Abstract
Purpose
The choice between liver transplantation (LT), liver resection (LR), and radiofrequency ablation (RFA) as initial therapy for early hepatocellular carcinoma (HCC) is controversial, yet little is known about how surgeons choose therapy for individual patients. We sought to quantify the impact of both clinical factors and surgeon specialty on surgical decision making in early HCC by using conjoint analysis.
Methods
Surgeons with an interest in liver surgery were invited to complete a Web-based survey including 10 case scenarios. Choice of therapy was then analyzed by using regression models that included both clinical factors and surgeon specialty (non-LT v LT).
Results
When assessing early HCC occurrences, non-LT surgeons (50% LR; 41% LT; 9% RFA) made significantly different recommendations compared with LT surgeons (63% LT; 31% LR; 6% RFA; P < .001). Clinical factors, including tumor number and size, type of resection required, and platelet count, had significant effects on the choice between LR, LT, and RFA. After adjusting for clinical factors, non-LT surgeons remained more likely than LT surgeons to choose LR compared with LT (relative risk ratio [RRR], 2.67). When the weight of each clinical factor was allowed to vary by surgeon specialty, the residual independent effect of surgeon specialty on the decision between LR and LT was negligible (RRR, 0.93).
Conclusion
The impact of surgeon specialty on choice of therapy for early HCC is stronger than that of some clinical factors. However, the influence of surgeon specialty does not merely reflect an across-the-board preference for one therapy over another. Rather, certain clinical factors are weighed differently by surgeons in different specialties.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer and, because of its poor prognosis, the third leading cause of cancer death worldwide.1 Surgical therapy offers the only means of cure, but the choice of appropriate surgical therapy for HCC is controversial, especially for early HCC in the setting of well-compensated cirrhosis.2–5 Multiple studies have demonstrated that acceptable long-term survival outcomes can be achieved in appropriately selected patients by using liver transplantation (LT),6–10 liver resection (LR),11–13 or radiofrequency ablation (RFA),14–16 and all of these options are used in clinical practice. Furthermore, surgeons who treat HCC come from a variety of training backgrounds and may perform only a subset of these procedures. As such, both clinical factors and surgeon specialty likely impact the choice of surgical therapy in early HCC. Although previous series have reported outcomes for treatment of early HCC with LT, LR, and RFA, no study has addressed how surgeons use clinical data and their own experience to choose the most appropriate surgical therapy for individual patients.
Conjoint analysis, a technique developed in mathematical psychology17 and then used widely in marketing research,18,19 allows healthcare decision making to be systematically studied.20,21 This technique consists of a choice experiment: key attributes of sample cases are varied, and the variation in respondents' choices in relation to these attributes can be quantified. Conjoint analysis has been used previously to study surgical decision making.22–30 We sought to use conjoint analysis to elucidate the factors that drive surgical decision making in early HCC. Our objectives were to characterize the therapeutic preferences of surgeons who treat HCC, to quantify the impact of clinical factors on the choice of surgical therapy, and to understand the interplay between surgeon specialty and clinical factors in determining choice of therapy for early HCC. We hypothesized that both clinical factors and surgeon factors would independently predict choice of therapy.
METHODS
Survey Instrument Design
For purposes of this study, early HCC was defined as HCC within the Milan criteria for LT (ie, single tumor < 5 cm or 2 to 3 tumors all < 3 cm, with no evidence of extrahepatic tumor)6 in the setting of well-compensated cirrhosis (ie, Child-Pugh class A, no varices, no ascites, and no encephalopathy). Standard conjoint analysis methodology was used to design, implement, and analyze the survey in five steps: defining attributes, assigning attribute levels, creating scenarios, obtaining preference data, and estimating model parameters.19 First, structured interviews were conducted with five academic and community surgeons who treat HCC to identify key clinical factors influencing choice of therapy as well as to determine a clinically meaningful range of values for each factor. Seven key clinical factors were thus identified and were then incorporated into 36 case scenarios by using a fractional factorial design that is based on an orthogonal array.31 A random subset of 10 case scenarios was chosen for each respondent. In each case, the respondent was asked which procedure he would recommend as initial curative-intent therapy: LR, RFA, LT without bridging therapy (LT − B), or LT with bridging therapy (LT + B). Bridging therapy is the use of an adjunct modality, such as chemoembolization, to reduce the risk of tumor progression while waiting for a transplantation. In addition to the case scenarios, the survey instrument included questions on practice characteristics and on attitudes toward surgical therapies for early HCC.
The resulting survey instrument was then pilot tested and iteratively refined. Survey invitations were e-mailed to surgeons who had an interest in liver surgery (including hepatobiliary surgery and liver transplantation); e-mail addresses were obtained from publicly available sources, such as Web sites and publications.32 Prespecified eligibility criteria specified that respondents must be practicing surgeons who had completed surgical training and who evaluated at least five patients with HCC per year.
