Abstract
Background
The role of surgical resection for patients with large or multifocal intrahepatic cholangiocarcinoma (ICC) remains unclear. This study evaluated the long-term outcome of patients who underwent hepatic resection for large (≥7 cm) or multifocal (≥2) ICC.
Methods
Between 1990 and 2013, 557 patients who underwent liver resection for ICC were identified from a multi-institutional database. Clinicopathologic characteristics, operative details, and long-term survival data were evaluated.
Results
Of the 557 patients, 215 (38.6 %) had a small, solitary ICC (group A) and 342 (61.4 %) had a large or multifocal ICC (group B). The patients in group B underwent an extended hepatectomy more frequently (16.9 vs. 30.4 %; P < 0.001). At the final pathology exam, the patients in group B were more likely to show evidence of vascular invasion (22.5 vs. 38.5 %), direct invasion of contiguous organs (6.5 vs. 12.9 %), and nodal metastasis (13.3 vs. 21.0 %) (all P < 0.05). Interestingly, the incidences of postoperative complications (39.3 vs. 46.8 %) and hospital mortality (1.1 vs. 3.7 %) were similar between the two groups (both P > 0.05). The group A patients had better rates for 5-year overall survival (OS) (30.5 vs. 18.7 %; P < 0.05) and disease-free survival (DFS) (22.6 vs. 8.2 %; P < 0.05) than the group B patients. For the patients in group B, the factors associated with a worse OS included more than three tumor nodules [hazard ratio (HR), 1.56], nodal metastasis (HR, 1.47), and poor differentiation (HR, 1.48).
Conclusions
Liver resection can be performed safely for patients with large or multifocal ICC. The long-term outcome for these patients can be stratified on the basis of a prognostic score that includes tumor number, nodal metastasis, and poor differentiation.
Intrahepatic cholangiocarcinoma (ICC) represents 10 to 15 % of all primary liver malignancies.1,2 The incidence of ICC has increased worldwide, from 3.2 per 1,000,000 in 1975 to 8.5 per 1,000,000 in 2000, and has continued to increase during the last decade.3–5 The prognosis of patients with ICC is poor, with an estimated median survival time of 18–39 months and a median 5-year survival rate of 25–40 %.6–8 Complete surgical resection is the only hope for the long-term survival of patients with ICC, but unfortunately, only 30–54 % of these patients are eligible for resection with curative intent at the time of presentation.9–12
Several clinicopathologic factors have an impact on the prognosis of patients undergoing surgical resection for ICC. As summarized in a recent prognostic nomogram, these factors include age at diagnosis, number of tumors, tumor size, presence of cirrhosis, lymph node metastasis, and vascular invasion.13 Whereas multifocal disease has been recognized as an important prognostic factor denoted by its incorporation into the American Joint Committee on Cancer (AJCC) staging system,14 the prognostic role of tumor size is more controversial.15 Some investigators have failed to note an association of tumor size with prognosis,16 but other data have suggested that large ICC tumor size does indeed confer a worse long-term outcome.13,17 However, more recently, our group, using a large multi-institutional data set, noted that the prognostic importance of tumor size had a nonlinear threshold effect on prognosis.13 In fact, the effect of tumor size on the risk of death was linear until the tumor was approximately 7 cm in diameter, after which the risk of death associated with any further incremental increase in size plateaued.13
Given that patients with multifocal or large ICC tumors may have a worse prognosis, the role of surgical resection for this group of patients remains unclear. For patients with advanced disease, the decision whether a patient is a candidate for surgery can be challenging from both technical and oncologic aspects. In particular, patients with large or multifocal ICC can require a technically complex operation with an associated increased risk of morbidity and an R1 margin.18 Nonsurgical options such as chemotherapy, however, are largely ineffective, leaving surgery as the only option with a potentially meaningful therapeutic effect.12,19,20
The current study therefore aimed to evaluate the disease-free survival (DFS) and long-term overall survival (OS) of patients who underwent hepatic resection for large (≥7 cm) or multifocal (≥2) ICCs. Specifically, we sought to define the therapeutic benefit of resection among patients with advanced ICC using a large multi-institutional cohort. In addition, we characterized the clinicopathologic factors associated with worse long-term outcomes among patients with large or multifocal ICC lesions undergoing surgical resection.
