Abstract
Purpose
Programmed death-1 (PD-1), an inhibitory receptor expressed on activated T cells, may suppress antitumor immunity. This phase I study sought to determine the safety and tolerability of anti–PD-1 blockade in patients with treatment-refractory solid tumors and to preliminarily assess antitumor activity, pharmacodynamics, and immunologic correlates.
Patients and Methods
Thirty-nine patients with advanced metastatic melanoma, colorectal cancer (CRC), castrate-resistant prostate cancer, non–small-cell lung cancer (NSCLC), or renal cell carcinoma (RCC) received a single intravenous infusion of anti–PD-1 (MDX-1106) in dose-escalating six-patient cohorts at 0.3, 1, 3, or 10 mg/kg, followed by a 15-patient expansion cohort at 10 mg/kg. Patients with evidence of clinical benefit at 3 months were eligible for repeated therapy.
Results
Anti–PD-1 was well tolerated: one serious adverse event, inflammatory colitis, was observed in a patient with melanoma who received five doses at 1 mg/kg. One durable complete response (CRC) and two partial responses (PRs; melanoma, RCC) were seen. Two additional patients (melanoma, NSCLC) had significant lesional tumor regressions not meeting PR criteria. The serum half-life of anti–PD-1 was 12 to 20 days. However, pharmacodynamics indicated a sustained mean occupancy of > 70% of PD-1 molecules on circulating T cells ≥ 2 months following infusion, regardless of dose. In nine patients examined, tumor cell surface B7-H1 expression appeared to correlate with the likelihood of response to treatment.
Conclusion
Blocking the PD-1 immune checkpoint with intermittent antibody dosing is well tolerated and associated with evidence of antitumor activity. Exploration of alternative dosing regimens and combinatorial therapies with vaccines, targeted therapies, and/or other checkpoint inhibitors is warranted.
INTRODUCTION
Genetic and epigenetic aberrations occur commonly in human tumors and produce altered antigenic profiles that can be selectively recognized by the adaptive immune response.1 A dynamic interplay exists between host and tumor, and the ability of the tumor to evade immune recognition often determines the clinical course of the disease.2 The successes of passive immunotherapies, such as monoclonal antibodies (mAbs) directed against tumor or vascular cell surface molecules or adoptive transfer of tumor-specific T cells, validate the potential of immunotherapy to eradicate established metastatic cancers. However, active immunotherapeutic strategies designed to enhance endogenous antitumor responses, such as cancer vaccines, have been far less successful.
Augmenting specific antitumor CD4+ and CD8+ T cell responses is a major goal of cancer immunotherapy. Important insights explaining the limitations of T cell–based cancer immunotherapies have come from the discovery of inhibitory coreceptors and pathways termed immune checkpoints, which restrain T cell functions in normal physiologic settings and may be exploited by tumors.3 Preclinical cancer models demonstrate that inhibitory signals mediated by coreceptors on tumor-specific T cells impede antitumor immunity and suggest that blockade of such interactions can release the brakes on immune responsiveness leading to tumor elimination. The most extensively studied inhibitory T cell coreceptor, CTLA-4 (CD152), has been evaluated in patients with advanced cancers. As originally predicted by murine models, anti–CTLA-4 therapy in humans resulted in objective tumor regressions including durable complete responses (CRs) in some patients. However, as anticipated from the uncontrolled lymphoproliferation observed in CTLA-4 null mice,4 anti–CTLA-4 therapy was associated with a significant frequency of serious immunologic adverse events (AEs).5 Thus, investigators have searched for new checkpoint blocking agents with more favorable therapeutic profiles.
Programmed death-1 (PD-1, CD279) is an inhibitory coreceptor expressed on antigen-activated and exhausted T and B cells.6 It bears homology to CTLA-4 but provides distinct immune-inhibitory signals. In contrast to early lethality in CTLA-4 knockout mice, PD-1 knockouts demonstrate modest late-onset strain- and organ-specific autoimmunity.7,8 There are two known ligands for PD1: B7-H1/PD-L1 (hereafter B7-H1), the predominant mediator of PD-1–dependent immunosuppression, and B7-DC/PD-L2. In murine tumor models, B7-H1 expression confers immune resistance, and interrupting PD-1:B7-H1 interactions has antitumor effects.9–11 B7-H1 is highly upregulated in many murine and human tumors (either in tumor cells or nontransformed cells in the tumor microenvironment such as antigen-presenting cells),12 and its expression is associated with poor outcome for patients with certain epithelial cancers.13,14 These findings have focused attention on PD-1:B7-H1 blockade as a strategy for cancer immunotherapy. On the basis of these considerations, we initiated a phase I clinical trial of PD-1 blockade with the fully human mAb MDX-1106 in 39 patients with advanced treatment-refractory solid tumors. We report here the safety, antitumor activity, pharmacodynamics, and correlative in vitro results from this trial.
PATIENTS AND METHODS
MDX-1106
MDX-1106 (BMS-936558/ONO-4538) is a genetically engineered, fully human immunoglobulin G4 (IgG4) mAb specific for human PD-1 (Appendix Fig A1A, online only). Mice transgenic for human Ig loci were immunized with Chinese hamster ovary cell PD-1 transfectants and a PD-1/human IgG1 Fc fusion protein. MDX-1106 contains an engineered hinge region mutation (S228P) designed to prevent exchange of IgG4 molecules; the IgG4 isotype minimizes cellular and complement-mediated cytolytic functions. MDX-1106 binds PD-1 with high affinity (KD = 2.6 nmol/L by Scatchard analysis to polyclonally activated human T cells), blocks its interactions with both B7-H1 and B7-DC (Appendix Fig A1B), and enhances tumor antigen-specific T cell proliferation and secretion of cytokines in vitro.15
Patients
Eligible patients had treatment-refractory metastatic melanoma, castrate-resistant prostate cancer, renal cell carcinoma (RCC), non–small-cell lung cancer (NSCLC), or colorectal cancer (CRC), and had no cancer therapy for at least 4 weeks before enrollment. Patients were ≥ 18 years old with a life expectancy of ≥ 12 weeks, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, adequate organ function, and no ongoing systemic infections or history of autoimmune disease. Concurrent antineoplastic therapies, systemic steroids, and prior treatment with anti–CTLA-4 were not permitted. Patients with treated stable brain metastases were eligible.
Study Design and Procedures
This multi-institutional, first in-human, open-label, phase I, dose-escalation study was approved by local institutional review boards. All participating patients signed informed consent. The primary objectives were to characterize the safety and tolerability of a single dose of MDX-1106 in patients with selected malignancies and to determine the maximum-tolerated dose (MTD) and pharmacokinetics. Secondary objectives included assessing antitumor activity, pharmacodynamics, and immunologic end points. Sequential cohorts of six patients received a 60-minute intravenous infusion of MDX-1106 at 0.3, 1, 3, or 10 mg/kg and were evaluated for toxicities on a weekly basis for 8 weeks. Dose-limiting toxicity (DLT) was defined as a treatment-related grade ≥ 3 AE or laboratory abnormality occurring ≤ 28 days postdose. The MTD was the highest dose at which no more than one of six patients experienced a DLT. Fifteen additional patients were planned to be enrolled at the MTD or the highest planned dose (10 mg/kg) to confirm safety.
