SUMMARY
This phase I/II study was conducted to determine the maximum tolerated dose, toxicity, and efficacy of clofarabine in combination with high dose cytarabine and granulocyte colony-stimulating factor (G-CSF) priming (GCLAC), in the treatment of patients with relapsed or refractory acute myeloid leukaemia (AML). Dose escalation of clofarabine occurred without dose-limiting toxicity, so most patients were treated at the maximum dose, 25 mg/m2/day with cytarabine 2 g/m2/day, each for 5 days, and G-CSF 5 μg/kg, beginning the day before chemotherapy and continuing daily until neutrophil recovery. The complete remission (CR) rate among the 46 evaluable patients was 46% (95% confidence interval [CI] 31–61%) and the CR + CR but with a platelet count <100 x 109/l rate was 61% (95% CI 45–75%). Multivariate analysis showed that responses to GCLAC were independent of age, cytogenetic risk category, and number of prior salvage regimens. GCLAC is highly active in relapsed and refractory AML and warrants prospective comparison to other regimens, as well as study in untreated patients.
Keywords: Multivariate analysis, salvage therapy, clinical trial phase 1, clinical trial phase 2, remission induction
INTRODUCTION
Although most patients with acute myeloid leukaemia (AML) enter complete remission (CR) with initial therapy, the majority of remissions are transient, typically lasting less than 1–2 years (Pulte et al, 2010). Moreover, patients who never achieve remission (Estey et al, 1996) or relapse within 6 months of attaining CR (Breems et al, 2005) are less likely to respond to any treatment regimen than those who have longer periods of remission. Consequently there has been interest in discovering new therapies for relapsed AML as well as AML that never enters CR (“primary refractory”); we will refer to such therapies as “salvage”. Patients who respond to salvage therapy may then be candidates for allogeneic haematopoietic cell transplant (HCT), the only known potentially curative treatment for relapsed or refractory AML.
A salvage regimen in common use is “FLAG”, which combines fludarabine, cytarabine (ara-C) and granulocyte colony-stimulating factor (G-CSF) priming (Estey et al, 1994; Visani et al, 1994; Estey et al, 1999; Jackson et al, 2001; Carella et al, 2001; Ferrara et al, 2002; Ossenkoppele et al, 2004; Bashey et al, 2006). Clofarabine is structurally related to fludarabine. However it appears to have more anti-AML activity at tolerable doses. As with fludarabine, metabolism of clofarabine to its triphosphate may increase synthesis of the triphosphate of cytarabine (ara-CTP) (Cooper et al, 2005). Formation and retention of ara-CTP may be important in response to ara-C. However unlike fludarabine, clofarabine inhibits ribonucleotide reductase (Parker et al, 1991; Xie & Plunkett, 1996). As a result, fewer deoxynucleotide triphosphates are available to compete with ara-CTP for incorporation into DNA. These observations suggest that combinations of clofarabine with ara-C may be superior to similar combinations of fludarabine. Indeed reports of the former’s effectiveness have appeared (Faderl et al, 2005; Faderl et al, 2006; Faderl et al, 2008). In untreated patients over age 50 years, the combination of clofarabine 40 mg/m2 daily days 2–6 with ara-C 1 g/m2 daily days 1–5 resulted in a CR rate of 52% (Faderl et al, 2006), while a CR rate of 63% was noted in similar patients age 60 years and older following clofarabine 30 mg/m2 for 5 days plus ara-C 20 mg/m2 daily for 14 days by subcutaneous injection (Faderl et al, 2008). Here we report the results of a Phase I/II trial that differed from the former by using (a) a higher dose of ara-C and (b) G-CSF priming. We refer to this regimen as GCLAC.
PATIENTS AND METHODS
Patient Selection
The GCLAC protocol was approved by the Fred Hutchinson Cancer Consortium Institutional Review Board; all patients gave informed consent. The study is listed as Clinical Trials.gov Identifier NCT00602225. Patients with relapsed or refractory AML (acute promyelocytic leukaemia excepted) were eligible if they were aged 18–70 years and had creatinine ≤ 88.4 μmol/l (or glomerular filtration rate > 60 ml/min), total bilirubin <33.34 μmol/l and alanine transaminase, aspartate transaminase, and alkaline phosphatase levels ≤2.5 times the upper limit of normal. The initial 32 patients on study were eligible up to the third salvage for initial treatment or for that relapse with no limit on the number of relapses. The last 18 patients were restricted to first salvage only.
Treatment with GCLAC
Patients began G-CSF, 5 μg/kg daily, rounded to the nearest vial size, by subcutaneous injection one day prior to chemotherapy, and received G-CSF daily until the absolute neutrophil count (ANC) rose to at least 2.0 x 109/l for two consecutive days. Clofarabine was administered intravenously over one hour, daily for five days at 15, 20, and 25 mg/m2. Ara-C 2 g/m2 was given over 2 h for five days, beginning 4 h after the start of the clofarabine infusion.
Response was initially evaluated by bone marrow 14 days after the start of GCLAC. Patients with > 5% blasts had a repeat bone marrow at day 21 and received a 2nd course if the blast count had not improved. CR was conventionally defined (Cheson et al, 2003), and CRp was defined by the same criteria as CR but with a platelet count <100 x 109/l (Faderl et al 2005). Patients who progressed with the first course (marked increase in the percentage of of blasts in a cellular marrow compared to pre-treatment marrow), or who did not achieve CR or CRp after a second course were removed from study. Patients in CR received GCLAC consolidation with each dose of clofarabine 5 mg/m2 less than their induction dose administered over one hour daily for five days, with ara-C given at 1 g/m2 daily, beginning 4 h after the start of clofarabine, over 2 h daily for 5 days. G-CSF was given in identical fashion as during induction. Fourteen patients received 1 cycle of consolidation and four received 2 cycles of consolidation.
