Abstract
Hepatocellular carcinoma (HCC) is one of the most lethal cancers worldwide because of metastasis. Epithelial‐mesenchymal transition (EMT) is widely considered to be crucial to the invasion‐metastasis cascade during cancer progression. Actin‐like 6A (ACTL6A) is initially verified important for cell proliferation, differentiation, and migration. In this study, we find that ACTL6A plays an essential role in metastasis and EMT of HCC. ACTL6A expression is up‐regulated in HCC cells and tissues. A high level of ACTL6A in HCCs is correlated with aggressive clinicopathological features and is an independent poor prognostic factor for overall and disease‐free survival of HCC patients. Ectopic expression of ACTL6A markedly promotes HCC cells migration, invasion, as well as EMT in vitro and promotes tumor growth and metastasis in the mouse xenograft model. Opposite results are observed when ACTL6A is knocked down. Mechanistically, ACTL6A promotes metastasis and EMT through activating Notch signaling. ACTL6A knockdown has the equal blockage effect as the Notch signaling inhibitor, N‐[N‐(3,5‐difluorophenacetyl)‐L‐alanyl]‐S‐phenylglycine t‐butylester, in HCC cells. Further studies indicate that ACTL6A might manipulate SRY (sex determining region Y)‐box 2 (SOX2) expression and then activate Notch1 signaling. Conclusions: ACTL6A promotes metastasis and EMT by SOX2/Notch1 signaling, indicating a prognostic biomarker candidate and a potential therapeutic target for HCC. (Hepatology 2016;63:1256–1271)
Abbreviations
- ACTL6A
actin‐like 6A
- AFP
alpha‐fetoprotein
- ANLT
adjacent nontumor liver tissue
- BAF
BRG1/brm‐associated factor
- BCLC
Barcelona Clinic Liver Cancer
- ChIP
chromatin immunoprecipitation
- DAPT
N‐[N‐(3,5‐difluorophenacetyl)‐L‐alanyl]‐S‐phenylglycine t‐butylester
- DFS
disease‐free survival
- EMT
epithelial‐mesenchymal transition
- HCC
hepatocellular carcinoma
- Hes1
Hes family BHLH transcription factor 1
- IF
immunofluorescence
- IHC
immunohistochemistry
- mRNA
messenger RNA
- NHCC
nodular hepatocellular carcinoma
- NICD
Notch intracellular domain
- OS
overall survival
- qPCR
quantitative PCR
- RT‐PCR
reverse‐transcription polymerase chain reaction
- SC
subcutaneous
- SHCC
small hepatocellular carcinoma
- shRNA
short hairpin RNA
- SLHCC
solitary large hepatocellular carcinoma
- SOX2
SRY (sex determining region Y)‐box 2
- TNM
tumor node metastasis
Hepatocellular carcinoma (HCC) in males is the fifth‐most common and second leading cause of cancer death in global. Approximately 782,500 new cases and 745,500 deaths occurred worldwide in 2012, with China accounting for more than half of the total number of cases and deaths.1 Although the treatments for HCC have been greatly improved, the outcome of HCC is still unfavorable, with an approximately 30% overall 5‐year survival rate after liver resection.2 Metastasis, the main reason for poor survival of HCC patients, is formed by a complicate succession of invasion‐metastasis cascades. In spite of the controversies, many recent studies proposed that epithelial‐mesenchymal transition (EMT) was one of the critical programs for invasion‐metastasis cascades.3 EMT is a biological process that enable an epithelial cell changes to a mesenchymal cell phenotype, which was initially described in embryonic development by Elizabeth Hay.4 Subsequently, a number of studies have found that EMT also endows epithelial cells with migratory and invasive capacity and also is involved in many cancer metastases, including HCC.5, 6 Notably, our previous studies have found and defined a specific HCC subtype with favorable prognosis, the solitary large HCC (SLHCC), in which gene expression profiles were different to nodular HCC (NHCC) with a poor prognosis.7, 8 Interestingly, some EMT promotion genes and microRNAs (e.g., miR‐331‐3p and SIN1) are highly expressed in NHCC, but poorly expressed in SLHCC.9, 10 Therefore, it is necessary to further understand the regulatory mechanisms of EMT in invasion and metastasis of HCC.
Actin‐like 6A (ACTL6A), also known as BAF53A, is a member of adenosine triphosphate‐dependent SWI/SNF‐like BRG1/brm‐associated factor (BAF) chromatin remodeling complexes and encodes a 53‐kDa subunit of the BAF complex in mammals.11 Previous studies indicated that ACTL6A was involved in diverse cellular processes, including transcriptional regulation, chromatin remodeling, and nuclear migration.12 Recently, some researches found that ACTL6A participated in neural progenitor proliferation, differentiation, and migration during gastrulation.13, 14 Another study found that ACTL6A could sustain epidermal self‐renewal and arrest cells at an undifferentiated progenitor state, as well as suppress epithelial properties.15 The role of ACTL6A involvement in proliferation, migration, and EMT gives rise to a hypothesis that ACTL6A plays an important role in HCC invasion and metastasis.
In this study, we found that a high ACTL6A expression level is closely correlated with poor prognosis of HCC patients and that ACTL6A promotes HCC progression and EMT. Mechanism studies show that ACTL6A enhances SRY (sex determining region Y)‐box 2 (SOX2) expression in HCC, which up‐regulates Notch1 expression and triggers Notch signaling in HCC.
Materials and Methods
HCC Samples and Patients
A total of 30 pairs of randomly selected snap‐frozen and 100 pairs of paraffin‐embedded HCCs and adjacent nontumor liver tissues (ANLTs) from consecutive patients who have received hepatectomy for HCC at Department of Surgery, Xiangya Hospital of Central South University (Changsha, China) from January 2006 to December 2009 were enrolled into the training cohort. Also, 63 pairs of randomly selected paraffin‐embedded HCCs and ANLTs from consecutive HCC patients who received a hepatectomy at Department of Abdominal Surgical Oncology, Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University from January 2005 to December 2010 were enrolled into the validation cohort (Supporting Fig. 1). Diagnosis of HCC was confirmed by two independent histopathologists. The surgical indication was an HCC patient with liver tumor of enough residual liver volume, and lack of distant metastasis, decompensated cirrhosis, and organic dysfunction. All researches on human materials were approved by the ethics committee of Xiangya Hospital of Central South University and Affiliated Cancer Hospital of Xiangya School of Medicine, respectively.
Follow‐up and Prognostic Study
Surveillance for tumor recurrence and metastasis was done using serum alpha‐fetoprotein (AFP), ultrasonography, and chest X‐ray every 3 months in the first 2 years, and twice a year in the next 5 years. Computed tomography scan or magnetic resonance imaging was performed if recurrence or metastasis was suspected clinically. Study endpoints were defined as: overall survival (OS); time from liver resection to death of HCC or to the date of the last follow‐up; or disease‐free survival (DFS): time from the date of hepatic resection to the date when recurrence or metastasis was detected.7 The complete clinical and pathological features of these patients were collected and stored in our database by a researcher fellow. Research protocols followed the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) recommendations for reporting prognostic biomarkers in cancer.16
Further details of materials and methods are described in the Supporting Materials and Methods.
