Abstract
Nanoparticles are being increasingly used in the field of cancer treatment due to their unique properties and advantages. The aim of the present research work was to prepare and characterize a polymeric albumin nanosystem for Cisplatin and evaluate its in-vitro efficacy against B16F10 melanoma. The developed nanoparticles were almost spherical in shape with a particle size in the range of 150–300 nm, low polydispersity values and about 80% drug entrapment efficiency. Albumin nanocarriers sustained the release of Cisplatin for more than 48 h, suggesting the reduction in dosing schedule for this drug. The results from in-vitro cell line studies indicated the dose dependent cytotoxic potential of drug loaded albumin nanoparticles, their potential to inhibit cell proliferation and induce morphological changes. In addition, these nanoparticles exhibited superiority to Cisplatin in hampering the cell migration. Developed nanoparticles caused cell cycle arrest along with time and concentration dependent cellular uptake in B16F10 cell line. These results signify that the prepared Cisplatin albumin nanoparticles could serve as a promising approach for B16F10 melanoma treatment.
Keywords: Cisplatin, Human serum albumin, B16F10 melanoma, Nanoparticles
1. Introduction
Metastasis is a terminology related to the diffusion of cancerous cells from their primary location to distant sites within the body (Fidler and Hart, 1982). Metastatic melanoma, owing to its aggressive nature, is regarded as one of the most severe types of skin cancer with high morality rates. These cells interact with healthy host cells in a stepwise fashion, with adhesion and invasion to the basement membrane along with extracellular matrix degradation (Lee et al., 2006, Cavallaro and Christofori, 2001). In United States alone, melanoma led to death of approximately 9000 patients in the year 2012, with threefold increase in number of cases since the year 1970 (Cancer Facts and Figures, 2012).
Numerous treatment options have been employed for melanoma such as surgery, radiation, chemotherapy and biologic therapeutics (Drugs for Melanoma, 2013). However, due to the high metastatic potential of these cancer cells and their resistance to conventional anticancer agents, in addition to poor diagnosis, the effective therapy of melanoma remains disappointing. Owing to the rise in number of melanoma cases, development of alternative methodologies with enhanced safety and efficacy, for rehabilitation of melanoma is the need of time.
Cisplatin, an alkylating agent has been successfully used since decades for the treatment of variety of cancers viz. bone cancer, lung cancer, head and neck cancer, sarcomas and ovarian tumors (Floria and Busselberg, 2011). This moiety intercalates with the DNA and causes conformational changes in its structure, thus leading to cell death. Cisplatin per se exhibits moderate activity against metastatic melanoma and is used as a single line treatment or in combination therapy with other antitumor agents. Alone, this drug has exhibited a response rate of about 10% in patients; however, combination of the same with dacarbazine, tamoxifen and amifostine has led to an increase in overall survival rate of melanoma patients (Del Prete et al., 1984, Glover et al., 1987). Conversely, Cisplatin because of its short term action on melanoma cells with associated nephrotoxicity and ototoxicity has proved to be futile for the patients in a long term perspective. One of the approaches to alter the pharmacokinetic profile and toxicity related to this drug is to formulate it in the form of novel delivery systems such as polymeric nanoparticles, micelles, liposomes, nanoemulsions, dendrimers and lipid nanocarriers.
Polymeric nanocarrier systems are of advantage in drug delivery arena due to their inherent biodegradability, ability to reduce drug toxicity, prevention of drug degradation and controlled release characteristics (Kreuter, 1994). Human serum albumin is one such biocompatible polymer approved by the food and drug administration for use in injectable preparations. Albumin is extensively used for nanoparticle synthesis due to its low cost, biocompatibility, ease of availability and ease of purification.
Due to the multiple advantages of albumin, incorporation of Cisplatin in these nanoparticles is expected to reduce its unwanted side effects with concomitant enhancement in efficacy. With this objective in mind, the present work was directed toward preparation of Cisplatin nanoparticles and evaluation of their anticancer potential in comparison with the plain drug in B16F10 melanoma cells.
2. Experimental
2.1. Materials
Cisplatin was obtained as a gift sample from Cipla Pvt Ltd, Mumbai, India. Sterile human serum albumin, 20% solution (Buminate, Baxter) was purchased from Lifeline Pharma, Mumbai, India. Absolute ethanol and Glutaraldehyde were obtained from Merck Pvt Ltd, India. B16F10 murine melanoma cell line was obtained from National center for cell sciences, Pune, India. The above cell line was maintained in 10% DMEM (Cell Clone, Genetix Biotech Asia Pvt Ltd, India) with Fetal Bovine serum (GIBCO BRL, MD, USA), penicillin (100 U/ml) and Streptomycin (100 g/ml). Cells were grown in humidified condition of 5% CO2 at a temperature of 37 °C. All other reagents and chemicals were of analytical grade.
2.2. Preparation of human serum albumin Cisplatin nanoparticles
Human serum albumin nanoparticles were prepared by a desolvation process as per the procedure described earlier in the literature with some modifications (Langer et al., 2008). Briefly, drug was added to 2% w/v aqueous human serum albumin solution and mixed on a magnetic stirrer for 30 min. Absolute ethanol was added to the above solution to desolvate the protein in the ratio of 1:2. Formation of coacervates was indicated by slight turbidity in the solution. The formed nanoparticles were hardened with 8% glutaraldehyde and stirred for 4 h for covalent crosslinking. Formed nanoparticles were subjected to a purification process by ultracentrifugation (Optima L-100 XP, Beckman Coulter, Inc) at 30,000 rpm. Blank batches for the nanoparticles were prepared in a similar manner except for the drug addition step. Purified nanoparticles were subjected to lyophilization with 5% sucrose and stored under refrigeration until further use.