Using National Cancer Institute Common Terminology Criteria for Adverse Events v3.0, (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf) dose-limiting toxicity was defined as grade 3 or higher toxicity of the lungs, heart, bladder, kidneys, or central nervous system, or grade 4 toxicity of the liver, skin, oropharynx and gastrointestinal tract, occurring within 21 days of start of therapy. Exceptions were grade 3 lung toxicity if probably due to infection, grade 3 or higher skin rash or anorexia, transient elevation of hepatic transaminases or alkaline phosphatase, and grade 3 or higher nausea, vomiting, diarrhoea or mucositis that resolved to < grade 3 within 48 h of onset.
Statistical analysis
We used a 3+3 Phase 1 design to identify the clofarabine maximum tolerated dose (MTD), with a plan for a Phase 2 expansion at the maximal tolerated dose. An amendment was approved to further expand the original Phase 2 cohort from 13 to 31 for the explicit purpose of obtaining more data in first salvage patients, as this group has been previously studied in regard to features predictive of response to other regimens. Table I lists the pre-treatment characteristics of the enrolled patients.
Table I.
Characteristics of patients enrolled on study. Definition of cytogenetic risk: Favourable: inv16, t(8;21); Intermediate: trisomy 8, normal, −Y, +6, −12p; Unfavourable: −5/−5q, −7/−7q, abnormal 3q, 9q, 17p, 20q, 21q, t(6;9), t(9;22), complex karyotype (Slovak et al 2000) [Note: t(15;17) patients were excluded from study.] AML, acute myeloid leukaemia; CR, complete remission
| GCLAC
Patients N=50 |
|||
|---|---|---|---|
| Number | % | ||
| Sex | Female | 14 | 28% |
| Male | 36 | 72% | |
|
| |||
| AML Onset | De novo | 32 | 64% |
| Secondary | 18 | 36% | |
|
| |||
| Relapsed (median first CR duration) | 32 (26 wk) | 60% | |
| First salvage | 32 | 64% | |
| Second or greater salvage | 18 | 36% | |
| Refractory | 18 | 36% | |
| Cytogenetics (at initial diagnosis) | |||
| Favourable | 3 | 6% | |
| Intermediate | 27 | 54% | |
| Unfavourable | 20 | 40% | |
|
| |||
| Median | Range | ||
|
| |||
| Age (years) | 53 | 19–69 | |
The principal efficacy outcomes of interest were response and survival. Criteria for CR and CRp were as typically specified (Cheson et al, 2003). Survival was calculated from the start of salvage therapy to death from any cause or to time of last follow up. Survival probabilities were estimated using the method of Kaplan and Meier. Cumulative incidence was calculated for overall mortality and for mortality prior to transplant, treating transplant as a competing event. Differences in binary variables, including CR or CRp, were assessed with the Fisher exact test and differences in continuous variables with the Kruskal-Wallis test.
Univariate and multivariate analyses were used to evaluate prognostic factors affecting CR and survival. Logistic regression was used for CR analysis and Cox regression for analysis of survival. Covariates examined included age, cytogenetics [with cytogenetic risk defined by Southwest Oncology Group criteria (Slovak et al, 2000)], duration of first CR (CR1), number of salvage regimens, and secondary vs de novo leukaemia. All p-values are 2-sided and were considered significant when <0.05.
RESULTS
Fifty patients were treated (Table I). Their median age was 53 years. Sixty-four percent had relapsed after a median CR duration of 26 weeks and 36% had failed initial induction therapy. Befitting these characteristics, only 6% had favourable cytogenetics.
Dose finding
During the Phase 1 part of the trial daily X 5 clofarabine doses were 15 mg/m2, 20 mg/m2 and 25 mg/m2 in 9,7, and 3 patients, respectively. More than 6 patients each received the 15 and 20 mg/m2 doses because 2 patients who had received an allogeneic HCT and were on immunosuppressive therapy for graft-versus-host disease (GVHD) prior to treatment with GCLAC had fatal, multi-organ toxicity and infections at these levels. After Institutional Review Board approval, we subsequently excluded such patients from participation and added extra patients. Considering only patients who had not received an allogeneic HCT and who were not on immunosuppressive therapy, we did not observe dose limiting toxicity at any dose. Because we had seen no suggestion of a relationship between dose and response (below and Table II), we decided to forego further dose escalation and treated subsequent patients at 25 mg/m2. Ultimately 34 patients received this dose in either phase 1 or 2.
Table II.
Response Rates by Cytogenetic Risk Category or Clofarabine Dose
| Cytogenetic Risk | Complete Remissions |
|---|---|
| Favourable | 2/3 |
| Intermediate | 10/25 |
| Unfavourable | 9/18 |
| Cytogenetic Risk | Clofarabine dose | ||
|---|---|---|---|
| 15 mg/m2 | 20 mg/m2 | 25 mg/m2 | |
| Favourable | -- | -- | 2/3 |
| Intermediate | 2/4 | 3/4 | 5+4CRp/17 |
| Unfavourable | 2/4 | 1/2 | 6+3CRp/12 |
CRp, complete remission but with a platelet count <100 x 109/l
Response Rate and Time to Blood Cell Recovery (Tables II, III)
Table III.
