Amphibians are susceptible to a disease-causing fungus known as Bd. We experimentally infected lowland leopard frogs to determine if Bd infection is associated with altered immune function in the blood. Our study suggests that Bd infected frogs with less active defense proteins in the blood are more susceptible to this deadly fungus.
Keywords: Amphibian, bacterial killing ability, Batrachochytrium dendrobatidis, chytridiomycosis, immune response, Lithobates yavapaiensis
Abstract
The relationship between amphibian immune function and disease susceptibility is of primary concern given current worldwide declines linked to the pathogenic fungus Batrachochytrium dendrobatidis (Bd). We experimentally infected lowland leopard frogs (Lithobates yavapaiensis) with Bd to test the hypothesis that infection causes physiological stress and stimulates humoral and cell-mediated immune function in the blood. We measured body mass, the ratio of circulating neutrophils to lymphocytes (a known indicator of physiological stress) and plasma bacterial killing ability (BKA; a measure of innate immune function). In early exposure (1–15 days post-infection), stress was elevated in Bd-positive vs. Bd-negative frogs, whereas other metrics were similar between the groups. At later stages (29–55 days post-infection), stress was increased in Bd-positive frogs with signs of chytridiomycosis compared with both Bd-positive frogs without disease signs and uninfected control frogs, which were similar to each other. Infection decreased growth during the same period, demonstrating that sustained resistance to Bd is energetically costly. Importantly, BKA was lower in Bd-positive frogs with disease than in those without signs of chytridiomycosis. However, neither group differed from Bd-negative control frogs. The low BKA values in dying frogs compared with infected individuals without disease signs suggests that complement activity might signify different immunogenetic backgrounds or gene-by-environment interactions between the host, Bd and abiotic factors. We conclude that protein complement activity might be a useful predictor of Bd susceptibility and might help to explain differential disease outcomes in natural amphibian populations.
Introduction
The pathogenic fungus Batrachochytrium dendrobatidis (Bd) was first identified as the causative agent of chytridiomycosis and definitively linked to declines and extinctions of natural amphibian populations in 1998 (Berger et al., 1998). Five years earlier, immunosuppression had been suggested as a potential explanation for enigmatic amphibian declines (Carey, 1993). Yet only recently has it been demonstrated that host immune function plays a role in Bd susceptibility (Ribas et al., 2009; Savage and Zamudio, 2011; Rosenblum et al., 2012) and that the pathogen actively suppresses host immunity by inducing lymphocyte apoptosis (Fites et al., 2013). These findings highlight the need to gain a better understanding of amphibian immune responses, particularly in the context of Bd infections.
The genomic basis of immunity is highly conserved across vertebrates (Flajnik and Kasahara, 2001; Nonaka and Kimura, 2006; Ohta et al., 2006). Many of the major structural and functional elements of innate and acquired immunity in mammals are also present in amphibians (Robert and Ohta, 2009; Zimmerman et al., 2010a,b), although certain aspects of this system, such as lymph transport, are unique to anurans (Hedrick et al., 2013). Vertebrates share a complex network of serum proteins, known as the complement system, that plays a key role in both innate (Koppenheffer, 1987) and acquired immunity (Nielsen and Leslie, 2002). Through a co-ordinated cascade of reactions, these proteins trigger local inflammatory responses, promote the uptake and destruction of pathogens by phagocytes, and form membrane-attack complexes that destroy certain pathogens directly (Janeway et al., 2001).
Innate immunity in general, and serum complement in particular, appears to be more diverse and functionally more important for immunity in ectothermic compared with endothermic vertebrates (Sunyer et al., 1998; Zhu et al., 2005). Crocodilian serum complement activity has a broader spectrum of bacterial killing ability (BKA) compared with human serum (Merchant et al., 2003), as also in teleost fish, which express at least five isoforms of the C3 serum complement protein component (Sunyer et al., 1997). In the frog Bombina maxima, whole-transcriptome profiling resulted in the identification of 157 highly expressed complement transcripts, including C2, C3, C3d, C4a, C5, C6, factor B, factor D and factor I (Zhao et al., 2014), confirming that anuran complement proteins also show expanded diversity compared with endotherms. Functional studies reveal a more complex pattern; complement gene expression in catfish infected with two bacterial species was up-regulated in response to Edwardsiella ictaluri but down-regulated in response to Flavobacterium columnare (Jiang et al., 2015), whereas these genes were down-regulated among Rana muscosa and Rana sierrae infected with Bd (Rosenblum et al., 2012). Thus, complement activity is an important immune defense for ectothermic vertebrates, but its role in combating infection is clearly pathogen and host specific.
