Abstract
Non-pharmacologic approaches to preserve or increase bone mineral density (BMD) include whole body vibration (WBV), but its efficacy in elderly persons is not clear. Therefore, we conducted the Vibration to Improve Bone in Elderly Subjects (“VIBES”) trial, a randomized, placebo-controlled trial of 10 minutes of daily WBV (0.3g at 37 Hz) in seniors recruited from 16 independent living communities. The primary outcomes were volumetric BMD of the hip and spine measured by quantitative computed tomography (QCT), and biochemical markers of bone turnover. We randomized 174 men and women (89 active, 85 placebo) with T-scores −1 to −2.5 who were not taking bone active drugs and had no diseases affecting the skeleton (mean age 82 ± 7 yrs, range 65–102). Participants received daily calcium (1,000 mg) and vitamin D (800 IU). Study platforms were activated using radio frequency ID cards providing electronic adherence monitoring; placebo platforms resembled the active platforms. In total, 61% of participants in the active arm and 73% in the placebo arm completed 24 months. The primary outcomes, median percent changes (inter-quartile range; IQR) in total volumetric femoral trabecular BMD (active group (2.2% [−0.8%, 5.2%]) vs. placebo 0.4% [−4.8%, 5.0%]), and in median mid-vertebral trabecular BMD of L1 and L2 (active group (5.3% [−6.9%, 13.3%]) vs. placebo (2.4% [−4.4%, 11.1%]), did not differ between groups (all p-values > 0.1). Changes in biochemical markers of bone turnover (P1NP and sCTX) also were not different between groups (p=0.19 and p=0.97, respectively). In conclusion, this placebo-controlled randomized trial of daily WBV in older adults did not demonstrate evidence of significant beneficial effects on volumetric BMD or bone biomarkers; however, the high variability in vBMD changes limited our power to detect small treatment effects. The beneficial effects of WBV observed in previous studies of younger women may not occur to the same extent in elderly individuals.
INTRODUCTION
Osteoporosis, a disease characterized by compromised bone strength predisposing to an increased risk of fracture, results in more than 2 million incident fractures and costs the United States health care system over $17 billion per year.1 While pharmacologic treatment of osteoporosis has advanced, safety concerns related to commonly used drugs has resulted in recommendations to limit the duration of use. Furthermore, concern of “polypharmacy” in elderly persons has renewed interest in using non-pharmacologic approaches to preserve or even increase bone density.
One such strategy has been exercise, with the goal of harnessing the skeleton’s sensitivity to mechanical signals to promote bone formation.2 Exercising may be contraindicated or difficult in some elderly individuals, and must be approached with caution in those with osteoporosis.3 Mechanical signals introduced via vibration have been shown to stimulate bone formation4 and suppress bone resorption5 in animal models of osteoporosis, possibly serving as a surrogate for exercise. These signals, when introduced at very low intensities, are considered safe by the International Organization for Standardization and the National Institute for Occupational Safety and Health6 and thus represent a possible means of introducing a stimulus to the skeleton without risking serious adverse events.7, 8
While there is some evidence that low intensity vibration signals (<1.0g, where 1g = Earth’s gravitational field) may improve some skeletal outcomes in younger individuals,9, 10 it remains unclear if this strategy would be successful in elderly persons, given the suppressed responsiveness of the aging skeleton to mechanical signals,11 the diminished viability of the bone marrow progenitor pool12 and loss of osteocytes with aging.13
Indeed, a recent meta-analysis concluded that whole body vibration had little overall treatment effect on bone mineral density (BMD) in older women.14 Another publication concluded that “claims about whole-body vibration therapy for the prevention and treatment of osteoporosis cannot be made without further research.”15 Thus, the purpose of this randomized, double blinded, placebo-controlled trial was to determine if up to three years of brief daily exposure to low magnitude mechanical stimulation using low intensity whole body vibration in older adults demonstrated any benefit to the hip and spine, areas of high susceptibility to fracture. The “VIBES” trial (Vibration to Improve Bones in Elderly Subjects) was registered at www.clinicaltrials.gov in 2006.
Role of the Funding Source
The National Institute on Aging (NIA) provided funding for this study and the role of the NIA was to approve members for the Data and Safety Monitoring Board and to review the progress and safety of the study at 6 monthly intervals. The investigators retained control of the study’s design, conduct and reporting.
METHODS
Design Overview
This was a randomized, double blinded, placebo-controlled clinical trial of whole body vibration to improve bone density. Participants in this two-year trial with a one year extension, were recruited from independent living communities in and around Boston, Massachusetts after approvals by Institutional Review Boards and consenting of participants.
Setting and Participants
Detailed recruitment and eligibility criteria have been described previously.16 Briefly, participants had to be 60 years of age or older, under 250 pounds, free of a known terminal disease, and cognitively intact (score < 12 on the Short Blessed Test).17 Exclusion criteria included: immobilization of the axial or lower appendicular skeleton within the last year; non-ambulatory; malignancy other than cured thyroid or skin cancer; hip replacement or internal fixation, total knee replacement, or lower limb fracture within the last year; use of bone active medications; known Paget's disease, rheumatoid arthritis or other connective tissue disorders requiring systemic treatment; history of Cushing's syndrome; fragility fracture within the last five years unless pharmacologic therapy was considered not an option by the participant’s physician. Participants were recruited from 16 independent living communities in and around Boston, MA, ranging in size from 150 to 1,695 residents.16
Residents who were judged eligible obtained a dual-energy X-ray absorptiometry (DXA) scan of the hip and spine using a Discovery A QDR 4500 densitometer (Hologic Inc., Waltham, MA) in the array (fan beam) mode. Further eligibility required that the BMD T-score at the hip or spine be < −1.0 and > −2.5. Some individuals were enrolled with lower BMD or fracture histories if they had no other options for approved osteoporosis therapies because of medication intolerance or personal preference. Following the scan, participants were screened for 25 hydroxy-vitamin D (25OHD) concentration (<15 ng/mL), renal and liver function, and thyroid stimulating hormone concentration. If the 25 (OH) D was < 15 ng/mL, supplements were given and participants enrolled if their values exceeded 15 ng/mL.