Response rates by duration first complete remission (CR1) and salvage number
| CR with GCLAC (21/46) | |
|---|---|
| Duration CR1 (months) | |
| 0 | 12/18 |
| 1–6 | 4/12 |
| >6–12 | 2/11 |
| > 12 | 3/5 |
| Salvage number | |
| 1 | 16/33 |
| 2 | 5/8 |
| ≥3 | 0/5 |
Of the 50 patients, 4 were excluded from analysis of response: the 2 aforementioned patients with GVHD, 1 patient who received only 1 g/m2 ara-C, and 1 patient who did not have a marrow confirming remission status prior to beginning a preparative regimen for allogeneic HCT. CRs were seen in 21 of the 46 evaluable patients (46%; 95% confidence interval (CI) 31–61%) and the CR + CRp rate was 61% (95% CI 45–75%). CR and CRp rates were 49% and 65% among the 43 evaluable patients without prior HCT.
While CR rates were considerably higher among relapsed patients whose first CR durations had exceeded 6 months (3/5 vs. 6/23), patients who had not previously entered CR (12/18) had a CR rate similar to that seen in patients with first CR > 6 months. CR rates appeared to be affected by salvage number (64% first salvage, 36% second or greater salvage) but not by cytogenetics or clofarabine dose; in particular, CR rates in evaluable patients according to clofarabine dose were: 4/8, 4/6, and 13/32 at daily doses of 15, 20, and 25 mg/m2, respectively (p =0.48, Fisher exact test).
The presence of > 5% blasts (by morphology) in a day-14 bone marrow was an unreliable guide to ultimate response. Thus, 12/19 patients who still had >5% blasts at day 14 subsequently achieved CR without a 2nd course, with a day 21 marrow showing <5% blasts in most cases, although in 1 case CR was not observed until 7 weeks from the start of therapy. Nineteen (90%) of the CRs were observed after the first course of therapy. The CR + CRp rate was 4/8 in patients who received a 2nd induction course.
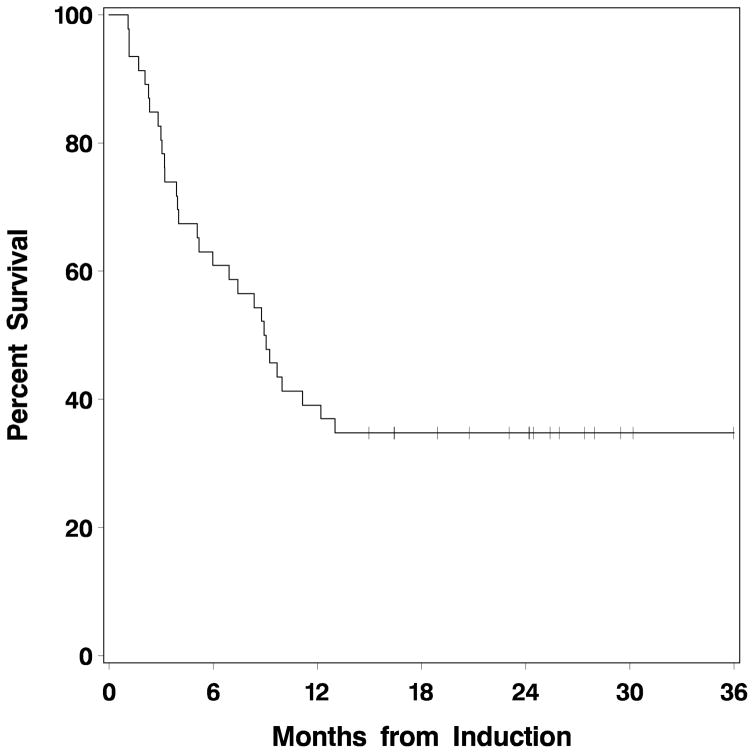
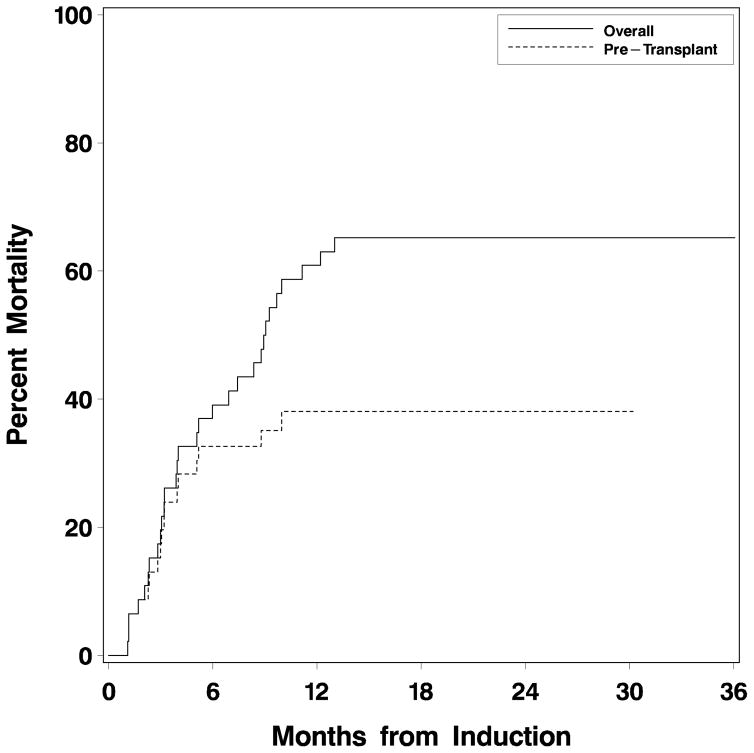
Including only patients whose neutrophil count recovered (41/50 patients), median time to neutrophil recovery (ANC>0.5 x 109/l) after first induction course was 21 days (range 17 to 35), and to platelet recovery (platelet count >100 x 109/l), 30 days (range 22–42). Nine patients did not recover neutrophils >0.5 x 109/l, and 26 did not recover platelet count>100 x 109/l, typically reflecting persistent AML in the marrow. Median times to neutrophil and platelet recovery were 21 days (range 17–27) and 34 days (range 26–73), respectively after the first consolidation course, and 19 (range 18–20) and 40 days (range 29–40) after the second consolidation course. Twenty-five patients underwent allogeneic HCT subsequent to GCLAC treatment, 20 in CR. Median survival for all 50 patients was 9 months (95% CI 5.2–13.0 with 17 patients remaining alive after a median follow up of 1.9 years since beginning GCLAC (Fig 1). Thirteen of these 17 received HCT after treatment with GCLAC. Figure 2 depicts the cumulative mortality occurring prior to transplant, as well as overall mortality. The difference between the two represents mortality occurring after transplant.