METHODS
Patient Population and Data Collection
A multi-institutional database consisting of 557 patients with ICC who underwent liver resection from 1990 to 2013 at 12 major hepatobiliary centers in the United States, Europe, Australia, and Asia was used for this study. The 12 medical centers included Johns Hopkins Hospital; Medical College of Wisconsin; Stanford University; University of Virginia; Emory University; University of Pittsburgh; Fundeni Clinical Institute of Digestive Disease; Curry Cabral Hospital; Hopitaux Universitaires De Geneve; Ospedale San Raffaele; Royal Prince Alfred Hospital, University of Sydney; and Eastern Hepatobiliary Surgery Hospital. The institutional review boards of the participating institutions approved the study. Only patients with histologically confirmed ICC were included in the cohort.
All the patients underwent hepatic resection with curative intent. The patients with metastatic disease, represented by American Join Committee on Cancer (AJCC) stage 4b, were excluded from the study, whereas the patients with AJCC T4 N0 M0 or T any N1 M0 (AJCC stage 4a) were included in the study cohort.
Standard demographic and clinicopathologic data were collected including sex, age, and tumor characteristics. Tumor size was defined as the maximal diameter of the tumor in the resected specimen, and the largest lesion was used as the index lesion in cases of patients with multiple tumors. For the purposes of analyses, tumor size was stratified using a 7-cm threshold based on the cutoff value used to define “large” tumor in the ICC nomogram published by Hyder et al.13 Histologic grade was categorized as well, moderate, or poor based on the grade of differentiation. If tumor grade varied for a specific specimen, the “worst” grade was used as the index tumor grade. Data on tumor stage also were collected according to the 7th edition of the AJCC staging system.14
Major hepatectomy was defined as the resection of three or more liver segments according to Couinaud’s classification. Nodal status was ascertained based on the final pathologic assessment. Perioperative complications and mortality within 90 days after the operation were obtained, and morbidity was defined based on the Clavien–Dindo classification system.21 The date of the last follow-up visit and vital status were collected for all the patients.
Statistical Analysis
Descriptive statistics are reported as the median and interquartile range (IQR) for continuous variables and as whole numbers and percentages for categorical variables. The baseline characteristics of the study population were summarized according to the following stratification: group A (patients with a small [<7 cm] solitary ICC versus group B (patients with large [≥7 cm] or multifocal [>1 nodule] ICC). Comparative analysis was performed using χ2, Student’s t test, and the Wilcoxon rank-sum test as appropriate. The Kaplan–Meier method was used to generate DFS and OS, and differences in survival were examined with the log-rank test. The association of relevant clinicopathologic variables with prognosis among the patients in group B was assessed using Cox proportional hazards models. Variables with statistical significance (P < 0.05) in the univariable analysis were included in the multivariable model. The prognostic power of covariates was expressed by calculating hazard ratios (HRs) with 95 % confidence intervals (CIs).
For statistical analyses, P values lower than 0.05 (two-tailed) were deemed significant. All analyses were performed with STATA version 12.0 (StataCorp LP, College Station, TX, USA).
RESULTS
Demographic and Clinicopathologic Characteristics
We identified 557 patients who underwent liver resection for ICC with curative intent and met the inclusion criteria of the study. The baseline characteristics of the population are summarized in Table 1.
TABLE 1.