Randomization and Interventions
Eligible participants were randomized to either the active or placebo platform group, with randomization stratified by independent living community, sex, and body mass index (< 24 vs ≥ 24) to ensure balance between treatment groups. Once enrolled, participants underwent a baseline quantitative computed tomography (QCT) scan of the hip and spine. All participants were provided with daily calcium (1,000 mg) and vitamin D (800 IU) tablets for the duration of the trial. The trial duration was initially planned for two years, but near the end of year two, participants were invited to enroll for a third year and provide consent accordingly. All relevant study procedures were approved by the Institutional Review Board at Hebrew SeniorLife and Beth Israel Deaconess Medical Center.
-
1.1.1.1.1
The intervention consisted of low magnitude mechanical stimulation (LMMS) using a platform that delivered sinusoidal accelerations of 0.3g at 37Hz, vibration which is barely perceptible. Accelerations of this intensity produced displacement of the top plate of less than 90 microns. Human exposure to vibration at this frequency and intensity are considered safe for up to four hours each day, as determined by the International Standards Organization (IS0-2631).18 Both active and placebo devices emitted a 500Hz sound to mask noise that an active device might make, and to diminish guessing about active or placebo status. In pilot studies using this device, participants had difficulty discriminating between the active and placebo platforms when queried at the end of a six-month trial.19
Each participant was instructed during the first week of participation to stand erect, in a relaxed stance, with feet shoulder-width apart and knees straight, for one ten-minute session each day, seven days per week, although daily sessions were not monitored by research staff. In young healthy adults, this relaxed posture transmits approximately 65–70% of the plantar-based acceleration up through the axial skeleton.20.The devices were available for use at any time, and activated by the user-specific radio-frequency identification card that activated only the device to which the individual was assigned. This card identified the participant and recorded the time, date, and duration of use. Only one 10-minute ‘dose’ of the device was possible a day. Regular calibrations confirmed that the proper acceleration signal was sustained over time.
Outcomes and Follow-up
Participants were evaluated at baseline, 1-, 2- and, when applicable, 3-year follow-up visits, obtaining information on demographic characteristics (baseline only), anthropometrics, medical history, physical activity, dietary intake, and skeletal status. All assessments were obtained by examiner/technologists blinded to treatment group.
QCT Measures
The primary outcome variables were changes in i) volumetric trabecular BMD of the total hip and ii) volumetric trabecular BMD of the lumbar vertebrae. Secondary outcomes included femoral neck trabecular and integral volumetric BMD, as well as integral volumetric BMD of the spine. Participants underwent QCT scanning of the proximal femur and lumbar spine (L1, L2) at baseline, 12, 24, and in some cases, 36 months using a helical CT scanner operating at 120 kVp, 150 mAs, 48 mm field of view and 1 mm slice thickness. A bone mineral reference phantom (Image Analysis, Columbia, KY) that allows conversion of Hounsfield units to BMD in mg/cm3 hydroxyapatite was scanned with each participant. If L1 and L2 were not evaluable due to fracture or anatomic technical issues, we averaged two consecutive vertebrae from T11 to L3 that were evaluable. If two evaluable lumbar vertebrae were not available, we used a single vertebral body that was consistently evaluable across all time points. All QCT data were evaluated using semi-automated methodology, previously described.21–24 The reported short-term coefficients of variation (CVs) for the total hip trabecular volumetric BMD and for the vertebral trabecular volumetric BMD are 0.72% and 2.93%.25 In our study, three repeated analyses of 10 scans produced CVs of 1.97% and 2.88% for total hip trabecular volumetric BMD and vertebral trabecular volumetric BMD, respectively.
Lumbar spine scans at any visit were excluded if both vertebrae in the scan region were fractured, image artifacts obscured vertebrae (metal or motion), or technical limitations in the analysis program led to inaccurate analysis regions. Proximal hip scans were excluded if the image acquisition was incomplete, or there was a metal or motion artifact. A review of outliers, blinded to treatment arm, was conducted to check analysis regions for correct segmentation, resulting in some additional scans being excluded.
Biochemical Markers of Bone Turnover
Serum samples were collected from participants after an overnight fast during the same two hour window at baseline, 1, 3, 6, 12 and 24 months. The samples were processed and frozen within 2 hours at −70°C until analysis. CTX and P1NP were measured in the Elecsys 2010 immunoassay analyzer (Roche Diagnostics, Indianapolis, IN) in a single batch using sandwich electrochemiluminescence immunoassays. All samples from each participant were analyzed in the same test run to minimize between-run variation. Precision of CTX measures has been previously published.26 The intra-run precision of P1NP gave CVs of 2.1%, 1.9% and 1.8% at 31 ng/mL, 111 ng/mL and 299 ng/mL respectively. The batch-specific inter-run imprecision for P1NP gave CVs of 3.0%, 4.2% and 5.0% at 57 ng/mL, 187 ng/mL and 385 ng/mL respectively.
Adverse Events
Quarterly adverse event (AE) ascertainment by phone began 2 weeks after randomization. Serious AEs were categorized using the Medical Dictionary for Regulatory Activities (MedDRA) standards. Non-serious AEs were grouped into pre-specified body system categories (e.g., musculoskeletal, neurologic, cardiovascular).
All AEs were reported to both IRBs that had oversight of the study; SAEs were reported as they occurred. A data and safety monitoring board (DSMB) met every six months and reviewed all AEs and SAEs.
Statistical analysis
Comparisons of baseline participant characteristics between active and placebo groups used t-tests for continuous characteristics and chi-square tests for categorical (and binary) characteristics.27 The primary analysis consists of participants who had completed up to two years; however, results for a subset of this group which completed the third year are also reported. We also performed two post hoc tests for interaction of the treatment effect by sex and by age.