Figure 1. Overall survival of patients treated on GCLAC.
The median survival was 9 months (95% confidence interval 5.2 – 13).
Figure 2. Cumulative mortality after GCLAC for patients who had not yet undergone transplant (“pre-transplant”) vs. mortality of all patients, including those who received allogeneic stem cell transplant after GCLAC.
Cumulative mortality was higher after patients had undergone allogeneic stem cell transplant.
Univariate and Multivariate Analysis of Prognostic Factors
Univariate and multivariate analyses (Table IV) were performed to assess the effect of clinical parameters on the complete remission rate and overall survival. There was no significant association of age or cytogenetic risk category with achievement of CR or overall survival. Patients with a CR1 duration of less than 38 weeks had a reduced CR rate (multivariate OR 0.1, 95% CI 0.0–1.0, p=0.05), and higher mortality (multivariate HR 4.4, 95% CI 1.5–13, p=0.007) than those with a CR1 duration greater than or equal to 38 weeks. There was no statistically significant difference between those who never achieved CR and those who had CR1 duration ≥ 38 weeks in either CR rate or mortality. Mortality, but not CR rate, was affected by the prior number of salvage chemotherapy regimens, with higher mortality for patients for whom GCLAC was second salvage or greater. Secondary leukaemia was associated with a lower CR rate (multivariate OR 0.2, 95% CI 0.0–1.1, p=0.06), but not with mortality.
Table IV.
Univariate and Multivariate Analysis of Complete Remission and Overall Mortality.
|
|
Complete Remission |
Mortality |
||||||
|---|---|---|---|---|---|---|---|---|
|
|
Univariate |
Multivariate |
Univariate |
Multivariate |
||||
| Characteristic |
OR (95%CI) |
p |
OR (95%CI) |
p |
HR (95% CI) |
p |
HR (95% CI) |
p |
| Age | ||||||||
| <60 years | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| ≥ 60 years | 2.0 (0.5–8.8) | 0.36 | 2.6 (0.5–15) | 0.29 | 1.8 (0.8–4.0) | 0.14 | 1.8 (0.8–4.1) | 0.18 |
|
| ||||||||
| Cytogenetics | ||||||||
| Good/Intermediate | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Worst | 1.5 (0.4–5.1) | 0.52 | 1.9 (0.4–9.5) | 0.45 | 1.3 (0.6–2.6) | 0.55 | 1.4 (0.6–3.1) | 0.41 |
|
| ||||||||
| Duration of First CR | ||||||||
| ≥ 38 weeks | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 1–37 vs ≥ 38 weeks | 0.2 (0.0–1.0) | 0.04 | 0.1 (0.0–1.0) | 0.05 | 3.4 (1.3–8.9) | 0.01 | 4.4 (1.5–13) | 0.007 |
| 0 | 0.6 (0.1–3.6) | 0.56 | 0.5 (0.0–6.0) | 0.56 | 1.0 (0.3–2.6) | 0.93 | 1.4 (0.4–4.4) | 0.60 |
|
| ||||||||
| Salvage number | ||||||||
| 1st | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 2nd | 0.6 (0.1–2.6) | 0.46 | 0.5 (0.1–3.0) | 0.44 | 3.2 (1.4–7.4) | 0.006 | 3.6 (1.4–9.1) | 0.008 |
| >2nd | 0.1 (0.0–1.2) | 0.07 | 0.1 (0.0–1.6) | 0.09 | 4.9 (1.7–14) | 0.003 | 7.3 (2.2–24) | 0.001 |
| Secondary AML | ||||||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Yes | 0.3 (0.1–0.9) | 0.04 | 0.2 (0.0–1.1) | 0.06 | 1.3 (0.6–2.8) | 0.48 | 1.4 (0.6–3.3) | 0.40 |
| Characteristic |
CR Odds ratio (95% CI)
|
Mortality Hazard ratio
(95%CI) |
Univariate p |
Multivariate p |
||||
|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
CR |
Mortality |
CR |
Mortality |
|
| Age | ||||||||
| <60 years | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| ≥ 60 years | 2.0 (0.5–8.8) | 2.6 (0.5–15) | 1.8 (0.8–4.0) | 1.8 (0.8–4.1) | 0.36 | 0.14 | 0.29 | 0.18 |
|
| ||||||||
| Cytogenetics | ||||||||
| Good/Intermediate | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Worst | 1.5 (0.4–5.1) | 1.9 (0.4–9.5) | 1.3 (0.6–2.6) | 1.4 (0.6–3.1) | 0.52 | 0.55 | 0.45 | 0.41 |
|
| ||||||||
| Duration of First CR | ||||||||
| ≥ 38 weeks | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 1–37 vs ≥ 38 weeks | 0.2 (0.0–1.0) | 0.1 (0.0–1.0) | 3.4 (1.3–8.9) | 4.4 (1.5–13) | 0.04 | 0.01 | 0.05 | 0.007 |
| 0 | 0.6 (0.1–3.6) | 0.5 (0.0–6.0) | 1.0 (0.3–2.6) | 1.4 (0.4–4.4) | 0.56 | 0.93 | 0.56 | 0.60 |
|
| ||||||||
| Salvage number | ||||||||
| 1st | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 2nd | 0.6 (0.1–2.6) | 0.5 (0.1–3.0) | 3.2 (1.4–7.4) | 3.6 (1.4–9.1) | 0.46 | 0.006 | 0.44 | 0.008 |
| >2nd | 0.1 (0.0–1.2) | 0.1 (0.0–1.6) | 4.9 (1.7–14) | 7.3 (2.2–24) | 0.07 | 0.003 | 0.09 | 0.001 |
| Secondary AML | ||||||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Yes | 0.3 (0.1–0.9) | 0.2 (0.0–1.1) | 1.3 (0.6–2.8) | 1.4 (0.6–3.3) | 0.04 | 0.48 | 0.06 | 0.40 |
Adverse Events (Table V)
Table V.