Statistical Analysis
Descriptive statistics were compared by using Fisher's exact test or the rank-sum test, as appropriate. Choice data were analyzed by using multinomial logistic regression models with robust variance estimates, yielding relative risk ratios (RRRs) that reflected the change in the probability of choosing a particular therapy over an alternative.33 It was specified a priori that the LT − B and LT + B options would be analyzed both as a combined choice and as separate choices. Interaction terms were included in an all-or-none fashion to assess differences in decision making between groups of respondents. All tests of statistical significance were two sided, and statistical significance was established at P < .05. Statistical analyses were performed with Stata/MP 10.1 for Windows (StataCorp LP, College Station, TX). The study protocol was deemed exempt from review by the Johns Hopkins Bloomberg School of Public Health institutional review board.
RESULTS
A total of 1,032 e-mail invitations were sent; 336 eligible and complete responses were received (response rate, 33%). Practice characteristics of the respondents are given in Table 1. Approximately half (54%) of respondents indicated that they currently performed LT for HCC, and these data were used to designate surgeon specialty (non-LT or LT). Of the 155 non-LT surgeons, 149 performed LR, and 130 performed LR as well as RFA for HCC. Of the 181 LT surgeons, 166 also performed LR, and 130 performed all three therapies. Respondents annually evaluated a median of 30 patients with HCC and annually performed a median of 20 surgical procedures for HCC.
Table 1.
Practice Demographics and Clinical Characteristics of Survey Respondents
| Characteristic | No. of Patients(N = 336) | % |
|---|---|---|
| Practice type | ||
| Academic practice or university hospital staff | 284 | 85 |
| Private practice or community hospital staff | 52 | 15 |
| Years in practice | ||
| Median | 10 | |
| IQR | 4-17 | |
| Fellowship training | ||
| HPB with LT | 112 | 33 |
| HPB without LT | 35 | 10 |
| Surgical oncology | 86 | 26 |
| Transplantation | 142 | 42 |
| None of the above | 22 | 7 |
| No. of patients annually evaluated for HCC | ||
| Median | 30 | |
| IQR | 20-60 | |
| Perform liver resection for HCC | ||
| Yes | 315 | 94 |
| No | 21 | 6 |
| Perform radiofrequency ablation for HCC | ||
| Yes | 267 | 79 |
| No | 69 | 21 |
| Perform liver transplantation for HCC | ||
| Yes | 181 | 54 |
| No | 155 | 46 |
| Annual liver procedure volumes for HCC* | ||
| Liver resection | ||
| Median | 5 | |
| IQR | 4-15 | |
| Radiofrequency ablation | ||
| Median | 5 | |
| IQR | 3-15 | |
| Liver transplantation | ||
| Median | 10 | |
| IQR | 8-20 | |
| HCC cases discussed at multidisciplinary tumor board | ||
| Yes | 311 | 93 |
| No | 25 | 7 |
Abbreviations: IQR, interquartile range; HPB, hepato-pancreato-biliary; LT, liver transplantation; HCC, hepatocellular carcinoma.
For non-zero volumes only.
When asked to indicate their general preferences for initial surgical therapy for early HCC on a Likert scale, there were significant differences by surgical specialty (Fig 1). Non-LT surgeons preferred LR rather than both LT and RFA, whereas non-LT surgeons were in aggregate neutral in their preference for LT versus RFA. In contrast, LT surgeons preferred LT rather than LR and RFA; however, LT surgeons were strongly in favor of LR compared with RFA. These differences were also manifested in the choice of therapy in case scenarios. In aggregate, without adjustment for surgical specialty or clinical factors, LT was chosen in 53% of cases (41% LT + B; 12% LT − B); LR, in 40%; and RFA, in 7%. However, there was a significant difference in the choices made by non-LT surgeons (50% LR; 41% LT; 9% RFA) and LT surgeons (63% LT; 31% LR; 6% RFA; P < .001). That RFA was uncommonly chosen was consistent with the fact that only 43% of respondents considered RFA to be a potentially curative treatment modality for HCC.
Fig 1.
Preferences for initial therapy for early hepatocellular carcinoma (HCC), stratified by surgeon specialty. (“In general, do you prefer A or B as initial surgical therapy for patients with well-compensated cirrhosis and HCC within the Milan criteria?”) Median response on Likert scale indicated for each group. LR, liver resection; LT, liver transplantation; RFA, radiofrequency ablation.
The clinical factors used to generate case scenarios are listed in Table 2. Respondents were asked to assume that all patients had HCC within the Milan criteria in the setting of well-compensated cirrhosis. Figure 2 depicts a sample case scenario. In regression analyses, all clinical factors demonstrated statistically significant effects on the choice among LR, LT, and RFA (Table 3). The type of resection required, tumor number and size, and platelet count had the largest effects on choice of therapy. LT was more likely to be recommended for patients who would require a major hepatic resection, for those who had multifocal disease, and for those who had low platelet count. Additional analyses were performed to identify factors important in choice of bridging therapy when LT was chosen. The presence of a solitary 4.5-cm tumor versus a solitary 2.5-cm tumor (RRR, 1.59; 95% CI, 1.13 to 2.23) or versus three tumors ≤ 2.5 cm (RRR, 1.85; 95% CI, 1.24 to 2.77) increased the choice of LT + B versus LT − B (P < .001). Similarly, longer LT waiting times increased the choice of LT + B v LT − B (5 v 2 months: RRR, 3.70; 95% CI, 2.66 to 5.14; 8 v 2 months: RRR, 11.9; 95% CI, 7.41 to 19.1; P < .001). No other clinical factor significantly affected the choice of LT + B versus LT − B.