Box plots were used to display the percent change from baseline for each of the major outcomes at the 12-, 24-, and 36-month visits. We used intention-to-treat principles for all analyses. Analyses and graphics were generated using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
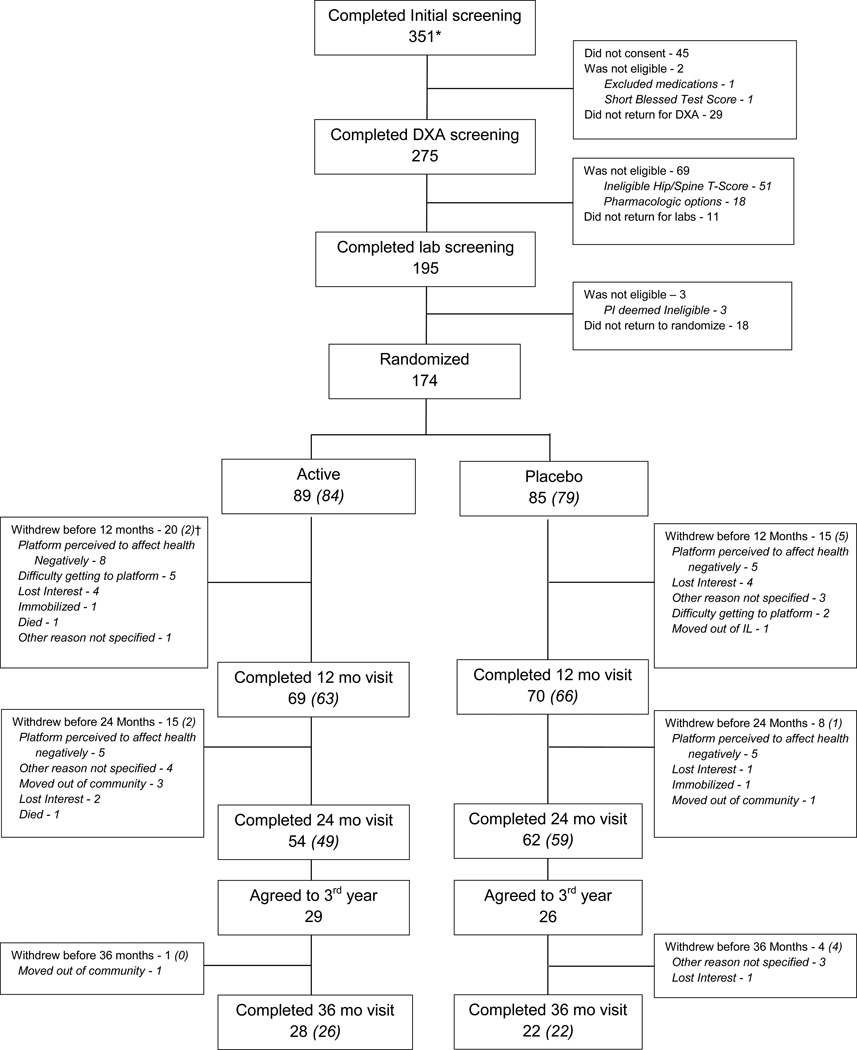
Figure 1 presents the CONSORT diagram of residents’ flow into the study. Of the 351 individuals identified from the 16 participating independent living communities, 174 individuals were randomized (89 to active and 85 to placebo platform group), with 61% of participants in the active group, and 73% in the placebo arm, completing the 24 month trial (p=0.08). Of the 116 participants who completed 24 months, 55 (47%) agreed to continue for a third year, with 50 (91%) completing the third year. Table 1 provides the baseline characteristics of the 174 randomized participants by group assignment. There were no significant differences at baseline for any measure.
Figure 1.
Consort diagram showing progress of participants through the study
*As previously described,16 these 351 individuals expressed an interest in participating in the trial following group information sessions and mailings.
† The numbers in parentheses represent technically valid CT scans for the primary total hip volumetric trabecular bone mineral density outcome analyses. For the spine, there were a total of 166 valid scans at baseline, 133 at 12 months, 107 at 24 months and 49 at 36 months.
Table 1.
Baseline characteristics of participants in the active and placebo arms of the VIBES clinical trial
| Characteristic | Active | Placebo | p-value |
|---|---|---|---|
| N=89 | N=85 | ||
| Gender | 0.31 | ||
| Female | 63 (70.8%) | 54 (63.5%) | |
| Male | 26 (29.2%) | 31 (36.5%) | |
| BMI (kg/m2) | 0.18 | ||
| <27 | 55 (61.8%) | 44 (51.8%) | |
| ≥27 | 34 (38.2%) | 41 (48.2%) | |
| Education | 0.38 | ||
| College or above | 66 (74.2%) | 67 (79.8%) | |
| High school or below | 23 (25.8%) | 17 (20.2%) | |
| Mean PASE* Score ± SD | 84 ± 50 | 82 ± 50 | 0.78 |
