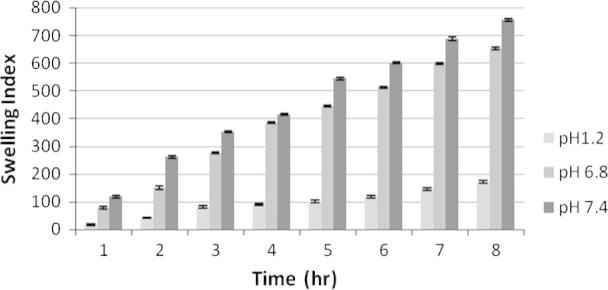
Swelling index study of F2 at different physiological pH. Each observation is the mean ± SD (n = 3).
Figure 6.
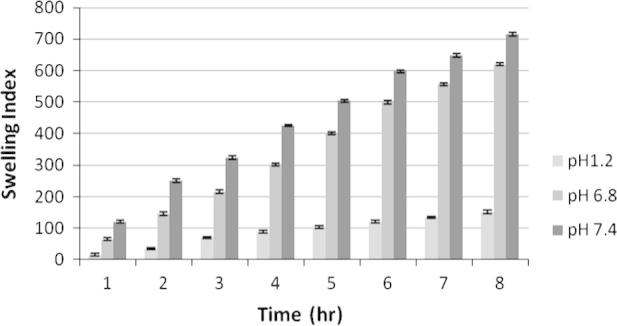
Swelling index study of F3 at different physiological pH. Each observation is the mean ± SD (n = 3).
3.2.2. Analysis reports of imaging through high resolution digital scanning microscope
This imaging technology provided us a rough thought on the shape and surface morphology of the microspheres without scaling of the particles in a magnification of 30×. Figure 7, Figure 8, Figure 9 show the images of F1, F2 and F3 microparticles enlightening that the particles of F2 and F3 were having a superior shape and surface morphology whereas the microparticles of F1 having a lesser chitosan concentration could not be competent enough to create an even polymer matrix reasoning the lower drug entrapment efficiency of F1 than other two formulations.
Figure 7.
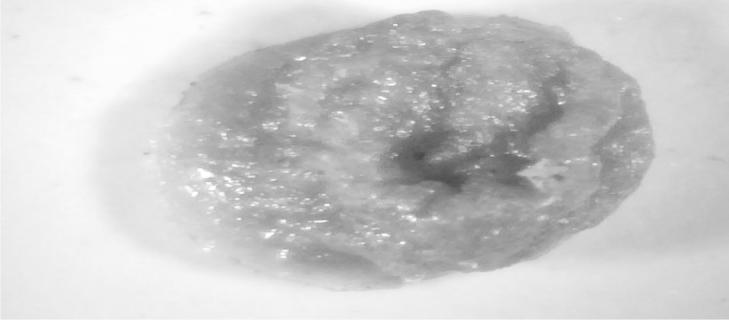
High resolution digital imaging of F1 microparticle at 30× magnification.
Figure 8.

High resolution digital imaging of F2 microparticle at 30× magnification.
Figure 9.

High resolution digital imaging of F3 microparticle at 30× magnification.