Patients were restaged radiographically (Response Evaluation Criteria in Solid Tumors [RECIST] 1.0) at 8 and 12 weeks. Patients with progressive disease were taken off study. Those with stable disease or evidence of lesional tumor regression, no AE grade ≥ 3, and no evidence of human antihuman Ab at a 1:10 serum dilution received additional doses of MDX-1106 at weeks 12 and 16 and were then observed for 3 months and restaged. Those with continued clinical benefit could receive two more doses, spaced by 4 weeks. Each re-treatment phase was 16 weeks. Patients with objective partial responses (PRs) or CRs were observed, with optional re-treatment on progression. Responses are reported as of January 2010.
Pharmacokinetics
Serum samples were collected serially before and up to 85 days after the first dose of MDX-1106. MDX-1106 serum concentrations were determined with a quantitative enzyme-linked immunosorbent assay (ELISA) capable of detecting ≥ 1.2 μg/mL, using 96-well plates coated with chimeric PD-1/human IgG1 Fc protein (R&D Systems, Minneapolis, MN).
Tumor Biopsies
Sections of formalin-fixed, paraffin-embedded tumor specimens archived before protocol entry or core-needle or excisional biopsies obtained immediately pre- and post-therapy were subjected to hematoxylin and eosin staining and immunohistochemistry (IHC) to detect lymphoid infiltrates (anti-CD3, anti-CD4, and anti-CD8) and B7-H1 expression (murine antihuB7-H1, clone 5H1; previously described13). Pigmented melanoma samples were bleached before staining and visualized with a red chromogen. Tumors were considered B7-H1–positive if ≥ 5% of tumor cells showed membranous staining with 5H1.
Immunologic Assessments
Delayed-type hypersensitivity reactions to Candida albicans and tetanus toxoid were assessed along with viral antigen recall reactions (details are included in the Appendix, online only). Peripheral blood lymphocyte (PBL) phenotypes were also assessed. Serially collected blood was analyzed for the presence and activation status of various lymphocyte subsets, as detailed in the Appendix.
PD-1 Receptor Occupancy (pharmacodynamics)
MDX-1106 binding to PD-1 molecules on circulating CD3+ PBLs was investigated with flow cytometric analysis of serially collected blood samples (see PBL phenotyping schedule in the Appendix). Peripheral blood mononuclear cells were preincubated (30 minutes at 4°C) with a saturating concentration (20 μg/mL) of either unlabeled huIgG4 (isotype control) or MDX-1106, washed extensively, and then costained with anti-CD3 fluorescein isothiocyanate and murine antihuIgG4 biotin (Invitrogen, Carlsbad, CA) plus streptavidin-phycoerythrin. PD-1 occupancy by infused MDX-1106 was estimated as the ratio of the percent of CD3+ cells stained with antihuIgG4 after in vitro saturation with isotype control Ab (indicating in vivo binding) to that observed after MDX-1106 saturation (indicating total available binding sites).
RESULTS
Patients and Treatments
Thirty-nine patients with advanced metastatic NSCLC, melanoma, castrate-resistant prostate cancer, RCC, or CRC received MDX-1106 in four escalating dose cohorts of 0.3 to 10 mg/kg and an expansion cohort at 10 mg/kg, from October 2006 through June 2009 (Tables 1 and 2). Their median age was 62 years. All had progressive treatment-refractory disease, and they had undergone a median of four prior therapies.
Table 1.
Patient Characteristics
| Characteristic | No. of Patients | % |
|---|---|---|
| Sex | ||
| Male | 22 | 56.4 |
| Female | 17 | 43.6 |
| Age, years | ||
| Median | 62 | |
| Range | 42-84 | |
| Tumor histology | ||
| Colorectal cancer | 14 | 35.9 |
| Melanoma | 10 | 25.6 |
| Prostate cancer | 8 | 20.5 |
| NSCLC | 6 | 15.4 |
| Renal cell carcinoma | 1 | 2.6 |
| ECOG PS | ||
| 0 | 13 | 33.3 |
| 1 | 26 | 66.7 |
| Prior therapies | ||
| Median | 4 | |
| Range | 1-13 | |
| Chemotherapy* | 36 | 92.3 |
| Radiation therapy | 13 | 33.3 |
| Surgery | 39 | 100 |
| Immunotherapy | 14 | 35.9 |
| Biologics† | 26 | 66.7 |
| Hormonal therapy | 8 | 20.5 |
Abbreviations: NSCLC, non–small-cell lung cancer; ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Includes molecularly targeted therapies.
Includes monoclonal antibody therapies.
Table 2.
Treatment Characteristics and Clinical Response to Therapy
| Dose (mg/kg) | No. of Patients | Total No. of Doses |
Best Response (duration in months)* | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | 11 | |||
| 0.3 | 6 | 6 | 0 | 0 | 0 | 0 | N/A |
| 1 | 6 | 3 | 1 | 1 | 1 | 0 | 1 MXR (1) |
| 3 | 6 | 3 | 0 | 2 | 1 | 0 | 1 CR (21+)† |
| 10 | 21 | 15 | 1 | 4 | 0 | 1 | 2 PR (3+, 16+)ठ|
| 1 MXR (1) | |||||||
| Total | 39 | 27 | 2 | 7 | 2 | 1 | 1 CR, 2 PR, 2 MXR |
Abbreviations: N/A, not applicable; MXR, mixed response defined as regression in some lesions but concomitant progression in others; CR, complete response; PR, partial response.
CR and PR by Response Evaluation Criteria in Solid Tumors 1.0 criteria.
This patient with stage IV colorectal cancer had previously shown progressive disease after receiving chemotherapy regimens including bevacizumab and cetuximab.
PR duration of 3+ months was preceded by an MXR in this patient with melanoma lasting 20 months. Previous therapies that were ineffective included high-dose interleukin-2 and temozolomide.
PR duration of 16+ months was preceded by an MXR in this patient with renal cell carcinoma lasting 4 months. Previous therapies that were ineffective included sunitinib, sorafenib, and an experimental histone deacetylase inhibitor.
Treatment-Related Toxicities
MDX-1106 was well-tolerated: no DLTs were observed after one dose, and an MTD was not defined in this study. Grade ≥ 2 adverse clinical and laboratory events are summarized in Appendix Table A1 (online only). Most frequent were decreased CD4+ lymphocyte counts (14 patients, 35.9%), lymphopenia (10 patients, 25.6%), fatigue and musculoskeletal events (six patients each, 15.4%). No patient developed human antihuman Ab, even after multiple doses.