Patient and tumor characteristics of the entire cohort and of the two groups
| Variable | Total (n = 557) n (%) |
Group A (n = 215) n (%) |
Group B (n = 342) n (%) |
P value |
|---|---|---|---|---|
| Median age: years (IQR) | 60.1 (51.2–69.1) | 60.8 (53.2–69.9) | 59.7 (49.6–69.0) | 0.09 |
| Gender (n = 554) | 0.19 | |||
| Female | 260 (46.9) | 93 (43.5) | 167 (49.1) | |
| Male | 294 (53.1) | 121 (56.5) | 173 (50.9) | |
| Race (n = 505) | 0.85 | |||
| White | 412 (81.6) | 152 (80.9) | 260 (82.0) | |
| Black | 15 (3.0) | 5 (2.7) | 10 (3.2) | |
| Others | 78 (15.4) | 31 (16.5) | 47 (14.8) | |
| Median size: cm (IQR) | 6.3 (4.5–9.0) | 4.6 (3.5–5.6) | 8.5 (7.0–11.0) | <0.001 |
| Median no. lesions: n (IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–1.0) | 2.0 (1.0–2.0) | <0.001 |
| Cirrhosis | 53 (9.8) | 24 (11.2) | 29 (8.9) | 0.38 |
| Median CEA: n (IQR) | 2.4 (1.4–4.2) | 2.5 (1.4–4.0) | 2.4 (1.2–4.4) | 0.90 |
| Median CA 19–9: n (IQR) | 84.0 (25.8–280.6) | 63.4 (19.6–237.0) | 91.0 (28.0–300.0) | 0.11 |
| Vascular invasion | 172 (32.1) | 48 (22.5) | 124 (38.5) | <0.001 |
| Perineural invasion | 102 (19.4) | 44 (21.0) | 58 (18.4) | 0.46 |
| Biliary invasion | 77 (14.2) | 25 (11.6) | 52 (15.9) | 0.16 |
| Direct invasion of contiguous organs | 56 (10.4) | 14 (6.5) | 42 (12.9) | 0.02 |
| Nodal status | 0.04 | |||
| Positive | 94 (17.9) | 28 (13.3) | 66 (21.0) | |
| Negative | 245 (46.6) | 97 (46.0) | 148 (47.0) | |
| Not harvested | 186 (35.4) | 85 (40.3) | 101 (32.1) | |
| AJCC T stage | <0.001 | |||
| T1 | 229 (43.6) | 147 (69.7) | 82 (26.1) | |
| T2 | 191 (36.4) | 38 (18.0) | 153 (48.7) | |
| T3 | 71 (13.5) | 11 (5.2) | 60 (19.1) | |
| T4 | 34 (6.5) | 15 (7.1) | 19 (6.1) | |
| AJCC (n = 324) | <0.001 | |||
| 1 | 120 (37.0) | 68 (55.7) | 52 (25.7) | |
| 2 | 84 (25.9) | 17 (13.9) | 67 (33.2) | |
| 3 | 22 (6.8) | 2 (1.6) | 20 (9.9) | |
| 4a | 98 (30.2) | 35 (28.7) | 63 (31.2) | |
| Type of surgical resection | <0.001 | |||
| Less than hemihepatectomy | 168 (31.0) | 89 (41.8) | 79 (24.0) | |
| Hemihepatectomy | 238 (43.9) | 88 (41.3) | 150 (45.6) | |
| Extended hemihepatectomy | 136 (25.1) | 36 (16.9) | 100 (30.4) | |
| Tumor grade | 0.03 | |||
| Well | 62 (11.5) | 31 (14.6) | 31 (9.5) | |
| Moderate | 339 (63.0) | 139 (65.3) | 200 (61.5) | |
| Poor | 137 (25.5) | 43 (20.2) | 94 (28.9) | |
| R0 margin | 452 (83.2) | 186 (86.5) | 266 (81.1) | 0.10 |
| Lymphadenectomy | 267 (49.5) | 85 (39.5) | 182 (56.2) | <0.001 |
| Median no. of lymph node harvested: n (IQR) | 2.0 (1.0–5.0) | 1.0 (0.0–4.0) | 2.0 (1.0–6.0) | <0.001 |
| Postoperative complications | 240 (43.9) | 84 (39.3) | 156 (46.8) | 0.08 |
| Grade of complications | 0.21 | |||
| Grades 1 & 2 | 146 (63.8) | 56 (69.1) | 90 (60.8) | |
| Grades 3 & 4 | 83 (36.2) | 25 (30.9) | 58 (39.2) | |
| Perioperative mortality | 13 (2.7) | 2 (1.1) | 11 (3.7) | 0.09 |
| Adjuvant chemotherapy | 260 (50.3) | 102 (49.5) | 158 (50.8) | 0.77 |
| Adjuvant radiotherapy | 57 (11.5) | 25 (12.5) | 32 (10.8) | 0.57 |
| Recurrence | 384 (68.9) | 130 (60.5) | 254 (74.3) | 0.001 |
| Intrahepatic | 234 (60.3) | 80 (60.6) | 154 (60.2) | 0.93 |
| Extrahepatic or both | 154 (39.7) | 52 (39.4) | 102 (39.8) |
IQR interquartile range, CEA carcinoembryonic antigen, CA carbohydrate antigen, AJCC American Joint Committee on Cancer.