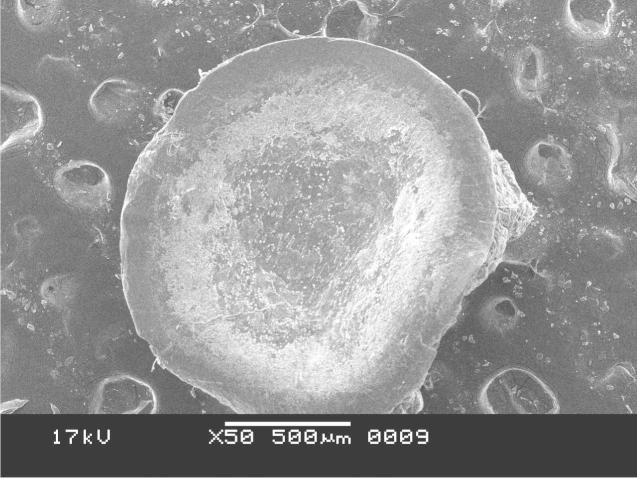
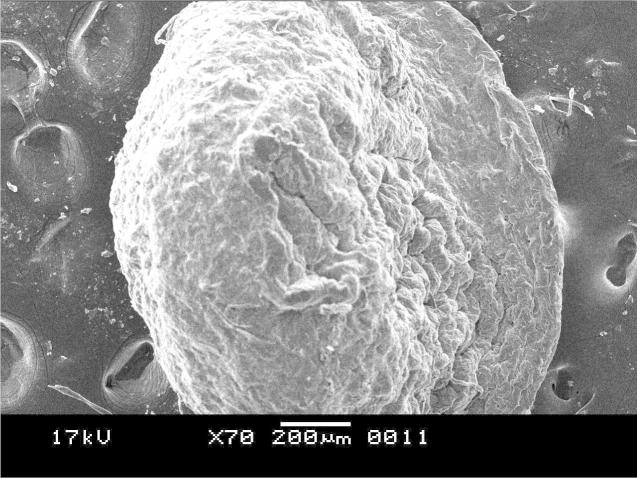
3.2.3. Results of scanning electron microscopy
The SEM photomicrograph shown in Figure 10, Figure 11 was the images of drug loaded microspheres of F1 and F2 respectively. F2 microspheres were almost spherical in shape with rough and nonporous surface. The micrographs of F1 were not good enough than that of the other two formulations showing almost flat beads instead of a spherical one. The result shows that the polymer concentration in the formulations actually affects the surface morphology of the microspheres reasoning for the uneven pellet type of shape for the Formualtion1 where the polymer concentration was less. Formulation2 having a polymer to drug ratio as (D:C:A = 2:10:30) has shown a more spherical shape and smooth surface morphology with optimum results in drug release and swelling index profiling which is supported by the work of Motlekar and Youan (2008). However formulation3 with a polymer to drug ratio as (D:C:A = 2:15:30) has shown a more rigid structure for which the drug release profile results were not reasonable.
Figure 10.
SEM photomicrograph of drug-loaded microparticle of F1.
Figure 11.
SEM photomicrograph of drug-loaded microparticle of F2.
3.2.4. Drug excipients interaction study results by Fourier Transforms Infrared Spectroscopy
Chitosan flakes, Na-alginate and their physical mixture along with the formulations have shown characteristic peaks around 3400 cm−1 for –OH and –NH2 stretching. CH stretching at 2973 cm−1 has been seen very significantly in formulation1 but is not significant in other formulations. In sodium alginate some distinct peaks such as carboxyl group showed strong absorption bands at 1642 cm−1 due to carboxyl anions asymmetric and symmetric stretching vibrations disappearing or becoming weak in the microparticle formulations which have shown the evidence of multi-interactions (hydrogen binding and electrostatic interaction) among chitosan and alginate molecules supporting the previous work of Zhang et al. (2008). However all of the FTIR reports have shown –OH stretching at 3420 cm−1 and –OH bending at around 1467 cm−1. Also there were significant peaks from 1600 to 1500 cm−1 due to the presence of amine group present in chitosan. This observation indicated that there was no significant intermolecular interaction occurred in the physical mixtures and also in the formulations. The details about the spectral lines which have been found in the FTIR graph have been tabulated in Table 2.
Table 2.
Details about the spectral lines which have been found in the FTIR graph.