| Mean days in study ± SD (Range) |
702 ± 368 (8 – 1,156) |
730 ± 308 (59 – 1,135) |
0.59 |
| Mean age (Range) |
82.5 ± 8.1 65.4 – 102.9 |
82.3 ± 6.2 67.3 – 97.7 |
0.84 |
| Race | 0.32 | ||
| Asian | 2 (2.2%) | 0 (0.0%) | |
| African American | 2 (2.2%) | 1 (1.2%) | |
| Caucasian | 85 (95.5%) | 84 (98.8%) | |
| Marital Status | 0.74 | ||
| Divorced | 10 (11.2%) | 9 (10.7%) | |
| Married | 29 (32.6%) | 32 (38.1%) | |
| Never married | 6 (6.7%) | 3 (3.6%) | |
| Widow or widower | 44 (49.4%) | 40 (47.6%) | |
| History of Falls (past 6 months) | 14 (15.7%) | 12 (14.3%) | 0.79 |
| Mean daily calcium intake ± SD (mg) | 911 ± 440 | 988 ± 507 | 0.28 |
| Mean 25 (OH) Vitamin D ± SD (ng/ml) | 37 ± 11 | 38 ± 11 | 0.65 |
| Mean total femoral trabecular BMD (g/cm3) | 0.083 ± 0.029 | 0.079 ± 0.027 | 0.37 |
| Mean volumetric femoral neck trabecular BMD (g/cm3) | 0.045 ± 0.036 | 0.040 ± 0.031 | 0.36 |
| Mean volumetric femoral neck integral BMD (g/cm3) | 0.241 ± 0.037 | 0.237 ± 0.032 | 0.45 |
| Percent cortical bone in total femur | 31.98 ± 3.68 | 31.55 ± 3.80 | 0.46 |
| Percent cortical bone in femoral neck | 35.49 ± 4.44 | 35.05 ± 3.84 | 0.50 |
| Mean mid-vertebral trabecular BMD (g/cm3) | 0.086 ± 0.029 | 0.084 ± 0.030 | 0.67 |
| Mean volumetric integral BMD in vertebral body (g/cm3) | 0.155 ± 0.032 | 0.15 ± 0.036 | 0.21 |
| sCTX ng/mL | 0.42 ± 0.21 | 0.45 ± 0.23 | 0.40 |
| P1NP ng/mL | 47 ± 23 | 51 ± 21 | 0.20 |
Physical activity scale for the elderly
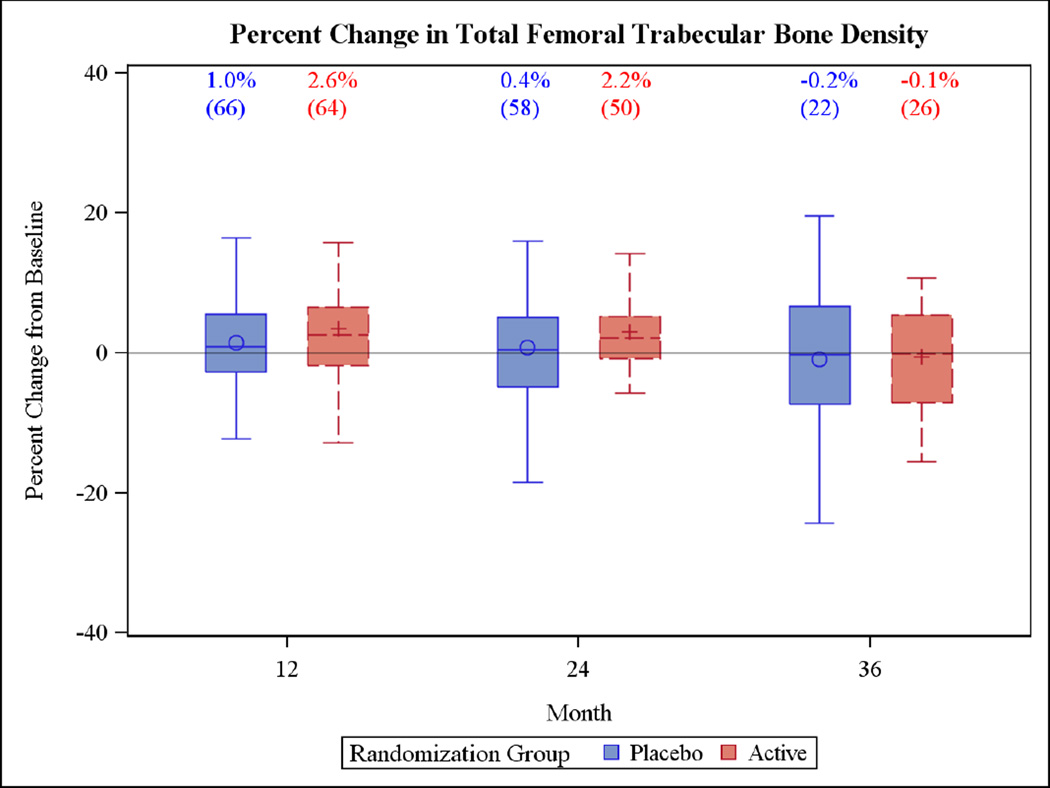
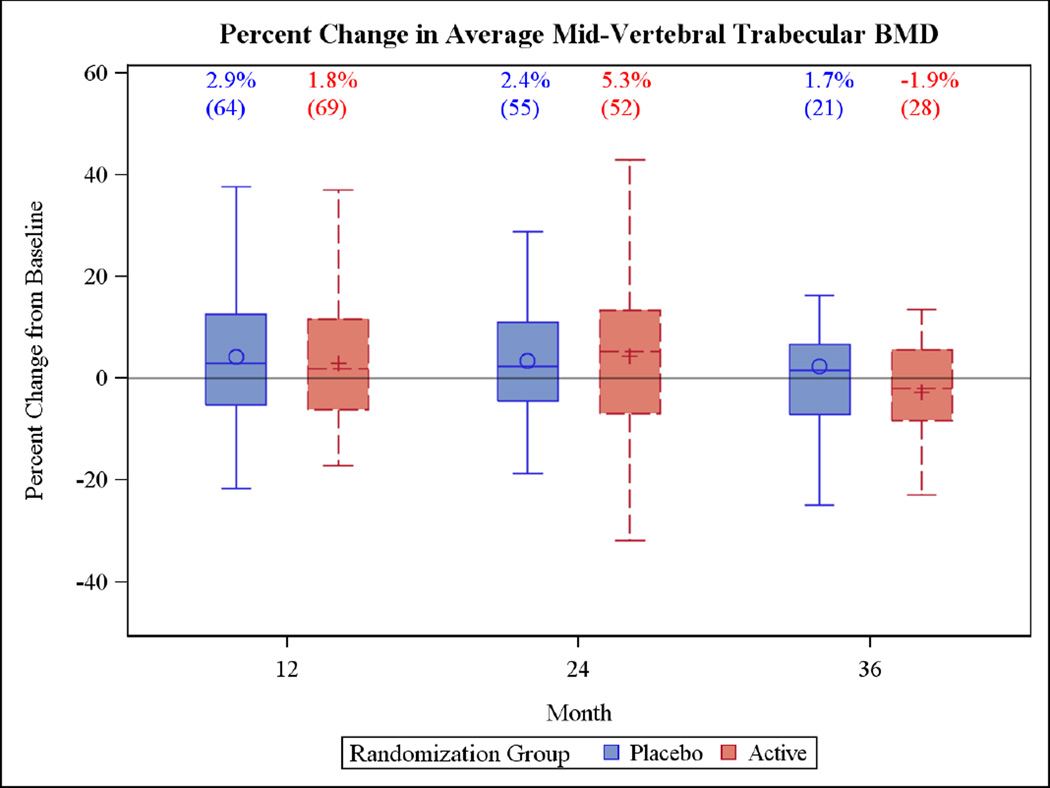
The primary outcomes at 24 months showed that the median (IQR) percent change in total femoral trabecular volumetric BMD of the active group (2.2% [−0.8%, 5.2%]) was not significantly different from changes in the placebo group (0.4% [−4.8%, 5.0%]) (Figure 2), with similarly non-significant differences for vertebral trabecular volumetric BMD (active 5.3% [−6.9%, 13.3%]; placebo 2.4% [−4.4%, 11.1%]) (Figure 3). No differences were detected at the end of the third year.