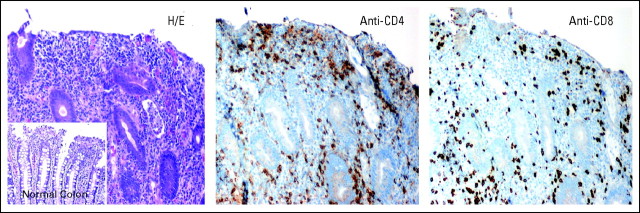
Immune-related AEs (irAEs) were of special interest because of the presumed mechanism of action of anti–PD-1 and prior experience with anti–CTLA-4.5 No grade ≥ 3 irAE occurred in the 28-day period following the first dose of anti–PD-1. One patient with metastatic ocular melanoma developed grade 3 inflammatory colitis following five doses (1 mg/kg) administered over 8 months (Appendix Fig A2, online only), which responded to steroids and infliximab. One patient (10 mg/kg) experienced grade 2 hypothyroidism requiring hormone replacement. Two patients (at 3 and 10 mg/kg) developed grade 2 polyarticular arthropathies requiring oral steroids and were not further treated; in retrospect, both had potentially contributory predisposing factors, one with a history of Lyme arthritis and polymyalgia rheumatica, the other with a preexisting antinuclear antibody titer > 1:1000.
Antitumor Activity
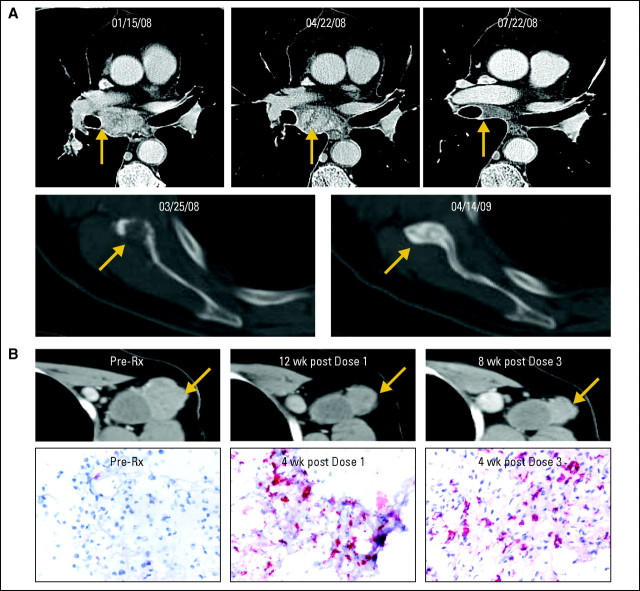
One patient with CRC (3 mg/kg) achieved a CR, and two patients with RCC (10 mg/kg) and melanoma (10 mg/kg) experienced PRs to therapy. A 67-year-old male with CRC metastatic to intra-abdominal lymph nodes received five doses of MDX-1106 and experienced a CR persisting 21+ months. A 72-year-old male with multiorgan metastatic RCC had a mixed response after one dose of MDX-1106, with progression in a pancreatic metastasis but regression in other sites; this evolved to an overall PR after two additional doses, lasting 16+ months without further therapy (Fig 1A). A 51-year-old female with melanoma metastatic to multiple lymph nodes and liver sites initially experienced a mixed response, with regression at all sites except an enlarging subpectoral lymph node, and achieved a PR after receiving 11 doses of MDX-1106 over 24 months (Fig 1B). Two additional patients had significant lesional or mixed tumor regressions (defined as regression in individual lesions with concomitant progression at other sites), including one with NSCLC (1 mg/kg) and another with melanoma (10 mg/kg). In total, 12 patients with stable disease or lesional tumor regressions at the first disease assessment received multiple doses of MDX-1106 (Table 2).
Fig 1.
Objective tumor responses in patients with metastatic renal cell carcinoma (RCC) and melanoma after repeated dosing with anti–programmed death-1 monoclonal antibody (MDX-1106) at 10 mg/kg. (A) Patient 4033 with RCC experienced a partial response (PR) after receiving three doses of MDX-1106. Regression of metastases in mediastinal lymph nodes and bone (scapula) demonstrated on contrast-enhanced computed tomography scans are representative of lesions at other sites including lung, muscle, pancreas, and pericolic lymph node. Date of first treatment was January 29, 2008. (B) Patient 3019 experienced a PR after receiving 11 doses of MDX-1106. Serial core-needle biopsies of a regressing axillary lymph node metastasis were stained with anti-CD8, revealing a moderate post-treatment infiltrate. Infiltration of CD4+ cells was not observed (not shown). 20× objective. Rx, treatment; wk, week.
Tumor Biopsies
B7-H1 expression on tumors may affect the ability to respond to PD-1 blockade. To explore this, tumor biopsies from nine patients undergoing treatment with MDX-1106 were analyzed for B7-H1 expression with IHC (Appendix Table A2, online only). In two cases, both pre- and post-treatment samples were available; in the others, only pretreatment (six cases) or post-treatment (one case) samples were accessible. Tumor cell staining for B7-H1 expression fell into three patterns: negative, intracytoplasmic, or membranous (cell surface). Among nine patients studied, four exhibited membranous B7-H1 staining (Fig 2): three of these patients experienced tumor regressions following MDX-1106 therapy; the fourth was treated in the 0.3-mg/kg cohort where no responses were observed. Conversely, among five patients whose tumors failed to express B7-H1 at the cell surface, there was no evidence of clinical response. B7-H1 staining patterns were consistent in five patients from whom multiple biopsies were available (AppendixTable A2). In this small sample size, the correlation between membranous B7-H1 expression on tumor cells and the likelihood of tumor regression following PD-1 blockade suggested potential significance (two-sided P = .0476; Fisher's exact test).
Fig 2.
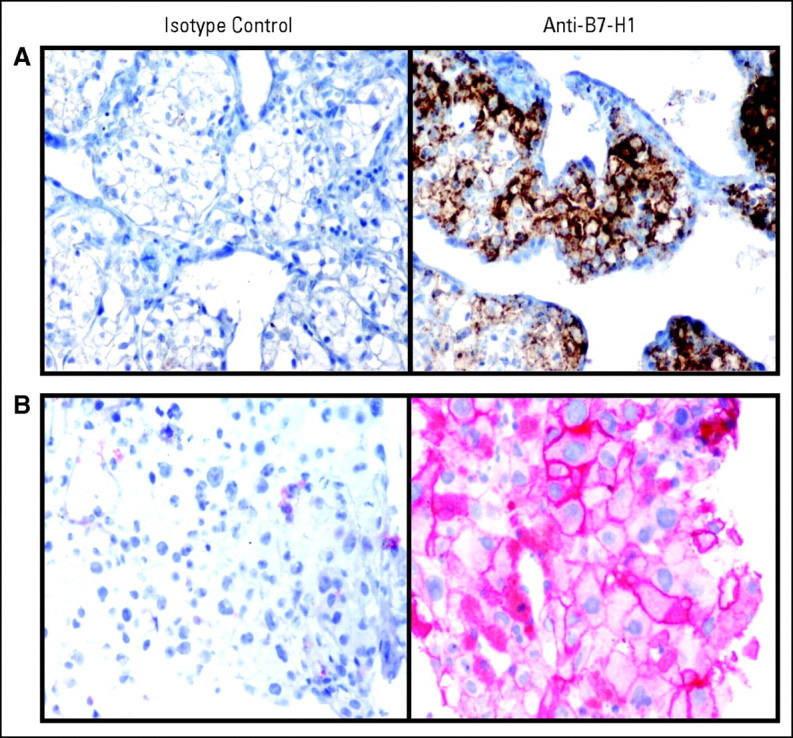
Membranous pattern of B7-H1 expression demonstrated on (A) renal cell carcinoma cells in a tumor thrombus from patient 4033, and (B) melanoma cells in an axillary lymph node metastasis from patient 3019. Both patients experienced partial responses after anti–programmed death-1 monoclonal antibody (MDX-1106) therapy. 40× objective.