Of the 557 patients, 215 (38.6 %) had a small, solitary ICC (group A), whereas 342 (61.4 %) had a large or multifocal ICC (group B) (Table 1). The patients in group A had a single-nodule ICC measuring 4.6 cm (IQR, 3.5–5.6), whereas the patients in group B had a median tumor burden of 2 (IQR, 1–2) and a median tumor size of 8.5 cm (IQR, 7.0–11.0 cm). The two groups did not differ in terms of age, sex, race, presence of cirrhosis, or level of tumor markers (all P > 0.05). According to the final pathology, the patients in group B had a higher proportion of tumors with vascular invasion (22.5 vs. 38.5 %; P < 0.001), poor differentiation (20.2 vs. 28.9 %; P = 0.03), direct invasion of contiguous organs (6.5 vs. 12.9 %; P = 0.02), and nodal metastasis (13.3 vs. 21.0 %; P = 0.04). Group B had fewer patients with T1 tumors (69.7 vs. 26.1 %; P < 0.001) and a lower proportion of patients with AJCC stage 1 disease (55.7 vs. 25.7 %; P < 0.001).
Concerning the treatment strategy, the patients in group B underwent an extended hepatectomy more often than the patients in group A (16.9 vs. 30.4 %), whereas the group A patients underwent less than a hemihepatectomy more often than the group B patients (41.8 vs. 24.0 %; P < 0.001). The patients in group B also were more likely to undergo a lymphadenectomy (39.5 vs. 56.2 %; P < 0.001). The incidence and grade of postoperative complications were similar between the two groups (both P > 0.05). Notably, even the type of complication was comparable (Table 2). The two groups showed no difference in the incidence of perioperative mortality (group A, 1.1 % vs. group B, 3.7 %; P = 0.08).
TABLE 2.
Postoperative complications in the entire cohort and in the two groups
| Complication | Total (n = 557) n (%) |
Group A (n = 215) n (%) |
Group B (n = 342) n (%) |
P value |
|---|---|---|---|---|
| Wound infection/ surgical-site infection |
19 (3.4) | 9 (4.2) | 10 (2.9) | 0.42 |
| Wound/fascia dehiscence |
9 (1.6) | 4 (1.9) | 5 (1.5) | 0.72 |
| Intraabdominal abscess (excluding liver abscess) |
12 (2.2) | 6 (2.8) | 6 (1.8) | 0.41 |
| Sepsis/multiorgan failure |
6 (1.1) | 3 (1.4) | 3 (0.9) | 0.56 |
| Bleeding/hemorrhage | 4 (0.7) | 2 (0.9) | 2 (0.6) | 0.64 |
| Gastrointestinal bleeding |
2 (0.4) | 2 (0.9) | 0 (0.0) | 0.07 |
| Bowel perforation | 2 (0.4) | 1 (0.5) | 1 (0.3) | 0.74 |
| Ileus | 9 (1.6) | 4 (1.9) | 5 (1.5) | 0.72 |
| Anastomotic leakage | 4 (0.7) | 1 (0.5) | 3 (0.9) | 0.58 |