Table 2.
Factors Used in Case Scenarios
| Factor by levels |
|---|
| Age, years |
| 50 |
| 65 |
| Tumor number and size |
| 3 tumors; largest, 2.5 cm |
| 1 tumor; 4.5 cm |
| 1 tumor; 2.5 cm |
| Type of resection required |
| Left hemi-hepatectomy (segments 2, 3, and 4); FLR, 60% |
| Right posterior sectionectomy (segments 6 and 7); FLR, 70% |
| Left lateral sectionectomy (segments 2 and 3); FLR, 85% |
| Etiology of cirrhosis* |
| Chronic hepatitis C |
| Chronic hepatitis B |
| Past alcohol abuse (abstinent > 1 year) |
| Biologic MELD score |
| 10 (INR, 1.3; TB, 1.3 mg/dL; SCr, 1.0 mg/dL) |
| 8 (INR, 1.1; TB, 1.3 mg/dL; SCr, 1.0 mg/dL) |
| 6 (INR, 1.0; TB, 1.0 mg/dL; SCr, 1.0 mg/dL) |
| Platelet count, per μL |
| 90,000 |
| 150,000 |
| Anticipated waiting time for liver transplantation, months |
| 2 |
| 5 |
| 8 |
Abbreviations: FLR, future liver remnant; MELD, model for end-stage liver disease; INR, international normalized ratio; TB, total serum bilirubin; SCr, serum creatinine.
All patients were described as having Child's A cirrhosis.
Fig 2.
Sample case scenario. HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease; INR, international normalized ratio.
Table 3.
Determinants of Choice of Therapy: Clinical Factors
| Factor | LR v LT |
LR v RFA |
RFA v LT |
P | |||
|---|---|---|---|---|---|---|---|
| RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | ||
| Age, years | |||||||
| 50 | Ref | Ref | Ref | .046 | |||
| 65 | 1.11 | 0.96 to 1.29 | 0.78 | 0.57 to 1.05 | 1.44 | 1.06 to 1.95 | |
| Tumor number and size | |||||||
| 3 tumors; largest, 2.5 cm | Ref | Ref | Ref | < .001 | |||
| 1 tumor; 4.5 cm | 1.72 | 1.35 to 2.19 | 3.23 | 1.57 to 6.64 | 0.53 | 0.26 to 1.07 | |
| 1 tumor; 2.5 cm | 2.27 | 1.76 to 2.93 | 0.40 | 0.24 to 0.68 | 5.65 | 3.48 to 9.16 | |
| Type of resection required | |||||||
| Left hemi-hepatectomy, FLR 60% | Ref | Ref | Ref | < .001 | |||
| Right posterior section, FLR 70% | 1.54 | 1.23 to 1.94 | 1.34 | 0.89 to 2.02 | 1.15 | 0.80 to 1.65 | |
| Left lateral section, FLR 85% | 4.19 | 3.31 to 5.31 | 5.79 | 3.61 to 9.30 | 0.72 | 0.47 to 1.12 | |
| Etiology of cirrhosis* | |||||||
| Chronic hepatitis C | Ref | Ref | Ref | < .001 | |||
| Chronic hepatitis B | 1.18 | 0.95 to 1.47 | 0.75 | 0.50 to 1.12 | 1.58 | 1.08 to 2.32 | |
| Past alcohol abuse | 1.47 | 1.21 to 1.80 | 1.08 | 0.74 to 1.59 | 1.36 | 0.94 to 1.97 | |
| Biologic MELD score | |||||||
| 10 | Ref | Ref | Ref | < .001 | |||
| 8 | 1.44 | 1.18 to 1.77 | 1.46 | 0.94 to 2.28 | 0.99 | 0.65 to 1.49 | |
| 6 | 1.66 | 1.37 to 2.02 | 1.56 | 1.03 to 2.37 | 1.06 | 0.71 to 1.59 | |
| Platelet count, per μL | |||||||
| 90,000 | Ref | Ref | Ref | < .001 | |||
| 150,000 | 2.32 | 1.93 to 2.79 | 1.97 | 1.43 to 2.73 | 1.18 | 0.87 to 1.59 | |
| Liver transplantation waiting time, months | |||||||
| 2 | Ref | Ref | Ref | < .001 | |||
| 5 | 1.30 | 1.08 to 1.57 | 0.98 | 0.60 to 1.60 | 1.33 | 0.83 to 2.11 | |
| 8 | 1.70 | 1.39 to 2.09 | 1.20 | 0.77 to 1.88 | 1.41 | 0.93 to 2.15 | |
Abbreviations: LR, liver resection; LT, liver transplantation; RFA, radiofrequency ablation; RRR, relative risk ratio; Ref, referent; FLR, future liver remnant; MELD, model for end-stage liver disease.