Adverse Events
| Adverse event (CTCAEv3) |
Gr 3 #(%) |
Gr 4 #(%) |
|---|---|---|
| Skin | 5 (10%) | 0 |
| Hepatic transaminases | 5 (10%) | 3 (6%) |
| Pulmonary | 18 (36%) | 5 (10%) |
| Infection | 12 (24%) | 8 (16%) |
| Gastrointestinal | 6 (12%) | 0 |
| Hyperbilirubinaemia | 3 (6%) | 1 (2%) |
| Renal | 2 (4%) | 0 |
| Pain | 1 (2%) | 0 |
| Neuropathy | 1 (2%) | 0 |
| Tumour lysis | 1 (2%) | 0 |
CTCAEv3, Common Terminology Criteria for Adverse Events v3.0
There were 6 treatment-related deaths: 4 after receiving the first and 1 after receiving the second course of induction and 1 after the first consolidation. These occurred 30, 33, 35, and 39 days after 1st induction, 28 days after 2nd induction and 33 days after 1st consolidation. All treatment-related deaths were associated with infection. Three of the deaths occurred in prior allogeneic HCT recipients. The overall treatment related mortality was 12%.
The most frequent adverse event was infection with ≥ grade 3 bacterial or fungal infections seen in 40% of patients (Table V). Although observed in 46% of patients, ≥ grade 3 pulmonary toxicity was invariably associated with infection.
DISCUSSION
The activity of clofarabine + ara-C as salvage therapy for AML has been previously described (Faderl et al, 2005). Response to prior therapy is typically the major predictor of response to AML salvage therapy (Estey et al, 1996; Breems et al, 2005). Here, however, while relapsed patients with CR1 exceeding either 38 or 52 weeks had higher response rates than relapsed patients with shorter CRs, univariate and multivariate analyses suggested no difference between the former and primary refractory patients. It is quite plausible that this reflected selection bias such that our primary refractory patients were more favourable than our relapsed patients. On the other hand, the difference between expected CR rates (15% by criteria of Estey et al, 1996) and our observed CR rate (12/18) is sufficiently large to suggest that GCLAC is qualitatively different than previous regimens in primary refractory patients. For example, assuming patients who do not respond to a first course of 3+7 have a 50% chance of responding to a second (Fernandez et al, 2009), we would expect 7–8 of the15 patients given GCLAC after failing one 3+7 to respond to a second 3+7 whereas 10 of these 15 entered CR after receiving GCLAC. Furthermore, among 5 patients who had previously not responded to fludarabine-containing therapy (FA 1 [fludarabine + ara-C], FLAG-AMSA 1 [FLAG + 4′-(9-Acridinylamino) methanesulfon-m-anisidide], FLAG-Ida 2 [FLAG + idarubicin], FLAG 1), 4 entered CR after receiving GCLAC. Another demonstration that GCLAC is qualitatively different from prior regimens arises from our multivariate analysis indicating that adverse cytogenetics was not a risk factor for response, in contrast to the finding of Breems et al, (2005). A lack of association between cytogenetics and response has also been made by others using clofarabine + ara-C (Faderl et al, 2008; Burnett et al, 2010).
The use of G-CSF in AML has been extensively studied (Ohno et al, 1994; Dombret et al, 1995; Heil et al, 1997; Godwin et al, 1998; Kern et al, 1998; Löwenberg et al, 2003; Hofmann et al, 2004). Although patients given G-CSF post-chemotherapy have had fewer days with neutropenia and in hospital, it has been more difficult to demonstrate improvements in remission rate and survival in patients who received growth factor priming, although one trial (Lowenberg et al, 2003) demonstrated improvement in progression-free survival in patients randomized to G-CSF priming. A different randomized study of granulocyte-macrophage colony-stimulating factor (GM-CSF) priming in conjunction with induction chemotherapy, found that younger patients benefitted from priming if they had a FLT3 internal tandem duplication or MLL rearrangement (Thomas et al, 2010). The role of G-CSF in GCLAC is uncertain, as seemingly encouraging results have been reported with clofarabine + high-dose ara-C without G-CSF (Powell et al, 2008). The effects of G-CSF might be mediated by1) mobilization of leukaemia cells out of the bone marrow to enhance chemotherapy sensitivity, 2) driving cells into active cell cycle with increased susceptibility to ara-C, 3) increasing formation of ara-CTP (Braess et al, 2000) or 4) promoting incorporation of ara-CTP into DNA in proliferating cells (Braess et al, 2001).