Whether the complement system of amphibians is activated in response to Bd infection is unclear. Complement proteins are present in human epidermis (Dovezenski et al., 1992), and complement activation plays a key role in inflammatory responses to certain epidermal fungal infections in mice (Ray and Wuepper, 1978) and humans (Tagami, 1992). Amphibians with chytridiomycosis typically demonstrate minimal skin inflammation (Pessier et al., 1999; Berger et al., 2000, 2005; Densmore and Green, 2007), suggesting that Bd does not trigger a sufficient immune response to activate complement pathways. However, immunological responses to Bd infection vary among amphibian species, and dermal inflammation was observed in up to 40% of Litoria caerulea with chytridiomycosis (Berger et al., 2005). Furthermore, experimental infection of Rana cascadae with Bd increased BKA, a measure of combined cellular and humoral immune function (including complement activity; (Gervasi et al., 2013). Yet the opposite effect was observed in Pseudacris regilla, where Bd infection decreased blood BKA (Gervasi et al., 2013). In contrast to whole blood, analysis of plasma or serum bactericidal ability can provide more specific information about complement activity, because these fluids contain only humoral components of the immune system. Plasma bactericidal assays have been used to identify significant associations between environmental factors and innate immune function in snakes (Sparkmann and Palacio, 2009), alligators (Merchant et al., 2003), turtles (Zimmerman et al., 2010a,b), fish (Hayman et al., 1992), salamanders (Terrell et al., 2013) and frogs (Maniero and Carey, 1997; Venesky et al., 2012).
Cellular mediators of immunity (e.g. lymphocytes) are also present in the epidermal layer and are known to be influenced by Bd infection (Fites et al., 2013). In addition to inhibiting lymphocyte function, Bd may alter the distribution/production of amphibian leucocytes, although this topic is largely unexplored. Percentages of circulating neutrophils and eosinophils were significantly reduced in Bd-infected tree frogs (Litoria chloris) compared with uninfected individuals (Woodhams et al., 2007). Circulating leucocytes represent a primary line of defense against invading pathogens (Jain, 1993). Numbers and proportions of leucocytes in peripheral blood are commonly used indicators of infection and physiological stress in amphibians and other vertebrates (Davis et al., 2008; Campbell, 2015). Neutrophils and lymphocytes constitute the majority of leucocytes in amphibian peripheral blood (Bennett et al., 1972; Cathers et al., 1997). Across vertebrates, the relative proportion of these two cell types (the N/L ratio) changes predictably in response to stress (Bennett and Alspaugh, 1964; Bennett et al., 1972; Turner, 1988) or infection (Davis et al., 2010; Gervasi et al., 2013). Specifically, proportions of neutrophils increase, whereas proportions of lymphocytes decrease, reflecting changes in leucocyte production/distribution (Davis et al., 2008). Differentiating the causes of increased N/L ratios can be challenging, particularly given that stress and disease are closely related (Blaustein et al., 2012). Nonetheless, leucocyte profiles provide valuable information on immune physiology, particularly when only small blood volumes are available for analysis. Additionally, these cells respond to stressors more slowly than glucocorticoids, reducing the likelihood that animal handling will confound stress measurements based on leucocyte profiles compared with hormone assays (Davis et al., 2008).
The lowland leopard frog (Lithobates yavapaiensis) is a good candidate model species for investigating the determinants of chytridiomycosis susceptibility. Natural populations have persisted in the southwestern USA with Bd infection and associated mortality for at least 25 years (Bradley et al., 2002; Sredl and Jennings, 2005). Mortality is ongoing in some populations but not others (Savage et al., 2011), a pattern that is predicted by population genetic variability (Savage et al., 2015). Host immunogenetic variation among L. yavapaiensis individuals is associated with survival in experimental Bd infections (Savage and Zamudio, 2011), implicating variation in immune function as a predictor of Bd infection outcomes. However, functional immunity has not been measured in L. yavapaiensis. Thus, immunogenetic variation might be indirectly linked to disease through a correlation with total genetic variation rather than a direct, functional relationship. An exploration of immune physiology in L. yavapaiensis is an important step in resolving functional relationships among disease, genetics and immunity.