All patients were described as having Child's A cirrhosis.
Additional regression analyses were then performed to assess the impact of surgeon specialty, relative to clinical factors, on choice of therapy. First, a variable denoting surgeon specialty was added to the previously described regression model, allowing for an overall difference in the propensity to choose each therapy (Appendix Table A1, online only). Although the effects of clinical factors did not change in this model compared with the previous model, there was a separate, independent effect of surgeon specialty on choice of therapy (P < .001). Non-LT surgeons were significantly more likely than LT surgeons to choose LR rather than LT (RRR, 2.67; 95% CI, 2.00 to 3.55) and RFA rather than LT (RRR, 2.64; 95% CI, 1.62 to 4.30). Surgeon specialty did not impact the choice of LR versus RFA (RRR, 1.01; 95% CI, 0.63 to 1.62). With respect to choice of bridging therapy, non-LT surgeons were significantly less likely than LT surgeons to choose LT + B rather than LT − B (RRR, 0.38; 95% CI, 0.25 to 0.58).
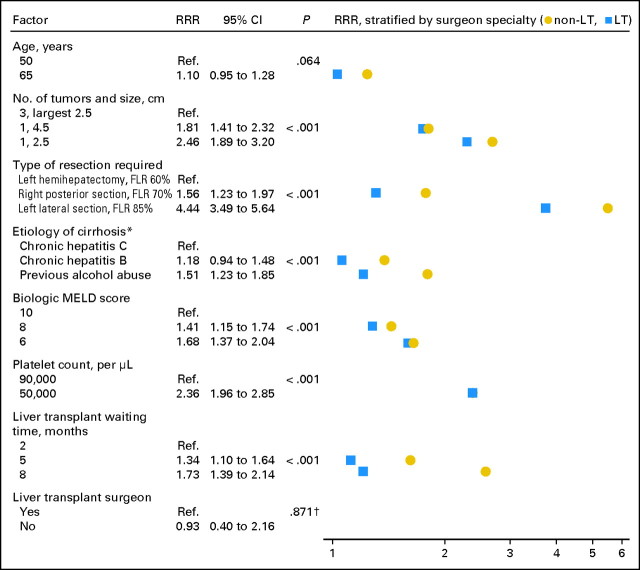
To additionally explore the differences in choice of therapy related to surgeon specialty, we examined a third model that allowed the impact of each clinical factor to vary by surgeon specialty (Figure 3). By doing so, the surgeon specialty effect could be split into two components: one part related to how different specialists weigh clinical data, and another part that was unrelated to the clinical data presented in our scenarios. Again, the overall impact of surgeon specialty (including both components of the effect) was significant (P < .001). This analysis revealed that several clinical factors were weighed differently by non-LT and LT surgeons, most notably type of resection required, etiology of cirrhosis, and LT waiting time (Figure 3, with differences by specialty visualized as the horizontal spread of points). When the weight of each clinical factor was allowed to vary by surgeon specialty in the model, the residual effect of surgeon specialty, independent of clinical factors, on the decision between LR and LT was negligible (RRR, 0.93; 95% CI, 0.40 to 2.16). Similarly, there was no significant residual effect on the decision between LT and RFA (RRR, 3.91; 955 CI, 0.82 to 18.7). These results indicated that the effect of surgeon specialty was completely explained by differences in how the two specialties weigh clinical data.
Fig 3.
Determinants of choice of liver resection (LR) versus liver transplantation (LT), stratified by and including surgeon specialty. (*) All patients were described as having Child's A cirrhosis. (†) P value for joint significance of all surgeon specialty-related variables < .001. RRR, relative risk ratio; Ref, referent; FLR, future liver remnant; MELD, model of end-stage liver disease.
When the choice of LT + B versus LT − B was examined in this fashion, the overall impact of surgeon specialty was again significant (P < .001). The impact of LT waiting time varied by surgeon specialty. Non-LT surgeons were sensitive to longer LT waiting times in choosing LT + B v LT − B (5 v 2 months: RRR, 6.77; 95% CI, 4.05 to 11.3; 8 v 2 months: RRR, 20.7; 95% CI, 10.1 to 42.4). In comparison, LT surgeons were relatively less influenced by LT waiting time (LT + B: 5 v 2 months; RRR, 2.32; 95% CI, 1.49 to 3.60; LT − B: 8 v 2 months; RRR, 7.70; 95% CI, 4.01 to 14.8). Notably, even when the weight of each clinical factor was allowed to vary by surgeon specialty, there was still a residual effect of surgeon specialty on the decision between LT + B and LT − B, with non-LT surgeons favoring bridging therapy one third as often as LT surgeons (RRR, 0.31; 95% CI, 0.13 to 0.75).