| Bileleak/bileduct obstruction |
49 (8.8) | 14 (6.5) | 35 (10.2) | 0.13 |
| Liver abscess | 8 (1.4) | 2 (0.9) | 6 (1.8) | 0.43 |
| Cholangitis | 4 (0.7) | 0 (0.0) | 4 (1.2) | 0.11 |
| Liver failure | 15 (2.7) | 4 (1.9) | 11 (3.2) | 0.34 |
| Pneumonia | 10 (1.8) | 3 (1.4) | 7 (2.0) | 0.57 |
| Pleural effusion | 49 (8.8) | 18 (8.4) | 31 (9.1) | 0.78 |
| Pulmonary embolism | 10 (1.8) | 1 (0.5) | 9 (2.6) | 0.06 |
| Respiratory insufficiency |
6 (1.1) | 3 (1.4) | 3 (0.9) | 0.56 |
| Pneumothorax | 1 (0.2) | 0 (0.0) | 1 (0.3) | 0.43 |
| Cardiac | 14 (2.5) | 8 (3.7) | 6 (1.8) | 0.15 |
| Cerebrovascular accident |
1 (0.2) | 0 (0.0) | 1 (0.3) | 0.43 |
| Renal failure | 1 (0.2) | 0 (0.0) | 1 (0.3) | 0.43 |
| Urinary tract infection |
6 (1.1) | 2 (0.9) | 4 (1.2) | 0.79 |
| Other | 40 (7.2) | 19 (8.8) | 21 (6.1) | 0.23 |
Long-Term Outcome
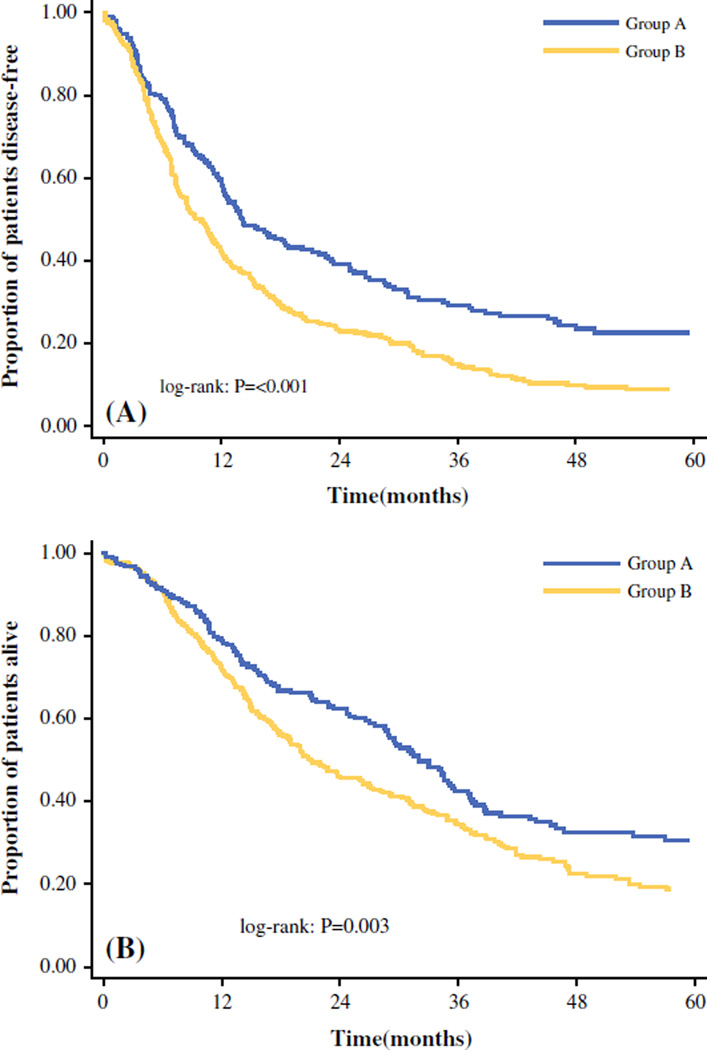
During a median follow-up period of 22.3 months, the majority of the patients experienced recurrence (n = 384, 68.9 %). Most of the patients (n = 234, 60.3 %) experienced recurrence only at an intrahepatic site, whereas 154 (39.7 %) patients had recurrence with an extrahepatic site as a component of disease recurrence. The median 1-, 3-, and 5-year DFS for the entire cohort was 11.8 months, 48.9, 20.2 and 13.8 %, respectively. Group A had a median DFS of 14.1 months (95 % confidence interval [CI], 12.2–18.7 months), whereas group B had a median DFS of 9.5 months (95 % CI, 7.9–11.3 months) (P < 0.001; Fig. 1a). The DFS was 59.6 % at 1 year, 29.1 % at 3 years, and 22.6 % at 5 years in Group A versus 41.8, 14.6, and 8.2 %, respectively, in group B.
FIG. 1.
a Disease-free survival (DFS) curves of patients undergoing hepatic resection for intrahepatic cholangiocarcinoma (ICC): group A (small solitary ICC) and group B (large or multifocal ICC). b Overall survival (OS) curves of patients undergoing hepatic resection for ICC: group A (small solitary ICC) and group B (large or multifocal ICC)
Most recurrences occurred within 24 months after surgery. Notably, short-term recurrence was more common among the group B patients than among the group A patients (76.2 vs. 66.0 %; P = 0.06). The pattern of recurrence did not differ between the two groups (intrahepatic recurrence only: group A, 60.6 % vs. group B, 60.2 %; extrahepatic recurrence as a component of failure: group A, 39.4 % vs. group B, 39.8 %; P = 0.93).