The CLASSIC1 trial was a Phase III trial comparing ara-C alone to clofarabine plus ara-C in relapsed or refractory AML patients aged 55 years or older (Faderl et al 2011). Although the CR rate was 35% in the clofarabine-containing arm compared to 18% in the ara-C alone arm p=0.004, the overall survival was not different, probably related to the 30-day mortality of 16% in the clofarabine-containing arm compared to 5% in the ara-C alone arm. The 30-day mortality for GCLAC was 0%, despite use in all our patients of a higher ara-C dose (2g/m2 vs. 1 g/m2). Of course, the difference in 30-day mortality rate may have resulted merely from inclusion of patients with a higher propensity for 30-day mortality in CLASSIC1. While the median age of our patients was only 53 years, age is not the principal predictor of 30-day mortality (Walter et al,2010) and it would be interesting to use more sophisticated predictors of this outcome to compare the CLASSIC1 and GCLAC populations. In the absence of such an analysis, we propose that G-CSF may have improved survival by reducing the days of neutropenia or by improving the function of circulating neutrophils, thus, reducing mortality due to infection.
In conclusion, GCLAC is active in relapsed and refractory AML, and may be qualitatively different from fludarabine-containing regimens. There were no dose-limiting toxicities observed for the studied dose range of clofarabine, other than in patients who had received prior allogeneic HCT and were on immunosuppression. In contrast, GCLAC appears as permissive of subsequent HCT as other salvage regimens. Prospective comparison of GCLAC with other salvage regimens is warranted, as well as study in untreated AML or high risk myelodysplastic syndrome. We have embarked on such a study using a clofarabine dose of 30 mg/m2 daily X 5 together with ara-C at 2g/m2 daily X 5.
Acknowledgments
NIH grant numbers: P30 CA015704, R01 CA138720 and UL1 RR025014.
This work was supported by a grant from Genzyme and investigator support from the Translational Research Program of the Leukemia and Lymphoma Society (P.S.B.). We are grateful for the assistance of Zeny Sisk and Donna Salins with preparation of the manuscript. We thank all patients for participation in this trial.
Footnotes
Presented in abstract form at the 50th annual meeting of the American Society of Hematology, San Francisco, CA, December 6–9, 2008, at the 51st annual meeting in New Orleans, LA, December 5–8, 2009, and at the 52nd annual meeting in Orlando, FL, Dec. 4–7, 2010.
AUTHOR CONTRIBUTIONS
Drs. Becker and Estey wrote the manuscript. Drs. Appelbaum, Becker, Estey, Hendrie, Pagel, Petersdorf, Stirewalt, Shustov contributed to the study design and recruitment and care of patients.. Dr. Storer performed the statistical analysis. Ms. Harrington was the study coordinator who performed data collection and interpretation, and managed regulatory documents for the trial. All authors performed a critical review of the manuscript.
CONFLICT OF INTEREST
Drs. Becker, Faderl, and Kantarjian receive research support from Genzyme. Drs. Faderl and Petersdorf are consultants for Genzyme. The other authors declare no conflict of interest.
References
- Bashey A, Liu L, Ihasz A, Medina B, Corringham S, Keese K, Carrier E, Castro JE, Holman P, Lane TA, Hassidim K, Ball ED. Non-anthracycline based remission induction therapy for newly diagnosed patients with acute myeloid leukemia aged 60 or older. Leukemia Research. 2006;30:503–506. doi: 10.1016/j.leukres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Braess J, Wegendt C, Jahns-Streubel G, Kern W, Keye S, Unterhalt M, Schleyer E, Hiddemann W. Successful modulation of high-dose cytosine arabinoside metabolism in acute myeloid leukaemia by haematopoietic growth factors: no effect of ribonucleotide reductase inhibitors fludarabine and gemcitabine. British Journal of Haematology. 2000;109:388–395. doi: 10.1046/j.1365-2141.2000.02056.x. [DOI] [PubMed] [Google Scholar]
- Braess J, Jahns-Streubel G, Schoch C, Haase D, Haferlach T, Fiegl M, Voss S, Kern W, Schleyer E, Hiddemann W. Proliferative activity of leukaemic blasts and cytosine arabinoside pharmacodynamics are associated with cytogenetically defined prognostic subgroups in acute myeloid leukaemia. British Journal of Haematology. 2001;113:975–982. doi: 10.1046/j.1365-2141.2001.02866.x. [DOI] [PubMed] [Google Scholar]
- Breems DA, Van Putten WL, Huijgens PC, Ossenkoppele GJ, Verhoef GE, Verdonck LF, Vellenga E, De Greef GE, Jacky E, Van der Lelie J, Boogaerts MA, Löwenberg B. Prognostic index for adult patients with acute myeloid leukemia in first relapse. Journal of Clinical Oncology. 2005;23:1969–1978. doi: 10.1200/JCO.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Burnett AK, Russell NH, Kell J, Dennis M, Milligan D, Paolini S, Yin J, Culligan D, Johnston P, Murphy J, McMullin MF, Hunter A, Das-Gupta E, Clark R, Carr R, Hills RK. European development of clofarabine as treatment for older patients with acute myeloid leukemia considered unsuitable for intensive chemotherapy. Journal of Clinical Oncology. 2010;28:2389–2395. doi: 10.1200/JCO.2009.26.4242. [DOI] [PubMed] [Google Scholar]