Figure 2.
Box plots of the percent change in the primary outcome variables for the two year trial and the one year extension period. Each box plot shows the mean (“○ or +”), the median (horizontal line) and the interquartile range. The vertical bars indicate the 5th and 95th percentiles
Figure 3.
Box plots of the percent change in the primary outcome variables for the two year trial and the one year extension period. Each box plot shows the mean (“○ or +”), the median (horizontal line) and the interquartile range. The vertical bars indicate the 5th and 95th percentiles
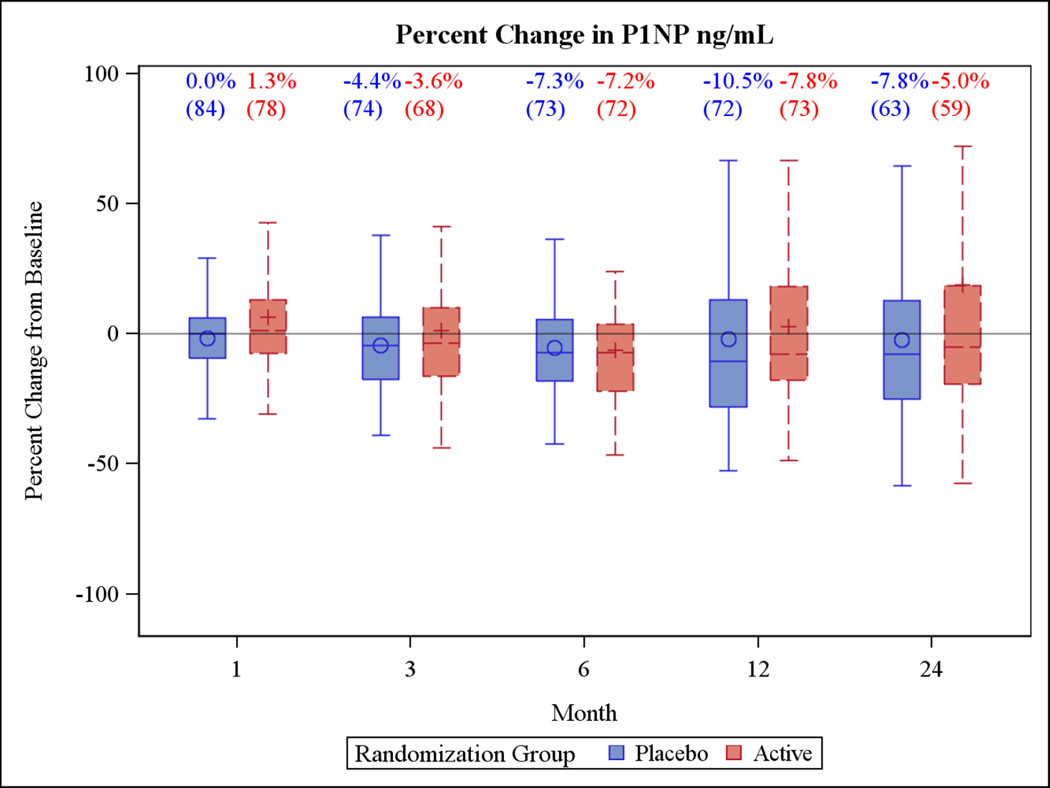
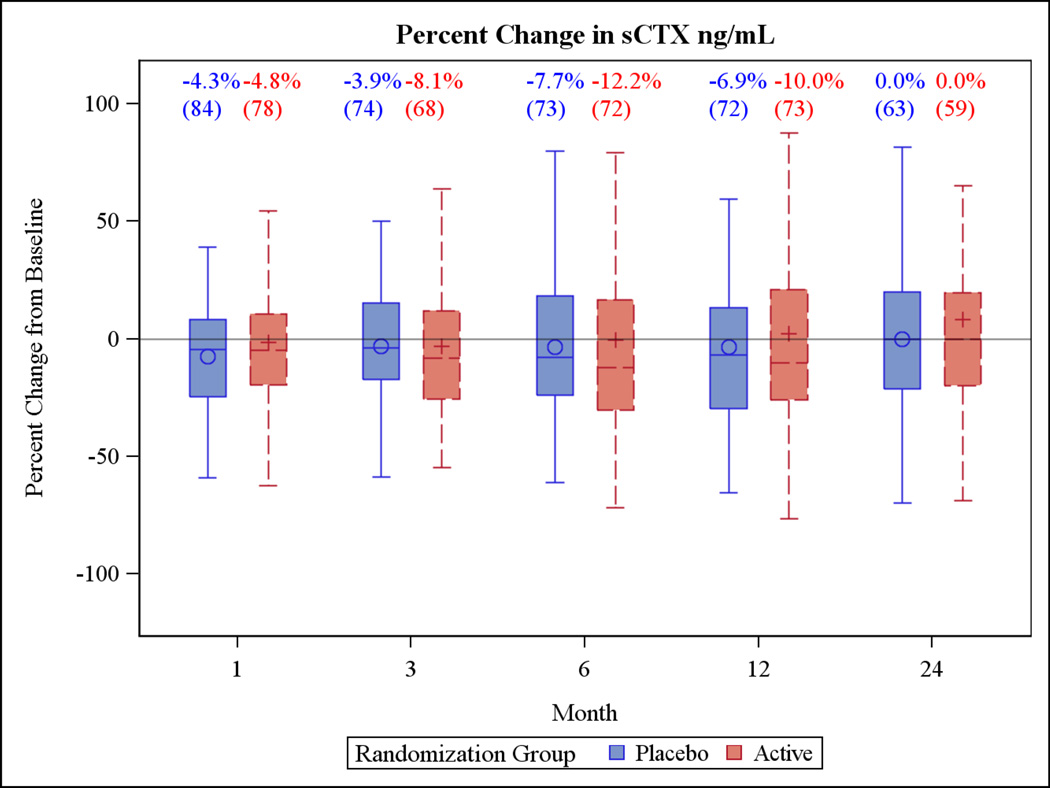
Changes in bone turnover markers at 24 months also did not differ by treatment group. For P1NP, median (IQR) percent change in the active group was −5.7% (−20.4%, 14.2%) versus −6.4% (−24.6%, 12.8%) in the placebo group at (Figure 4). For CTX, the median (IQR) percent change was −0.9% (−21.3%, 15.8%) in the active group compared with 0.0% (−20.0%, 20.0%) in the placebo group (Figure 5).