2.3. Determination of particle size, polydispersity index and zeta potential
Average particle size of developed albumin nanoparticles was analyzed by photon correlation spectroscopy using Malvern Zetasizer instrument, 90S (Ver 6.12.). The sample (Quantity-20 μl) was diluted with double distilled water in the polystyrene cuvette and was placed in the path of scattered light. The scattered intensity measured at an angle of 90° was determined by the software to give the hydrodynamic diameter. Zeta potential was measured using a dip cell at a temperature of 25 °C.
2.4. Determination of entrapment efficiency of Cisplatin in nanoparticles
Entrapment efficiency of the developed nanoparticles was determined after ultracentrifugation at 30,000 rpm (4 °C, 1 h). Amount of Cisplatin entrapped in the nanoparticles was calculated by subtracting the amount of free drug in the supernatant from the initial concentration of Cisplatin used in the preparation of formulation. Amount of free Cisplatin present in the supernatant was estimated using UV spectrophotometer by a colorimetric method after reaction with o-phenylenediamine at 703 nm with minor modifications (Golla and Ayres, 1973).
2.5. Determination of morphology of the developed nanoparticles
2.5.1. Scanning electron microscopy (SEM)
A drop of nanoformulation was placed on a specimen holder and kept for drying. The dried sample was then coated with platinum by high vacuum evaporation and placed inside the specimen chamber for viewing of particles.
2.5.2. Transmission electron microscopy (TEM)
Morphology of the nanoparticles was investigated using Transmission electron microscopy (CM200 machine, PHILIPS Model) operated at voltage of 20–200 kV with a resolution of 2.4 Å. A drop of nanoparticle solution was placed on the copper slide of the instrument provided with carbon grids. The sample was allowed to dry on the slide, introduced into the instrument and scanned under the microscope for viewing of particles.
2.6. In-vitro drug release studies
Cisplatin albumin nanoparticle dispersion (2 ml) was placed inside a dialysis bag with molecular weight cutoff of 12–14 kDa (Himedia Laboratories Pvt Ltd, Mumbai). Dialysis bag was tied and placed in a beaker containing 100 ml of phosphate buffer saline 7.4, maintained at 37 °C and stirring under 100 rpm. Dissolution medium (2 ml) was withdrawn at suitable time intervals and replaced with same volume of fresh dissolution medium. Amount of Cisplatin in the release medium was determined by a colorimetric method as described above. All the dissolution measurements were done in triplicate.
2.7. In-vitro studies on B16F10 cell line
2.7.1. Determination of cell viability by MTT assay
Cell viability for B16F10 cells was measured as per the procedure described earlier (Dua and Gude, 2006). B16F10 cells were seeded in 96 well plates at a concentration of 4 × 103 cells/100 μl/well and incubated for 24 h. These cells were then treated with Cisplatin, Blank nanoparticles and Cisplatin polymeric nanoparticles for 24, 48, 72 and 96 h at concentrations ranging from 0.1 μg/ml to 100 μg/ml for determination of IC50. After treatment, the cells were washed with phosphate buffer saline and MTT (Sigma Aldrich, USA) was added in the plates at a concentration of 5 mg/ml. The formed formazan crystals were solubilized in DMSO and the optical density was measured using ELISA microplate reader (Molecular devices, Spectra Max 190) at 540 nm after background correction at 690 nm.
2.7.2. Colony formation assay
For colony formation assay, 35 mm petri plates were seeded with 600 cells/plate and allowed to proliferate for 48 h. Later, these cells were treated for 24 h with the sub-toxic concentrations of pure drug, blank and drug loaded formulation. Complete DMEM medium was added after PBS washing of the treated cells. Petri plates were incubated for approximately 3–4 days, and cells were fixed using methanol and stained with 0.2% crystal violet solution for visual observation of colonies. Colonies with 50 or more number of cells were counted and percent inhibition potential for the colonies by the developed formulations was calculated.
2.7.3. Wound healing assay
Wound healing assay was carried out as per an earlier procedure with minor modifications (Dua and Gude, 2008). B16F10 cells were seeded at a concentration of 4 × 104 cells/ml in 35 mm petri plates and allowed to grow up to 60–70% confluency. Sub-toxic concentrations of above mentioned formulations were added in the above plates and incubated for 24 h. Later, wounds were created in the above plates using a microtip. The cells were washed with PBS for 2–3 times to remove the peeled cells from the surface and initial wound width was measured using an ocular grid. After incubation period of 24 h, the cells were fixed with methanol, observed for migration and the images were taken using an inverted microscope (Axiovision, Zeiss). Twenty-five measurements were recorded for each formulation and percent migration for each of the formulations was calculated considering migration in untreated control as 100%.
2.7.4. Determination of cellular morphology by leighton tube assay
Leighton tube assay was carried out as per the procedure described earlier (Shenoy et al., 2013). B16F10 melanoma cells were grown in 35 mm petri plates on coverslips in DMEM medium. Sub-toxic concentrations of pure drug, blank albumin nanocarriers and drug loaded formulations were added in the above plates and incubated for 48 h. Later, these cells were fixed with 70% ethanol and stained with hematoxylin and eosin. The coverslips were dipped lightly in xylene to remove the excess stain and fixed on glass slides using DPX mounting medium. The changes in B16F10 morphology cells after treatment were observed under the inverted light microscope.
2.7.5. Cell cycle analysis by flow cytometry
For cell cycle analysis, sub-toxic amounts of pure Cisplatin and developed nanoformulations were added to 35 mm plates containing sub-confluent B16F10 cells after 48 h incubation period. These cells were collected, cleaned 2–3 times with PBS and fixed with chilled 70% ethanol. The obtained cell pellet was subjected to treatment with RNAse (MBI Fermentas, USA) and stained with propidium iodide. Cell cycle analysis was performed on Becton–Dickinson FACS scan and data were analyzed using MODFIT software.