Finally, respondents were asked to self-characterize their decision-making strategy for early HCC. Among LT surgeons, 60% reported that they typically decide for or against LT first and then consider other treatment options. Fewer (30%) reported that they consider LR first; 3%, RFA; and 6%, some other strategy. Among non-LT surgeons, 42% reported that they consider LT first; 47%, LR; 3%, RFA; and 7%, some other strategy. Again, there was a significant difference by specialty (P = .009).
DISCUSSION
Variation in choice of therapy in HCC is well documented34 and likely reflects a collective sense of equipoise regarding the optimal therapy of patients with early HCC.2–5 Choice of therapy may depend on clinical factors as well as surgeon characteristics. The factors influencing surgeons' choices of therapy for early HCC have not, however, been previously studied. This study is, to our knowledge, the first to analyze surgical decision making in HCC. By using conjoint analysis, we quantified the relative impact of clinical factors on choice of therapy. Interestingly, we also noted a significant impact of surgeon specialty on decision making, and we were able to quantify the relative contribution of surgeon specialty versus clinical factors to early HCC decision making. In fact, we noted that the impact of surgeon specialty was larger in magnitude than the impact of several clinical factors.
In this study, the choice of surgical therapy for early HCC varied widely, with roughly equal numbers of respondents choosing LR or LT as initial therapy. Unlike in Asia, where RFA is viewed more favorably as a treatment modality for early HCC,15,16 only 43% of respondents from our American cohort of surgeons considered RFA to be potentially curative for HCC, choosing it in only 7% of cases. Our study identified the need for major hepatic resection, the presence of multifocal disease, and thrombocytopenia as the clinical factors most influencing the choice of LT over LR. The influence of these factors on surgical decision making is consistent with published data that implicate them as important predictors of surgical outcome.5 For example, more extensive liver resection, especially in the setting of thrombocytopenia and portal hypertension,35 is associated with increased postoperative morbidity and mortality.36 Furthermore, LR for multifocal early HCC has been associated with inferior oncologic results.13 As such, LT may be a better option for patients with these clinical factors.
Of note, surgeon specialty played a role that was at least as important as clinical factors in determining preference for initial therapy. When asked for their general attitudes towards surgery for early HCC, non-LT surgeons indicated a preference for LR, and LT surgeons, for LT. Similarly, analysis of choice data showed that non-LT surgeons were much more likely than LT surgeons to choose LR compared with LT. Such findings may seem self evident and lead one to assume that the impact of surgeon specialty is merely an example of Maslow's hammer37 (ie, that surgical specialists use the techniques available to them). Our analyses revealed, however, that the effect of specialty was entirely explained by the fact that certain clinical variables were weighed differently by non-LT versus LT surgeons. For example, in choosing between LR and LT, non-LT surgeons were more likely than LT surgeons to be influenced by LT waiting time and extent of resection required. As such, variations in choice of therapy for early HCC do not appear to reflect dogmatic, specialty-specific preferences for one therapy versus another. Rather, the effect of surgeon specialty on decision making is mediated by the differential impact of certain clinical factors. A notable exception was found, however, in the choice of bridging therapy, for which specialty-specific differences persisted despite accounting for differential weighting of clinical factors.
These data are important for several reasons. First, they begin to shed light on the underlying root causes for variation in choice of therapy for early HCC. They also highlight a potential mechanism for variation in choice of therapy that may be relevant in other cancers. Second, identification of factors that are weighed differently by non-LT and LT surgeons can focus debate and consensus building regarding the appropriate roles of these factors in driving choice of therapy. Our data should not serve as a guide to choosing surgical therapy for early HCC, but they do highlight areas of agreement and disagreement among experts that should stimulate future work. Finally, because these differences in decision making are likely the result of both surgical training and subsequent surgical experience, these data highlight the potential for surgical specialization to result in the formation of intellectual silos, even in closely related disciplines, and even in an era when presentation of cases at multidisciplinary tumor boards is routine (93% in this study). Reconciling differences in surgical decision making will require high-quality data, not just multidisciplinary discussion, to demonstrate the optimal treatment strategies for specific subgroups of patients.
A particular strength of our study was the use of conjoint analysis to assess provider decision making. Although it has been used extensively to elicit the preferences of patients and communities for health care,20,21 conjoint analysis has also been used to study decision making by physicians,38–41 including surgeons.22–30 Conjoint analysis is a robust technique with the benefits of a strong theoretical underpinning and rigorous, quantitative methodology.18,19 Conjoint analysis allows clinical factors of interest to be explored in a clean context that is free from constraints, such as institutional policies or insurance regulations, that would influence interpretation of practice data. The Delphi method, which relies on experts' own introspections regarding their decision-making strategies, has been advocated as a means of forging consensus in areas of surgical oncology with substantial practice variation.42 However, clinical judgment analysis, such as conjoint analysis, generates assessments of decision-making strategies that are more accurate than decision makers' own perceptions of how they use information,22,38,40 providing a useful reality check of expert opinions.