Melanoma patient 3019, who experienced a PR to anti–PD-1 therapy, underwent pre- and post-treatment biopsies of an axillary lymph node metastasis for characterization of intratumoral lymphoid infiltrates by IHC. Whereas the pretreatment biopsy contained only sparse lymphoid cells, subsequent tumor regression was accompanied by a moderate infiltration of CD8+, but not CD4+, T cells (Fig 1B).
Reactivity to Recall Antigens and PBL Phenotypes
No significant effects of MDX-1106 therapy on delayed-type hypersensitivity responses against C albicans or tetanus toxoid or antiviral recall responses by interferon gamma ELISpot analysis were observed in 15 and six patients examined, respectively. The effects of a single 10-mg/kg dose of MDX-1106 on PBL numbers, subset profiles, and activation status were analyzed in 17 patients (Fig 3). Twenty-four hours postdose, total lymphocyte as well as CD3, CD4, and CD8 numbers declined and then rebounded from days 2 through 29 and declined again from days 29 through 85. These trends were not observed for CD19 (B lymphocyte) or CD56 (natural killer) cells (not shown), suggesting a selective effect on T cells. Percentages of CD3, CD4, and CD8 cells declined steadily over the 85-day observation period, with a reciprocal increase in CD19 and CD56 cell percentages (Appendix Fig A3A, online only). No significant changes were observed in expression of the T cell activation markers CD25, CD45RO, or HLA-DR by CD4+ or CD8+ PBLs (Appendix Fig A3B).
Fig 3.
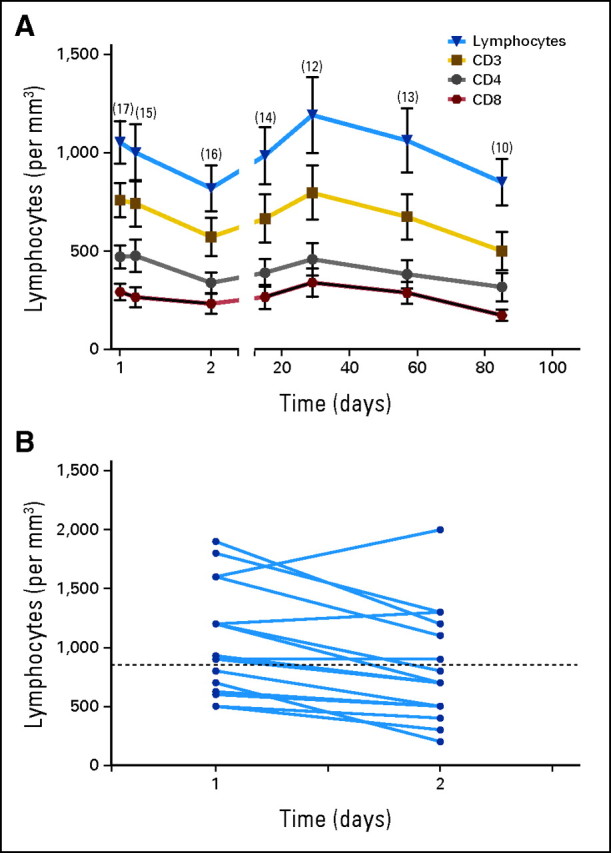
Effects of a single dose of anti–programmed death-1 monoclonal antibody (MDX-1106; 10 mg/kg) on circulating lymphocyte numbers. (A) Twenty-four hours postdose, a decline in total lymphocyte as well as CD3, CD4, and CD8 numbers was observed (two-sided P = .004, .002, < .001, and .01 respectively; Wilcoxon signed rank test). These parameters followed similar trends, rebounding from days 2 through 29 (two-sided P < .001; two-sided P = .01 for CD8), and declining again from days 29 through 85 (two-sided P < .001; mixed model test for trend with knots [changes in slopes] and repeated measures). Means ± standard error of mean are shown; numbers of patients studied at each time point are indicated in parentheses. (B) Paired analysis of total lymphocyte numbers in 16 patients, comparing immediate pretreatment samples (day 1) with 24-hour post-treatment samples (day 2). A significant decline at day 2 was observed (two-sided P = .004; Wilcoxon signed rank test). Dotted line indicates the lower limit of normal lymphocyte counts.
PD-1 Receptor Occupancy (pharmacodynamics)
PD-1 has predominant cell surface expression, unlike CTLA-4, which is displayed only transiently at the T cell surface. Thus, it was possible to develop flow cytometric methods to evaluate the pharmacodynamics of infused MDX-1106, estimating PD-1 occupancy on circulating T cells over time. Standard pharmacokinetic measurements of MDX-1106 serum concentrations yielded an approximate serum half-life (t1/2) of 12 days (0.3-, 1-, or 3-mg/kg dose) to 20 days (10 mg/kg), with maximum concentration (Cmax) and AUC directly related to dose. However, pharmacokinetics and pharmacodynamics were unexpectedly discordant in 15 patients studied (Fig 4). PD-1 occupancy appeared to be dose-independent, with a mean peak occupancy of 85% (range, 70% to 97%) and a mean plateau occupancy of 72% (range, 59% to 81%) observed at 4 to 24 hours and ≥ 57 days, respectively, after one infusion (Fig 4A). These data are consistent with the high affinity of MDX-1106 for PD-1—in vitro, 0.04 μg/mL MDX-1106 is sufficient to occupy > 70% PD-1 molecules on T cells (not shown)—suggesting that even when serum levels are undetectable (< 1.2 μg/mL), sufficient concentrations persist to maintain plateau PD-1 occupancy. Occupancy eventually decayed after 85 days (Fig 4B, top and middle panels). In patients receiving repeated infusions of MDX-1106 at 10 mg/kg, troughs and peaks of PD-1 occupancy around each dose were observed (Fig 4B, middle and bottom panels), although 100% occupancy was not achieved. In vitro experiments indicate that PD-1 occupancy analyses of cryopreserved PBLs may underestimate occupancy on fresh PBLs (not shown). It is unknown whether these findings in circulating lymphocytes reflect PD-1 occupancy on lymphocytes in the tumor, secondary lymphoid organs, and/or tissues.
Fig 4.