2.7.6. Determination of qualitative cellular uptake of nanoparticles by confocal microscopy
For this particular study, the nanoparticles were loaded with FITC dye in the concentration of 0.2 mg/ml. B16F10 cells were allowed to reach 60% confluency and were treated with the plain dye and dye loaded albumin nanoparticles respectively for 2 h. These cells were fixed with 1% paraformaldehyde subsequent to PBS washings. Further, the cells were treated with DAPI and washed thrice with PBS. The coverslips containing B16F10 cells were observed under the microscope after treatment with 2.5% DABCO and were sealed by utilizing nail paint. Acquisition was performed using confocal microscope at 63X. (LSM 510, Zeiss). Analysis of the data was done using LSM image browser software.
2.7.7. Determination of quantitative cellular uptake of nanoparticles in B16F10 cells by flow cytometry
Quantitative assessment was performed for uptake of nanoparticles loaded with coumarin-6 dye (0.6 mg/ml). B16F10 cells were grown in 35 mm plates till they attained 60–70% confluency. Confluent cells were treated with plain dye and dye loaded nanocarriers for specific time limit as in a particular study (Jain et al., 2013). After harvesting, these cells were fixed using 1% PFA solution. Cells were suspended in PBS solution subsequent to washings with the same solvent. Analysis was performed on FACS Calibur using CellQuest software.
2.8. Statistical analysis
All the measurements for the present work were performed in triplicate. Statistical analysis was performed using the software Graphpad Instat, Version 3.10. Results of the same are expressed as mean ± S.D. One-way ANOVA was used to determine the significant difference between the treatment groups and untreated control (p < 0.001).
3. Results and discussion
3.1. Particle size, polydispersity index and zeta potential measurements for developed nanoparticles
Human serum albumin nanoparticles were synthesized using a desolvation method. Developed albumin nanoparticles were found to exhibit particle size in the range of 150–300 nm with low polydispersity values indicating homogeneity in the formed nanocarriers. The results for particle size analysis are as given in Table 1. Thus, the prepared nanoparticles were ideal candidates for enhanced permeability and retention mechanism for tumor delivery. There was a slight increase in the particle size and polydispersity index in the drug loaded formulations as compared to blank nanoparticles due to the presence of the drug. However, there was not much change in the zeta potential values of both the formulations due to the unionized nature of the drug moiety. Zeta potential values were in the negative range due to the acidic albumin, which exhibited negative potential values at pH 7.4.
Table 1.
Determination of particle size, polydispersity index and zeta potential for the developed albumin nanocarriers.
| Formulation | Particle size (nm) | Polydispersity index | Zeta potential (mV) |
|---|---|---|---|
| Blank nanoparticles | 193.34 ± 2.416 | 0.1266 ± 0.05 | −16.96 ± 1.05 |
| Cisplatin HSA nanoparticles | 265 ± 1.1476 | 0.115 ± 0.013 | −15.6 ± 1.285 |
Human serum albumin nanoparticles exhibit poor stability per se in solution form. Also, it is well known that during the freezing process of a particular sample, there is a phase separation into ice and cryo-concentrated solution. Furthermore, the crystallization of ice may exert a mechanical stress, leading to destabilization of nanoparticles (Abdelwahed et al., 2006). For these reasons, sucrose was added to the suspension of nanoparticles before freezing. There was only a minor increase in the particle size of the nanoformulations after lyophilization, indicating suitability and rigidity of the freeze drying process.
3.2. Determination of entrapment efficiency of Cisplatin in nanoparticles by colorimetry
Entrapment efficiency of Cisplatin in nanoparticles was found to be in the range of 70–80%. Although Cisplatin is a hydrophobic moiety, it showed good entrapment in albumin nanoparticles. This phenomenon may be attributed to the high plasma protein binding efficiency of Cisplatin in the blood (Ivanov et al., 1998).
3.3. Transmission and scanning electron microscopy imaging
TEM images of developed nanoparticles showed the presence of irregular and discrete particles whereas SEM image showed presence of irregular to almost spherical particles. Particle size of the nanoparticles obtained with TEM and SEM was in well correlation to the particle size data obtained by zetasizer. The results for TEM and SEM are as depicted in Figure 1, Figure 2 respectively.
Figure 1.
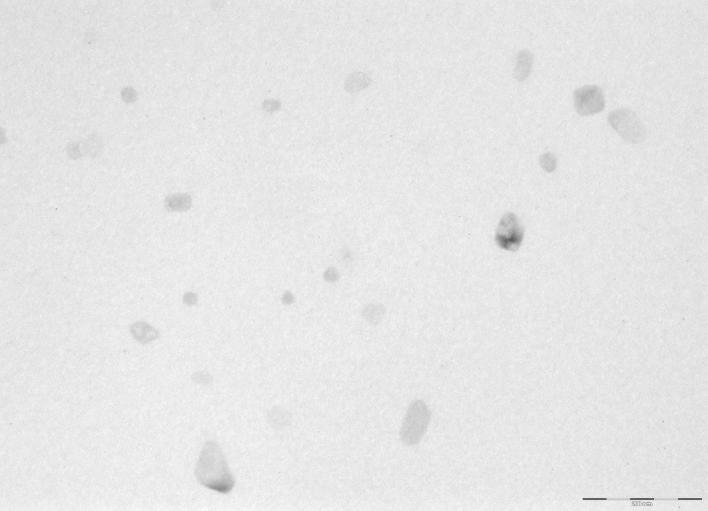
Transmission electron microscopy imaging of albumin nanoparticles.
Figure 2.