Several limitations of our study should also be considered. First, this study assessed stated preferences for surgical therapy as opposed to surgeons' actual practice patterns. This approach has the advantage of standardizing case scenarios and reducing confounding, but it has the disadvantage of assessing decision making in a relatively idealized context. For example, referring physicians, such as hepatologists, who were not included in this study, may also play an important role in choice of surgical therapy. Nevertheless, previous work has demonstrated that this approach results in valid assessments of clinical decision making that are predictive of future decisions.26,38,43 Second, because we do not know the characteristics of survey nonrespondents, we cannot verify that our respondents are representative of all surgeons who treat HCC. Finally, our survey focused on a subgroup of patients with HCC—those with Milan-criteria tumors and well-compensated cirrhosis—who are the focus of greatest controversy in choice of therapy. Although many patients with HCC will not fall into this group, decision making is often simpler in those cases, because fewer surgical options are generally available to them. Also, because this study focused on initial surgical therapy, other therapeutic strategies, such as LR followed by salvage LT, were not considered.
In conclusion, choice of surgical therapy for early HCC varies widely as the result of both clinical factors and surgeon specialty. The impact of surgeon specialty on choice of therapy is stronger than that of some clinical factors. However, the influence of surgeon specialty results, for the most part, from the differential impact of certain clinical factors rather than an across-the-board preference for one therapy over another. These data should inform future initiatives to understand choice of therapy for patients with HCC as well as other malignancies. High-quality clinical data must be complemented by a deeper understanding of clinical decision making if we are to ensure delivery of consistent, high-quality care for all patients with cancer.
Acknowledgment
We thank H. Richard Alexander, Jr, MD, and Mark Fraiman, MD, for their assistance with development of the survey instrument. We also thank the survey respondents who made this work possible.
Appendix
Table A1.
Determinants of Choice of Therapy, Including Surgeon Specialty
| Factor | LR v LT |
LR v RFA |
RFA v LT |
P | |||
|---|---|---|---|---|---|---|---|
| RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | ||
| Age, years | |||||||
| 50 | Ref | Ref | Ref | .064 | |||
| 65 | 1.10 | 0.95 to 1.28 | 0.77 | 0.57 to 1.05 | 1.43 | 1.05 to 1.95 | |
| Tumor number and size | |||||||
| 3 tumors; largest, 2.5 cm | Ref | Ref | Ref | < .001 | |||
| 1 tumor; 4.5 cm | 1.81 | 1.41 to 2.32 | 3.26 | 1.58 to 6.74 | 0.55 | 0.27 to 1.12 | |
| 1 tumor; 2.5 cm | 2.46 | 1.89 to 3.20 | 0.40 | 0.24 to 0.68 | 6.11 | 3.71 to 10.1 | |
| Type of resection required | |||||||
| Left hemi-hepatectomy, FLR 60% | Ref | Ref | Ref | < .001 | |||
| Right posterior section, FLR 70% | 1.56 | 1.23 to 1.97 | 1.34 | 0.89 to 2.01 | 1.17 | 0.82 to 1.67 | |
| Left lateral section, FLR 85% | 4.44 | 3.49 to 5.64 | 5.85 | 3.64 to 9.41 | 0.76 | 0.49 to 1.18 | |
| Etiology of cirrhosis* | |||||||
| Chronic hepatitis C | Ref | Ref | Ref | < .001 | |||
| Chronic hepatitis B | 1.18 | 0.94 to 1.48 | 0.75 | 0.50 to 1.11 | 1.59 | 1.08 to 2.33 | |
| Past alcohol abuse | 1.51 | 1.23 to 1.85 | 1.08 | 0.74 to 1.58 | 1.40 | 0.97 to 2.02 | |
| Biologic MELD score | |||||||
| 10 | Ref | Ref | Ref | < .001 | |||
| 8 | 1.41 | 1.15 to 1.74 | 1.44 | 0.92 to 2.24 | 0.98 | 0.65 to 1.49 | |
| 6 | 1.68 | 1.37 to 2.04 | 1.56 | 1.03 to 2.37 | 1.07 | 0.71 to 1.61 | |
| Platelet count, per μL | |||||||
| 90,000 | Ref | Ref | Ref | < .001 | |||
| 150,000 | 2.36 | 1.96 to 2.85 | 2.00 | 1.45 to 2.77 | 1.18 | 0.87 to 1.60 | |
| Liver transplantation waiting time, months | |||||||
| 2 | Ref | Ref | Ref | < .001 | |||
| 5 | 1.34 | 1.10 to 1.64 | 0.99 | 0.61 to 1.60 | 1.36 | 0.85 to 2.18 | |
| 8 | 1.73 | 1.39 to 2.14 | 1.20 | 0.77 to 1.88 | 1.44 | 0.94 to 2.20 | |
| Liver transplantation surgeon | |||||||
| Yes | Ref | Ref | Ref | < .001 | |||
| No | 2.67 | 2.00 to 3.55 | 1.01 | 0.63 to 1.62 | 2.64 | 1.62 to 4.30 | |
Abbreviations: LR, liver resection; LT, liver transplantation; RFA, radiofrequency ablation; RRR, relative risk ratio; Ref, referent; FLR, future liver remnant; MELD, model of end-stage liver disease.
