Abstract
Renal cell carcinoma (RCC) is common with more than 60,000 new cases in the United States yearly. No curative therapies are available for metastatic RCC. Improved methods of imaging metastatic RCC would be of value in identifying sites of occult disease and potentially for judging response to therapy. A 58-year-old male with known metastatic clear cell RCC was imaged with both 18F-FDG and 18F-DCFPyL PET/CT. 18F-DCFPyL is a small molecule inhibitor of the prostate-specific membrane antigen (PSMA), a target known to be highly expressed on solid tumor neovasculature. Relative to 18F-FDG, 18F-DCFPyL identified more lesions and demonstrated higher tumor radiotracer uptake.
Keywords: Metastatic renal cell carcinoma, prostate-specific membrane antigen (PSMA), PET/CT
Figure 1.
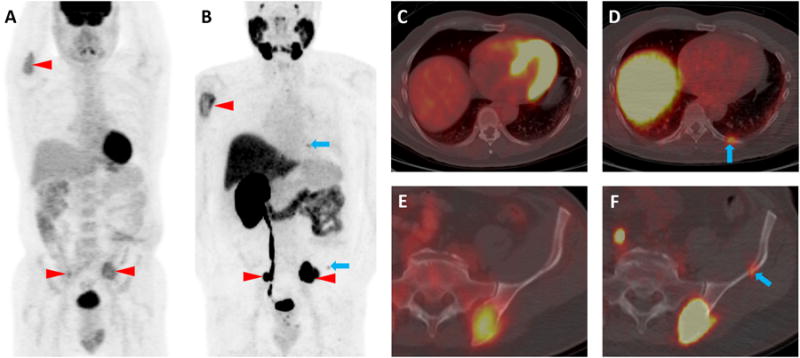
A 58-year-old male patient with known clear cell RCC metastases to the skeleton was imaged contemporaneously with two PET/CT examinations, one with 18F-FDG and a second with 18F-DCFPyL, a small molecule inhibitor of PSMA. The maximum intensity projection images from the two examinations (18F-FDG, A, and 18F-DCFPyL, B) demonstrate concordance of multiple radiotracer-avid lesions including the proximal right humerus and both iliac bones (red arrowheads). However, additional subtle sites of 18F-DCFPyL uptake are noted that do not have corresponding 18F-FDG uptake (blue arrows). These sites include subtle endosteal scalloping of the left posterior ninth rib and the left iliac bone without accompanying 18F-FDG uptake (C and E, blue arrows). In contrast, the axial 18F-DCFPyL PET/CT images demonstrated moderate radiotracer uptake at these sites (D, SUVmax (lean body mass corrected) 3.2, blue arrow and F, 18F-DCFPyL SUVmax 2.7, blue arrow).
Figure 2.
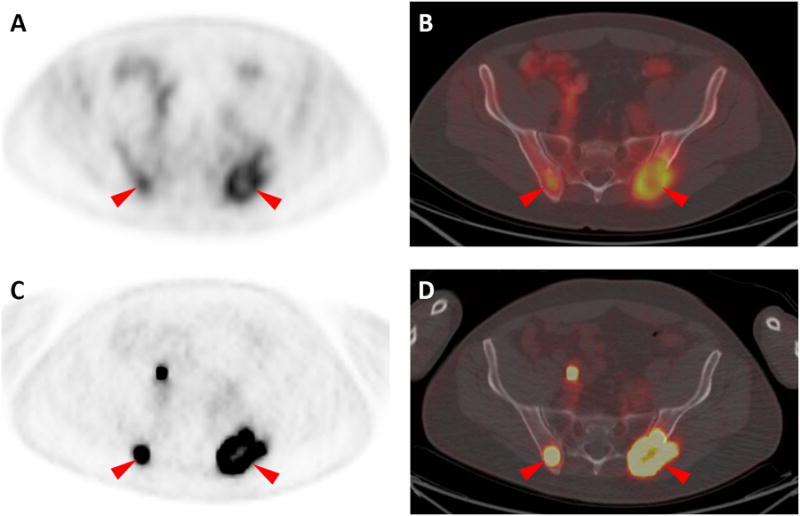
Large lytic lesions were present in the posterior aspects of both iliac bones in this patient, and both sites were found to have intense uptake of both radiotracers (18F-FDG in A and B and 18F-DCFPyL in C and D, red arrowheads). However, 18F-DCFPyL uptake was both visually and quantitatively higher than 18F-FDG. For the right iliac lesion, 18F-FDG uptake yielded SUVmax of 3.3, while for the same lesion 18F-DCFPyL uptake generated SUVmax of 16.6. For the left iliac, 18F-FDG SUVmax was 4.0 while 18F-DCFPyL SUVmax was 13.9. In aggregate, our findings in this patient with metastatic clear cell RCC are suggestive that 18F-DCFPyL may able to identify more lesions and has higher tumor uptake than 18F-FDG. Although a significant body of work has examined the role of 18F-DCFPyL and other small molecule inhibitors targeted against PSMA in the detection of prostate cancer [1-4], the use of such radiotracers for non-prostate applications has been limited to date [5, 6]. This is despite the fact that PSMA is highly expressed on the tumor neovasculature of many solid tumors, including RCC [7, 8]. Indeed, a previous case report has demonstrated the ability of a 68Ga-labeled small molecule inhibitor of PSMA (68Ga-PSMA) to identify sites of disease in a patient with metastatic clear cell RCC [5]. In that report, the authors noted concordance between 18F-FDG and 68Ga-PSMA uptake for all described lesions, though the 68Ga-PSMA PET acquisition was notable for improved lesion conspicuity. In combination with the earlier findings utilizing 68Ga-PSMA, this report confirms that further study with PSMA radiotracers in metastatic RCC is warranted.
Acknowledgments
Funding: Buerger Family Scholarship Fund, EB006351, CA134675, CA184288, CA103175, CA183031.
Footnotes
Disclosures: No conflicts of interest to disclose by any of the authors.
References
- 1.Cho SY, Gage KL, Mease RC, et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J Nucl Med. 2012;53:1883–1891. doi: 10.2967/jnumed.112.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eiber M, Maurer T, Souvatglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:688–674. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 3.Szabo Z, Mena E, Rowe SP, et al. Initial evaluation of [18F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015 doi: 10.1007/s11307-015-0850-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowe SP, Gage KL, Faraj SF, et al. 18F-DCFBC PET/CT for PSMA-based detection and characterization of primary prostate cancer. J Nucl Med. 2015 doi: 10.2967/jnumed.115.154336. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demirci E, Ocak M, Kabasakal L, et al. 68Ga-PSMA PET/CT imaging of metastatic clear cell renal cell carcinoma. Eur J Nucl Med Mol Imaging. 2014;41:1461–1462. doi: 10.1007/s00259-014-2766-y. [DOI] [PubMed] [Google Scholar]
- 6.Sathekge M, Modiselle M, Vorster M, et al. 68Ga-PSMA imaging of metastatic breast cancer. Eur J Nucl Med Mol Imaging. 2015 doi: 10.1007/s00259-015-3066-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Chang SS, O'Keefe DS, Bacich DJ, Reuter VE, Heston WD, Gaudin PB. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin Cancer Res. 1999;5:2674–2681. [PubMed] [Google Scholar]
- 8.Baccala A, Sercia L, Li J, Heston W, Zhou M. Expression of prostate-specific membrane antigen in tumor-associated neovasculature of renal neoplasms. Urology. 2007;70:385–390. doi: 10.1016/j.urology.2007.03.025. [DOI] [PubMed] [Google Scholar]