The median, 1-, 3- and 5-year OS for the entire cohort was 26.9 months, 74.7, 37.6, and 23.4 %, respectively. The OS was worse for the group B patients than for the group A patients (Fig. 1b). Specifically, whereas the patients in group A had a median OS of 32.0 months (95 % CI, 28.6–35.6 months), the patients in group B had a median survival of 21.1 months (95 % CI, 18.6–26.3 months) (P = 0.003). The 1-, 3-, and 5-year OS was 79.3 % at 1 year, 42.4 % at 3 years, and 30.5 % at 5 years in group A versus 71.7, 34.5, and 18.7 %, respectively, in group B.
Prognostic Factors
The patients with large or multifocal ICC (group B) were further analyzed with respect to the prognostic factors affecting DFS and OS (Table 3). In the multivariable analyses, the factors associated with DFS included more than three tumor nodules (hazard ratio [HR], 1.72; 95 % CI, 1.10–2.69; P = 0.02), vascular invasion (HR, 1.60; 95 % CI, 1.20–2.15; P = 0.001), nodal metastasis (HR, 1.76; 95 % CI, 1.29–2.40; P < 0.001), and poor tumor differentiation (HR, 1.37; 95 % CI, 1.02–1.83; P = 0.03). Similarly, in the univariable analysis, more than three tumor nodules, nodal metastasis, and poor tumor differentiation were associated with worse OS. In the multivariable analysis, more than three tumor nodules (HR, 1.56; 95 % CI, 1.09–2.23; P = 0.02), nodal metastasis (HR, 1.47; 95 % CI, 1.05–2.07; P = 0.02), and poor tumor differentiation (HR, 1.48; 95 % CI, 1.11–1.96; P = 0.01) remained as independent predictors of worse survival.
TABLE 3.
Significant prognostic factors for overall survival of group B patients
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | P value | HR | 95 % CI | P value | |
| Age >60 years | 1.06 | (0.82–1.38) | 0.63 | |||
| Male gender | 1.07 | (0.83–1.39) | 0.59 | |||
| No. of lesions >3 | 1.7 | (1.22–2.37) | <0.001 | 1.56 | (1.09–2.23) | 0.02 |
| Cirrhosis | 1.24 | (0.79–1.95) | 0.34 | |||
| Vascular invasion | 1.31 | (1.00–1.72) | 0.05 | |||
| Perineural invasion | 0.88 | (0.61–1.27) | 0.49 | |||
| Biliary invasion | 1.41 | (0.99–2.01) | 0.05 | |||
| Direct invasion of contiguous organs | 1.39 | (0.95–2.03) | 0.09 | |||
| Nodal metastasis | 1.71 | (1.25–2.34) | <0.001 | 1.47 | (1.05–2.07) | 0.02 |
| Poor tumor differentiation | 1.53 | (1.16–2.02) | <0.001 | 1.48 | (1.11–1.96) | 0.01 |
| Major hepatectomy | 1.05 | (0.79–1.41) | 0.73 | |||
| R1 margins | 1.29 | (0.90–1.85) | 0.16 | |||
| No. lymphadenectomy | 1.07 | (0.83–1.39) | 0.60 | |||
| No. of lymph node harvested <3 | 0.85 | (0.60–1.21) | 0.36 | |||
HR hazard ratio, CI confidence interval.
For the patients with large multifocal ICC undergoing resection, prognosis was stratified based on the number of risk factors present (Fig. 2). The patients without any prognostic risk factors had the most favorable 5-year survival rate (28.8 %), which was better than the prognosis of the patients with one risk factor (12.8 %; P < 0.001). The patients with two or more risk factors had the worst prognosis (5-year survival rate, 3.2 %). Notably, among the group B patients, the median survival time was 29.2 months for the patients with a single tumor 7 cm in size or larger who had no other risk factors, whereas the median survival time for the patients with more than three tumor nodules was 14.9 months. Conversely, the median survival time was 17.8 months for the patients with nodal metastasis and 26 months for the patients with poor tumor differentiation.
FIG. 2.
Overall survival curves of group B patients stratified by the number of risk factors present.
DISCUSSION
The management of patients with advanced ICC is ill defined. Whether the treatment of choice should be hepatic resection, systemic chemotherapy, or intraarterial therapy remains controversial. This study evaluated the short- and long-term outcomes for patients with large or multifocal ICC after curative hepatic resection. Although large tumor size and multiple tumor nodules are reported to be negative prognostic factors for patients undergoing liver resection with curative intent,22 surgery in many cases can be the only hope of cure for patients with large or multifocal ICC. For this reason, it is important to analyze the short- and long-term outcomes for this group of patients to determine whether surgical treatment is justified. To the best of our knowledge, this is the first international multi-institutional study that specifically examined survival and defined prognostic factors after hepatic resection for large or multifocal ICC.