Here, we experimentally exposed L. yavapaiensis to Bd in order to identify changes in humoral and cellular measures of immune function during Bd infection. We measured survival, zoospore load, body mass, plasma BKA and circulating leucocyte profiles to test the following hypotheses: (i) immune responses to Bd infection are energetically costly and include elevated complement activity and changes in leucocyte distribution/abundance; (ii) frogs that survive infection are able to mount an effective immune response and demonstrate increased complement activity, greater body mass, elevated N/L ratios and lower infection intensities compared with frogs dying of chytridiomycosis.
Materials and methods
Animal husbandry
We collected L. yavapaiensis individuals from the Muleshoe Ranch Cooperative Management Area in Graham County, AZ, USA. This population has high chytridiomycosis susceptibility in controlled infections (Savage and Zamudio, 2011) but low morbidity and mortality in the wild owing to a geothermal spring that limits chytridiomycosis (Schlaepfer et al., 2007; Forrest and Schlaepfer, 2011; Savage et al., 2015). We collected partial egg masses (50–75 eggs per clutch) on 15 September 2012 and shipped them overnight to the National Zoological Park (NZP) in Washington, DC, USA. Clutches 1 and 2 were collected from the Secret Spring pond [Universal Transverse Mercators (UTMs): 571093E, 3578368N], clutches 3–5 were collected from the upper thermal spring pools (UTMs: 571502E, 3570135N), and clutches 6 and 7 were collected from the main thermal spring pool (UTMs: 571681E, 3578002N). Upon hatching, larvae spent 30 days in quarantine and were confirmed by NZP veterinary staff and Genetics Laboratory staff to be in good health and free of Bd and ranavirus by PCR testing. We then reared individuals through metamorphosis in an animal holding room at the NZP’s Reptile Discovery Center at 24 ± 3°C with a 12 h–12 h light–dark cycle and 5% UVB during lighted hours. Animals were reared in plastic mouse containers filled with reconstituted water (reverse osmosis water with salts added) in groups of 10–15 individuals. We rotated clutch mates among cages to equalize numbers per cage owing to moderate levels of tadpole mortality (∼20% of tadpoles died over 7 months). Cage locations were rotated bi-weekly to avoid any potential micro-environmental effects. Tadpoles were fed a diet of 90% dried spirulina algae and 10% fish food flakes, egg whites and bee pollen ad libitum.
When all frogs had reached or exceeded Gosner stage 41, we transferred them to a climate chamber held at 20 ± 2°C with a 12 h–12 h light–dark cycle and 5% UVB during lighted hours. Metamorphosed juvenile frogs were housed individually in plastic mouse cages held at a slight angle, with a reservoir of water at one end and a plastic shelter with wet paper towels at the other end. Frogs were fed vitamin-dusted crickets, mealworms and wingless flies daily ad libitum.
Experimental B. dendrobatidis infections
On 4 April 2013 (2–4 weeks post-metamorphosis for all individuals, weight range 1.1–2.9 g), we placed all frogs in individual plastic infection chambers (sterile plastic containers 12 cm in diameter, with holes poked in the lids) with a film of water at the bottom and either ∼100 000 zoospores of Bd in 1 ml of reconstituted water (n = 49 Bd-exposed frogs) or 1 ml of reconstituted water only (n = 28 sham-exposed frogs). We exposed frogs to Bd strain JEL423, isolated from an Agalychnis lemur individual in Panama in 2004 and cryopreserved until 7 days before infections. After 24 h in the infection chambers, we returned all frogs to their plastic mouse cages and resumed husbandry as described above. Gloves were worn while handling frogs and, subsequent to infection, were changed between cages to avoid pathogen spread.