Figure 4.
Box plots of the percent change in the primary outcome variables for the two year trial and the one year extension period. Each box plot shows the mean (“○ or +”), the median (horizontal line) and the interquartile range. The vertical bars indicate the 5th and 95th percentiles
Figure 5.
Box plots of the percent change in the primary outcome variables for the two year trial and the one year extension period. Each box plot shows the mean (“○ or +”), the median (horizontal line) and the interquartile range. The vertical bars indicate the 5th and 95th percentiles
As shown in Supplemental Figures 1–3, there were no significant differences in the percent changes between groups in other BMD outcomes (femoral neck trabecular and integral volumetric BMD, and volumetric integral vBMD of the spine).
When we performed post hoc tests for interaction between the treatment effect and sex and age, we did observe a significant interaction of gender and treatment at 24 months, with women in the active treatment group having 10% (compared to baseline) greater trabecular BMD of the total femur region compared to the combined group of all men (regardless of treatment group) and women in the placebo group, and the interaction was significant only at the 24-month measurement (Pinteraction = 0.01). We did not observe a significant interaction in the lumbar spine (Pinteraction=0.66), and we did not observe any interactions with sex at any skeletal site when considering all participants regardless of the length of followup time (Pinteraction for the hip =0.11, Pinteraction for the spine 0.52).
Adherence for the active group was 68% versus 70% for the placebo group. There were no differences in any of the categories of SAEs between active and placebo groups and none was attributed to use of the device (Table 2). Similarly, for non-serious AEs, there were no differences between groups. Of the sixteen participants who withdrew from the trial and had symptomatic musculoskeletal adverse events such as leg or back pain, seven were in the placebo group and nine were in the active group.
Table 2.
Serious and non-serious adverse events occurring in more than five participants among active and placebo platform groups*
| Serious adverse event | Active (N=89) | Placebo (N=85) | Total (N=174) | P-value |
|---|---|---|---|---|
| Infections and Infestations | 5 | 8 | 13 | 0.34 |
| Injury, Poisoning, and Procedural Complications | 8 | 4 | 12 | 0.27 |
| Gastrointestinal Disorders | 7 | 4 | 11 | 0.39 |
| Nervous System Disorders | 7 | 4 | 11 | 0.39 |
| Cardiac Disorders | 7 | 3 | 10 | 0.22 |
| Respiratory, Thoracic and Mediastinal Disorders* | 7 | 1 | 8 | 0.06 |
| Vascular Disorders* | 5 | 2 | 7 | 0.44 |
| Musculoskeletal and Connective Tissue Disorders* | 4 | 3 | 7 | 1.00 |
| Metabolism and Nutrition Disorders* | 4 | 2 | 6 | 0.68 |
| Non serious adverse event | ||||
| Musculoskeletal | 37 | 30 | 67 | 0.39 |
| Respiratory | 21 | 18 | 39 | 0.70 |
| Other | 15 | 14 | 29 | 0.95 |
| Cardiovascular | 12 | 10 | 22 | 0.73 |
| Ocular/Visual | 7 | 12 | 19 | 0.19 |
| Neurologic | 8 | 9 | 17 | 0.72 |
| Skin | 8 | 8 | 16 | 0.92 |
| Gastrointestinal | 9 | 6 | 15 | 0.47 |
| Genitourinary | 7 | 6 | 13 | 0.84 |
See methods for description of adverse event classification systems
All differences in serious and non-serious adverse events were non-significant p>0.10
DISCUSSION
This two-year randomized, double-blinded, placebo-controlled trial was designed to determine if low magnitude, high frequency mechanical signals would increase volumetric BMD of the hip and spine in older adults. In 174 randomized participants with a mean age of 82 years, after two years of ten minutes per day whole body vibration, there were no significant differences in any of the BMD measures or biochemical markers of bone turnover between the active and placebo groups, despite adherence close to 70%. Similar results were observed in the subset of individuals who continued for a third year. After 3 years, there was no significant loss of bone, even in the placebo group, as compared to baseline.
Previous smaller-scale studies in children with disabling conditions9 and in young women with osteoporosis10 demonstrated that similar low intensity vibration used in this trial was anabolic to the skeleton. In a randomized, placebo-controlled study of recently post-menopausal women,28 there was less bone loss in the active group of women with high adherence and low body weight. In contrast, Slavotska and colleagues, reporting on healthy postmenopausal women ages 44–79 years (mean age 60 years) randomized to 12 months of whole body vibration showed no effects on changes in BMD or microarchitecture when compared to a group who received no intervention.29 While the lack of a placebo control in that study, as well as a twenty year difference in mean age, represent major differences from our trial, it is unlikely that simply “standing” for ten minutes per day was responsible for the lack of bone lost in the placebo/control arm of our study. In our study, despite the longer duration and use of placebo platforms, there were still no significant effects of whole body vibration on the change in any outcome measures.
Other whole body vibration trials in older individuals, including those using high magnitude vibration, have combined the whole body vibration with exercise performed while standing on the platforms, making it difficult to disentangle the influence of the vibration from the exercises. In one of these trials, 113 women living in long term care residences (mean age 77 years) were randomized to either six months of a whole body vibration “training program,” 800 IU of vitamin D per day, or 1,600 IU vitamin D per day.30 The whole body vibration group exercised with static and dynamic exercises while on a platform. The vibrations delivered to the participants were progressively increased in frequency and magnitude, with the magnitude (1.6 g to 2.2 g) ultimately exceeding the current ISO safety recommendations,31 yet the whole body vibration program did not provide additional benefit over vitamin D supplementation for hip BMD, muscle mass or strength. In 2011, a meta-analysis of whole body vibration therapy in older adults reported that in 13 randomized trials involving 896 participants, whole body vibration had no overall treatment effect on BMD in older women.14 Finally, a 2011 technical brief from the Agency for Healthcare Research and Quality (AHRQ) concluded that there was insufficient evidence to draw firm conclusions about the efficacy of whole body vibration on skeletal health.32 Thus, our findings are consistent with the results from these recently reported clinical trials and the meta-analysis; however, it is important to also recognize that the age of the participants, the vibration regimens, the variability of the measures in both groups, the skeletal measures of efficacy, and the durations of the studies all varied markedly.