Results
ACTL6A Is Significantly Up‐Regulated in HCC Cell Lines and Tissues
To study expression of ACTL6A in HCC cells and tissues, real‐time polymerase chain reaction (PCR), reverse‐transcription PCR (RT‐PCR), and western blotting were performed. Compared with L02 cells, which are immortalized human normal liver cells, ACTL6A messenger RNA (mRNA) and protein were highly expressed in HCC cells (Fig. 1A). Then, expression of ACTL6A in HCCs and their corresponding ANLTs were also detected. Thirty pairs of HCCs (SHCC [single nodule <5 cm] = 10; SLHCC = 10; NHCC = 10) and corresponding ANLTs were used here and compared with normal liver tissue from a hepatic hemangioma patient (L495). Consistent with the increased ACTL6A expression in HCC cell lines, ACTL6A expression in HCC tissues was markedly higher than ANLTs and normal liver tissue (Fig. 1B). ACTL6A expression in HCC was analyzed by immunohistochemistry (IHC), which suggested that ACTL6A protein was highly expressed in HCC (Fig. 1C). Comparing samples with metastasis to nonmetastasis samples and ANLTs, metastasis samples have the highest intensities of ACTL6A staining, whereas ANLTs have the lowest intensities (Supporting Fig. 2A). Notably, ACTL6A expression in high‐metastasis potential cell lines, such as HCCLM3, MHCC97‐H, and Hep3B was higher than in low‐metastasis potential cell lines PLC/PRF5, SMMC7721, and HepG2 (Fig. 1A,B). ACTL6A expression in SHCC, SLHCC, and NHCC was also step‐increased (Supporting Fig. 2B). These data indicated that ACTL6A was up‐regulated in HCCs and it might be correlated with HCC invasion and metastasis.
Figure 1.
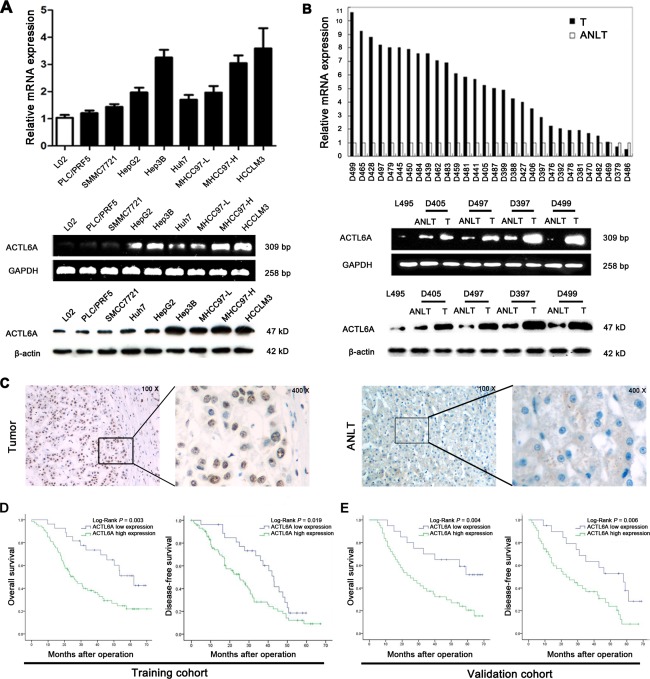
ACTL6A expression is up‐regulated in HCC and indicates poor prognosis. (A) ACTL6A expression is up‐regulated in human HCC cell lines and tumor tissues (B) analyzed by real‐time PCR, RT‐PCR, and western blotting. (C) Representative IHC images of ACTL6A expression in HCC and ANLT. (D) OS and DFS of HCC patients with high or low ACTL6A expression in the training cohort and validation cohort (E). Survival curve was calculated with the log‐rank test. Abbreviations: bp, base pairs; T, HCC tissue.
High ACTL6A Expression Correlates With Poor Clinicopathological Features and Survival of HCC Patients
We then estimated the association of ACTL6A expression with clinicopathological features and survival of HCC patients using the training cohort and validation cohort (Supporting Table 1). In the training cohort, it showed that a high expression level of ACTL6A was significantly associated with serum AFP level, tumor nodule number, Edmondson‐Steiner grade, microvascular invasion, Barcelona Clinic Liver Cancer (BCLC) stage, and tumor node metastasis (TNM) stage (all P < 0.05; Table 1). HCC patients in the high ACTL6A expression group had shorter OS (1‐, 3‐, and 5‐year OS: 96.3%, 73.5%, and 51.0% vs. 80.8%, 38.1%, and 21.9%; P = 0.003) and DFS rates (1‐, 3‐, and 5‐year DFS: 96.3%, 64.6%, and 18.5% vs. 75.6%, 28.2%, and 9.1%; P = 0.019) than patients in the low‐expression group (Fig. 1D). Furthermore, uni‐ and multivariate analysis revealed that high ACTL6A expression was an independent risk factor for both OS and DFS of HCC patients after liver resection (Table 2). ACTL6A was an independent prognosis marker, and its high expression associated with poor survival of HCC patients was further verified in the validation cohort (Fig. 1E; Supporting Tables 2 and 3). Then, survival analysis for the overall cohort and different HCC subtypes also demonstrated that high ACTL6A expression level of patients had shorter OS and DFS (Supporting Fig. 3A‐D). These results fully demonstrated that ACTL6A was closely correlated with poor survival and could be used as a novel independent prognosis biomarker for HCC patients after hepatic resection.
Table 1.
Correlation Between ACTL6A Expression With Clinicopathological Characteristics of HCC in Training and Validation Cohort
| Training Cohort | Validation Cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinicopathological Variables | ACTL6A Expression | ACTL6A Expression | ||||||
| n | High (73) | Low (27) | P Value | n | High (43) | Low (20) | P Value | |
| Sex | ||||||||
| Female | 31 | 22 | 9 | 22 | 16 | 6 | ||
| Male | 69 | 51 | 18 | 0.759 | 41 | 27 | 14 | 0.576 |
| Age, years | ||||||||
| <50 | 62 | 43 | 19 | 37 | 24 | 13 | ||
| ≥50 | 38 | 30 | 8 | 0.294 | 26 | 19 | 7 | 0.491 |
| AFP, ng/mL | ||||||||
| <20 | 43 | 26 | 17 | 25 | 15 | 10 | ||
| ≥20 | 57 | 47 | 10 | 0.014 | 38 | 28 | 10 | 0.254 |
| HBsAg | ||||||||
| Negative | 7 | 6 | 1 | 6 | 4 | 2 | ||
| Positive | 93 | 67 | 26 | 0.731 | 57 | 39 | 18 | 0.583 |
| Liver cirrhosis | ||||||||
| Absence | 35 | 24 | 11 | 21 | 13 | 8 | ||
| Presence | 65 | 49 | 16 | 0.464 | 42 | 30 | 12 | 0.444 |
| Liver function | ||||||||
| Child‐Pugh A | 91 | 66 | 25 | 56 | 38 | 18 | ||
| Child‐Pugh B | 9 | 7 | 2 | 0.464 | 7 | 5 | 2 | 0.534 |
| Tumor size, cm | ||||||||
| ≤5 | 38 | 25 | 13 | 19 | 12 | 7 | ||
| >5 | 62 | 48 | 14 | 0.204 | 44 | 31 | 13 | 0.568 |
| Tumor nodule number | ||||||||
| Solitary | 57 | 36 | 21 | 30 | 16 | 14 | ||
| Multiple (≥2) | 43 | 37 | 6 | 0.011 | 33 | 27 | 6 | 0.015 |
| Capsulation formation | ||||||||
| Presence | 42 | 29 | 13 | 26 | 15 | 11 | ||
| Absence | 58 | 44 | 14 | 0.449 | 37 | 28 | 9 | 0.131 |
| Edmondson‐Steiner grade | ||||||||
| I & II | 64 | 42 | 22 | 39 | 24 | 15 | ||
| III & IV | 36 | 31 | 5 | 0.014 | 24 | 19 | 5 | 0.082 |
| Microvascular invasion | ||||||||
| Absence | 34 | 19 | 15 | 25 | 13 | 12 | ||
| Presence | 66 | 54 | 12 | 0.006 | 38 | 30 | 8 | 0.025 |
| BCLC stage | ||||||||
| 0 & A | 28 | 16 | 12 | 18 | 7 | 11 | ||
| B & C | 72 | 57 | 15 | 0.026 | 45 | 36 | 9 | 0.002 |
| TNM stage | ||||||||
| Early (I & II) | 67 | 44 | 23 | 35 | 21 | 14 | ||
| Late (III & IV) | 33 | 29 | 4 | 0.010 | 28 | 22 | 6 | 0.116 |
No patients with Child‐Pugh C were included. ACTL6A low expression: IHC score 0‐1; ACTL6A high expression: IHC score 2‐4.