One main objective of the current study was to determine the safety of hepatic resection for patients with advanced ICC. The data strongly suggest that hepatic resection can indeed be performed safely for patients with large or multifocal ICC. In fact, although the group B patients underwent a higher proportion of major hepatic resections, the perioperative morbidity and mortality were similar between the two groups (Table 2). Similarly, even the grade and type of complications were comparable between the two groups.
The patients in group B had worse DFS and OS than the group A patients. Whereas the 5-year survival time for the patients in group A was 30.5 %, the patients with large or multifocal ICC had a 4-year survival time of only 18.7 %. This finding perhaps was not surprising because large or multifocal tumors are more likely to have an aggressive tumor biology.13,17 In fact, tumor size and number were included in the nomogram created by Hyder et al.13 to predict the survival of ICC after resection. In the current study, we expanded on this previous study and specifically defined recurrence and survival outcomes for patients with advanced disease. Although the risk of short-term recurrence was high among all the patients (i.e., >50 %), we noted that it was particularly high among the group B patients, with more than three fourths of these patients having a recurrence within 2 years. It was sobering to note that less than 10 % of the group B patients survived disease-free for 5 years after hepatic resection versus more than 20 % of the group A patients. Nonetheless, the pattern of recurrence was similar between the two groups, which differed from data from Ng et al.23 who reported that large, multifocal hepatocellular lesions were more likely to recur within the liver. These data serve to emphasize that whereas hepatocellular carcinoma tends to be more a locoregional disease process, the natural history of advanced ICC tends to more systemic.12
Although the survival after surgery in group B was inferior to the survival in group A, hepatic resection was able to achieve a 5-year survival for nearly 1 in 5 patients with large or multifocal ICC. In addition, the prognosis of the group B patients was not homogeneous but varied based on several prognostic factors. The three independent factors identified as affecting OS adversely included more than three tumor nodules, nodal metastasis, and poor tumor differentiation. These findings are consistent with a recent metaanalysis reporting prognostic factors among a pooled cohort of 2,132 patients with ICC.24 In the current study, we noted that the group B patients without any of these risk factors had a 5-year OS rate of 28.8 %, which was comparable with the 30.5 % 5-year survival rate for the patients in group A. The patients with one risk factor had less favorable outcomes, whereas the patients with two or more risk factors had the worst survival (Fig. 2). Notably, the patients with a single tumor measuring 7 cm or larger and no other risk factors in group B had a median survival comparable with the survival of the group A patients. In contrast, the patients with more than three tumor nodules or nodal metastasis fared particularly poorly, with a median survival period shorter than 2 years. Collectively, the data suggest that surgical resection may provide a potential benefit for certain subsets of patients with advanced ICC (i.e., large solitary tumor, no nodal metastasis). In contrast, the overall poor DFS and OS for other patients with advanced ICC (>3 tumors, nodal metastasis) need to be considered in deciding whether to proceed with operative management or not. Although surgery may benefit some of these patients, other neoadjuvant approaches such as chemotherapy or intraarterial therapy should be considered to allow the tumor biology to declare itself better before initiation of resection.12,25
The current study had several limitations that should be considered. As with all retrospective studies, this investigation may have had a selection bias regarding the diagnosis and treatment of the patients with ICC. We used a surgical database, so only the data for patients who underwent resection were collected. As such, we cannot comment specifically on the patients who did not undergo resection or on what factors may have determined their inoperability. Of interest, when data from the current surgical series were compared with data from a previous reported series of ICC patients who underwent intraarterial therapy, the latter nonsurgical group seemed to be slightly older and to have a higher incidence of extrahepatic extension and nodal metastasis.25 Another possible limitation of the current study was that the findings reflect the experience of major hepatobiliary centers and might not be generalizable to low-volume centers. Although the multicenter nature of the study is a considerable strength conferring a larger sample and more generalizability, it also could lead to some heterogeneity in the therapeutic approach to patients.
In conclusion, we report the first multi-institutional study that specifically examined the outcomes of patients with large or multifocal ICC treated with hepatic resection. The findings show that liver resection can be performed safely and yields acceptable short-term outcomes. This study was able to stratify the long-term outcomes of patients with large or multifocal ICC on the basis of a prognostic score that included factors such as more than three tumors, nodal metastasis, and poor tumor differentiation. The use of these factors may help to identify subsets of patients with advanced ICC who might benefit the most from surgical management.