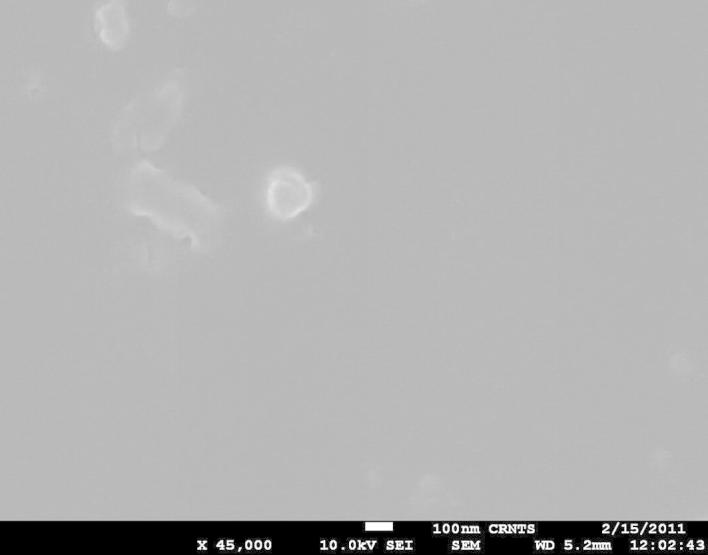
Scanning electron microscopy of developed nanoparticles.
3.4. In-vitro drug release studies
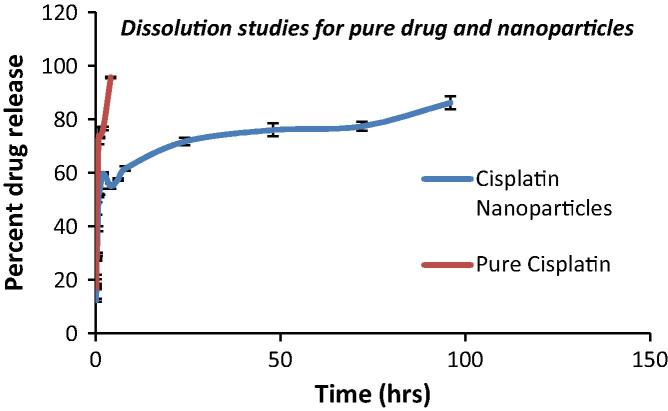
Pure Cisplatin exhibited complete release within 2 h due to its short half life. Drug release for the nanoparticulate formulation depicted a biphasic pattern wherein the nanoparticles exhibited a sustained release pattern for more than 48 h. It was observed that around 30% of the drug released within first 2 h followed by sustained release of Cisplatin from polymer matrix. These results suggest that the cytotoxicity of Cisplatin would be reduced due to slow release of the drug from the nanoparticles with concomitant reduction in frequency of dosing and increase in patient compliance. The results for the dissolution studies are as depicted in Fig. 3.
Figure 3.
Comparative in-vitro dissolution profiles of pure drug and nanoparticles.
3.5. In-vitro studies on B16F10 cell line
3.5.1. Developed nanoparticles exert cytotoxicity & inhibit the proliferation of B16F10 cells
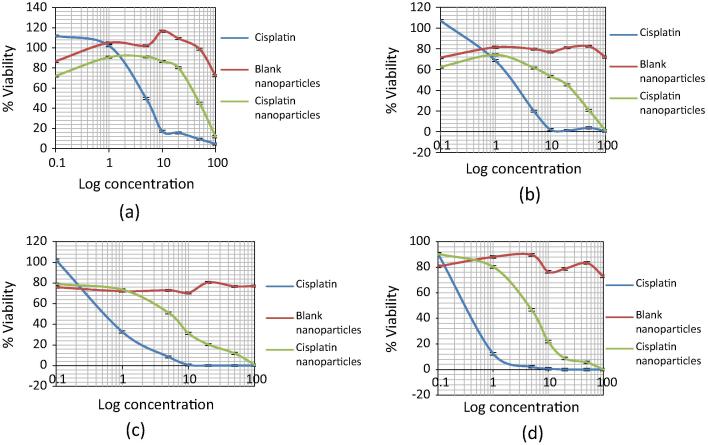
MTT assay was performed to determine the B16F10 cell viability after treatment with the plain drug as well as drug loaded nanoparticles based on time and concentration. The IC50 values for Cisplatin after 24, 48, 72 and 96 h were found to be 5 μg/ml, 2 μg/ml, 0.5 μg/ml and 0.4 μg/ml respectively. Hence it was seen that Cisplatin was able to hamper the cell growth in a time and concentration dependent manner wherein almost 100% of the cells were killed after 96 h. IC50 values for Cisplatin loaded nanocarriers after 24, 48, 72 and 96 h were found to be 45 μg/ml, 15 μg/ml, 5 μg/ml and 4 μg/ml respectively. Developed nanoparticles were able to inhibit the cell proliferation slowly over 96 h time period due to the slow release of Cisplatin from the albumin nanoparticles as exemplified from the dissolution profile. Also, the blank nanoparticle formulation presented an IC50 value greater than 1000 μg/ml in B16F10 cells, proving the safety of formulation excipients and absence of any background toxicity. The results for MTT assay are as presented in Fig. 4. Based on the results of MTT assay, the sub-toxic IC20 and IC50 concentrations of Cisplatin and Cisplatin albumin nanoparticles were selected for further studies and are as shown in Table 2.
Figure 4.
Effects of Cisplatin and Cisplatin albumin nanoparticles on B16F10 cell viability (a) 24 h viability, (b) 48 h viability, (c) 72 h viability and (d) 96 h viability.
Table 2.