* Significant results (P < 0.05) are given in bold.
Abbreviation: HBsAg, hepatitis B surface antigen.
Table 2.
Uni‐ and Multivariate Analyses of Risk Factors Associated With OS and DFS of HCC Patients in Training Cohort
| OS | DFS | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | ||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Sex | ||||||||
| Female | 1 | 1 | ||||||
| Male | 1.138 (0.671‐1.929) | 0.632 | NA | 1.021 (0.629‐1.657) | 0.934 | NA | ||
| Age, years | ||||||||
| <50 | 1 | 1 | ||||||
| ≥50 | 1.011 (0.587‐1.741) | 0.968 | NA | 1.196 (0.717‐1.992) | 0.493 | NA | ||
| AFP, ng/mL | ||||||||
| <20 | 1 | 1 | 1 | |||||
| ≥20 | 2.282 (1.353‐3.847) | 0.002 | 1.832 (1.064‐3.157) | 0.029 | 1.826 (1.137‐2.774) | 0.013 | NS | |
| HBsAg | ||||||||
| Negative | 1 | 1 | ||||||
| Positive | 2.029 (0.634‐6.490) | 0.233 | NA | 2.294 (0.828‐6.359) | 0.110 | NA | ||
| Liver cirrhosis | ||||||||
| Absence | 1 | 1 | 1 | |||||
| Presence | 1.415 (0.839‐2.385) | 0.193 | NA | 2.023 (1.221‐3.350) | 0.004 | 1.992 (1.139‐3.484) | 0.016 | |
| Liver function | ||||||||
| Child‐Pugh A | 1 | 1 | ||||||
| Child‐Pugh B | 1.952 (0.930‐4.096) | 0.077 | NS | 1.298 (0.561‐3.000) | 0.542 | NA | ||
| Tumor size(cm) | ||||||||
| ≤5 | 1 | 1 | ||||||
| >5 | 1.669 (0.997‐2.795) | 0.051 | NS | 1.223 (0.768‐1.948) | 0.397 | NA | ||
| Tumor nodule number | ||||||||
| Solitary | 1 | 1 | 1 | 1 | ||||
| Multiple (≥2) | 4.133 (2.457‐6.950) | <0.001 | 2.399 (1.300‐4.426) | 0.005 | 1.795 (1.130‐2.850) | 0.013 | 2.024 (1.204‐3.402) | 0.008 |
| Capsulation formation | ||||||||
| Presence | 1 | 1 | ||||||
| Absence | 1.576 (1.041‐3.193) | 0.028 | NS | 1.413 (0.863‐2.126) | 0.092 | NS | ||
| Edmondson‐Steiner grade | ||||||||
| I & II | 1 | 1 | ||||||
| III & IV | 1.866 (1.137‐3.063) | 0.014 | NS | 1.823 (1.133‐2.931) | 0.013 | NS | ||
| Microvascular invasion | ||||||||
| Absence | 1 | 1 | 1 | 1 | ||||
| Presence | 5.866 (2.209‐8.674) | <0.001 | 2.707 (1.209‐6.604) | 0.015 | 3.786 (2.207‐6.495) | <0.001 | 3.304 (1.844‐5.920) | <0.001 |
| BCLC stage | ||||||||
| 0 & A | 1 | 1 | 1 | |||||
| B & C | 2.703 (1.266‐5.101) | <0.001 | 2.434 (1.016‐5.831) | 0.046 | 2.753 (1.602‐4.731) | <0.001 | NS | |
| TNM stage | ||||||||
| Early (I & II) | 1 | 1 | 1 | 1 | ||||
| Late (III & IV) | 3.978 (2.408‐6.572) | <0.001 | 1.965 (1.133‐3.409) | 0.016 | 2.215 (1.331‐3.685) | 0.002 | 1.732 (1.013‐2.962) | 0.045 |
| ACTL6A expression | ||||||||
| Negative | 1 | 1 | 1 | 1 | ||||
| Positive | 3.476 (1.340‐5.974) | 0.002 | 2.775 (1.301‐5.918) | 0.008 | 3.312 (1.942‐5.647) | <0.001 | 2.332 (1.285‐4.233) | 0.005 |
Abbreviations: HR, hazard risk ratio; CI, confidence interval; NA, not applicable; NS, not significant.
ACTL6A Promotes HCC Cell Proliferation, Migration, and Invasion In Vitro
To understand the function of ACTL6A in HCC cells, we manipulated ACTL6A expression in cells by ectopic expression and short hairpin RNA (shRNA) knockdown. Ectopic ACTL6A was constituently expressed in PLC/PRF5 cells named as PLC/PRF5‐ACTL6A subsequently. Meanwhile, three shRNAs (shRNA1, shRNA2, and shRNA3) were designed to silence ACTL6A expression in Hep3B cells named as Hep3B‐shACTL6A subsequently. Expression level of ACTL6A was identified by real‐time PCR and western blotting; shRNA3 was the most effective one and was chosen for further study (Supporting Fig. 4A,B). Compared to PLC/PRF5, PLC/PRF5‐ACTL6A had a higher absorbance in methyl thiazol tetrazolium assay, which indicated a higher proliferation rate (Fig. 2A). Consistently, PLC/PRF5‐ACTL6A cells also formed more colonies in colony formation assay (Fig. 2B and Supporting Fig. 4C). In contrast, Hep3B‐shACTL6A cells had decreased absorbance, cell proliferation rate, and clonogenicity capacity (Fig. 2A,B and Supporting Fig. 4C). The wound‐healing and transwell assays were used to investigate migration and invasion capacity. Results showed that PLC/PRF5‐ACTL6A cells had a faster wound closure rate and more invasion cells than PLC/PRF5 cells, whereas Hep3B‐shACTL6A cells had markedly reduced migratory and invasive capacity (Fig. 2C,D and Supporting Fig. 4D,E). It suggests that ACTL6A promotes HCC cell proliferation, migration, and invasion capacity in vitro.
Figure 2.