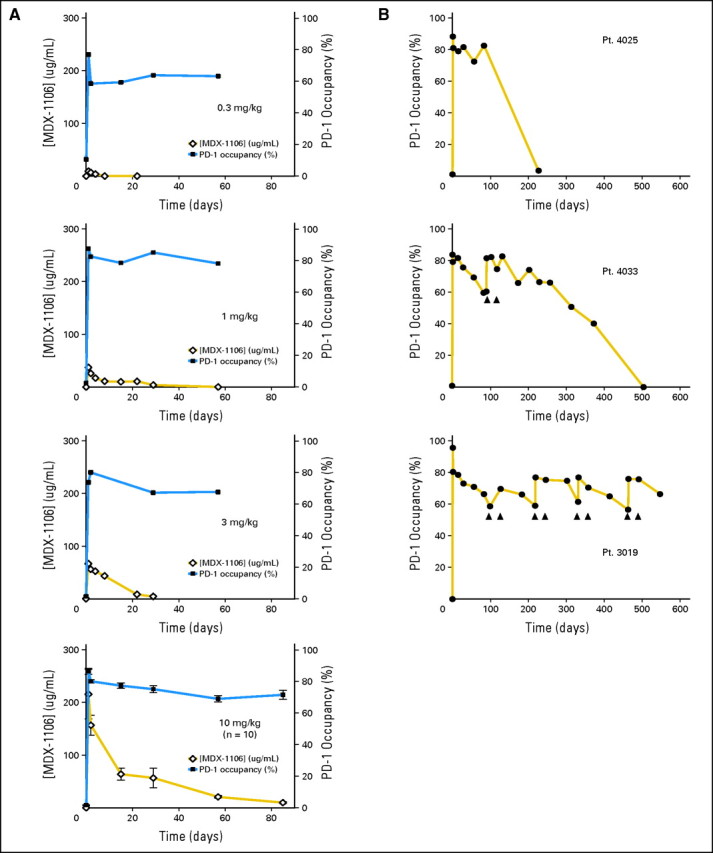
Pharmacodynamics of anti–programmed death-1 (PD-1) monoclonal antibody (MDX-1106). (A) PD-1 occupancy on circulating CD3+ T cells after one infusion of MDX-1106 is shown for single patients (Pts.) each receiving 0.3, 1, or 3 mg/kg, and for 10 patients receiving 10 mg/kg (mean ± standard error of mean; solid squares). Serum concentrations of MDX-1106 at the same time points are indicated (open diamonds). (B) Long-term PD-1 occupancy analysis in patients receiving one (top panel) or multiple doses (middle and bottom panels) of MDX-1106 at 10 mg/kg. All patients received infusions at day 1; additional infusions are indicated by arrows. Results in (B) middle and bottom panels are representative of five patients receiving multiple doses.
DISCUSSION
We report results from a dose-escalation trial of a fully human anti–PD-1 mAb, MDX-1106, in 39 patients with refractory metastatic cancers. MDX-1106 was well tolerated to the maximum planned dose of 10 mg/kg. While both anti–PD-1 and anti–CTLA-4 therapies are associated with irAEs, as predicted by preclinical models and consistent with the physiologic roles of these molecules, these toxicities appear to be less frequent and milder in patients receiving anti–PD-1. Among 39 patients treated with MDX-1106, a single attributable serious AE, inflammatory colitis, occurred. Low-grade irAEs occurred in two patients with polyarticular arthropathies requiring oral steroids and one patient with hypothyroidism requiring hormone replacement. However, expanded experience with multidose anti–PD-1 treatment will be necessary to make direct comparisons with anti–CTLA-4.
While efficacy was not a primary end point of this trial, we found evidence of antitumor activity, with one CR (ongoing at 21 months) and two PRs (ongoing at 3 and 16 months) in patients with CRC, melanoma, and RCC; significant lesional regressions were seen in two additional patients with melanoma and NSCLC. The observed clinical activity of MDX-1106 is most likely exerted through immunologic mechanisms rather than direct tumoricidal effects, since nonhematologic tumors do not express PD-1. Importantly, tumor regressions were seen in patients with colon and lung cancer, suggesting that the capacity of anti–PD-1 to enhance antitumor immunity extends beyond the classically immunogenic tumor types of melanoma and RCC.
Two possible scenarios for T cell inhibition via PD-1:B7-H1 ligation have been proposed in the context of cancer.16,17 First, lymphocytes may encounter B7-H1 expressed on activated antigen-presenting cells during priming or reactivation in lymphoid organs. In addition, tumor-specific effector lymphocytes may be inhibited on contact with B7-H1–positive tumor cells. In the latter case, direct tumor assessment for B7-H1 expression may provide a powerful predictive biomarker for responsiveness to anti–PD-1. Analysis of serial tumor biopsies may provide additional insights into specific antitumor mechanisms resulting from PD-1 blockade. A regressing lesion in one melanoma patient on this study revealed a significantly increased and selective CD8+ T cell infiltrate post-therapy. While this kind of analysis needs to be performed on much larger numbers of patients, in light of the acute decline in CD3+ T cell numbers observed post-treatment, it is interesting to speculate that anti–PD-1 may cause redistribution of lymphocyte subsets from the blood into tumor and tissue sites.
There is a single previous report18 of cancer therapy with a different anti–PD-1 antibody, CT-011, in 17 patients with hematologic malignancies. One CR was reported in a non-Hodgkin's lymphoma patient. Because the CT-011 trial evaluated patients with a completely distinct and nonoverlapping set of cancers relative to this study, it is difficult to compare the two antibodies at this time.
The promising safety profile and evidence of durable clinical activity in this phase I trial is encouraging, and early results from a follow-up trial (NCT00730639) appear to confirm the tolerability and activity of anti–PD-1 in patients for whom multiple prior therapies failed. However, it is important to consider factors that might improve the response rate. Despite the consistently high level of PD-1 occupancy observed on PBLs and the fact that T cells continuously redistribute between blood, lymph, and tissue, repeated dosing of MDX-1106 at shorter intervals might increase occupancy and tissue penetration and should be explored. Patient selection is an important issue, since anti–PD-1 may have suboptimal effects in patients with compromised immune systems. This notion is supported by preclinical models demonstrating that anti–PD-1 is most effective against immunogenic tumors in intact mice. Another important factor in determining outcomes may be intratumoral expression of B7-H1, either by tumor cells or by nontransformed cells in the tumor microenvironment. As suggested by our preliminary biopsy analysis, tumor cell surface expression of B7-H1 may be a predictor of responsiveness to PD-1 blockade. Finally, on the basis of murine models showing that anti–PD-1 is highly synergistic in combination with tumor vaccines,19 we anticipate that more effective uses of this agent for cancer therapy will involve combinatorial therapies with other agents that boost endogenous antitumor immunity. Treatment regimens combining MDX-1106 with vaccines, molecularly targeted therapies, or other immunomodulators are already under evaluation in the laboratory for near-term clinical development.
Acknowledgment
We thank Ono Pharmaceuticals for development of MDX-1106/BMS-936558/ONO-4538; Nils Lonberg and Geoffrey Nichol (Medarex/Bristol-Myers Squibb) for their intellectual contributions and expert guidance; Peng Huang (Johns Hopkins University School of Medicine) for guidance in statistical analyses; Helen Xu (Johns Hopkins University School of Medicine) for B7-H1 immunohistochemistry; and the clinical research coordinators, research nurses, data managers, scientists, and patients at all institutions involved in this study.
Glossary Terms
- Antigen:
A substance that promotes, or is the target of, an immune response.
- Antigen-presenting cells:
Cells of the immune system that play a major role in adaptive immunity, APCs are responsible for binding and processing antigens for presentation to T lymphocytes and producing signals that lead to lymphocyte proliferation and differentiation. Dendritic cells and macrophages are examples of APCs.
- AUC (area under the curve):
A measure of the amount of drug in the blood over a set period of time (e.g., 24 hours) that can be used to determine drug exposure.