There may have been other reasons why we did not observe significant beneficial effects on the skeleton. If effects of the intervention differed by age, the wide age range of our participants may have contributed to the null findings of our study; however, we found no evidence for an interaction between treatment effect and age. Since we did not monitor posture during the daily sessions, if participants did not fully extend their legs during each 10 minute session, the signal from the platforms may not have been adequately transmitted to the hip and spine. Finally since we did not hypothesize that there would be different effects in men and women, we may have missed an effect that differed between sexes. In our post hoc test for interaction of the treatment effect with sex, we did observe a difference in treatment effect between men and women at 24 months in terms of trabecular BMD of the total femur region. We did not observe a significant interaction in the spine, and the significant interaction for the hip appeared only at the 24-month measurement. While this result is intriguing, we cannot rule out that this is a chance finding and that, at the very least, it should be replicated before a definitive statement can be made about it.
When the VIBES trial began in 2007, less was known about the potential benefits of whole body vibration over extended periods. Thus our study was designed for a two year period, the longest clinical trial of whole body vibration to date. There were several unique aspects of the study. First, we specifically recruited participants of advanced age to test this non-pharmacologic intervention to preserve or even increase BMD in elderly persons, i.e., the group of individuals at highest risk for fracture. Most osteoporosis trials do not include as many participants in the age group represented by this trial, offering insights into longitudinal changes in volumetric bone density in older persons. Second, we developed a placebo platform, which had not been used in all of the previous trials. Third, we used a platform that delivered vertical acceleration at a frequency and intensity that were well within the ISO safety recommendations, which contrasts with some commercially available platforms that exceed the safety recommendations.6 Fourth, our electronic adherence monitoring was state-of-the art, using radio frequency ID tags to ensure accurate compliance measures. While we successfully enrolled elderly participants, their advanced age contributed to an attrition rate that was not inconsequential. To examine potential differences between those who completed the trial and those who dropped out before two years, we compared baseline characteristics and observed that non-completers were on average three years older (p=0.01), had a greater BMI (p=0.02), were slightly less physically active (p=0.05), and had lower adherence during their participation compared to completers. Baseline measures of 25(OH) D and calcium intake, as well as the BMD and biochemical markers of bone turnover, did not differ between completers and non-completers.
One of the important considerations in the interpretation of our results is that the placebo group did not lose bone, contrary to our predictions defined during study planning. The reasons may include the calcium and vitamin D administered to participants, or the relatively active lifestyles of these well-resourced and educated seniors. Alternatively, it may be that these older individuals have already lost much of their trabecular bone, and do not have additional bone to lose,33 or have a depletion of bone cells in the progenitor pool to respond to such interventions.34 The sample size estimate for the primary outcome of trabecular vBMD assumed there would be an 8% difference in outcome between the treatment and control groups at the end of two years, based on a 3%/year increase in volumetric BMD (6% over 2 years) in the treated group and a 1%/year decrease in vBMD (2% over 2 years) in the control group, both with a standard deviation of 11.8%, using prior results from a clinical trial of parathyroid hormone in women with osteoporosis who had a mean age 12 years younger than our participants (range 55–85 years).35 Data from a randomized trial of raloxifene in 70 year old women was used to estimate the two-year loss of 2% in a placebo group treated with calcium and vitamin D.36 Assuming an attrition rate of 18%, we projected that 82 participants would complete the study per group (i.e., a total sample of 164 at the end of the study), providing 91% power to detect an 8% difference in bone density between groups (2-sided α = 0.05). The actual observed percent change in total hip trabecular volumetric BMD at 24 months was 3.09% (SD = 10.9, n=50) in the active group and 0.76% (SD = 9.5, n=58) in the placebo group, both results smaller than estimated a priori, and in the case of the placebo group, opposite to what was projected. Thus, for the magnitude of differences actually observed, we lacked sufficient statistical power (22%) to rule out the possibility that smaller skeletal benefits may have accrued.19
In conclusion, this trial of whole body vibration to improve bone density in elderly persons with osteopenia failed to demonstrate a statistically significant beneficial effect on the skeleton. We cannot exclude the possibility that the modest differences that we observed between the active and placebo groups would have been significant with a larger sample size, or longer duration. Nevertheless, it is not likely that whole body vibration will be shown to have large effects on the skeleton when administered in the same way as in our protocol. Future trials should use the information generated from this study and others to plan and enroll larger samples of participants to determine if there may be more modest beneficial effects on the skeleton, and if there may be a root cause for age-related differences in responsiveness of the skeleton to low magnitude mechanical signals. Future trials may also need to consider adjusting the signal to a given individual’s posture, and perhaps changing parameters of the mechanical signal such as the frequency, intensity or duration, as well as considering the incorporation of several bouts of mechanical stimulation per day, or even adding pharmacologic agents to whole body vibration.
Supplementary Material
Acknowledgments
The authors thank the participants and the Hebrew Rehabilitation Center VIBES research team for their hard work and contribution to the study and the members of the Data and Safety Monitoring Board (Dennis Black, PhD, Susan Greenspan, MD, Gail Greendale, MD, Ronald Zernicke, PhD, and Lawrence Raisz, MD), for their scientific guidance.
Funded by a grant from the National Institute on Aging R01 AG025489.