All patients were described as having Child's A cirrhosis.
Footnotes
Presented in part at the 63rd Annual Cancer Symposium of the Society of Surgical Oncology, March 4-7, 2010, St Louis, MO.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Hari Nathan, John F.P. Bridges, Richard D. Schulick, Andrew M. Cameron, Kenzo Hirose, Dorry L. Segev, Michael A. Choti, Timothy M. Pawlik
Financial support: John F.P. Bridges
Administrative support: John F.P. Bridges
Provision of study materials or patients: Hari Nathan, John F.P. Bridges, Dorry L. Segev, Timothy M. Pawlik
Collection and assembly of data: Hari Nathan, John F.P. Bridges, Dorry L. Segev, Timothy M. Pawlik
Data analysis and interpretation: Hari Nathan, John F.P. Bridges, Timothy M. Pawlik
Manuscript writing: Hari Nathan, John F.P. Bridges, Richard D. Schulick, Andrew M. Cameron, Kenzo Hirose, Barish H. Edil, Christopher L. Wolfgang, Dorry L. Segev, Michael A. Choti,Timothy M. Pawlik
Final approval of manuscript: Hari Nathan, John F.P. Bridges, Richard D. Schulick, Andrew M. Cameron, Kenzo Hirose, Barish H. Edil, Christopher L. Wolfgang, Dorry L. Segev, Michael A. Choti,Timothy M. Pawlik
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: Resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 3.Bellavance EC, Lumpkins KM, Mentha G, et al. Surgical management of early-stage hepatocellular carcinoma: Resection or transplantation? J Gastrointest Surg. 2008;12:1699–1708. doi: 10.1007/s11605-008-0652-2. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham SC, Tsai S, Marques HP, et al. Management of early hepatocellular carcinoma in patients with well-compensated cirrhosis. Ann Surg Oncol. 2009;16:1820–1831. doi: 10.1245/s10434-009-0364-1. [DOI] [PubMed] [Google Scholar]
- 5.Jarnagin WR. Management of small hepatocellular carcinoma: A review of transplantation, resection, and ablation. Ann Surg Oncol. 2010;17:1226–1233. doi: 10.1245/s10434-010-0978-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 7.Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 1999;19:311–322. doi: 10.1055/s-2007-1007120. [DOI] [PubMed] [Google Scholar]
- 8.Iwatsuki S, Dvorchik I, Marsh JW, et al. Liver transplantation for hepatocellular carcinoma: A proposal of a prognostic scoring system. J Am Coll Surg. 2000;191:389–394. doi: 10.1016/s1072-7515(00)00688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figueras J, Ibañez L, Ramos E, et al. Selection criteria for liver transplantation in early-stage hepatocellular carcinoma with cirrhosis: Results of a multicenter study. Liver Transpl. 2001;7:877–883. doi: 10.1053/jlts.2001.27856. [DOI] [PubMed] [Google Scholar]
- 10.Todo S, Furukawa H. Living donor liver transplantation for adult patients with hepatocellular carcinoma: Experience in Japan. Ann Surg. 2004;240:451–459. doi: 10.1097/01.sla.0000137129.98894.42. discussion 459–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou XD, Tang ZY, Yang BH, et al. Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer. 2001;91:1479–1486. doi: 10.1002/1097-0142(20010415)91:8<1479::aid-cncr1155>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Poon RT, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: Implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathan H, Schulick RD, Choti MA, et al. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg. 2009;249:799–805. doi: 10.1097/SLA.0b013e3181a38eb5. [DOI] [PubMed] [Google Scholar]
- 14.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho YK, Kim JK, Kim MY, et al. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453–459. doi: 10.1002/hep.22648. [DOI] [PubMed] [Google Scholar]
- 16.Cho YK, Kim JK, Kim WT, et al. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: A Markov model analysis. Hepatology. 2010;51:1284–1290. doi: 10.1002/hep.23466. [DOI] [PubMed] [Google Scholar]
- 17.Luce RD, Tukey JW. Simultaneous conjoint-measurement: A new type of fundamental measurement. J Math Psych. 1964;1:1–27. [Google Scholar]
- 18.Green PE, Rao VR. Conjoint measurement for quantifying judgmental data. J Market Res. 1971;8:355–363. [Google Scholar]
- 19.Louviere JJ, Hensher DA, Swait JD. Stated choice methods: Analysis and applications. Cambridge, United Kingdom, and New York, NY: Cambridge University Press; 2000. [Google Scholar]
- 20.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320:1530–1533. doi: 10.1136/bmj.320.7248.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bridges JF. Stated preference methods in health care evaluation: An emerging methodological paradigm in health economics. Appl Health Econ Health Policy. 2003;2:213–224. [PubMed] [Google Scholar]