- Cytokines:
Cell communication molecules that are secreted in response to external stimuli.
- ELISpot:
Enzyme-linked immunospot that is exquisitely sensitive to assay minute amounts of mediators that are produced by cells. Typically, cells are deposited on a membrane coated with an antibody specific for a given protein. The protein of interest is captured directly around the secreting cell and is detected with an antibody specific for a different epitope. Coupled with colorimetry, the cells are visualized by specialized plate readers. Thus, the molecule is assayed before it is diluted in the supernatant, captured by receptors of adjacent cells, or degraded.
- Enzyme-linked immunosorbent assay (ELISA):
An ELISA is a sensitive, quantitative immunochemical test that involves an enzyme linked to an antibody or antigen to allow detection of a specific protein. Generally, the specimen is added to a surface, on which are immobilized antibodies specific to the protein of interest. If the protein is present, it will bind to the attached antibody layer. The presence of the bound protein is then verified with antibodies that have been tagged with an enzyme, which causes the specimen to change color corresponding to the concentration of the target protein.
- Immunogenic:
Capable of inducing an immune response.
- Immunohistochemistry:
The application of antigen-antibody interactions to histochemical techniques. Typically, a tissue section is mounted on a slide and is incubated with antibodies (polyclonal or monoclonal) specific to the antigen (primary reaction). The antigen-antibody signal is then amplified using a second antibody conjugated to a complex of peroxidase-antiperoxidase (PAP), avidin-biotin-peroxidase (ABC) or avidin-biotin alkaline phosphatase. In the presence of substrate and chromogen, the enzyme forms a colored deposit at the sites of antibody-antigen binding. Immunofluorescence is an alternate approach to visualize antigens. In this technique, the primary antigen-antibody signal is amplified using a second antibody conjugated to a fluorochrome. On UV light absorption, the fluorochrome emits its own light at a longer wavelength (fluorescence), thus allowing localization of antibody-antigen complexes.
- Immunotherapy:
A therapeutic approach that uses cellular and/or humoral elements of the immune system to fight a disease.
Appendix
Methods
Delayed-type hypersensitivity reactions.
Skin tests for tetanus and Candida albicans were placed 1 to 6 days before the first dose of anti–programmed death-1 monoclonal antibody (MDX-1106), and injection site induration was measured immediately predose and 24 to 48 hours postdose. Skin tests were placed again 29 days postdose, and induration was measured 24 to 48 hours later.
Viral antigen recall reactions.
Serially collected peripheral blood mononuclear cells were stimulated with a pool of 32 major histocompatibility complex class I–restricted cytomegalovirus, Epstein-Barr virus, and influenza virus epitopes (CEF Peptide Pool Plus; Cellular Technology, Shaker Heights, OH). Reactive T cells were enumerated in interferon gamma enzyme-linked immunospot assays according to standard techniques.
Peripheral blood lymphocyte phenotypes.
Blood was collected predose and postdose at 4 and 24 hours, and at 15, 29, 57, and 85 days. Lymphocyte subsets (CD3, CD4, CD8, CD19, and CD56) were analyzed according to absolute cell numbers per microliter of whole blood, percent representation among all lymphocytes, and coexpression of the activation markers CD25, HLA-DR, and CD45RO, at a central laboratory using automated flow cytometric techniques (Quest Diagnostics, Exton, PA).
Fig A1.
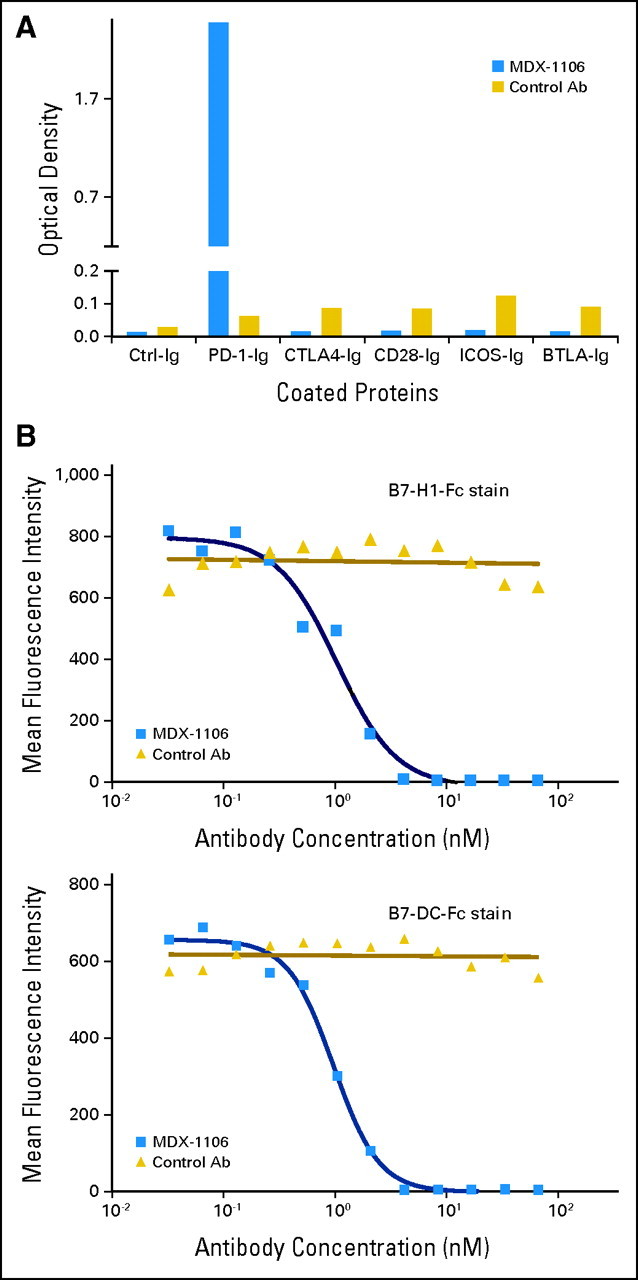
Binding specificity of anti–programmed death-1 (PD-1) monoclonal antibody (MDX-1106). (A) MDX-1106 (20 μg/mL) was tested for binding to various T cell immunogloblulin (Ig) family members by standard enzyme-linked immunosorbent assay using plate-coated Fc-fusion proteins (R&D Systems, Minneapolis, MN). Goat antihuman immunoglobulin G (IgG; kappa chain specific) polyclonal antibody conjugated with horseradish peroxidase (Jackson ImmunoResearch, West Grove, PA) was used as secondary antibody (Ab). (B) MDX-1106 inhibits binding of both B7-H1 and B7-DC to PD-1. Transfected Chinese hamster ovary cells expressing PD-1 molecules were incubated with serial dilutions of MDX-1106 or an isotype-matched control antibody, followed by addition of fluorescein isothyocyanate–conjugated recombinant human B7-H1-Fc or B7-DC-Fc fusion proteins and flow cytometric analysis.
Fig A2.
Inflammatory colitis in patient 1008 with metastatic ocular melanoma, after receiving five doses of anti–programmed death-1 monoclonal antibody (MDX-1106) at 1 mg/kg. Routine staining and immunohistochemistry of colonoscopic biopsy specimens demonstrated mucosal ulceration and dense interstitial CD4+ and CD8+ lymphoid infiltrates, with intraepithelial lymphocyte infiltration and cryptitis. A normal colonic biopsy from another patient is shown for comparison (see inset). H/E, hematoxylin and eosin. 20× objective.
