Abstract
Treatment of patients with newly diagnosed primary CNS lymphomas using high dose methotrexate regimens is reported to yield about 30% long term survivors with minimal neurotoxicity. As in other systemic large cell lymphomas, it is generally assumed that most relapses occur within 5 years of diagnosis. A retrospective review of the Johns Hopkins experience in 52 patients treated between 1995 and 2008 yielded 19 patients (37%) who achieved a complete response and were followed for over 5 years. Four of these patients remained progression-free for over 10 years. However, two of these long-term survivors have now relapsed over 10 years after their initial diagnosis. An analysis of progression and overall survival does not reveal a plateau suggesting that even patients who have not recurred for over 10 years remain at high risk for relapse after treatment with single agent high dose methotrexate.
PRACTICE POINTS .
High-dose methotrexate monotherapy (HD-MTX) is reported to provide 30% long-term survival in patients with newly diagnosed primary central nervous system lymphoma (PCNSL).
This report describes first relapses occurring over 10 years after initial diagnosis of PCNSL in patients achieving a durable complete response with HD-MTX.
Overall and progression-free survival curves in this population fail to demonstrate an obvious plateau in patients achieving durable complete responses with this therapy.
Retreatment of patients with late relapse yields additional complete responses.
Long term monitoring of patients in complete response is needed as are improved therapeutic approaches.
Background
Primary central nervous system lymphoma (PCNSL) constitutes about 3% of all newly diagnosed primary brain tumors and a similar percent of all lymphomas [1–3]. Treatment of this rare cancer has dramatically changed since the 1970s when whole brain radiation provided an overall median survival of 12–24 months with few long term survivors and significant neurotoxicity [4–8]. High dose-methotrexate (HD-MTX) without radiation is reported to yield excellent response rates and over 30% of patients live disease free for over 5 years with minimal neurotoxicity [9–11]. As with other large cell lymphomas, patients with PCNSL are frequently considered ‘cured’ if they remain progression-free beyond 5 years [8,12,13]. In this paper we report first recurrences of PCNSL that occurred in patients more than 10 years after their initial treatment with HD-MTX monotherapy.
Methods
This retrospective study was approved by the Institutional Review Board of the Johns Hopkins Medical Institutions. Patients with PCNSL diagnosed between 1995 and 2008 who were treated at Johns Hopkins with HD-MTX monotherapy to a radiographically confirmed complete response (CR) were identified using the Johns Hopkins Comprehensive Cancer Center’s tumor registry. Other eligibility criteria included age over 18 years and biopsy proven diffuse large B-cell lymphoma. Patients who were HIV positive or otherwise immunocompromised, had evidence of systemic lymphoma, received other concurrent chemotherapeutic agents or radiation, or were followed for less than 5 years were excluded from this analysis. Measurable contrast enhancing disease was present on MRI at diagnosis in all cases and CR was defined as disappearance of all contrast enhancing lesions. Relapse was defined as reappearance of measurable enhancing areas on brain MRI that were attributed to PCNSL. All imaging studies were reviewed centrally by a radiologist (D Bonekamp) to determine the duration of CR. Kaplan–Meier curves were generated to evaluate progression-free survival and overall survival outcomes for the 19 patients in this analysis.
Treatment: The standard regimen for patients with newly diagnosed PCNSL at Johns Hopkins Hospital during this time period consisted of HD-MTX (8 g/m2) administered every 2 weeks until CR, two additional biweekly cycles and then monthly HD-MTX treatments to complete one year of therapy as described in previous published reports [9].
Results
• Patients & outcomes
Nineteen of the 52 patients (37%) who received HD-MTX monotherapy during the period between 1995 and 2008 met the criteria described above to be included in this analysis (Figure 1). These 10 males and nine females had a median age of 57 years (range: 31–79) and received a median of five cycles of HD-MTX (range 1–15 doses) to attain CR. An average of 12 cycles (range: 8–21 cycles) of HD-MTX was administered as part of their initial treatment (Table 1). Seven of these 19 patients (37%) remained in a CR for over 5 years and four of 19 (21%) remained in a CR for over 10 years. None of the four long term survivors had a gross total resection on initial presentation. Two of the four 10-year disease-free survivors (50%) have now presented with seizures and evidence of new intraparenchymal contrast enhancement consistent with a first recurrence of their PCNSL more than 10 years (11.2 and 12.8 years, respectively) after initial diagnosis (Table 2). Two others remain symptom and relapse free at 13.8 and 14.1 years from initial diagnosis, respectively.
Figure 1. . Study schema.