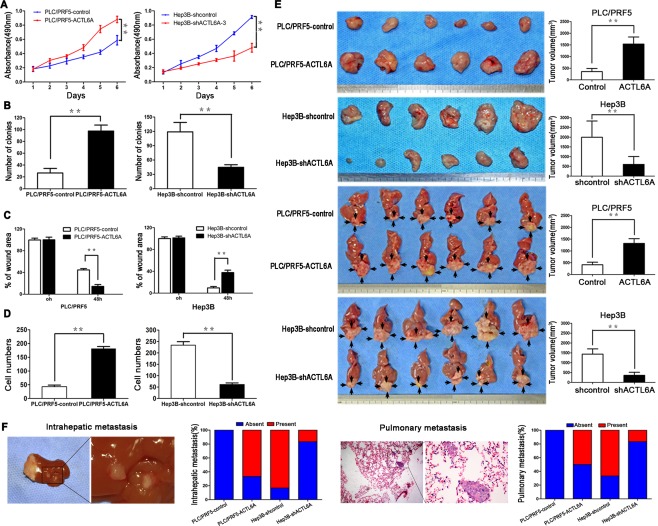
ACTL6A promotes proliferation and metastasis of HCC in vitro and in vivo. (A) Proliferation of PLC/PRF5‐ACTL6A, Hep3B‐shACTL6A‐3 cells and control cells was examined by MTT and colony formation assays (B). (C) Wound‐healing assay and (D) transwell invasion assay were subjected to detect the migration and invasion capacity of ACTL6A‐interfered cells. (E) SC tumors from PLC/PRF5‐ACTL6A and Hep3B‐shACTL6A cells and their control cells are shown in the upper two panels. Orthotopic tumors from each indicated groups are shown in the lower two panels (black arrows indicate tumors). Tumor volumes of tumors are shown in the right panels. (F) Representative pictures of intrahepatic and lung metastasis; metastatic nodules proportion of livers or lungs was calculated and compared.* P < 0.05; ** P < 0.01 based on the Student t test. Error bars, standard deviation.
ACTL6A Promotes HCC Growth and Metastasis In Vivo
To verify the above findings in vivo, we established subcutaneous (SC) xenograft tumor and orthotopic xenograft tumor models, as previously described.9 After 6 weeks, PLC/PRF5‐ACTL6A cell‐derived tumors at the SC implantation sites were larger and grew more rapidly than PLC/PRF5‐derived tumors, whereas Hep3B‐shACTL6A‐derived tumors were smaller and grew more slowly than Hep3B‐derived tumors (Fig. 2E and Supporting Fig. 5A). Consistently, liver orthotopic xenograft tumor also showed that ACTL6A promoted tumor growth, whereas ACTL6A knockdown inhibited tumor growth in vivo (Fig. 2E). ACTL6A expression in xenograft tumors was verified by IHC (Supporting Fig. 5B). We further detected the metastatic foci in livers and lungs. The intrahepatic and pulmonary metastasis rates in mice with tumors derived from PLC/PRF5‐ACTL6A cells were significantly higher than in mice with tumors derived from PLC/PRF5 cells. In contrast, metastasis rates were markedly decreased for tumors generated from Hep3B‐shACTL6A cells compared to Hep3B cells (Fig. 2F). Taking these results together, our studies demonstrated that ACTL6A could promote HCC growth and metastasis in vivo.
ACTL6A Promotes EMT in HCC
In this study, PLC/PRF5‐ACTL6A cells exhibited spindle‐like mesenchymal morphology, whereas PLC/RPF5 cells mostly looked like epithelial cobblestones in appearance. On the contrary, Hep3B‐shACTL6A cells changed to an epithelial phenotype as compared with mesenchymal‐like Hep3B cells (Fig. 3A). Considering the morphological change and the function of ACTL6A in HCC, we speculated that an abnormal high level of ACTL6A would trigger EMT.15, 17 This speculation was confirmed by cytological and histology experiments. Immunofluorescence (IF) analysis showed that PLC/PRF5‐ACTL6A cells presented an elongated cellular morphology and the appearance of F‐actin fibers compared with PLC/PRF5 cells. Inversely, Hep3B‐shACTL6A cells exhibited a cobblestone shape and shrinkable F‐actin fiber changes to Hep3B cells (Fig. 3B). The anchorage‐independent growth assay indicated that colonies that grew from PLC/PRF5‐ACTL6A cells were larger and more in soft agar than PLC/PRF5 cells, whereas colonies that grew from Hep3B‐shACTL6A cells were smaller and less than Hep3B cells (Fig. 3C). IF analysis further showed that ectopic expression of ACTL6A reduced expression of the epithelial marker, E‐cadherin, and increased the mesenchymal marker, vimentin, in PLC/PRF5‐ACTL6A cells, whereas knockdown ACTL6A in Hep3B cells increased E‐cadherin expression and decreased vimentin expression (Fig. 3D). Consistent with this, ACTL6A highly expressed cells showed decreased E‐cadherin and increased vimentin and snail (EMT transcriptional factor) expressions at both protein and mRNA levels, whereas ACTL6A knockdown induced the opposite results (Fig. 3E and Supporting Fig. 6). Moreover, IHC for HCC consecutive sections revealed that high ACTL6A expression was colocation with low E‐cadherin and high vimentin expression, and vice versa in ANLT (Fig. 3F). The correlation analysis revealed that ACTL6A expression was positively correlative with vimentin and negatively correlative with E‐cadherin expression in HCC samples in both the training and validation cohorts (Fig. 3F). These results indicated that ACTL6A played an important role in promoting EMT in HCC.
Figure 3.
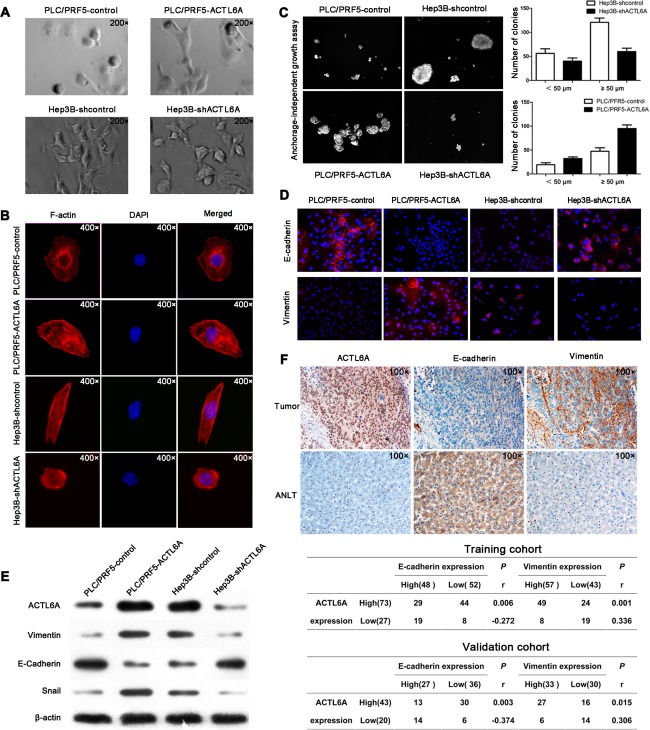
ACTL6A induces EMT in HCC. (A) Representative phase‐contrast images of PLC/PRF5‐ACTL6A, Hep3B‐shACTL6A, and their control cells. (B) Representative images of cytoskeleton, (C) anchor‐independent growth, and (D) IF for EMT markers showed that ACTL6A affected cellular morphology, anchor‐independent growth, and expressions of EMT markers. (E) Expressions of EMT markers mediated by ACTL6A were detected by western blotting. (F) Representative IHC images of ACTL6A, E‐cadherin, and vimentin expressions in consecutive tissue sections of HCC and ANLT, and their correlation in HCC samples, were analyzed by Spearman's rank correlation test in the training and validation cohorts.