Inhibitory concentrations (24 h) for developed formulation and Cisplatin as calculated from MTT assay.
| Formulation | IC20 (μg/ml) | IC50 (μg/ml) |
|---|---|---|
| Cisplatin | 1.3 | 5 |
| Cisplatin albumin nanoparticles | 14 | 45 |
3.5.2. Nanoparticles hamper the formation of colonies in B16F10 melanoma
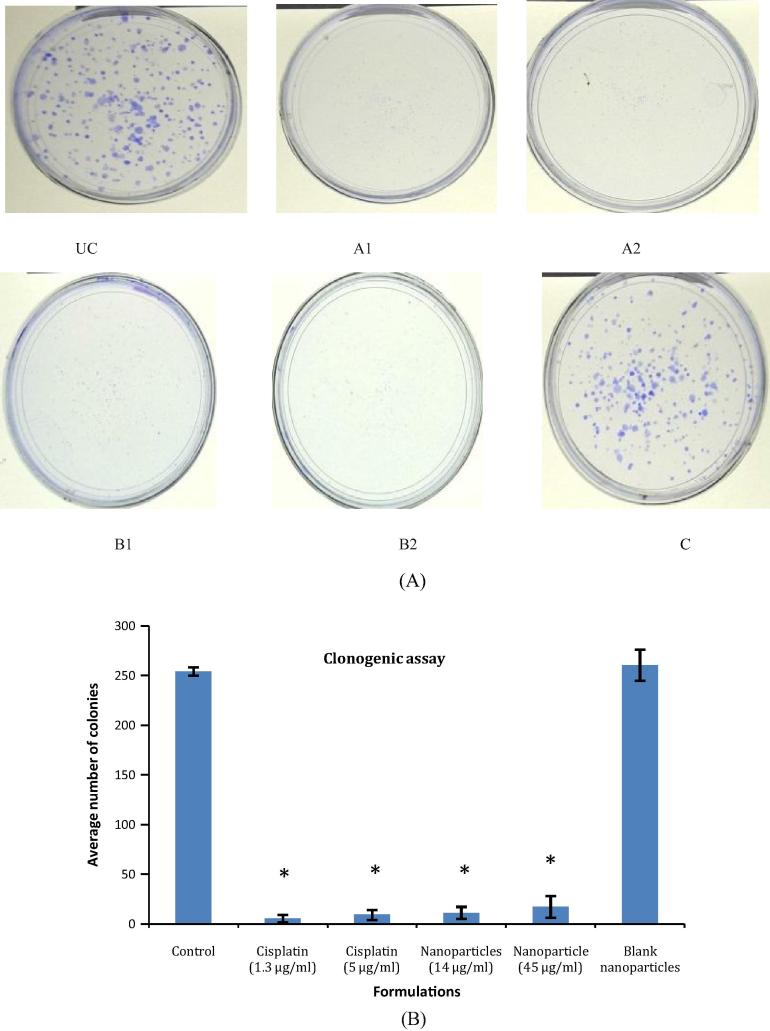
Cancerous cells are prone to formation of colonies form a single cell due to their inherent property of uncontrolled division and proliferation (Franken et al., 2006). In the present work, clonogenic assay was performed at IC20 and IC50 concentrations for the drug as well as formulations. A concentration dependent decrease in the formation of colonies was observed for both i.e. Cisplatin and Cisplatin loaded nanoparticles. Interestingly, the numbers of colonies were found to be less than 50 in number for the above groups. Hence, the nanoparticle formulation was comparable in terms of efficacy with Cisplatin in inhibiting the colony formation. The number of colonies in the blank formulation was found to be 261 ± 15.71, similar to the untreated control i.e. 254.66 ± 4.16. The results for clonogenic assay are as given in Fig. 5.
Figure 5.
(A) Pictographic representation of the clonogenic assay UC: untreated control, A: pure Cisplatin, B: Cisplatin albumin nanoparticles, C: blank albumin nanoparticles, 1: IC20 concentration, 2: IC50 concentration and (B) comparative evaluation of the reduction in the number of colonies for the treatment groups in comparison with control (∗P < 0.001).
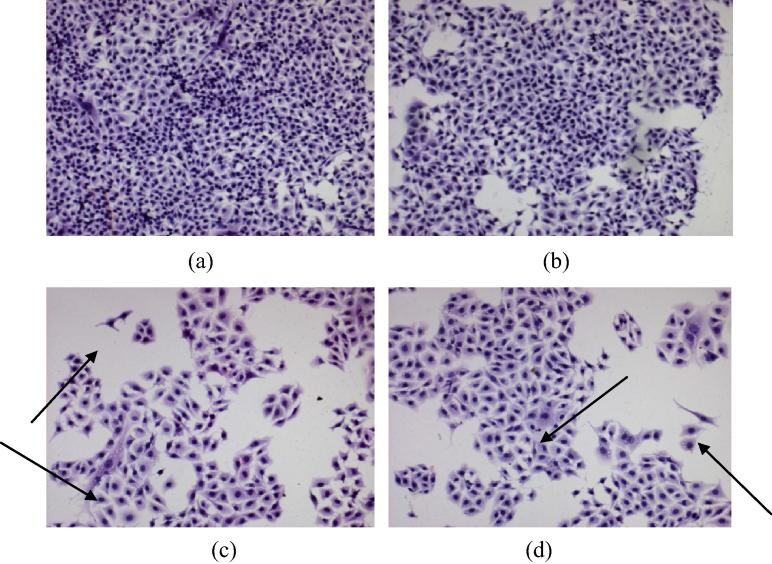
3.5.3. Nanoparticles inhibit cellular migration in wound scratch assay
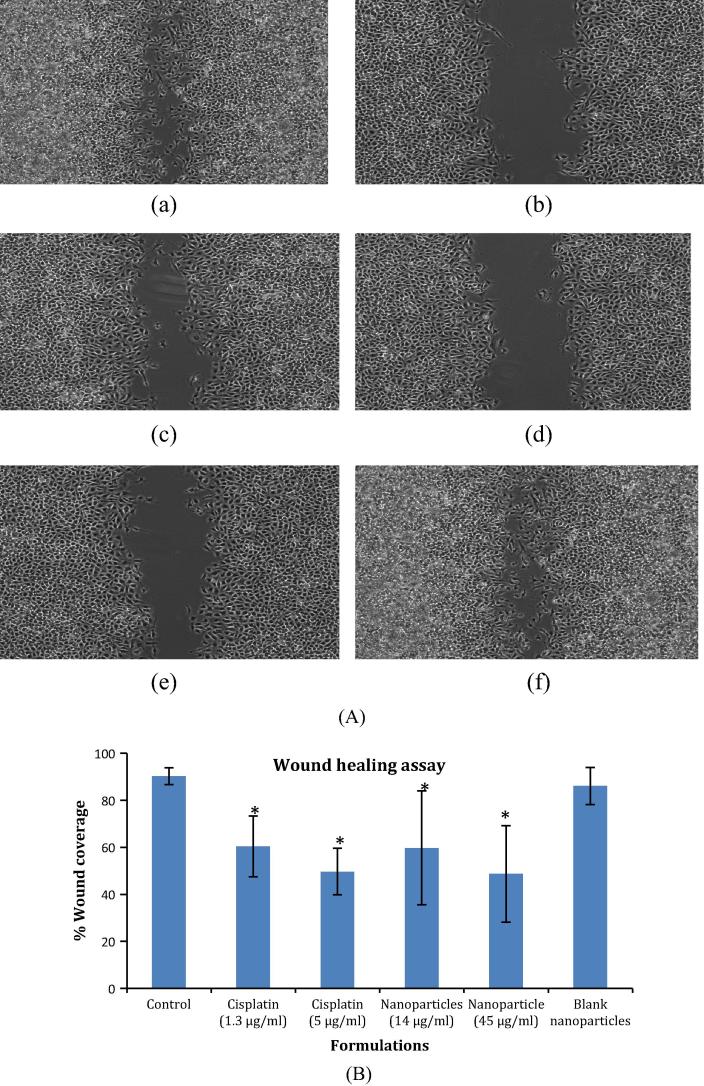
Cellular migration is an important phenomenon in cancer progression since the metastasis of tumor occurs in this manner. In the present study, wound healing assay was performed at IC20 and IC50 concentrations to examine the potential of Cisplatin and the developed nanocarriers to inhibit the above process. For pure Cisplatin, there was no significant difference in the cellular migration at IC20 and IC50 concentrations (79.45% at 1.3 μg/ml and 75.41% at 5 μg/ml respectively). However, the developed nanoparticles effectively reduced the cellular motility at both the sub-toxic levels as compared to Cisplatin alone i.e. the inhibition in cellular migration was 45% at 14 μg/ml and 22.865% at 45 μg/ml for the developed Cisplatin nanoformulation thus emphasizing its superiority over pristine drug for therapy of cancer. Additionally, the blank nanoparticles did not exhibit any reduction in B16F10 cell migration, indicating its non-toxicity and resemblance to untreated control (94.66% migration in comparison with control group). The results for wound scratch assay are presented in Fig. 6.