This figure describes the algorithm used to attain the study population. Patients who did not attain CR, received radiation or alternate treatment regimens or with inadequate follow-up, were excluded.
Table 1. . Demographics of patients who attained CR with single agent HD-MTX.
| Patient profile | Median (range), n = 19 |
|---|---|
| Age in years at diagnosis | 57 (31–79) |
| Gender distribution | Male: 10, females: 9 |
| Number of cycles of HD-MTX to CR | 5 (1–15) |
| Number of cycles of HD-MTX at initial treatment | 12 (5–21) |
| Median progression-free survival in years | 3.3 |
CR: Complete remission; HD-MTX: High-dose methotrexate
Table 2. . Information on patients who were relapse-free 10 years after initial diagnosis.
| Age (years) | Sex | Tumor location | HD-MTX cycles to CR (n) | Total cycles (n) | Years to first relapse | Site of first relapse | Treatment at relapse |
|---|---|---|---|---|---|---|---|
| 45 | M | Right temporal | 2 | 15 | Remains in CR at 14 years | - | - |
| 63 | F | Right frontal and right thalamus | 5 | 13 | Remains in CR at 14 years | - | - |
| 33 | M | Left hippocampus/parahippocampal gyrus | 4 | 16 | 11.4 years | Left occipital | HD-MTX+ R |
| 66 | M | Right basal ganglia, left hypothalamus, corpus callosum | 10 | 18 | 12.8 years | Left occipital, corpus callosum | HD-MTX+ R |
HD-MTX: High-dose methotrexate; R: Rituximab
• Case presentations
PCNSL first relapses more than 10 years after their initial diagnosis.
Case 1
This is a 33-year-old man who initially presented with generalized seizures in 2000 and was found to have an enhancing lesion in the left hippocampus and parahippocampal gyrus. Repeated lumbar punctures and systemic work-ups were inconclusive. A stereotactic biopsy confirmed the diagnosis of diffuse large B-cell lymphoma (DLBCL). He attained a CR after four cycles of HD-MTX and subsequently received 16 cycles of HD-MTX in one year without major complications. He remained relapse-free on routine surveillance MRI scans but presented in September 2012 (11.2 years after initial diagnosis) with a generalized seizure. An MRI revealed a new left occipital contrast enhancing lesion which was biopsied and found to be recurrent DLBCL. His systemic work-up was otherwise unremarkable. He was retreated with HD-MTX in combination with rituximab and again attained CR after two cycles. He went on to receive a total of 18 cycles as part of the retreatment and remains disease-free with an excellent performance status 19 months after his recurrence.
Case 2
This is a 66-year-old man who presented with a brief episode of confusion concerning for seizures in 2001. His MRI revealed multifocal enhancing lesions in the right basal ganglia, left hypothalamus and in the splenium of the corpus callosum. His systemic work up was negative and a stereotactic biopsy confirmed DLBCL. He was treated with HD-MTX monotherapy and attained a CR after 10 cycles. He received a total of 18 cycles of HD-MTX over one year and thereafter was closely followed with regular MRI scans and clinical examinations. In March 2013 (12.8 years after initial diagnosis) he presented with another brief episode of confusion and his MRI revealed new intraparenchymal enhancement in the corpus callosum and left occipital lobe consistent with recurrent disease. His lumbar puncture was inconclusive and he refused rebiopsy. Other potential causes were ruled out and he was retreated with HD-MTX in combination with rituximab. He attained a CR after four cycles. His methotrexate was discontinued after six cycles due to impaired renal function and he has remained on monthly rituximab without evidence of progressive disease and with an excellent performance status 13 months after his recurrence.
• Progression-free & overall survival analysis
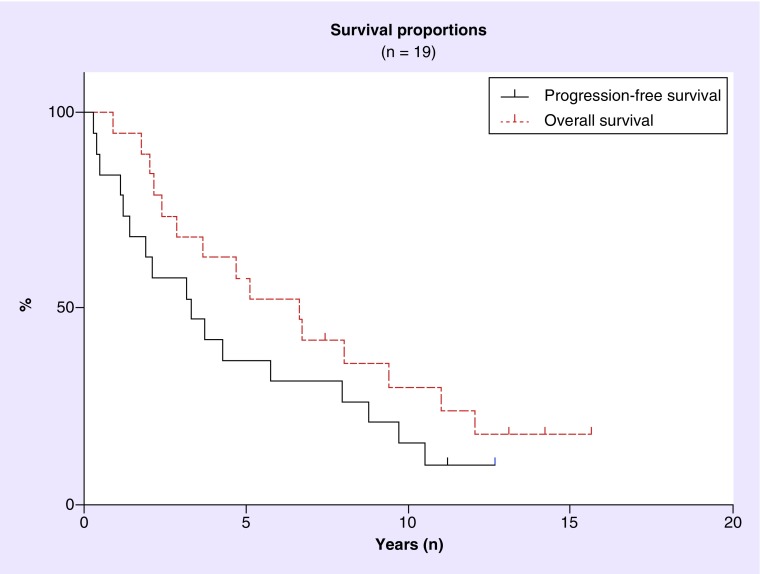
Kaplan–Meier survival curves for progression-free and overall survival are presented in Figure 2. The median progression-free survival was 3.3 years and the median overall survival was 6.6 years in this cohort. These curves demonstrate a distinct lack of a plateau in either progression-free or overall survival. Instead, there is a slow but continuing pattern of relapse and death from progressive disease.