ACTL6A Activates Notch Signaling in HCC
To systemically screen the potential signaling manipulated by ACTL6A, a Cignal Finder Cancer 10‐Pathway Reporter Array was used. ACTL6A significantly enhanced the activity of Notch signaling in PLC/PRF5 cells, and ACTL6A knockdown attenuated Notch signaling activity in Hep3B cells (Fig. 4A). Notch signaling is crucial for development and progression of HCC, and Notch receptor is the key role of Notch signaling activation.18, 19 However, which member of the four known Notch receptors (Notch1‐4) is regulated by ACTL6A in HCC was unknown.20, 21, 22 We detected Notch 1‐4 expression in PLC/PRF5, PLC/PRF5‐ACTL6A, Hep3B, and Hep3B‐shACTL6A cells. Ectopic expression of ACTL6A in PLC/PRF5 increased Notch1 expression, whereas knockdown of ACTL6 in Hep3B decreased Notch1 expression. No considerable change was observed for the other three Notch genes (Fig. 4B). Then, we focused on Notch1 signaling members in HCC cells. It showed that a high level of ACTL6A in PLC/PRF5 cells increased Notch intracellular domain (NICD)1, RBP‐Jκ, and Hes1 (Hes family BHLH transcription factor 1) expression. Consistently, knockdown of ACTL6A in Hep3B cells inhibited these gene expressions. Manipulation of ACTL6A expression in cells did not affect Jagged1 expression, which indicated that ACTL6A specifically regulated NICD1, RBP‐Jκ, and Hes1 in Notch1 signaling (Fig. 4C).
Figure 4.
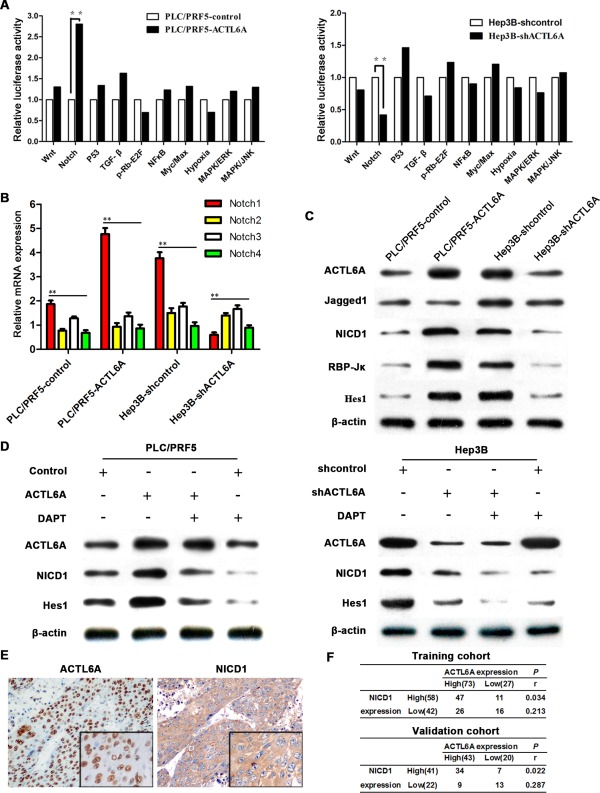
ACTL6A activates Notch signaling in HCC. (A) The 10‐Pathway Reporter Array showed the signaling change in ACTL6A‐interfered cells. (B) The four known Notch receptors' mRNA expression were detected by real‐time PCR. (C) Key members of Notch1 signaling expressions were detected by western blotting. (D) NICD1 and Hes1 expression in HCC cells after being treated by ACTL6A, shACTL6A, and/or DAPT for 72 hours were detected. (E) Representative IHC images of ACTL6A and NICD1 expression in HCC tissues; magnification: 100×, inset magnification: 400×. (F) Correlation of ACTL6A and NICD1 expression levels was analyzed by Spearman's rank correlation test. Abbreviations: ERK, extracelullar signal‐regulated kinase; JNK, c‐Jun N‐terminal kinase; MAPK, mitogen‐activated protein kinase; NF‐κB, nuclear factor kappa B; TGF‐β, transforming growth factor beta.
To confirm activation of Notch signaling in HCC cells, cells were treated by the gamma‐secretase inhibitor, N‐[N‐(3,5‐difluorophenacetyl)‐L‐alanyl]‐S‐phenylglycine t‐butylester (DAPT), for 72 hours, which prevented release of activated NICD. The appropriate dosage (5 μM) was selected based on cell viability (MTS assay) and NICD inhibition efficiency (Supporting Fig. 7A,B). Results showed that DAPT treatment did not affect ACTL6A expression in PLC/PRF5 cells, but it inhibited ACTL6A‐induced NICD1 release and Notch1 effector Hes1 expression. It confirms that ACTL6A activates Notch1, not its downstream molecules. In Hep3B cells, as DAPT treatment inhibited NICD1 release, ACTL6A knockdown also caused reduced NICD1 release. It proves that ACTL6A up‐regulates Notch1 signaling in HCC cells (Fig. 4D). IHC analysis also showed that both ACTL6A and NICD1 were high expression in HCC tissues (Fig. 4E). Spearman's rank correlation test indicated the significant, but moderate, positive correlation of NICD1 expression and ACTL6A expression (Fig. 4F). These results concluded that ACTL6A could activate Notch signaling as an upstream regulator in HCC.
ACTL6A Promotes Invasion, Metastasis, and EMT Through Notch Signaling
To further study the role of ACTL6A and Notch signaling in invasion, metastasis, and EMT of HCC, we first measured the invasive capacity of ACTL6A‐interfered HCC cells with or without DAPT treatment. Wound‐healing and transwell assays showed that DAPT treatment obviously decreased the migration and invasion capacity of ACTL6A high‐expression cells, but had none or less effect for PLC/PRF5 and Hep3B‐shACTL6A cells in which ACTL6A expression and Notch1 activity was low (Fig. 5A and Supporting Fig. 8A,B). IF analysis revealed that DAPT treatment increased E‐cadherin expression, and decreased vimentin expression in PLC/PRF5‐ACTL6A and Hep3B cells, but they were not significantly altered for PLC/PRF5 and Hep3B‐shACTL6A cells (Fig. 5B). Anchorage‐independent growth assay also indicated that DAPT treatment decreased the clonogenic capacity of cells with a high ACTL6A level (Supporting Fig. 8C). Western blotting analysis indicated that DAPT treatment increased epithelial marker (E‐cadherin) expression and decreased mesenchymal marker (vimentin and snail) expression in cells with a high level of ACTL6A, and ACTL6A expression had no considerable change (Fig. 5C). Moreover, IHC analysis of liver orthotopic transplantation tumor showed that NICD1, vimentin, and snail expression levels were high in tumors with a high level of ACTL6A and were low in tumors with poor expression of ACTL6A. Correspondingly, E‐cadherin expression was the opposite (Fig. 5D). Taken together, we concluded that ACTL6A promoted invasion, metastasis, and EMT through Notch signaling in HCC.
Figure 5.