Figure 6.
(A) Pictographic representation of effect of pure drug and developed nanoparticles on cellular motility (a) untreated control, (b) pure drug IC20, (c) pure drug IC50, (d) nanoparticles IC20, (e) nanoparticles IC50, (f) blank nanoparticles and (B) comparative evaluation of the percent wound coverage for the treatment groups in comparison with control (∗P < 0.001).
3.5.4. Nanoparticles instigate changes in the morphology of B16F10 cells
Leighton tube assay was carried out for the pure drug as well as the drug loaded nanoparticles to assess the potential of nanosystems to cause changes in the cellular morphology in comparison with the pure drug. From Fig. 7, it can be seen that both the drug and drug loaded nanoformulations caused changes in the cell structure, swelling of cells, spindle formation and consequent cell damage with equivalent efficacy. Additionally there is a decrease in the population of B16F10 cells in the treatment groups compared to untreated control. However, blank nanoparticles act parallel to the control group and do not depict any morphological changes.
Figure 7.
Changes in the B16F10 cellular morphology by treatment groups (a) untreated control, (b) blank formulation, (c) Cisplatin treated cells and (d) Cisplatin nanoparticulate formulation.
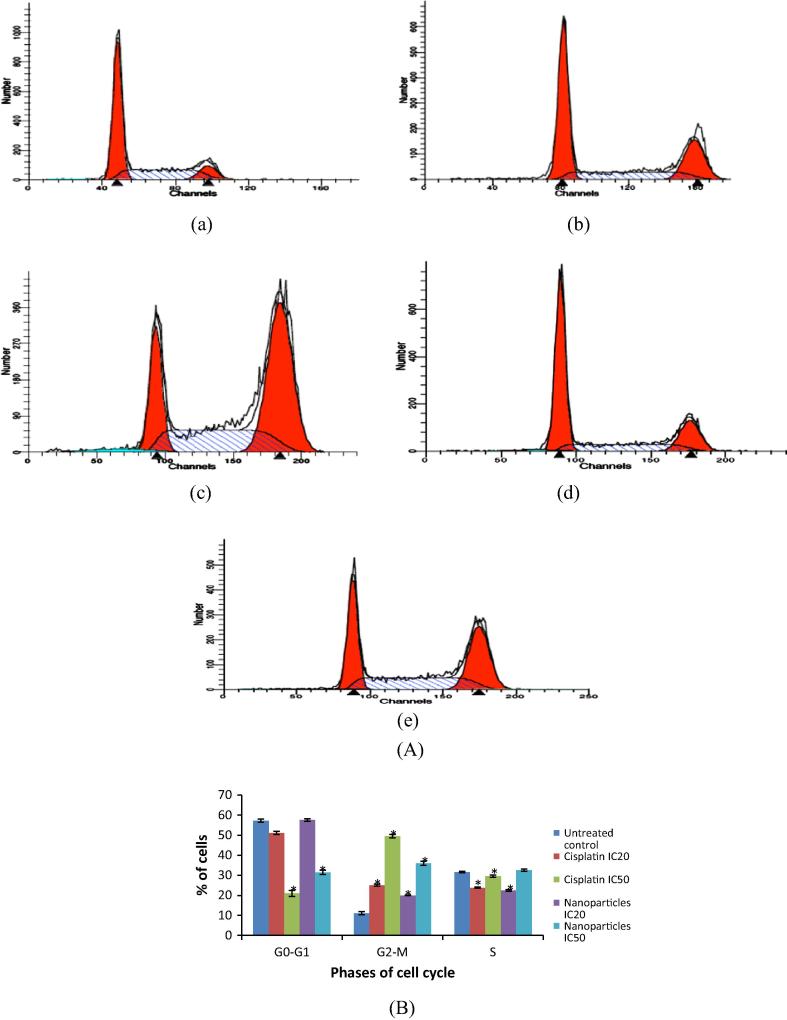
3.5.5. Developed nanoparticles cause cell cycle arrest in B16F10 melanoma
Alkylating agents like Cisplatin are known to cause programmed cell death by inhibiting the cells in G2 phase of cell cycle (Cepeda et al., 2007). These results were reinstated by the present study wherein dose dependent increase in Cisplatin induced G2/M phase arrest was seen in B16F10 melanoma (Fig. 8). The developed nanoparticles also exhibited dose dependent increase in the G2/M phase leading to cell death. The percentage of cells in the G2/M phase was found to be less for nanoparticles than the pure drug group probably because of the sustained release of drug from nanoparticles, leading to slower rate of action. Additionally, the concentration of nanoparticles was seen to increase in the S phase in nanoparticle formulation IC50 group, suggesting their ability to hinder the production of DNA in S phase of cell cycle in addition to the cell division process. Hence, the developed nanoparticles were found to be superior to pure drug by acting on different phases of cell cycle.