Figure 2. . Kaplan–Meier curve showing progression-free survival and overall survival in 19 patients who attained CR with HD-MTX monotherapy.
Discussion
We report first relapses in two of the four patients who had remained disease-free for greater than 10 years since their original diagnosis and who were thought to be ‘cured’ of their disease after treatment with HD-MTX monotherapy. In systemic diffuse large B cell lymphomas, a distinct plateau in progression-free survival and overall survival is seen in patients who attain CR [14,15]. As a result, it was assumed that similar plateaus in progression-free and overall survival were likely to occur in patients with PCNSL who had been in a complete response for over 5 years [6,10,16]. However, in this report we describe first relapses in two of our four patients (50%) who have remained disease free for over 10 years after treatment with HD-MTX monotherapy. In addition, the Kaplan–Meier progression-free and overall survival curves generated from this patient population strongly suggest that these patients are not being cured of their cancer and remain at very substantial risk of relapse even after they have been in complete response for many years (Figure 1).
It is highly likely that these late relapses represent the same cancer rather than new disease clones. Mono-clonality was evident in one of our patients who underwent biopsy at the time of relapse. In addition, this has been found in prior studies in PCNSL as well as in systemic DLBCL [12,13]. Also of note is that both patients with late relapses rapidly achieved a second complete remission with HD-MTX and rituximab and remain without evidence of recurrence 1.7 and 1.1 years since their relapse.
Our study is limited by its retrospective nature and its relatively small sample size in patients with a rare malignancy. Although it is not surprising that late relapses occur in PCNSL, our results clearly highlight the lack of a plateau in the progression-free and overall survival curves and hence the need for long term surveillance in patients treated with this regimen. Of note, retreatment with high dose methotrexate and rituximab was well tolerated and resulted in complete remissions in these two patients with late relapses. This report also emphasizes the need to be cautious regarding statements relating to ‘cure’ in this disease without follow-up of a decade or more as the timing of relapses appears to be different than in systemic DLBCL. Finally, our data strongly suggest that better therapies are needed for this disease. Current studies are evaluating the use of rituximab, novel agents and treatment schedules, low dose radiation and autologous hematopoietic stem cell transplantation [10,17–22].
Footnotes
Author contributions
Authorship responsibility criteria and contributions: conception and design: P Ambady, M Holdhoff, D Bonekamp, SA Grossman; acquisition, analysis and interpretation of data: P Ambady, M Holdhoff, F Wong, D Bonekamp, SA Grossman; drafting of manuscript: P Ambady, M Holdhoff, F Wong, D Bonekamp, SA Grossman; intellectual content: P Ambady, M Holdhoff, F Wong, D Bonekamp, SA Grossman; statistical analysis: F Wong; funding: NIH funding from the Johns Hopkins Comprehensive Cancer Center NCI grant # 5P30CA006973) PI: Nelson; administrative, technical, or materials support: none; supervision: none
Financial & competing interests disclosure
NCI grant support for cancer center (NCI: 5P30CA006973, PI: Nelson) was utilized for this manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
- 1.Panageas KS, Elkin EB, Deangelis LM, Ben-Porat L, Abrey LE. Trends in survival from primary central nervous system lymphoma, 1975– 1999: a population-based analysis. Cancer. 2005;104(11):2466–2472. doi: 10.1002/cncr.21481. [DOI] [PubMed] [Google Scholar]
- 2.Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br. J. Cancer. 2011;105(9):1414–1418. doi: 10.1038/bjc.2011.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering cancer center prognostic model. J. Clin. Oncol. 2006;24(36):5711–5715. doi: 10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- 4.Deangelis LM. Neuro-oncology: primary CNS lymphoma treatment – the devil is in the details. Nat. Rev. Neurol. 2015;11(6):314–315. doi: 10.1038/nrneurol.2015.64. [DOI] [PubMed] [Google Scholar]