Fig A3.
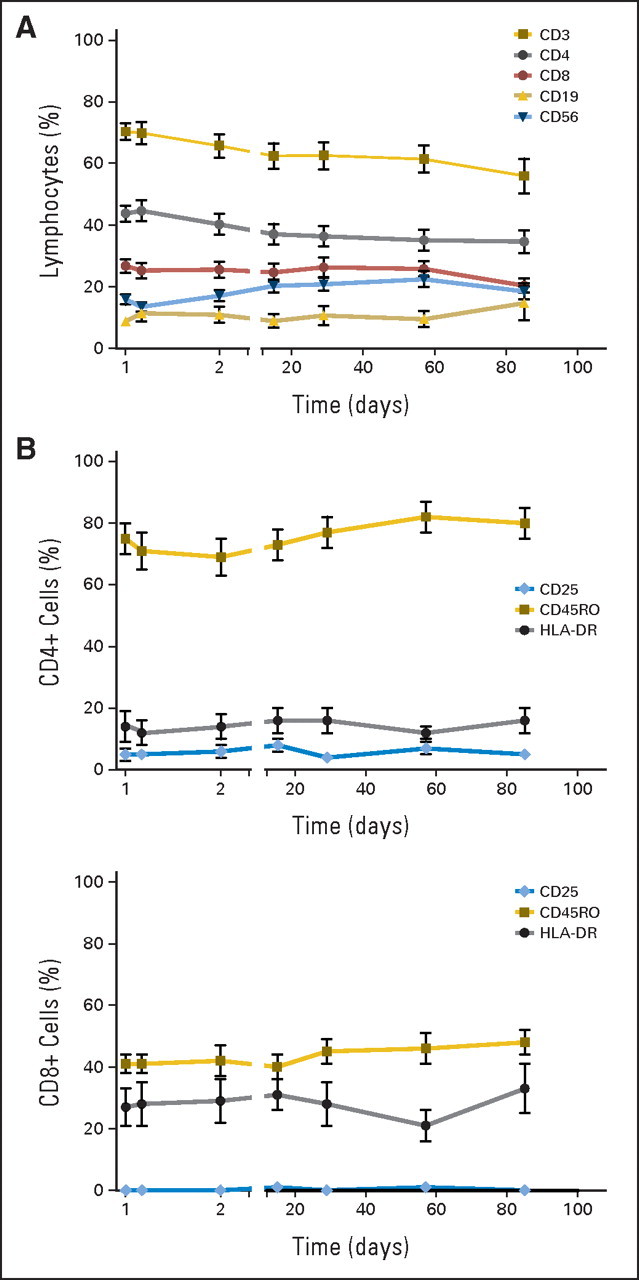
Peripheral blood lymphocyte phenotypes following a single dose of anti–programmed death-1 monoclonal antibody (MDX-1106; 10 mg/kg) on day 1. (A) Percentages of circulating CD3, CD4, and CD8 cells declined steadily (two-sided P < .001), while CD19 and CD56 percentages rose reciprocally (two-sided P = .01; mixed model test for trends) over the 85-day observation period. (B) Expression of the activation markers CD25, CD45RO, and HLA-DR on circulating CD4+ and CD8+ T cells did not change significantly after treatment (Wilcoxon signed rank test and test for trends).
Table A1.
Grade ≥ 2 Toxicities Attributed to MDX-1106
| Disorder | Dose (mg/kg) |
Total No. of Patients (N = 39) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.3 (n = 6) |
1.0 (n = 6) |
3 (n = 6) |
10 (n = 21) |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Blood | ||||||||||
| Anemia | ||||||||||
| Grade 2 | 0 | 1 | 16.7 | 0 | 1 | 4.8 | 2 | 5.1 | ||
| Grade 3 | 1 | 16.7 | 0 | 0 | 0 | 1 | 2.6 | |||
| CD4 decrease | ||||||||||
| Grade 2 | 0 | 2 | 33.3 | 1 | 16.7 | 4 | 19.0 | 7 | 17.9 | |
| Grade 3 | 1 | 16.7 | 0 | 0 | 6 | 28.6 | 7 | 17.9 | ||
| Lymphopenia | ||||||||||
| Grade 2 | 2 | 33.3 | 0 | 2 | 33.3 | 5 | 23.8 | 9 | 23.1 | |
| Grade 3 | 0 | 0 | 0 | 1 | 4.8 | 1 | 2.6 | |||
| Neutropenia | ||||||||||
| Grade 2 | 1 | 16.7 | 0 | 0 | 0 | 1 | 2.6 | |||
| Cardiovascular | ||||||||||
| Hypertension | ||||||||||
| Grade 2 | 1 | 16.7 | 0 | 0 | 0 | 1 | 2.6 | |||
| Endocrine and metabolic | ||||||||||
| Hypocalcemia | ||||||||||
| Grade 3 | 0 | 0 | 0 | 1 | 4.8 | 1 | 2.6 | |||
| Hyperglycemia | ||||||||||
| Grade 2 | 0 | 0 | 0 | 1 | 4.8 | 1 | 2.6 | |||
| Hypothyroidism | ||||||||||
| Grade 2 | 0 | 0 | 0 | 1 | 4.8 | 1 | 2.6 | |||
| Gastrointestinal | ||||||||||
| Ascites | ||||||||||
| Grade 3 | 1 | 16.7 | 0 | 0 | 0 | 1 | 2.6 | |||
| Colitis | ||||||||||
| Grade 3 | 0 | 1 | 16.7 | 0 | 0 | 1 | 2.6 | |||
| Stomatitis | ||||||||||
| Grade 2 | 0 | 0 | 0 | 1 | 4.8 | 1 | 2.6 | |||
| General | ||||||||||
| Fatigue | ||||||||||
| Grade 2 | 0 | 0 | 3 | 50.0 | 2 | 9.5 | 5 | 12.8 | ||
| Grade 3 | 0 | 0 | 0 | 1 | 4.8 | 1 | 2.6 | |||
| Infusion site reaction | ||||||||||
| Grade 2 | 0 | 0 | 0 | 2 | 9.5 | 2 | 5.1 | |||
| Pyrexia | ||||||||||
| Grade 2 | 0 | 1 | 16.7 | 0 | 0 | 1 | 2.6 | |||
| Hepatobiliary | ||||||||||
| Liver disorder | ||||||||||
| Grade 2 | 1 | 16.7 | 0 | 0 | 0 | 1 | 2.6 | |||
| Hypersensitivity | ||||||||||
| Grade 2 | 0 | 0 | 1 | 16.7 | 0 | 1 | 2.6 | |||
| Infections | ||||||||||
| Sinusitis/nasal polyps | ||||||||||
| Grade 2 | 0 | 0 | 0 | 1 | 4.8 | 1 | 2.6 | |||
| Musculoskeletal* | ||||||||||
| Grade 2 | 0 | 0 | 1 | 16.7 | 3 | 14.3 | 4 | 10.3 | ||
| Grade 3 | 1 | 16.7 | 0 | 0 | 1 | 4.8 | 2 | 5.1 | ||
| Neurologic | ||||||||||
| Headache | ||||||||||
| Grade 2 | 0 | 0 | 0 | 1 | 4.8 | 1 | 2.6 | |||
| Respiratory | ||||||||||
| Dyspnea | ||||||||||
| Grade 2 | 0 | 0 | 1 | 16.7 | 0 | 1 | 2.6 | |||
| Pleural effusion | ||||||||||
| Grade2 | 1 | 16.7 | 0 | 0 | 0 | 1 | 2.6 | |||
| Skin† | ||||||||||
| Grade 2 | 0 | 1 | 16.7 | 0 | 1 | 4.8 | 2 | 5.1 | ||
| Urinary tract | ||||||||||
| Proteinuria | ||||||||||
| Grade 2 | 0 | 1 | 16.7 | 0 | 0 | 1 | 2.6 | |||
Abbreviation: MDX-1106, anti–programmed death-1 monoclonal antibody.