REFERENCES
- 1.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 2.Sinaki M, Pfeifer M, Preisinger E, et al. The role of exercise in the treatment of osteoporosis. Curr Osteoporos Rep. 2010;8:138–144. doi: 10.1007/s11914-010-0019-y. [DOI] [PubMed] [Google Scholar]
- 3.Liu-Ambrose TY, Khan KM, Eng JJ, Gillies GL, Lord SR, McKay HA. The beneficial effects of group-based exercises on fall risk profile and physical activity persist 1 year postintervention in older women with low bone mass: follow-up after withdrawal of exercise. J Am Geriatr Soc. 2005;53:1767–1773. doi: 10.1111/j.1532-5415.2005.53525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism: Low mechanical signals strengthen long bones. Nature. 2001;412:603–604. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 5.Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J. 2001;15:2225–2229. doi: 10.1096/fj.01-0166com. [DOI] [PubMed] [Google Scholar]
- 6.Muir J, Kiel DP, Rubin CT. Safety and severity of accelerations delivered from whole body vibration exercise devices to standing adults. Journal of science and medicine in sport / Sports Medicine Australia. 2013 doi: 10.1016/j.jsams.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pel JJ, Bagheri J, van Dam LM, et al. Platform accelerations of three different whole-body vibration devices and the transmission of vertical vibrations to the lower limbs. Med Eng Phys. 2009;31:937–944. doi: 10.1016/j.medengphy.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Kiiski J, Heinonen A, Jarvinen TL, Kannus P, Sievanen H. Transmission of vertical whole body vibration to the human body. J Bone Miner Res. 2008;23:1318–1325. doi: 10.1359/jbmr.080315. [DOI] [PubMed] [Google Scholar]
- 9.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–369. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 10.Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-Level, High-Frequency Mechanical Signals Enhance Musculoskeletal Development of Young Women With Low BMD. J Bone Miner Res. 2006;21:1464–1474. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 11.Rubin CT, Bain SD, McLeod KJ. Suppression of the osteogenic response in the aging skeleton. Calcif Tissue Int. 1992;50:306–313. doi: 10.1007/BF00301627. [DOI] [PubMed] [Google Scholar]
- 12.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 13.Busse B, Djonic D, Milovanovic P, et al. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell. 2010;9:1065–1075. doi: 10.1111/j.1474-9726.2010.00633.x. [DOI] [PubMed] [Google Scholar]
- 14.Lau RW, Liao LR, Yu F, Teo T, Chung RC, Pang MY. The effects of whole body vibration therapy on bone mineral density and leg muscle strength in older adults: a systematic review and meta-analysis. Clin Rehabil. 2011;25:975–988. doi: 10.1177/0269215511405078. [DOI] [PubMed] [Google Scholar]
- 15.Wysocki A, Butler M, Shamliyan T, Kane RL. Whole-Body Vibration Therapy for Osteoporosis. Rockville, MD: University of Minnesota Evidence-based Practice Center; 2011. [November 2011]. Report No.: Technical Brief No. 10. [Google Scholar]
- 16.Kiel DP, Hannan MT, Barton BA, et al. Insights from the conduct of a device trial in older persons: low magnitude mechanical stimulation for musculoskeletal health. Clin Trials. 2010;7:354–367. doi: 10.1177/1740774510371014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 18.International Standards O. Evaluation of Human Exposure to Whole-Body Vibration. Geneva: 1985. [Google Scholar]
- 19.Hannan MT, Cheng DM, Green E, Swift C, Rubin CT, Kiel DP. Establishing the compliance in elderly women for use of a low level mechanical stress device in a clinical osteoporosis study. Osteoporos Int. 2004;15:918–926. doi: 10.1007/s00198-004-1637-y. [DOI] [PubMed] [Google Scholar]
- 20.Rubin C, Pope M, Chris FJ, Magnusson M, Hansson T, McLeod K. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine. 2003;28:2621–2627. doi: 10.1097/01.BRS.0000102682.61791.C9. [DOI] [PubMed] [Google Scholar]
- 21.Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 22.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–1012. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 23.Lang TF, Leblanc AD, Evans HJ, Lu Y. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J Bone Miner Res. 2006;21:1224–1230. doi: 10.1359/jbmr.060509. [DOI] [PubMed] [Google Scholar]
- 24.Marshall LM, Zmuda JM, Chan BK, et al. Race and ethnic variation in proximal femur structure and BMD among older men. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23:121–130. doi: 10.1359/JBMR.070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Sode M, Saeed I, Lang T. Automated registration of hip and spine for longitudinal QCT studies: integration with 3D densitometric and structural analysis. Bone. 2006;38:273–279. doi: 10.1016/j.bone.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garnero P, Borel O, Delmas PD. Evaluation of a fully automated serum assay for C-terminal cross-linking telopeptide of type I collagen in osteoporosis. Clin Chem. 2001;47:694–702. [PubMed] [Google Scholar]
- 27.Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991;78:691–692. [Google Scholar]
- 28.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 29.Slatkovska L, Alibhai SM, Beyene J, Hu H, Demaras A, Cheung AM. Effect of 12 months of whole-body vibration therapy on bone density and structure in postmenopausal women: a randomized trial. Ann Intern Med. 2011;155:668–679. W205. doi: 10.7326/0003-4819-155-10-201111150-00005. [DOI] [PubMed] [Google Scholar]
- 30.Verschueren SM, Bogaerts A, Delecluse C, et al. The effects of whole-body vibration training and vitamin D supplementation on muscle strength, muscle mass, and bone density in institutionalized elderly women: a 6-month randomized, controlled trial. J Bone Miner Res. 2011;26:42–49. doi: 10.1002/jbmr.181. [DOI] [PubMed] [Google Scholar]
- 31.Mechanical Vibration and Shock: Evaluation of human exposure to whole body vibration. Geneva: International Standards Organization; 1997. [1997-05-01]. Report No.: ISO 2631-1:1997(E). [Google Scholar]
- 32.Wysocki A, Butler M, Shamliyan T, Kane RL. Technical Brief No. 10. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Nov, Whole-Body Vibration Therapy for Osteoporosis. (Prepared by the University of Minnesota Evidence-based Practice Center under Contract No. HHSA 290 2007 10064 1.) [PubMed] [Google Scholar]
- 33.Zebaze RM, Ghasem-Zadeh A, Bohte A, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–1736. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 34.Nishida S, Endo N, Yamagiwa H, Tanizawa T, Takahashi HE. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab. 1999;17:171–177. doi: 10.1007/s007740050081. [DOI] [PubMed] [Google Scholar]
- 35.Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med. 2005;353:555–565. doi: 10.1056/NEJMoa050336. [DOI] [PubMed] [Google Scholar]
- 36.Genant HK, Lang T, Fuerst T, et al. Treatment with raloxifene for 2 years increases vertebral bone mineral density as measured by volumetric quantitative computed tomography. Bone. 2004;35:1164–1168. doi: 10.1016/j.bone.2004.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.