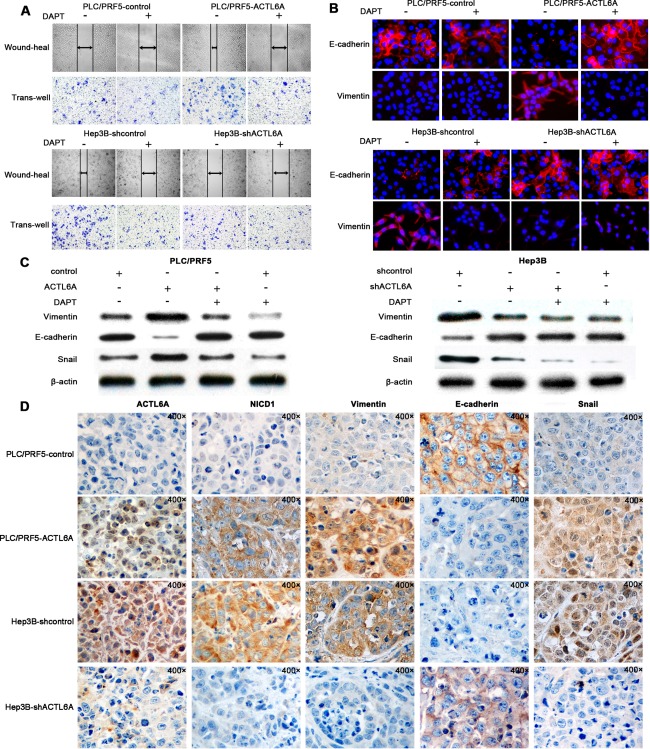
ACTL6A promotes invasion, metastasis, and EMT through Notch signaling. (A) Migration and invasion assays for PLC/PRF5‐ACTL6A, Hep3B‐shACTL6A and their control cells with/without DAPT treatment. (B) IF assay of E‐cadherin and vimentin expressions in PLC/PRF5‐ACTL6A, Hep3B‐shACTL6A, and their control cells with/without DAPT treatment. (C) EMT‐related protein expression in PLC/PRF5‐ACTL6A, Hep3B‐shACTL6A, and their control cells with/without DAPT treatment. (D) Representative IHC images of ACTL6A, NICD1, vimentin, E‐cadherin, and snail expression in liver orthotopic transplantation tumor generated from PLC/PRF5‐ACTL6A, Hep3B‐shACTL6A and their control cells; magnification: 400×.
ACTL6A Activates Notch Signaling by SOX2
It has proved that ACTL6A promotes Notch signaling in HCCs; however, how ACTL6A regulates Notch1 signaling in HCC is unknown. We have reviewed literature and searched the BioGrid 3.4 database, and found that ACTL6A could interact with the SOX2 promoter.17 SOX2 is an important transcription factor that could positively transcriptional regulate Notch1, and it also could be transcriptionally regulated by Jagged123; these findings led us to hypothesize that ACTL6A could activate Notch signaling by regulating SOX2 expression. To test this, we first determined the expression levels of SOX2, Jagged1, Notch1, and Hes1 in ACTL6A‐interfered HCC cells. It showed that ectopic expression of ACTL6A could enhance SOX2, Notch1, and Hes1 expression, whereas ACTL6A knockdown markedly decreased their expression both at mRNA and protein levels. However, Jagged1 expression was not affected by ectopic expression or knockdown of ACTL6A (Fig. 6A).
Figure 6.
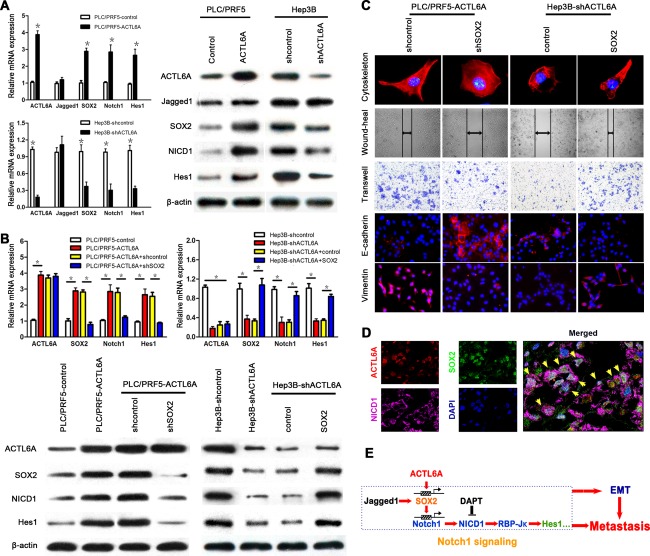
ACTL6A activates Notch signaling by SOX2. (A) mRNA and protein expressions of ACTL6A, SOX2, and key members of Notch1 signaling were detected in PLC/PRF5‐ACTL6A, Hep3B‐shACTL6A, and their control cells. (B) mRNA and protein expressions of ACTL6A, SOX2, Notch1, and Hes1 in ACTL6A‐interfered HCC cells with SOX2 knockdown or ectopic expression. (C) The cytoskeleton, migration, invasion, and EMT marker expression assays of ACTL6A‐interfered HCC cells with SOX2 knockdown or ectopic expression. (D) Representative triple IF images showed that ACTL6A, SOX2, and NICD1 were colocalized in HCC tissue detected by confocal laser scanning microscope (yellow arrows indicate colocalization cells). (E) The schematic diagram of ACTL6A activating Notch1 signaling by SOX2, and promoting metastasis and EMT of HCC. Abbreviation: DAPI, 4′,6‐diamidino‐2‐phenylindole.
To further test whether SOX2 was regulated by ACTL6A, SOX2‐shRNA was transfected into PLC/PRF5‐ACTL6A cells, and SOX2 ectopic expression plasmid was transfected into Hep3B‐shACTL6A cells. Ectopic expression and silence efficacy was identified by real‐time PCR and western blotting (Supporting Fig. 9A,B). Knockdown of SOX2 decreased Notch1 and Hes1 expression and had no significant effect on ACTL6A expression. Ectopic expression of SOX2 in Hep3B‐shACTL6A cells restored the poor Notch1 and Hes1 expression, which was caused by ACTL6A knockdown as shown earlier (Fig. 6B). The cytoskeleton, wound‐healing, transwell, and EMT marker assays indicated that knockdown of SOX2 in PLC/PRF5‐ACTL6A cells presented a cobblestone‐like morphology with shrinkable F‐actin fibers, decreased migration and invasion capacity, and induced E‐cadherin expression (Fig. 6C and Supporting Fig. 10A). In contrast, ectopic expression of SOX2 in Hep3B‐shCTL6A cells had the inverse effect (Fig. 6C and Supporting Fig. 10B). Triple IF and IHC analysis also revealed the colocalization of ACTL6A, SOX2, and NICD1 in HCC tissues (Fig. 6D and Supporting Fig. 11A). Correlation analysis showed a significant, but moderate, positive correlation between ACTL6A and SOX2 and NICD1 expression of HCC samples in the training and validation cohorts (Supporting Fig. 11B). We also performed chromatin immunoprecipitation/quantitative PCR (ChIP‐qPCR) using antibodies against endogenous ACTL6A or SOX2 in PLC/PRF5 and PLC/PRF‐ACTL6A cells. It indicated that enrichment of ACTL6A on SOX2 promoter regions was apparent, as well as SOX2 on Notch1 promoter regions (Supporting Fig. 12A). These results concluded that ACTL6A activated Notch1 signaling by SOX2 and promoted metastasis and EMT of HCC (Fig. 6E).
Discussion
Invasion and metastasis are responsible for the vast majority of cancer associated deaths, including HCC, and EMT may play an important role for them. In this study, we provided the first evidence that ACTL6A was highly expressed in HCC and associated with poor prognosis of HCC patients. Moreover, the in vitro and in vivo biological experiments also first demonstrated that ACTL6A promoted invasion, metastasis, and EMT through activating Notch1 signaling by SOX2. Thus, ACTL6A has a great clinical value for prediction of prognosis and targeted therapy.