Acknowledgments
The authors thank Donielle Neal, Maria Knoblich, Mafalda Sobral, Eduardo Barroso, Jorge Lamelas, Susana Rodrigues, Francesca Ratti, and Stéphanie Meyer.
Footnotes
CONFLICT OF INTEREST There are no conflicts of interest.
REFERENCES
- 1.Bektas H, Schrem H, Kleine M, et al. Primary liver tumours: presentation, diagnosis and surgical treatment. InTech. 2013:91–116. [Google Scholar]
- 2.Aljiffry M, Abdulelah A, Walsh M, Peltekian K, Alwayn I, Molinari M. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg. 2009;208:134–147. doi: 10.1016/j.jamcollsurg.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 4.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 5.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 6.Morimoto Y, Tanaka Y, Ito T, et al. Long-term survival and prognostic factors in the surgical treatment for intrahepatic cholangiocarcinoma. J Hepatobil Pancreat Surg. 2003;10:432–440. doi: 10.1007/s00534-002-0842-3. [DOI] [PubMed] [Google Scholar]
- 7.Konstadoulakis MM, Roayaie S, Gomatos IP, et al. Fifteen-year, single-center experience with the surgical management of intrahepatic cholangiocarcinoma: operative results and long-term outcome. Surgery. 2008;143:366–374. doi: 10.1016/j.surg.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Choi S-B, Kim K-S, Choi J-Y, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009;16:3048–3056. doi: 10.1245/s10434-009-0631-1. [DOI] [PubMed] [Google Scholar]
- 9.Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992– 2008. Hepatology. 2012;55:476–482. doi: 10.1002/hep.24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan H, Segev DL, Mayo SC, et al. National trends in surgical procedures for hepatocellular carcinoma: 1998–2008. Cancer. 2012;118:1838–1844. doi: 10.1002/cncr.26501. [DOI] [PubMed] [Google Scholar]
- 11.Cance WG, Stewart AK, Menck HR. The National Cancer Data Base Report on treatment patterns for hepatocellular carcinomas: improved survival of surgically resected patients, 1985–1996. Cancer. 2000;88:912–920. doi: 10.1002/(sici)1097-0142(20000215)88:4<912::aid-cncr23>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 12.Dodson RM, Weiss MJ, Cosgrove D, et al. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg. 2013;217:736–750. e734. doi: 10.1016/j.jamcollsurg.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Hyder O, Marques H, Pulitano C, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg. 2014;149(5):432–438. doi: 10.1001/jamasurg.2013.5168. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th. New York: Springer; 2010. pp. 143–164. [Google Scholar]
- 15.Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 16.Nathan H, Aloia TA, Vauthey JN, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:14–22. doi: 10.1245/s10434-008-0180-z. [DOI] [PubMed] [Google Scholar]
- 17.Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery. 2013;153:811–818. doi: 10.1016/j.surg.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140–3145. doi: 10.1200/JCO.2011.35.6519. [DOI] [PubMed] [Google Scholar]
- 19.Raderer M, Hejna MH, Valencak JB, et al. Two consecutive phase II studies of 5-fluorouracil/leucovorin/mitomycin C and of gemcitabine in patients with advanced biliary cancer. Oncology. 1999;56:177–180. doi: 10.1159/000011961. [DOI] [PubMed] [Google Scholar]
- 20.Kubicka S, Rudolph KL, Tietze MK, Lorenz M, Manns M. Phase II study of systemic gemcitabine chemotherapy for advanced unresectable hepatobiliary carcinomas. Hepatogastroenterology. 2001;48:783–789. [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6,336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spolverato G, Ejaz A, Kim Y, et al. Tumor size predicts vascular invasion and histologic grade among patients undergoing resection of intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2014;18:1284–1291. doi: 10.1007/s11605-014-2533-1. [DOI] [PubMed] [Google Scholar]
- 23.Ng KK, Vauthey JN, Pawlik TM, et al. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol. 2005;12:364–373. doi: 10.1245/ASO.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014 doi: 10.1001/jamasurg.2013.5137. [DOI] [PubMed] [Google Scholar]
- 25.Hyder O, Marsh JW, Salem R, et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol. 2013;20:3779–3786. doi: 10.1245/s10434-013-3127-y. [DOI] [PubMed] [Google Scholar]