Every 7 days, starting on day 1 post-infection, we swabbed the epidermis of each individual using sterile fine-tip swabs (Medical Wire and Equipment Co. MW113) following standardized protocols (Hyatt et al., 2007) and stored swabs dry in 2 ml tubes at −80°C. Frogs were euthanized following a staggered schedule to allow comparison of immune function during early (≤day 15) vs. late infection (≥day 29). Given the small body mass of each frog, immune function could be assessed only once in each individual, after euthanasia. On days 1, 4, 8, 15, 29 and 41 post-infection, eight frogs (five Bd exposed and three control) were swabbed and then euthanized. We selected these individuals semi-randomly; using a random number generator, we chose Bd-exposed and control individuals separately for euthaniasia, eliminating the entire clutch from the selection pool after one individual from that clutch was selected. Thus, we ensured that euthanized frogs represented the broadest genetic sampling possible, with paired Bd-exposed and control frogs from three clutches per euthanasia day. On day 55 after Bd exposure, we ended the experiment and euthanized all surviving frogs. At any time point during the experiment, an individual manifesting all signs of chytridiomycosis (ventral redness, skin shedding, lethargy, anorexia and loss of righting ability) was euthanized by an NZP veterinarian to prevent further suffering and ensure that fresh blood was collected before death. Immediately before euthanasia, we swabbed each frog for Bd quantification. We then injected each frog with ∼200 μl of MS-222 (10 g/l in sterile water buffered to a pH of 7 with sodium bicarbonate). Each frog was injected into the body wall on the right flank using a sterile 1 ml syringe and a 25 gauge ¾ inch needle. A new pair of sterile gloves was used to handle each frog.
Blood collection
We placed each euthanized frog on an ethanol-sterilized surface covered with fresh kimwipes and used sterilized scissors, tweezers and scalpels to open the body cavity and sever the aorta. We then used sterile heparinized capillary tubes to collect as much blood as possible (one to three tubes depending on the size of the animal). A new pair of sterile gloves was used to handle and collect blood from each frog. A small volume (5 µl) of blood was immediately smeared onto a glass microscope slide for differential leucocyte counts. The remainder of each blood sample was stored on ice and centrifuged (5 min, 2000g) within 2 h to isolate plasma for bacterial killing assays. Blood plasma was transferred to a 0.5 ml plastic tube and stored at −80°C until bacterial killing assays were performed. Corticosteroids are a more traditional indicator of stress (Davis et al., 2008), but we did not have sufficient blood from these small metamorphs to include both a corticosterone analysis and the BKA analysis; therefore, we prioritized the BKA analysis as an indicator more directly linked to immune function.
Quantification of B. dendrobatidis
We extracted DNA from swabs using Qiagen DNeasy blood and tissue kits, eluting into a volume of 200 µl for all samples. The number of Bd genome equivalents (GE) per swab, a measure of infection intensity, was determined using a Taqman quantitative PCR assay designed for Bd quantification (Boyle et al., 2004). All samples were run in duplicate; if replicate runs showed inconsistencies in infection status (infected vs. uninfected) or at least one order of magnitude difference in infection intensity, a third replicate was run, and the two most similar replicates were retained.
Leucocyte counts
After drying, blood smears were fixed in 100% methanol for 5 min, stained with DipQuick (MWI Veterinary Supply, Boise, ID, USA), and examined at ×1000 magnification using a standard light microscope. For each smear, 100 leucocytes were counted and identified as neutrophils (N), lymphocytes (L), eosinophils, monocytes or basophils (Heatley and Johnson, 2009). Numbers of each cell type were calculated as percentages of total leucocytes. The ratio of neutrophils to lymphocytes was calculated as N/L × 100.
Bacterial killing assay
Bacterial killing ability of L. yavapaiensis plasma was tested against Escherichia coli (ATCC8739) as described previously (Terrell et al., 2013). Briefly, a colony was isolated on a 5% blood agar plate (Fisher Scientific) and used to inoculate a working stock solution. The bacterial concentration of the stock solution was determined by plating serial dilutions (10−4, 10−5, 10−6 and 10−7) onto 5% blood agar. For each sample, 5 µl of thawed plasma was combined with 20 µl of bacteria (diluted to 106 colony-forming units ml−1) and 75 µl of amphibian phosphate-buffered saline (PBS) in duplicate in a 96-well plate. Plates were shaken and subsequently incubated (21°C, 1 h) to allow bacterial killing to occur. Tryptic soy broth (TSB; 200 µl) was then added to each well, and samples were mixed manually by pipetting to minimize contamination risk, given the relatively large reaction volumes. Plates were incubated for 12 h at 30°C to allow bacterial growth to occur. Given that red blood cell contamination can increase absorbance, a plasma blank (i.e. plasma and PBS only) was included for each sample. Negative (TSB and PBS only) and positive (plasma-free) controls were included in each plate in triplicate. Absorbance was read at 405 nm immediately after incubation. Bacterial killing ability (BKA) was calculated as follows:
where A is the absorbance at 405 nm.