Figure 8.
(A) Cell cycle analysis by Flow cytometry (a) untreated control, (b) Cisplatin IC20, (c) Cisplatin IC50, (d) nanoparticles IC20, (e) nanoparticles IC50 and (B) comparative evaluation of the percentage of cells after treatment in different cell cycle phases for the treatment groups in comparison with control (∗P < 0.001).
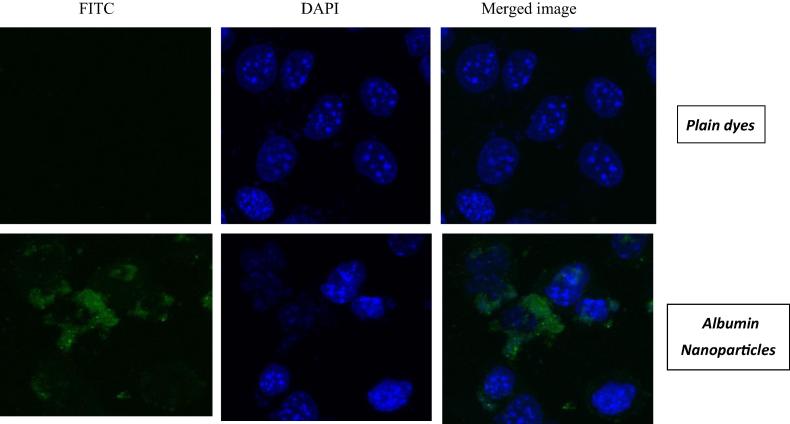
3.5.6. Nanoparticles are uptaken effectively by B16F10 cells in confocal microscopy
In this study, the nucleus of the cells was stained using DAPI and FITC just surrounded the cells in the overlay images. FITC per se is not able to transverse the cells due to its hydrophilic nature (Fig. 9). However, when the nanoparticles were loaded with this particular dye, fluorescence was observed in the B16F10 cells indicating that nanoparticles are able to enhance the penetration of FITC in the cancer cells. These results indicate that the nanoparticles are able to modulate the properties of antineoplastic drugs and enhance their uptake in cancer cells.
Figure 9.
Qualitative cell uptake by confocal microscopy.
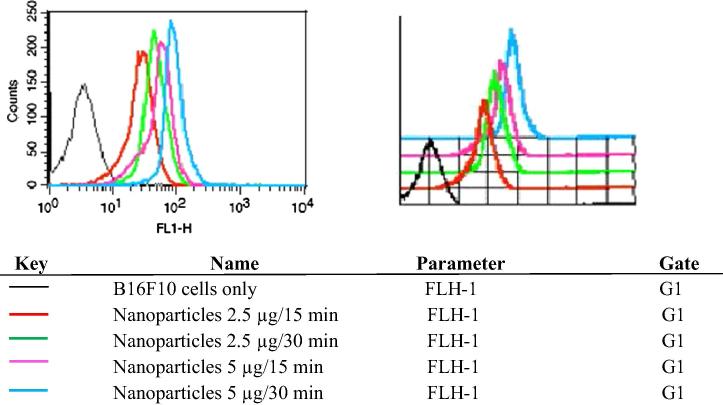
3.5.7. Nanoparticles show time and concentration dependent uptake in B16F10 cells in flow cytometry studies
Flow cytometry analysis was performed to evaluate the uptake of developed nanoparticles in B16F10 cells (Fig. 10). It was observed that albumin nanoformulations were uptaken in the B16F10 cells in a time and concentration dependent manner. MFI of the albumin nanoparticles at a concentration of 2.5 μg/ml for 15 min was found to be 28.67 units, which was enhanced to 47.89 units after 30 min. Similarly, the MFI was 53.79 units and 91.26 units for similar time points at a concentration of 5 μg/ml. Hence, the developed nanoformulations were readily taken up by the melanoma cells and would prove effective when used in the remedy of melanoma.
Figure 10.
Quantitative cellular uptake by flow cytometry.
4. Conclusion
Cisplatin, although a well known moiety for melanoma treatment, is associated with side effects during treatment and exhibits less activity against the same. Formulation of Cisplatin in the form of nanoparticles is expected to cause an improvement in its activity. Hence, the present work was undertaken to compare the efficacy of Cisplatin with its polymeric nanoparticle formulation in B16F10 melanoma. The developed nanoparticles exhibited a sustained release action in MTT assay suggesting great advantage over otherwise short acting Cisplatin. These results are also exemplified in clonogenic and wound scratch assays. Developed nanoparticles could cause a change in the morphology of cancerous cell lines similar to the pure drug. In cell cycle analysis, these nanocarriers proved to be superior to Cisplatin by causing cell cycle arrest in S phase in addition to G2/M phase along with superior cellular uptake as seen from qualitative and quantitative studies. Hence, this study paves a way for therapy of melanoma by formulating Cisplatin in novel drug delivery systems like nanoparticles.