Previous studies verified the important role of ACTL6A for transcriptional regulation, cell proliferation, and migration, indicating the potential role of prompting tumor progression.12, 13, 14, 24 Consistent with previous researches, our study confirmed the clinical significance of ACTL6A as an independent prognostic marker for HCC patients after liver resection. More interesting, ACTL6A had a different expression pattern in SLHCC, SHCC, and NHCC; this might be used to distinguish the clinical subtypes of HCCs. The results were also consistent with our previous studies that different clinical HCC subtypes always had distinct molecular characteristics.7, 8, 25, 26, 27, 28 The functional experiments revealed that ACTL6A overexpression could strongly promote invasion and metastasis of HCC, and HCC invasion and metastasis were effectively inhibited by ACTL6A knockdown. These results uncovered the role of ACTL6A in promoting HCC progression. There are also a few researches that referred the potential role of ACTL6A involvement with tumors; for instance, ACTL6A expression is critical for c‐Myc oncogenic activity and could suppress p53‐mediated gene transcription.29, 30 Furthermore, a recent study found that ACTL6A expression was essential for differentiation block in rhabdomyosarcoma.31 These studies further confirmed that ACTL6A played an important role in tumor progression, but the role of ACTL6A in tumors had not been validated in clinical samples.
Though controversial, EMT is now considered as a hallmark of cancer and plays a crucial part in facilitating cancer cell invasion and metastasis.5, 32, 33 ACTL6A expression is high in fibroblast and progenitor cells, and ectopic expression could suppress the epithelial characteristic of epidermal tissue.15, 17 The functional role of ACTL6A suppressing cell differentiation, sustaining self‐renewal and pluripotency is associated with stem cell‐like features.15, 17, 34 These characteristics are similar with the biological function of EMT. In this study, we found that ACTL6A ectopic expression changed the epithelial morphology cell to mesenchymal phenotype, decreased epithelial marker expression, and increased mesenchymal marker expression, as well as increased anchorage‐independent growth. ACTL6A knockdown showed opposite impacts. These findings can be confirmed by expressions of ACTL6A, E‐cadherin, and vimentin in HCC samples and xenografted tumors. Therefore, our results first illustrate that ACTL6A can induce EMT.
In this study, we also revealed the mechanism of ACTL6A promoting HCC progression. Our results demonstrated that ACTL6A facilitated HCC invasion, metastasis, and EMT through Notch1 signaling activation. Notch signaling is critical for tissue and organ development; recent advances have also confirmed that Notch played a very important role in progression of HCC.18, 35 However, which member of Notch1‐4 plays the predominant role in HCC is still controversial.20, 21, 22, 36 Our study indicated that Notch1 was highly expressed in HCC cells and samples (58.0% and 65.1% in the training and validation cohorts), which was consistent with former studies, and further confirmed the important role of Notch1 in HCC.22, 37 More significantly, DAPT is one of the most widely used and powerful Notch signaling inhibitors for tumors, but its inhibitory effect is nonselective for all Notch receptor paralogs and some other proteins.35, 38 However, it was revealed in the present study that ACTL6A knockdown had the equal blockage effect as DAPT, but only for Notch1 in vitro. Therefore, blocking ACTL6A may provide the similar inhibition for tumor metastasis activated by Notch1 signaling, but more specific than DAPT. Further intervention assessment is needed according to the Response Evaluation Criteria in Solid Tumors guideline.39
In this study, it was also demonstrated that SOX2 was the potential target of ACTL6A in HCC. ACTL6A ectopic expression up‐regulated SOX2 expression; on the other hand, ACTL6A knockdown decreased SOX2 expression. Meanwhile, SOX2 ectopic expression could recover Notch1 signaling activity, invasiveness, and mesenchymal phenotype in ACTL6A knockdown cells. Conversely, silencing SOX2 exhibited inverse effects in ACTL6A overexpression cells. Moreover, ACTL6A, SOX2, and NICD1 expressions in HCC samples were colocalization, and positive correlation further confirmed the relationship. SOX2 plays an essential role in the maintenance stemness of embryonic and adult stem cells, as well as implication in cancer stem cells and metastasis.23, 40 Previous studies found that SOX2 was involved in Notch signaling by interacting with Jagged1 and Notch1.23, 41 There are also some reports that link SOX2 expression to HCC progression and poor survival.42, 43 A recent study that used ChIP‐qPCR showed that ACTL6A could bind to the promoter of SOX2.17 Based on these researches, our study confirmed that ACTL6A not only activated Notch1 signaling by SOX2, but also affected the biological behavior of HCC cells by SOX2. Recently, there were studies that proposed some partners (e.g., SOX9 and insulin‐like growth factor 2) played a pivotal role in supporting the pro‐oncogenic functions of Notch1 in HCC.19, 44, 45 Further studies are needed to explore whether SOX2 acts in a similar role in HCC development and progression.
In conclusion, our study first demonstrated that ACTL6A was an independent prognosis indicator for HCC patients. In addition, the in vitro and in vivo assays verified the essential role of ACTL6A in promoting invasion, metastasis, and EMT through activating Notch1 signaling by SOX2. Meanwhile, ACTL6A knockdown had the similar effect as DAPT inhibiting Notch signaling. Therefore, our study suggests that ACTL6A can be used as a valuable prognostic biomarker and a potential therapeutic target in HCC.
Author names in bold designate shared co‐first authorship.
Supporting information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.28417/suppinfo.
Supporting Information
Acknowledgment
The authors thank Prof. Qiong‐qiong He and Prof. Geng‐Qiu Luo (Department of Pathology, Xiangya Hospital of Central South University) for the help of pathological diagnoses and guidance.
Potential conflict of interest: Nothing to report.
This work was supported by National Keystone Basic Research Program of China (2009CB521801), the Clinical Subjects' Key Project of Ministry of Health (no. 2010439), the National Science & Technology Major Projects (2009ZX091032681 and 2012ZX100020122011), and the Key Project of National Nature Science Foundation of China (81330057).