- Carella AM, Cascavilla N, Greco MM, Melillo L, Sajeva MR, Ladogana S, D’Arena G, Perla G, Carotenuto M. Treatment of “poor risk” acute myeloid leukemia with fludarabine, cytarabine and G-CSF (flag regimen): a single center study. Leukemia Lymphoma. 2001;40:295–303. doi: 10.3109/10428190109057928. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. Journal of Clinical Oncology. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- Cooper T, Ayres M, Nowak B, Gandhi V. Biochemical modulation of cytarabine triphosphate by clofarabine. Cancer Chemotherapy and Pharmacology. 2005;55:361–368. doi: 10.1007/s00280-004-0906-y. [DOI] [PubMed] [Google Scholar]
- Dombret H, Chastang C, Fenaux P, Reiffers J, Bordessoule D, Bouabdallah R, Mandelli F, Ferrant A, Auzanneau G, Tilly H, Yver A, Degos L for the AML Cooperative Study Group. A controlled study of recombinant human granulocyte colony-stimulating factor in elderly patients after treatment for acute myelogenous leukemia. AML Cooperative Study Group. New England Journal of Medicine. 1995;332:1678–1683. doi: 10.1056/NEJM199506223322504. [DOI] [PubMed] [Google Scholar]
- Estey E, Thall P, Andreeff M, Beran M, Kantarjian H, O’Brien S, Escudier S, Robertson LE, Koller C, Kornblau S, Pierce S, Freireich EJ, Deisseroth A, Keating M. Use of granulocyte colony-stimulating factor before, during, and after fludarabine plus cytarabine induction therapy of newly diagnosed acute myelogenous leukemia or myelodysplastic syndromes: comparison with fludarabine plus cytarabine without granulocyte colony-stimulating factor. Journal of Clinical Oncology. 1994;12:671–678. doi: 10.1200/JCO.1994.12.4.671. [DOI] [PubMed] [Google Scholar]
- Estey E, Kornblau S, Pierce S, Kantarjian H, Beran M, Keating M. A stratification system for evaluating and selecting therapies in patients with relapsed or primary refractory acute myelogenous leukemia. Blood. 1996;88:756. [PubMed] [Google Scholar]
- Estey EH, Thall PF, Pierce S, Cortes J, Beran M, Kantarjian H, Keating MJ, Andreeff M, Freireich E. Randomized phase II study of fludarabine + cytosine arabinoside + idarubicin +/− all-trans retinoic acid +/− granulocyte colony-stimulating factor in poor prognosis newly diagnosed acute myeloid leukemia and myelodysplastic syndrome. Blood. 1999;93:2478–2484. [PubMed] [Google Scholar]
- Faderl S, Gandhi V, O’Brien S, Bonate P, Cortes J, Estey E, Beran M, Wierda W, Garcia-Manero G, Ferrajoli A, Estrov Z, Giles FJ, Du M, Kwari M, Keating M, Plunkett W, Kantarjian H. Results of a phase 1–2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory acute leukemias. Blood. 2005;105:940–947. doi: 10.1182/blood-2004-05-1933. [DOI] [PubMed] [Google Scholar]
- Faderl S, Verstovsek S, Cortes J, Ravandi F, Beran M, Garcia-Manero G, Ferrajoli A, Estrov Z, O’Brien S, Koller C, Giles FJ, Wierda W, Kwari M, Kantarjian HM. Clofarabine and cytarabine combination as induction therapy for acute myeloid leukemia (AML) in patients 50 years of age or older. Blood. 2006;108:45–51. doi: 10.1182/blood-2005-08-3294. [DOI] [PubMed] [Google Scholar]
- Faderl S, Ravandi F, Huang X, Garcia-Manero G, Ferrajoli A, Estrov Z, Borthakur G, Verstovsek S, Thomas DA, Kwari M, Kantarjian HM. A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2008;112:1638–1645. doi: 10.1182/blood-2007-11-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faderl S, Wetzler M, Rizzieri D, Schiller GJ, Jagasia MH, Stuart RK, Ganguly S, Avigan D, Craig M, Collins R, Maris MB, Kovacsovics T, Goldberg S, Seiter K, Hari P, Ravandi F, Wang ES, Eckert S, Huebner D, Kantarjian H. Clofarabine plus cytarabine compared to cytarabine alone in older patients with relapsed or refractory (R/R) acute myelogenous leukemia (AML): Results from the phase III CLASSIC 1 trial. Journal of Clinical Oncology. 2011;29(suppl):2011. abstr 6503. doi: 10.1200/JCO.2011.37.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM, Racevskis J, Dewald GW, Ketterling RP, Bennett JM, Rowe JM, Lazarus HM, Tallman MS. Anthracycline dose intensification in acute myeloid leukemia. New England Journal of Medicine. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara F, Palmieri S, Pocali B, Pollio F, Viola A, Annunziata S, Sebastio L, Schiavone EM, Mele G, Gianfaldoni G, Leoni F. De novo acute myeloid leukemia with multilineage dysplasia: treatment results and prognostic evaluation from a series of 44 patients treated with fludarabine, cytarabine and G-CSF (FLAG) European Journal of Haematoogy. 2002;68:203–209. doi: 10.1034/j.1600-0609.2002.01651.x. [DOI] [PubMed] [Google Scholar]
- Godwin JE, Kopecky KJ, Head DR, Willman CL, Leith CP, Hynes HE, Balcerzak SP, Appelbaum FR. A double-blind placebo-controlled trial of granulocyte colony-stimulating factor in elderly patients with previously untreated acute myeloid leukemia: a Southwest oncology group study (9031) Blood. 1998;91:3607–3615. [PubMed] [Google Scholar]
- Heil G, Hoelzer D, Sanz MA, Lechner K, Liu Yin JA, Papa G, Noens L, Szer J, Ganser A, O’Brien C, Matcham J, Barge A. A randomized, double-blind, placebo-controlled, phase III study of filgrastim in remission induction and consolidation therapy for adults with de novo acute myeloid leukemia. The International Acute Myeloid Leukemia Study Group. Blood. 1997;90:4710–4718. [PubMed] [Google Scholar]