Acknowledgment
The authors are thankful to Cipla Pvt Ltd, India for funding the research project. We also acknowledge SAIF, IIT, Mumbai for TEM analysis and ACTREC, Navi Mumbai for their help in ultracentrifugation.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Shruti S. Shrikhande, Email: shrutishrikhande@gmail.com.
Darshana S. Jain, Email: darshanaj_cup@yahoo.com.
Rajani B. Athawale, Email: rajani.athawale@gmail.com.
Amrita N. Bajaj, Email: bajajamrita@gmail.com.
Peeyush Goel, Email: peeyushgoel29@gmail.com.
Zahid Kamran, Email: zbiotech@gmail.com.
Yuvraj Nikam, Email: ymnikam@rediffmail.com.
Rajiv Gude, Email: rgude@actrec.gov.in.
References
- Abdelwahed W., Degobert G., Fessi H. Investigation of nanocapsules stabilization by amorphous excipients during freeze-drying and storage. Eur. J. Pharm. Biopharm. 2006;63:87–94. doi: 10.1016/j.ejpb.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Cancer Facts and Figures, 2012. Atlanta: American Cancer Society. <http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acs c-031941.pdf> (accessed on 19.12.12).
- Cavallaro U., Christofori G. Cell adhesion in tumor invasion and metastasis: loss of the glue is not enough. Biochim. Biophys. Acta. 2001;1552:39–45. doi: 10.1016/s0304-419x(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Cepeda V., Fuertes M.A., Castilla J., Alonso G., Quevedo C., Perez J.M. Biochemical mechanisms of cisplatin cytotoxicity. Anti-Cancer Agents Med. Chem. 2007;7:3–18. doi: 10.2174/187152007779314044. [DOI] [PubMed] [Google Scholar]
- Del Prete S.A., Maurer L.H., O’Donnell J. Combination chemotherapy with cisplatin, carmustine, dacarbazine, and tamoxifen in metastatic melanoma. Cancer Treat. Rep. 1984;68:1403–1405. [PubMed] [Google Scholar]
- Drugs for Melanoma. National Cancer Institute at the National Institutes of Health. <http://www.cancer.gov/cancertopics/druginfo/melanoma> (Accessed 17.02.13).
- Dua P., Gude R.P. Antiproliferative and anti-proteolytic activity of pentoxifylline in cultures of B16f10 melanoma cells. Cancer Chemother. Pharmacol. 2006;58:195–202. doi: 10.1007/s00280-005-0155-8. [DOI] [PubMed] [Google Scholar]
- Dua P., Gude R.P. Pentoxifylline impedes migration in B16F10 melanoma by modulating Rho GTPase activity and actin organization. Eur. J. Cancer. 2008;44:1587–1595. doi: 10.1016/j.ejca.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Fidler I.J., Hart I.R. Biologic diversity in metastatic neoplasms-origins and implications. Science. 1982;217:998–1001. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- Floria A.-M., Busselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers. 2011;3:1351–1371. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken N.A., Rodermond H.M., Stap J., Haveman J., van Bree C. Clonogenic assay of cells in-vitro. Nat. Protoc. 2006;1(5):2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- Glover D., Glick J.H., Weiler C. WR-2721 and high-dose cisplatin: an active combination in the treatment of metastatic melanoma. J. Clin. Oncol. 1987;5:574–578. doi: 10.1200/JCO.1987.5.4.574. [DOI] [PubMed] [Google Scholar]
- Golla E.D., Ayres G.H. Spectrophotometric determination of platinum with o-phenylenediamine. Talanta. 1973;20:199–210. doi: 10.1016/0039-9140(73)80267-x. [DOI] [PubMed] [Google Scholar]
- Ivanov A.I., Christoduolou J., Parkinson J.A., Burnham K.J., Tucker A., Woodrow J., Sadler P.J. Cisplatin binding sites on human albumin. J. Biol. Chem. 1998;273:14721–14730. doi: 10.1074/jbc.273.24.14721. [DOI] [PubMed] [Google Scholar]
- Jain D.S., Athawale R.B., Bajaj A.N., Shrikhande S.S., Goel P.N., Nikam Y., Gude R.P. Poly lactic acid (PLA) nanoparticles sustain the cytotoxic action of temozolamide in C6 Glioma cells. Biomed. Aging Path. 2013;3(4):201–208. [Google Scholar]
- Kreuter J. Nanoparticles. In: Kreuter J., editor. Colloidal Drug Delivery Systems. Marcel Dekker; New York: 1994. pp. 219–342. [Google Scholar]
- Langer K., Anhorn M.G., Steinhauser I., Dreis S., Celebi D., Schrickel N., Faust S., Vogel V. Human serum albumin (HSA) nanoparticles: reproducibility of preparation process and kinetics of enzymatic degradation. Int. J. Pharm. 2008;347(1–2):109–117. doi: 10.1016/j.ijpharm.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Lee K.J., Kim J.Y., Choi J.H., Kim H.G., Chung Y.C., Roh S.H. Inhibition of tumor invasion and metastasis by aqueous extract of the radix of Platycodon grandiflorum. Food Chem. Toxicol. 2006;44:1890–1896. doi: 10.1016/j.fct.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Shenoy V.S., Gude R.P., Murthy R.S.R. In-vitro anticancer evaluation of 5-fluorouracil lipid nanoparticles using B16F10 melanoma cell lines. Int. Nano Lett. 2013;3:1–9. [Google Scholar]