| Functional groups | Wave number for chitosan (cm−1) | Wave number for Na-alginate (cm−1) | Wave number for quercetin (cm−1) | Wave number for chitosan Na-Alg physical mixture (cm−1) | Wave number for F1 (cm−1) | Wave number for F2 (cm−1) | Wave number for F3 (cm−1) |
|---|---|---|---|---|---|---|---|
| —OH stretching | 3407 | 3408 | 3415 | 3407 | 3419 | 3428 | 3428 |
| —OH bending | 1467 | 1466 | 1467 | 1467 | 1465 | 1467 | 1467 |
| CH stretching | 2973 | 2973 | 2973 | 2973 | 2973 (sharp peak) | 2973 | 2973 |
| CO stretching | – | 1642 | – | – | – | – | – |
| Aromatic rings | 1564 | 1564 | 1563 | 1564 | 1559 | 1563 | 1562 |
| —C C— | 2360 | 2360 | 2360 | 2360 | – | 2359 | 2359 |
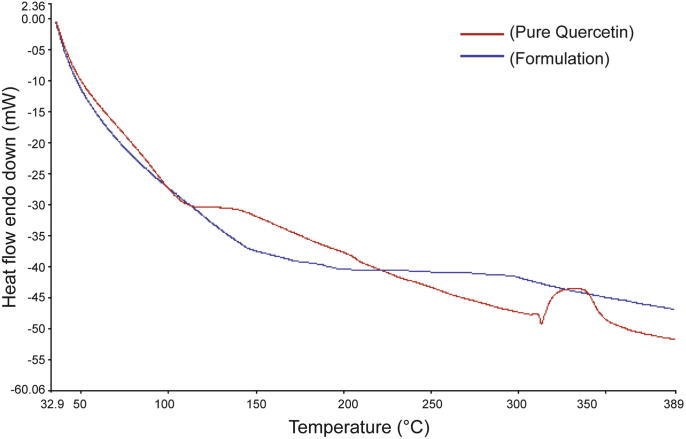
3.2.5. Differential scanning calorimetry report
DSC report in Fig. 12 shows the quercetin powder used in this study had a sharp melting endothermic peak (marked by the red line) at 316 °C followed by degradation supporting the melting point of quercetin. However, no melting point peak was exhibited in the curve of formulation (marked in blue line). A similar observation has been reported by Jung et al. (1999). These results suggested that the drug was dispersed throughout the polymers forming a high-energy amorphous state.
Figure 12.
DSC curves of formulation (in blue) and pure drug (in red).
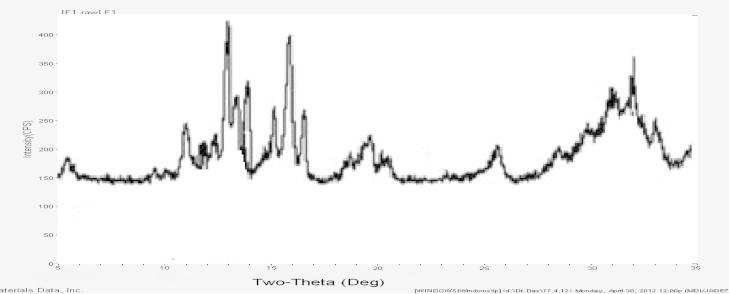
3.2.6. X-ray diffraction analysis
The characteristic peaks of quercetin exhibited at a diffraction angle of 2θ, 10.73°, 12.33°, 15.87°, 24.41°, 26.50°, and 27.40° Fig. 13, can be inferred to traits of a high crystalline structure. However, there were no characteristic peaks appearing on the patterns of the formulation Fig. 14. One explanation for this is that the molecule of quercetin has been encapsulated or dispersed into chitosan–alginate polymers when undergoing the ionic cross linking technique, thus forming an amorphous complex with intermolecular interaction occurring within the matrix. A similar phenomenon has been observed for oridonin dispersed in poly(d,l-lactic acid) (PLA) in which the XRD pattern of oridonin-loaded PLA nanoparticles showed that the drug was in its amorphous state by Xing et al. (2007). Findings of the present study were also in accordance with the above results and also from the DSC analysis providing evidence has been found that the crystal structure of quercetin was indeed converted to an amorphous state.
Figure 13.
X-ray diffraction patterns of pure quercetin.
Figure 14.
X-ray diffraction patterns of formulations.
4. Conclusion
Quercetin appears to have limited therapeutic window through oral route, which is demanding to produce an ideal delivery technique to increase the therapeutic concentration in vivo. The present study has shown that chitosan-coated alginate microspheres could be a challenging controlled drug delivery system for quercetin with more therapeutic bioavailability. Similar result has been observed by Sarmento et al. (2007) for the oral delivery of insulin through alginate/chitosan nanoparticles. Hence, it can be concluded that using these cost effective polymers can develop a novel drug delivery system of herbal drug quercetin which can increase the bioavailability of the compound for several degenerative diseases.
Acknowledgment
The authors are thankful to Prof. (Dr.) Vivekananda Mandal and the entire team of Gupta College of Technological Sciences, West Bengal, India for their valuable contribution to make this research work possible.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Moumita Hazra, Email: moumita.hazra@gmail.com.
Dalia Dasgupta Mandal, Email: daliadasguptamandal@gmail.com.