- Hofmann WK, Heil G, Zander C, Wiebe S, Ottmann OG, Bergmann L, Hoeffken K, Fischer JT, Knuth A, Kolbe K, Schmoll HJ, Langer W, Westerhausen M, Koelbel CB, Hoelzer D, Ganser A. Intensive chemotherapy with idarubicin, cytarabine, etoposide, and G-CSF priming in patients with advanced myelodysplastic syndrome and high-risk acute myeloid leukemia. Annals of Hematology. 2004;83:498–503. doi: 10.1007/s00277-004-0889-0. [DOI] [PubMed] [Google Scholar]
- Jackson G, Taylor P, Smith GM, Marcus R, Smith A, Chu P, Littlewood TJ, Duncombe A, Hutchinson M, Mehta AB, Johnson SA, Carey P, MacKie MJ, Ganly PS, Turner GE, Deane M, Schey S, Brookes J, Tollerfield SM, Wilson MP. A multicentre, open, non-comparative phase II study of a combination of fludarabine phosphate, cytarabine and granulocyte colony-stimulating factor in relapsed and refractory acute myeloid leukaemia and de novo refractory anaemia with excess of blasts in transformation. British Journal of Haematology. 2001;112:127–137. doi: 10.1046/j.1365-2141.2001.02551.x. [DOI] [PubMed] [Google Scholar]
- Kern W, Aul C, Maschmeyer G, Kuse R, Kerkhoff A, Grote-Metke A, Eimermacher H, Kubica U, Wörmann B, Büchner T, Hiddemann W. Granulocyte colony-stimulating factor shortens duration of critical neutropenia and prolongs disease-free survival after sequential high-dose cytosine arabinoside and mitoxantrone (S-HAM) salvage therapy for refractory and relapsed acute myeloid leukemia. German AML Cooperative Group. Annals of Hematology. 1998;77:115–122. doi: 10.1007/s002770050425. [DOI] [PubMed] [Google Scholar]
- Löwenberg B, van Putten W, Theobald M, Gmür J, Verdonck L, Sonneveld P, Fey M, Schouten H, de Greef G, Ferrant A, Kovacsovics T, Gratwohl A, Daenen S, Huijgens P, Boogaerts M Dutch-Belgian Hemato-Oncology Cooperative Group & Swiss Group for Clinical Cancer Research. Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. New England Journal of Medicine. 2003;349:743–752. doi: 10.1056/NEJMoa025406. [DOI] [PubMed] [Google Scholar]
- Ohno R, Naoe T, Kanamaru A, Yoshida M, Hiraoka A, Kobayashi T, Takanori U, Minami S, Morishima Y, Saito Y, Furusawa S, Imai K, Takemoto Y, Miura Y, Teshima H, Hamajima N the Kohseisho Leukemia Study Group. A double-blind controlled study of granulocyte colony-stimulating factor started two days before induction chemotherapy in refractory acute myeloid leukemia. Kohseisho Leukemia Study Group. Blood. 1994;83:2086–2092. [PubMed] [Google Scholar]
- Ossenkoppele GJ, Graveland WJ, Sonneveld P, Daenen SM, Biesma DH, Verdonck LF, Schaafsma MR, Westveer PH, Peters GJ, Noordhuis P, Muus P, Selleslag D, van der Holt B, Delforge M, Löwenberg B, Verhoef GE Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) The value of fludarabine in addition to ARA-C and G-CSF in the treatment of patients with high-risk myelodysplastic syndromes and AML in elderly patients. Blood. 2004;103:2908–2913. doi: 10.1182/blood-2003-07-2195. [DOI] [PubMed] [Google Scholar]
- Parker WB, Shaddix SC, Chang CH, White EL, Rose LM, Brockman RW, Shortnacy AT, Montgomery JA, Secrist JA, 3rd, Bennett LL., Jr Effects of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)adenine on K562 cellular metabolism and the inhibition of human ribonucleotide reductase and DNA polymerases by its 5′-triphosphate. Cancer Research. 1991;51:2386–2394. [PubMed] [Google Scholar]
- Powell B, D’Agostino R, Levitan D, Ellis L, Lyerly S, Skiles M, Manuel M, Harrelson R, Kimbrough C, Hurd DD. High dose cytarabine and clofarabine (HiDAC CLOF) in relapsed or refractory acute myeloid leukemia; a phase 2 trial [abstract] Blood. 2008;112:1936. [Google Scholar]
- Pulte D, Gondos A, Brenner H. Expected long-term survival of patients diagnosed with acute myeloblastic leukemia during 2006–2010. Annals of Oncology. 2010;21:335–341. doi: 10.1093/annonc/mdp309. [DOI] [PubMed] [Google Scholar]
- Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM, Forman SJ, Appelbaum FR. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- Thomas X, Raffoux E, Renneville A, Pautas C, de Botton S, Terre C, Gardin C, Hayette S, Preudhomme C, Dombret H. Which AML subsets benefit from leukemic cell priming during chemotherapy? Long-term analysis of the ALFA-9802 GM-CSF study. Cancer. 2010;116:1725–1732. doi: 10.1002/cncr.24943. [DOI] [PubMed] [Google Scholar]
- Visani G, Tosi P, Zinzani PL, Manfroi S, Ottaviani E, Testoni N, Clavio M, Cenacchi A, Gamberim B, Carrara P, Gobbi M, Tura S. FLAG (fludarabine + high-dose cytarabine + G-CSF): an effective and tolerable protocol for the treatment of ‘poor risk’ acute myeloid leukemias. Leukemia. 1994;8:1842–1846. [PubMed] [Google Scholar]
- Walter RB, Othus M, Borthakur G, Ravandi F, Cortes JE, Pierce SA, Appelbaum FR, Kantarjian H, Estey EH. Quantitative effect of age in predicting empirically-defined treatment-related mortality and resistance in newly diagnosed AML: Case against age alone as primary determinant of treatment assignment. Blood. 2010;116:Abstract 2191. [Google Scholar]
- Xie KC, Plunkett W. Deoxynucleotide pool depletion and sustained inhibition of ribonucleotide reductase and DNA synthesis after treatment of human lymphoblastoid cells with 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl) adenine. Cancer Research. 1996;56:3030–3037. [PubMed] [Google Scholar]