- 5.Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a Phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11(11):1036–1047. doi: 10.1016/S1470-2045(10)70229-1. [DOI] [PubMed] [Google Scholar]
- 6.Abrey LE, Deangelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J. Clin. Oncol. 1998;16(3):859–863. doi: 10.1200/JCO.1998.16.3.859. [DOI] [PubMed] [Google Scholar]
- 7.Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin’s lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the radiation therapy oncology group (RTOG): RTOG 8315. Int. J. Radiat. Oncol. Biol. Phys. 1992;23(1):9–17. doi: 10.1016/0360-3016(92)90538-s. [DOI] [PubMed] [Google Scholar]
- 8.Hottinger AF, Deangelis LM, Yahalom J, Abrey LE. Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma. Neurology. 2007;69(11):1178–1182. doi: 10.1212/01.wnl.0000276986.19602.c1. [DOI] [PubMed] [Google Scholar]
- 9.Batchelor T, Carson K, O’Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96–07. J. Clin. Oncol. 2003;21(6):1044–1049. doi: 10.1200/JCO.2003.03.036. [DOI] [PubMed] [Google Scholar]
- 10.Herrlinger U, Kuker W, Uhl M, et al. NOA-03 trial of high-dose methotrexate in primary central nervous system lymphoma: final report. Ann. Neurol. 2005;57(6):843–847. doi: 10.1002/ana.20495. [DOI] [PubMed] [Google Scholar]
- 11.Gerstner ER, Carson KA, Grossman SA, Batchelor TT. Long-term outcome in PCNSL patients treated with high-dose methotrexate and deferred radiation. Neurology. 2008;70(5):401–402. doi: 10.1212/01.wnl.0000300671.37279.0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Jong D, Glas AM, Boerrigter L, et al. Very late relapse in diffuse large B-cell lymphoma represents clonally related disease and is marked by germinal center cell features. Blood. 2003;102(1):324–327. doi: 10.1182/blood-2002-09-2822. [DOI] [PubMed] [Google Scholar]
- 13.Nayak L, Hedvat C, Rosenblum MK, Abrey LE, Deangelis LM. Late relapse in primary central nervous system lymphoma: clonal persistence. Neuro Oncol. 2011;13(5):525–529. doi: 10.1093/neuonc/nor014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larouche JF, Berger F, Chassagne-Clement C, et al. Lymphoma recurrence 5 years or later following diffuse large B-cell lymphoma: clinical characteristics and outcome. J. Clin. Oncol. 2010;28(12):2094–2100. doi: 10.1200/JCO.2009.24.5860. [DOI] [PubMed] [Google Scholar]
- 15.Moller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation – a population-based study of 1575 cases. Br. J. Haematol. 2004;124(2):151–159. doi: 10.1046/j.1365-2141.2003.04749.x. [DOI] [PubMed] [Google Scholar]
- 16.Pels H, Schmidt-Wolf IG, Glasmacher A, et al. Primary central nervous system lymphoma: results of a pilot and Phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J. Clin. Oncol. 2003;21(24):4489–4495. doi: 10.1200/JCO.2003.04.056. [DOI] [PubMed] [Google Scholar]
- 17.Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (alliance 50202) J. Clin. Oncol. 2013;31(25):3061–3068. doi: 10.1200/JCO.2012.46.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omuro A, Correa DD, Deangelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary cns lymphoma. Blood. 2015;125(9):1403–1410. doi: 10.1182/blood-2014-10-604561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neuwelt EA, Schiff D. Primary CNS lymphoma: a landmark trial and the next steps. Neurology. 2015;84(12):1194–1195. doi: 10.1212/WNL.0000000000001407. [DOI] [PubMed] [Google Scholar]
- 20.Deangelis LM. Whither whole brain radiotherapy for primary CNS lymphoma? Neuro Oncol. 2014;16(8):1032–1034. doi: 10.1093/neuonc/nou122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibamoto Y, Ogino H, Hasegawa M, et al. Results of radiation monotherapy for primary central nervous system lymphoma in the 1990s. Int. J. Radiat. Oncol. Biol. Phys. 2005;62(3):809–813. doi: 10.1016/j.ijrobp.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 22.Shibamoto Y, Sumi M, Onodera S, et al. Primary CNS lymphoma treated with radiotherapy in japan: a survey of patients treated in 2005–2009 and a comparison with those treated in 1985–2004. Int. J. Clin. Oncol. 2014;19(5):963–971. doi: 10.1007/s10147-013-0644-4. [DOI] [PubMed] [Google Scholar]