Statistical analyses
Frogs euthanized early (≤15 days) after Bd exposure were classified only as Bd negative or Bd positive for statistical analyses, because adequate time had not passed to determine infection or chytridiomycosis susceptibility. In contrast, Bd-infected individuals euthanized on or after day 29 were classified, based on observed phenotypes and prior knowledge of infection and susceptibility dynamics from previous experimental and field-based chytridiomycosis studies in L. yavapaiensis (Savage and Zamudio, 2011; Savage et al., 2011, 2015), into the following two groups: individuals dying of chytridiomycosis (all signs of chytridiomycosis manifesting at the time of euthanasia); and individuals surviving Bd infection (no signs of chytridiomycosis manifesting at any time in the 29–55 days before euthanasia). The average daily growth rate was calculated as the total change in body mass (from week 1 to euthanasia) divided by the number of days between the initial and final measurement. In uninfected frogs, there was no significant effect of euthanasia period (i.e. early vs. late) on growth rates (t = 1.87, P = 0.095), BKA (t = −1.60, P = 0.124) or N/L ratios (t = −1.35, P = 0.194); therefore, these control animals were pooled to increase statistical power. Data were analysed using R statistical software (R Development Core Team, 2008). Quantile–quantile plots were used to verify approximate normality in transformed data. Percentage data (i.e. bacterial killing activity and individual leucocyte counts) were arcsine transformed before analysis. All data were back-transformed for presentation in figures. Comparisons made between two groups (i.e. Bd exposed vs. unexposed or early vs. late Bd exposure) were performed using unpaired Student’s t-tests. Differences among susceptible, tolerant and uninfected groups were evaluated by ANOVA and Tukey’s honest significant difference (HSD) test. Principal components analyses were conducted to visualize relationships among response variables (infection load, body mass, BKA and N/L ratios) and animal groups using the prcomp function in the base package of R. Only components with eigenvalues >1 were retained in the final analysis (Kaiser, 1960).
Results
Experimental infections
Among 49 Bd-exposed frogs, 37 individuals had not developed clinical signs of chytridiomycosis on their scheduled euthanasia date [ranging from 1 to 41 days post-infection (DPI); n = 27] or at the end of the experiment (55 DPI; n = 10 frogs). Eleven of the remaining 12 infected frogs developed all signs of chytridiomycosis and were euthanized immediately (ranging from 34 to 50 DPI) to collect fresh samples before they died; the 12th frog died before euthanasia could take place (31 DPI), and we were unable to collect any viable samples.
All infected frogs shed visible sections of epidermis three or four times per week from 14 DPI onwards, but only the ‘dying’ category of frogs developed other signs of chytridiomycosis. None of the uninfected control frogs (n = 28) shed visible sections of epidermis or developed any other signs of chytridiomycosis. Hereafter, Bd exposed frogs (n = 49) are referred to as ‘early infection’ if they were euthanized ≤15 DPI (before susceptibility could manifest; n = 18), ‘surviving’ if they did not develop disease signs by their scheduled ≥29 DPI euthanasia date (n = 19) or ‘dying’ if they developed all chytridiomycosis disease signs before euthanasia (n = 12). Given that the 12 dying frogs manifested all signs of chytridiomycosis over a 21 day period (ranging from 29 to 50 DPI; median = 40 DPI), some of the ‘surviving’ group of frogs that were euthanized between 29 and 55 DPI may have been Bd susceptible but not yet manifesting signs. Thus, the surviving group is likely to have included some susceptible frogs.