References
- Davis W., et al. Antioxidants and cancer III: quercetin. Altern. Med. Rev. 2000;5(3):196–208. [PubMed] [Google Scholar]
- Frijlink H.W., et al. The pharmacokinetics of beta-cyclodextrin and hydroxypropyl-beta-cyclodextrin in the rat. Pharm. Res. 1990;7(12):9–16. doi: 10.1023/a:1015929720063. [DOI] [PubMed] [Google Scholar]
- Gombotz W.R., Wee S.F. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998:267–285. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- Hamidi M., et al. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008;60(15):1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Hertog M.G.L., et al. Optimization of a quantitative HPLC determination of potentially anticarcinogenic flavonoids in vegetables and fruits. J. Agric. Food Chem. 1992;40:1591–1598. [Google Scholar]
- Inal M.E., Kahraman A. The protective effect of flavonol quercetin against ultraviolet A induced oxidative stress in rats. Toxicology. 2000;154:21–29. doi: 10.1016/s0300-483x(00)00268-7. [DOI] [PubMed] [Google Scholar]
- Jessy S., Sneha I. Double-loaded liposomes encapsulating quercetin and quercetin beta-cyclodextrin complexes: preparation, characterization and evaluation. Asian J. Pharm. 2012;6(3):18–26. [Google Scholar]
- Jung J.Y., et al. Enhanced solubility and dissolution rate of itraconazole by a solid dispersion technique. Int. J. Pharm. 1999;187:209–218. doi: 10.1016/s0378-5173(99)00191-x. [DOI] [PubMed] [Google Scholar]
- Kanadaswami C., Lee L.T., Lee P.P., Hwang J.J., Ke F.C., Huang Y.T., Lee M.T. The antitumor activities of flavonoids. In Vivo. 2005;19:895–909. [PubMed] [Google Scholar]
- Kotze A.F., et al. Enhancement of paracellular drug transport with highly quaternized N-trimethyl chitosan chloride in neutral environments: in vitro evaluation in intestinal epithelial cells (Caco-2) J. Pharm. Sci. 1999:253–257. doi: 10.1021/js980233c. [DOI] [PubMed] [Google Scholar]
- Motlekar N., Youan B. Optimization of experimental parameters for the production of LMWH-loaded polymeric microspheres. J. Drug Des. Devel. Ther. 2008:39–47. [PMC free article] [PubMed] [Google Scholar]
- Murata Y., et al. The drug release profile from calcium-induced alginate gel beads coated with an alginate hydrolysate. Molecules. 2007;12(11):2559–2566. doi: 10.3390/12112559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmento B., Ribeiro A., Veiga F., Sampaio P., et al. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm. Res. 2007;24(12):2198–2206. doi: 10.1007/s11095-007-9367-4. [DOI] [PubMed] [Google Scholar]
- Shi J., et al. Chitosan coated alginate beads containing poly(N-isopropylacrylamide) for dual-stimuli-responsive drug release. J. Biomed. Mater. Res. B Appl. Biomater. 2008;84(2):595–603. doi: 10.1002/jbm.b.30907. [DOI] [PubMed] [Google Scholar]
- Smidsrod O., Skjakbraek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8:71–78. doi: 10.1016/0167-7799(90)90139-o. [DOI] [PubMed] [Google Scholar]
- Sultana Y., et al. Preparation and in vitro characterization of diltiazem hydrochloride loaded alginate microspheres. Pharm. Dev. Technol. 2009;14(3):321–331. doi: 10.1080/10837450802626304. [DOI] [PubMed] [Google Scholar]
- Wittaya-areekul S., et al. Preparation and in vitro evaluation of mucoadhesive properties of alginate/chitosan microparticles containing prednisolone. Int. J. Pharm. 2006;312(1–2):113–118. doi: 10.1016/j.ijpharm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Xing J., Zhang D., Tan T. Studies on the oridonin-loaded poly(d, l-lactic acid) nanoparticles in vitro and in vivo. Int. J. Biol. Macromol. 2007;40:153–158. doi: 10.1016/j.ijbiomac.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Zhang J., et al. Preparation and characterization of tamarind gum/sodium alginate composite gel beads. Iran. Polym. J. 2008;17(12):899–906. [Google Scholar]