Musculoskeletal disorders include arthralgia, arthritis, myalgia, extremity pain, joint stiffness, back pain, bone pain, muscular weakness, and polymyalgia rheumatica.
Skin disorders include rash, erythema, pruritis, dry skin, alopecia, hyperhidrosis, lichenoid keratosis, and skin discoloration.
Table A2.
Correlation of B7-H1 Expression by Tumor Cells With Tumor Regression Following MDX-1106 Therapy
| Patient No. | Diagnosis | MDX-1106 Dose (mg/kg) | Biopsy Site | Biopsy Pre-/Post-Therapy | B7-H1 Staining Pattern* | Clinical Response |
|---|---|---|---|---|---|---|
| 0006 | NSCLC | 0.3 | Pleural fluid | Pre- | Membranous | NR |
| 2014 | CRPC | 3.0 | Pelvic LN | Pre- | Negative | NR |
| Inguinal LN | Post- | Negative | ||||
| 3019 | Melanoma | 10.0 | Axillary LN | Pre- | Membranous | PR |
| Axillary LN | Post- | Membranous | ||||
| 3021 | Melanoma | 10.0 | Parotid gland | Pre- | Membranous | MXR |
| 4025 | CRPC | 10.0 | Epidural mass #1 | Post- | Cytoplasmic | NR |
| Epidural mass #2 | Post- | Cytoplasmic | ||||
| 4033 | RCC | 10.0 | Renal primary | Pre- | Membranous | PR |
| Tumor thrombus | Pre- | Membranous | ||||
| 4036 | Melanoma | 10.0 | Subcutaneous | Pre- | Cytoplasmic | NR |
| 4038 | Melanoma | 10.0 | Brain | Pre- | Cytoplasmic | NR |
| 4039 | Melanoma | 10.0 | Small bowel | Pre- | Negative | NR |
| Intra-abdominal mass | Pre- | Cytoplasmic |
Abbreviations: MDX-1106, anti–programmed death-1 monoclonal antibody; NSCLC, non–small-cell lung cancer; NR, no response; CRPC, castrate-resistant prostate cancer; LN, lymph node; PR, partial response; MXR, mixed response; RCC, renal cell carcinoma.
Determined by immunohistochemistry.
Footnotes
Supported in part by a grant from the Melanoma Research Alliance Foundation (L.C., D.M.P., S.L.T.).
Presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology (ASCO) May 30-June 3, 2008, Chicago, IL, and the 45th Annual Meeting of ASCO, May 29-June 2, 2009, Orlando, FL.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00441337.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Elizabeth Stankevich, Medarex (C); Changyu Wang, Medarex (C); Mark Selby, Medarex (C); Alan J. Korman, Medarex (C); Israel Lowy, Medarex (C) Consultant or Advisory Role: Julie R. Brahmer, Medarex (U); Charles G. Drake, Medarex (C), Amplimmune (C), Bristol-Myers Squibb (C), Dendreon (C); Suzanne L. Topalian, Millennium Pharmaceuticals (C), Bristol-Myers Squibb (U) Stock Ownership: Charles G. Drake, Amplimmune; Mark Selby, Bristol-Myers Squibb; Alan J. Korman, Bristol-Myers Squibb; Israel Lowy, Bristol-Myers Squibb Honoraria: Charles G. Drake, Institute for Medical Education and Research; Drew M. Pardoll, Medarex; Suzanne L. Topalian, Medarex Research Funding: John D. Powderly, Medarex; Joel Picus, Medarex; Drew M. Pardoll, Medarex; Suzanne L. Topalian, Medarex Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Julie R. Brahmer, Drew M. Pardoll, Israel Lowy, Suzanne L. Topalian
Provision of study materials or patients: Julie R. Brahmer, Charles G. Drake, Ira Wollner, John D. Powderly, Joel Picus, William H. Sharfman, Changyu Wang, Mark Selby, Alan J. Korman, Israel Lowy
Collection and assembly of data: Julie R. Brahmer, Charles G. Drake, John D. Powderly, Elizabeth Stankevich, Alice Pons, Theresa M. Salay, Tracee L. McMiller, Changyu Wang, Janis M. Taube, Robert Anders, Alan J. Korman, Israel Lowy, Suzanne L. Topalian
Data analysis and interpretation: Julie R. Brahmer, Charles G. Drake, Joel Picus, Theresa M. Salay, Tracee L. McMiller, Marta M. Gilson, Changyu Wang, Janis M. Taube, Robert Anders, Lieping Chen, Alan J. Korman, Drew M. Pardoll, Israel Lowy, Suzanne L. Topalian
Manuscript writing: Julie R. Brahmer, Charles G. Drake, Drew M. Pardoll, Israel Lowy, Suzanne L. Topalian
Final approval of manuscript: Julie R. Brahmer, Charles G. Drake, Ira Wollner, John D. Powderly, Joel Picus, William H. Sharfman, Elizabeth Stankevich, Alice Pons, Theresa M. Salay, Tracee L. McMiller, Marta M. Gilson, Changyu Wang, Mark Selby, Janis M. Taube, Robert Anders, Lieping Chen, Alan J. Korman, Drew M. Pardoll, Israel Lowy, Suzanne L. Topalian
REFERENCES
- 1.Segal NH, Parsons DW, Peggs KS, et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68:889–892. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- 2.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: The roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 3.Topalian SL, Chen L, Taube J, et al. Immunology. In: Balch C, Houghton AN, Sober AJ, et al., editors. Cutaneous Melanoma. ed 5. St. Louis, MO: Quality Medical Publishing; 2009. pp. 865–882. [Google Scholar]
- 4.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 5.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 9.Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strome SE, Dong H, Tamura H, et al. B7-H1 blockade augments T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 11.Blank C, Brown I, Peterson AC, et al. PD-L1/B7-H1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 12.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nature Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 13.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 14.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong RM, Scotland RR, Lau RL, et al. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol. 2007;19:1223–1234. doi: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]
- 16.Tsushima F, Yao S, Shin T, et al. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110:180–185. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg MV, Maris CH, Hipkiss EL, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 19.Li B, VanRoey M, Wang C, et al. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor–secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin Cancer Res. 2009;15:1623–1634. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]