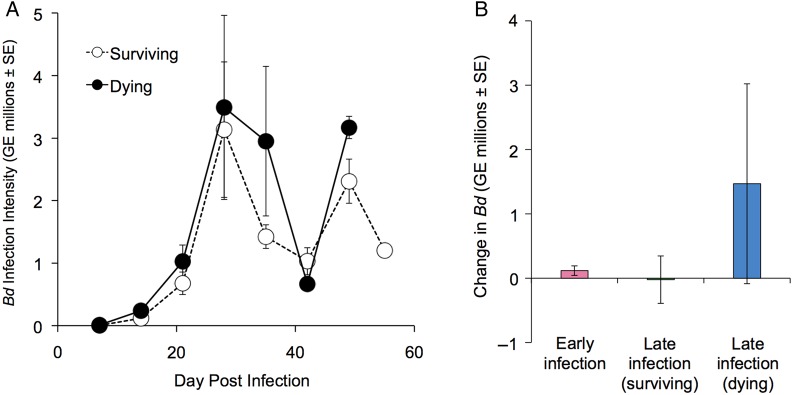
Infection intensity rose steadily over time, remained elevated throughout the remainder of the experiment, and was not significantly different between surviving and dying frogs (Fig. 1A; Supplementary Appendix 1 and Supplementary Fig. 1), consistent with field studies of Bd in natural L. yavapaiensis populations (Savage et al., 2011, 2015). From day 29 until 55 DPI, dying frogs showed a non-significant trend towards higher infection loads compared with surviving frogs, with the exception of 42 DPI when all infected frogs had a significant reduction in infection intensity (q ≥ 5.20, P ≤ 0.02; Fig. 1A). Based on our qualitative observations that skin shedding was most severe in the week before 42 DPI, we hypothesize that this reduction in zoospore load resulted from a high turnover of epidermis. However, we did not collect quantifiable information on skin shedding. Infection intensity did not change significantly during the week before euthanasia for any infected group (Fig. 1B), consistent with previous observations that Bd zoospore load does not reliably predict host mortality in L. yavapaiensis (Savage et al., 2011, 2015).
Figure 1:
(A) Mean Batrachochytrium dendrobatidis (Bd) infection intensity [measured as genome equivalents (GE)] in dying (filled circles, n = 12) and surviving frogs (open circles, n = 37) measured weekly from 7 to 55 days post-infection (DPI). (B) Change in Bd infection intensity (value at time of euthanasia minus value at the most recent weekly sampling) for Bd-exposed frogs euthanized 1–15 DPI (pink, n = 18) and Bd-infected frogs surviving 29–55 DPI with (blue, n = 12) or without (green, n = 19) signs of chytridiomycosis. All values are shown ±SEM.
Leucocyte counts
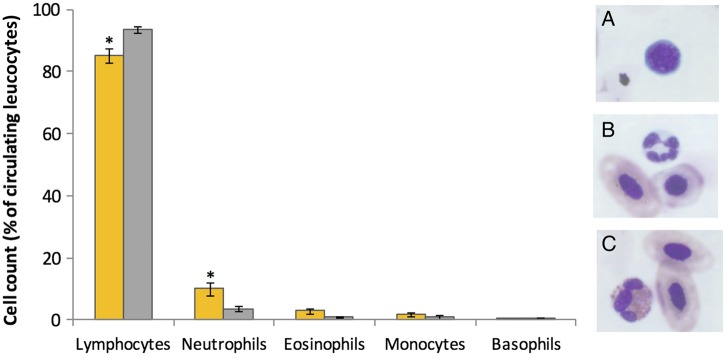
Blood samples were obtained in sufficient volume and quality (i.e. without clotting) to permit leucocyte counts in 38 individuals (n = 12 control, n = 8 early infection, n = 12 surviving and n = 6 dying). Across all samples (n = 38; Supplementary Appendix 1), lymphocytes were the predominant leucocyte in circulation (mean ± SEM, 88.0 ± 1.8%) followed by neutrophils (8.0 ± 1.5%; Fig. 2). Percentages of neutrophils were increased (t = −2.47, P = 0.018) and percentages of lymphocytes decreased (t = 2.85, P = 0.007) in infected vs. control animals. Given that changes in both cell types were consistent with a response to stress or infection, we focused subsequent analysis on N/L ratios, a single metric that is commonly used to quantify stress in vertebrates (Davis et al., 2008). These ratios differed (F = 6.38, P = 0.002) among uninfected, early infection, dying and surviving groups (Fig. 3). Specifically, post hoc comparisons revealed that the N/L ratio was increased in early infection Bd-positive frogs (q = 4.77, P = 0.010) and in dying frogs (q = 4.02, P = 0.036) compared with uninfected control animals. In contrast, surviving frogs demonstrated N/L ratios that were lower than other Bd-positive groups (q ≥ 3.91, P ≤ 0.043) and similar to uninfected control frogs. Other cell types were observed infrequently (eosinophils, 2.2 ± 0.6%; monocytes, 1.5 ± 0.4%; and basophils, 0.3 ± 0.1%), and proportions did not differ among all groups (Fig. 2).