- 22.Kee F, McDonald P, Kirwan JR, et al. The stated and tacit impact of demographic and lifestyle factors on prioritization decisions for cardiac surgery. QJM. 1997;90:117–123. doi: 10.1093/qjmed/90.2.117. [DOI] [PubMed] [Google Scholar]
- 23.Timmermans DR, Gooszen AW, Geelkerken RH, et al. Analysis of the variety in surgeons' decision strategies for the management of left colonic emergencies. Med Care. 1997;35:701–713. doi: 10.1097/00005650-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Kee F, McDonald P, Kirwan JR, et al. Urgency and priority for cardiac surgery: A clinical judgment analysis. BMJ. 1998;316:925–929. doi: 10.1136/bmj.316.7135.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouma BJ, van der Meulen JH, van den Brink RB, et al. Variability in treatment advice for elderly patients with aortic stenosis: A nationwide survey in the Netherlands. Heart. 2001;85:196–201. doi: 10.1136/heart.85.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouma BJ, van der Meulen JH, van den Brink RB, et al. Validity of conjoint analysis to study clinical decision making in elderly patients with aortic stenosis. J Clin Epidemiol. 2004;57:815–823. doi: 10.1016/j.jclinepi.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Lamme B, Boermeester MA, de Vos R, et al. Survey among surgeons on surgical treatment strategies for secondary peritonitis. Dig Surg. 2004;21:387–394. doi: 10.1159/000081883. discussion 394–395. [DOI] [PubMed] [Google Scholar]
- 28.Caldon LJ, Walters SJ, Ratcliffe J, et al. What influences clinicians' operative preferences for women with breast cancer? An application of the discrete choice experiment. Eur J Cancer. 2007;43:1662–1669. doi: 10.1016/j.ejca.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 29.Langenhoff BS, Krabbe PF, Ruers TJ. Computer-based decision making in medicine: A model for surgery of colorectal liver metastases. Eur J Surg Oncol. 2007; 33(suppl 2):S111–S117. doi: 10.1016/j.ejso.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Danishevski K, McKee M, Sassi F, et al. The decision to perform caesarean section in Russia. Int J Qual Health Care. 2008;20:88–94. doi: 10.1093/intqhc/mzm070. [DOI] [PubMed] [Google Scholar]
- 31.Hedayat A, Sloane NJA, Stufken J. Orthogonal arrays: Theory and Applications. New York, NY: Springer; 1999. [Google Scholar]
- 32.Kucirka LM, Namuyinga R, Hanrahan C, et al. Formal policies and special informed consent are associated with higher provider utilization of CDC high-risk donor organs. Am J Transplant. 2009;9:629–635. doi: 10.1111/j.1600-6143.2008.02523.x. [DOI] [PubMed] [Google Scholar]
- 33.Long JS, Freese J. Regression models for categorical dependent variables using Stata (ed 2) College Station, TX: StataCorp LP; 2006. [Google Scholar]
- 34.El-Serag HB, Siegel AB, Davila JA, et al. Treatment and outcomes of treating of hepatocellular carcinoma among Medicare recipients in the United States: A population-based study. J Hepatol. 2006;44:158–166. doi: 10.1016/j.jhep.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Cucchetti A, Ercolani G, Vivarelli M, et al. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl. 2006;12:966–971. doi: 10.1002/lt.20761. [DOI] [PubMed] [Google Scholar]
- 36.Cescon M, Vetrone G, Grazi GL, et al. Trends in perioperative outcome after hepatic resection: Analysis of 1500 consecutive unselected cases over 20 years. Ann Surg. 2009;249:995–1002. doi: 10.1097/SLA.0b013e3181a63c74. [DOI] [PubMed] [Google Scholar]
- 37.Maslow AH. The Psychology of Science: a Reconnaissance (ed 1) New York, NY: Harper and Row; 1966. [Google Scholar]
- 38.Kirwan JR, Chaput de Saintonge DM, Joyce CR, et al. Inability of rheumatologists to describe their true policies for assessing rheumatoid arthritis. Ann Rheum Dis. 1986;45:156–161. doi: 10.1136/ard.45.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wigton RS, Hoellerich VL, Patil KD. How physicians use clinical information in diagnosing pulmonary embolism: An application of conjoint analysis. Med Decis Making. 1986;6:2–11. doi: 10.1177/0272989X8600600102. [DOI] [PubMed] [Google Scholar]
- 40.Wigton RS. Use of linear models to analyze physicians' decisions. Med Decis Making. 1988;8:241–252. doi: 10.1177/0272989X8800800404. [DOI] [PubMed] [Google Scholar]
- 41.Bachmann LM, Muhleisen A, Bock A, et al. Vignette studies of medical choice and judgment to study caregivers' medical decision behaviour: Systematic review. BMC Med Res Methodol. 2008;8:50. doi: 10.1186/1471-2288-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robson N, Rew D. Collective wisdom and decision making in surgical oncology. Eur J Surg Oncol. 2010;36:230–236. doi: 10.1016/j.ejso.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Rovner DR, Rothert ML, Holmes MM. Validity of structured cases to study clinical decision-making. Clin Res. 1986;34:A834–A834. [Google Scholar]