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol 2014;28:753‐770. [DOI] [PubMed] [Google Scholar]
- 3. Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell 2011;147:275‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalluri R, Weinberg RA. The basics of epithelial‐mesenchymal transition. J Clin Invest 2009;119:1420‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial‐mesenchymal transitions in development and disease. Cell 2009;139:871‐890. [DOI] [PubMed] [Google Scholar]
- 6. Jou J, Diehl AM. Epithelial‐mesenchymal transitions and hepatocarcinogenesis. J Clin Invest 2010;120:1031‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang LY, Fang F, Ou DP, Wu W, Zeng ZJ, Wu F. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg 2009;249:118‐123. [DOI] [PubMed] [Google Scholar]
- 8. Wang W, Yang LY, Huang GW, Lu WQ, Yang ZL, Yang JQ, et al. Genomic analysis reveals RhoC as a potential marker in hepatocellular carcinoma with poor prognosis. Br J Cancer 2004;90:2349‐2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang RM, Yang H, Fang F, Xu JF, Yang LY. MicroRNA‐331‐3p promotes proliferation and metastasis of hepatocellular carcinoma by targeting PH domain and leucine‐rich repeat protein phosphatase. Hepatology 2014;60:1251‐1263. [DOI] [PubMed] [Google Scholar]
- 10. Xu J, Li X, Yang H, Chang R, Kong C, Yang L. SIN1 promotes invasion and metastasis of hepatocellular carcinoma by facilitating epithelial‐mesenchymal transition. Cancer 2013;119:2247‐2257. [DOI] [PubMed] [Google Scholar]
- 11. Zhao K, Wang W, Rando OJ, Xue Y, Swiderek K, Kuo A, et al. Rapid and phosphoinositol‐dependent binding of the SWI/SNF‐like BAF complex to chromatin after T lymphocyte receptor signaling. Cell 1998;95:625‐636. [DOI] [PubMed] [Google Scholar]
- 12. Krasteva V, Buscarlet M, Diaz‐Tellez A, Bernard MA, Crabtree GR, Lessard JA. The BAF53a subunit of SWI/SNF‐like BAF complexes is essential for hemopoietic stem cell function. Blood 2012;120:4720‐4732. [DOI] [PubMed] [Google Scholar]
- 13. Ronan JL, Wu W, Crabtree GR. From neural development to cognition: unexpected roles for chromatin. Nat Rev Genet 2013;14:347‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lessard J, Wu JI, Ranish JA, Wan M, Winslow MM, Staahl BT, et al. An essential switch in subunit composition of a chromatin remodeling complex during neural development. Neuron 2007;55:201‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bao X, Tang J, Lopez‐Pajares V, Tao S, Qu K, Crabtree GR, et al. ACTL6a enforces the epidermal progenitor state by suppressing SWI/SNF‐dependent induction of KLF4. Cell Stem Cell 2013;12:193‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Altman DG, Mcshane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. BMC Med 2012;10:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu W, Fang L, Ouyang B, Zhang X, Zhan S, Feng X, et al. Actl6a protects embryonic stem cells from differentiating into primitive endoderm. Stem Cells 2015;33:1782‐1793. [DOI] [PubMed] [Google Scholar]
- 18. Geisler F, Strazzabosco M. Emerging roles of Notch signaling in liver disease. Hepatology 2015;61:382‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Villanueva A, Alsinet C, Yanger K, Hoshida Y, Zong Y, Toffanin S, et al. Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in mice. Gastroenterology 2012;143:1660‐1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giovannini C, Gramantieri L, Chieco P, Minguzzi M, Lago F, Pianetti S, et al. Selective ablation of Notch3 in HCC enhances doxorubicin's death promoting effect by a p53 dependent mechanism. J Hepatol 2009;50:969‐979. [DOI] [PubMed] [Google Scholar]
- 21. Huntzicker EG, Hotzel K, Choy L, Che L, Ross J, Pau G, et al. Differential effects of targeting Notch receptors in a mouse model of liver cancer. Hepatology 2015;61:942‐952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, et al. Yes‐associated protein up‐regulates Jagged‐1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology 2013;144:1530‐1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Surzenko N, Crowl T, Bachleda A, Langer L, Pevny L. SOX2 maintains the quiescent progenitor cell state of postnatal retinal Muller glia. Development 2013;140:1445‐1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, et al. MicroRNA‐mediated conversion of human fibroblasts to neurons. Nature 2011;476:228‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fang F, Chang RM, Yu L, Lei X, Xiao S, Yang H, et al. MicroRNA‐188‐5p suppresses tumor cell proliferation and metastasis by directly targeting FGF5 in hepatocellular carcinoma. J Hepatol 2015;63:874‐885. [DOI] [PubMed] [Google Scholar]
- 26. Yang H, Fang F, Chang R, Yang L. MicroRNA‐140‐5p suppresses tumor growth and metastasis by targeting transforming growth factor beta receptor 1 and fibroblast growth factor 9 in hepatocellular carcinoma. Hepatology 2013;58:205‐217. [DOI] [PubMed] [Google Scholar]
- 27. Wu F, Yang LY, Li YF, Ou DP, Chen DP, Fan C. Novel role for epidermal growth factor‐like domain 7 in metastasis of human hepatocellular carcinoma. Hepatology 2009;50:1839‐1850. [DOI] [PubMed] [Google Scholar]
- 28. Wang W, Wu F, Fang F, Tao Y, Yang L. RhoC is essential for angiogenesis induced by hepatocellular carcinoma cells via regulation of endothelial cell organization. Cancer Sci 2008;99:2012‐2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang M, Gu C, Qi T, Tang W, Wang L, Wang S, et al. BAF53 interacts with p53 and functions in p53‐mediated p21‐gene transcription. J Biochem 2007;142:613‐620. [DOI] [PubMed] [Google Scholar]
- 30. Park J, Wood MA, Cole MD. BAF53 forms distinct nuclear complexes and functions as a critical c‐Myc‐interacting nuclear cofactor for oncogenic transformation. Mol Cell Biol 2002;22:1307‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taulli R, Foglizzo V, Morena D, Coda DM, Ala U, Bersani F, et al. Failure to downregulate the BAF53a subunit of the SWI/SNF chromatin remodeling complex contributes to the differentiation block in rhabdomyosarcoma. Oncogene 2014;33:2354‐2362. [DOI] [PubMed] [Google Scholar]
- 32. Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol 2011;27:347‐376. [DOI] [PubMed] [Google Scholar]
- 33. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646‐674. [DOI] [PubMed] [Google Scholar]
- 34. Yoo AS, Staahl BT, Chen L, Crabtree GR. MicroRNA‐mediated switching of chromatin‐remodelling complexes in neural development. Nature 2009;460:642‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morell CM, Strazzabosco M. Notch signaling and new therapeutic options in liver disease. J Hepatol 2014;60:885‐890. [DOI] [PubMed] [Google Scholar]
- 36. Zhang Q, Lu C, Fang T, Wang Y, Hu W, Qiao J, et al. Notch3 functions as a regulator of cell self‐renewal by interacting with the beta‐catenin pathway in hepatocellular carcinoma. Oncotarget 2015;6:3669‐3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou L, Zhang N, Song W, You N, Li Q, Sun W, et al. The significance of Notch1 compared with Notch3 in high metastasis and poor overall survival in hepatocellular carcinoma. PLoS One 2013;8:e57382. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Sjolund J, Johansson M, Manna S, Norin C, Pietras A, Beckman S, et al. Suppression of renal cell carcinoma growth by inhibition of Notch signaling in vitro and in vivo. J Clin Invest 2008;118:217‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer , National Cancer Institute of the United States , National Cancer Institute of Canada . J Natl Cancer Inst 2000;92:205‐216. [DOI] [PubMed] [Google Scholar]
- 40. Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, et al. SOX2 controls tumour initiation and cancer stem‐cell functions in squamous‐cell carcinoma. Nature 2014;511:246‐250. [DOI] [PubMed] [Google Scholar]
- 41. Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR. Sox2 and JAGGED1 expression in normal and drug‐damaged adult mouse inner ear. J Assoc Res Otolaryngol 2008;9:65‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun C, Sun L, Li Y, Kang X, Zhang S, Liu Y. Sox2 expression predicts poor survival of hepatocellular carcinoma patients and it promotes liver cancer cell invasion by activating Slug. Med Oncol 2013;30:503. [DOI] [PubMed] [Google Scholar]
- 43. Huang P, Qiu J, Li B, Hong J, Lu C, Wang L, et al. Role of Sox2 and Oct4 in predicting survival of hepatocellular carcinoma patients after hepatectomy. Clin Biochem 2011;44:582‐589. [DOI] [PubMed] [Google Scholar]
- 44. Strazzabosco M, Fabris L. Notch signaling in hepatocellular carcinoma: guilty in association!. Gastroenterology 2012;143:1430‐1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dominguez M. Oncogenic programmes and Notch activity: an ‘organized crime’? Semin Cell Dev Biol 2014;28:78‐85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.28417/suppinfo.
Supporting Information