Figure 2:
Leucocyte profiles of frogs infected with Batrachochytrium dendrobatidis (Bd; orange bars, n = 26) compared with uninfected control animals (grey bars, n = 12) pooled across all time points and survival categories. Representative images of lymphocytes (A), neutrophils (B) and eosinophils (C) in L. yavapaiensis are illustrated. Bars with an asterisk (*) differed from control values (P < 0.05).
Figure 3:
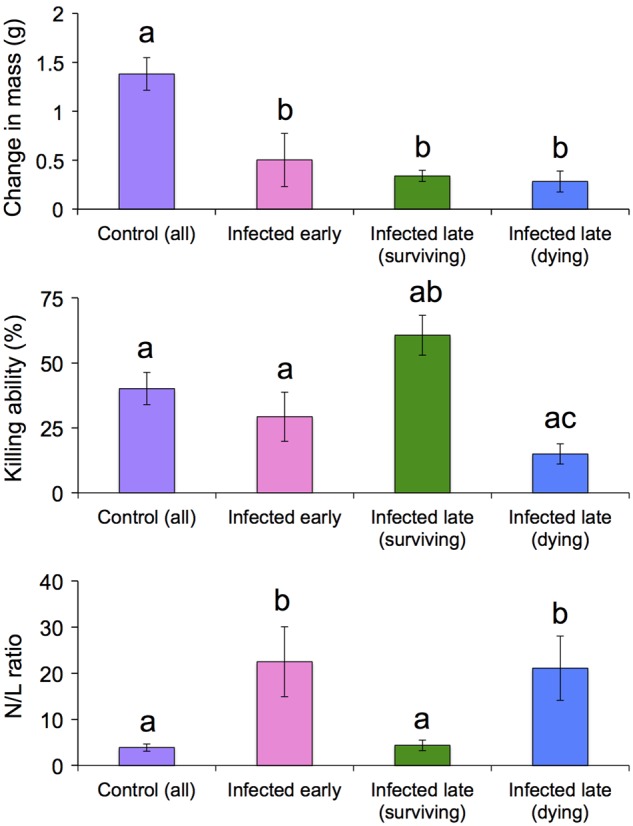
Physiological metrics among frogs exposed to Batrachochytrium dendrobatidis (Bd) and uninfected control frogs euthanized at early time points [1–15 days post-infection (DPI)] compared with late time points (29–55 DPI). Sample sizes vary among these metrics owing to lack of growth data for week 1 and limited blood volumes for many frogs. Top panel: change in mass in control frogs (n = 26), Bd-exposed frogs euthanized 1–15 DPI (n = 13) and Bd-infected frogs surviving 29–55 DPI with (n = 10) or without (n = 19) signs of chytridiomycosis. Middle panel: bacterial killing ability (BKA) of blood plasma from control frogs (n = 26), Bd-exposed frogs euthanized early (1–15 DPI, n = 18) and Bd-infected frogs surviving 29–55 DPI with (n = 10) or without (n = 17) signs of chytridiomycosis. Bottom panel: ratios of circulating neutrophils to lymphocytes (N/L) in control frogs (n = 12), Bd-exposed frogs euthanized early (1–15 DPI, n = 8) and Bd-infected frogs surviving 29–55 DPI with (n = 6) or without (n = 12) signs of chytridiomycosis. Groups with different letters represent statistically significant differences (P < 0.05). All values are shown ±SEM.
Average daily growth rate
Given that initial mass was recorded on the first day of the experiment, growth rates were not calculated for animals euthanized in the first 7 days (n = 3 control, n = 5 Bd exposed). Average daily growth rates differed (F = 11.26, P < 0.0001) among groups. Specifically, growth was reduced (q ≥ 5.23, P ≤ 0.003) in Bd-exposed frogs (n = 42, including early infection, dying and surviving groups) compared with uninfected control frogs (n = 26; Fig. 3). Growth rates were similar among early infection (n = 13), dying (n = 10) and surviving (n = 19) groups.
Bacterial killing ability
Sufficient volumes of blood were obtained to assay BKA in 72 individuals (n = 27 control, n = 18 early infection, n = 17 surviving and n = 10 dying). Animal groups differed (F = 5.96, P = 0.001) with respect to BKA. Post hoc analysis revealed that BKA of surviving frogs was increased compared with dying frogs (q ≥ 4.57, P ≤ 0.01). However, neither group differed from uninfected control animals or frogs euthanized within 15 days of Bd exposure (Fig. 3), consistent with a sorting of surviving and dying frogs associated with initial BKA.
Principal components analysis
Frogs with missing data were excluded from PCA, resulting in smaller sample sizes for uninfected (n= 11), early infection (n = 5), surviving (n = 10) and dying (n = 6) frogs. Missing data were the result of limited blood volumes, which precluded N/L or BKA analysis for some frogs. Principal components 1 and 2 collectively accounted for 77% of the observed variation in the data set (Fig. 4). Visualization of these components revealed that dying frogs, survivors and control animals represented three distinct physiological groups. Frogs surviving infection were associated with high BKA, low N/L ratios, intermediate body mass gain and high zoospore loads. In contrast, frogs dying of chytridiomycosis exhibited low BKA, high N/L ratios, low body mass gain and high zoospore loads. Control frogs were associated with high body mass gain, but substantial variation in immune function.
Figure 4:
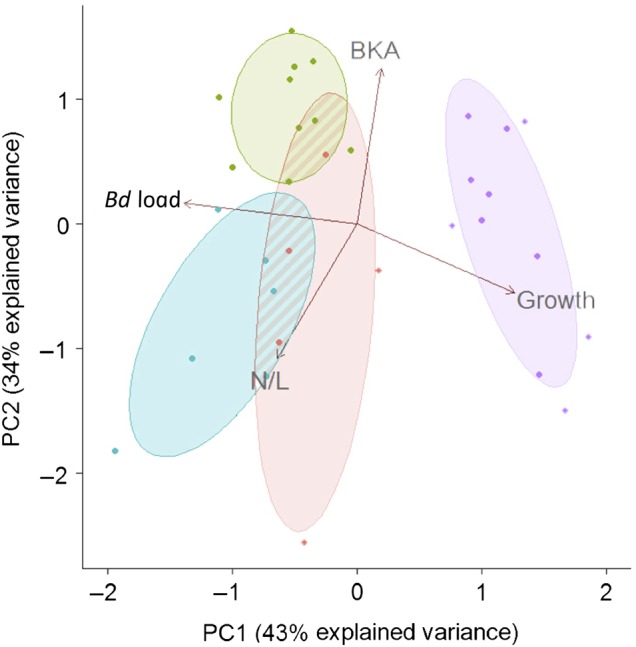
Principal components analysis (PCA) illustrating relationships among Batrachochytrium dendrobatidis (Bd) load, bacterial killing ability (BKA), growth rates and neutrophil/lymphocyte (N/L) ratios in uninfected frogs (n = 11, purple), Bd-infected individuals that did not develop chytridiomycosis (n = 10, green), Bd-infected individuals with chytridiomycosis (n = 6, blue) or Bd-exposed individuals euthanized during early infection, before disease signs occurred (n = 5, pink). Corresponding ellipses represent 68% confidence intervals for each group. Individuals with missing data for any of the above parameters were excluded from the PCA by necessity, resulting in smaller sample sizes.
Discussion
Hallmarks of effective immunity against a spectrum of pathogens are well described among endothermic vertebrates. In contrast, amphibian immunology is a relatively nascent field, with little known about protective immune response characteristics outside of the model system Xenopus/Silurana (Du Pasquier et al., 1989). In particular, few studies have examined anuran haematological responses to Bd infection and chytridiomycosis (Gervasi et al., 2013). In the present study, we found that the complement pathway of the innate immune system, as inferred by plasma BKA against E. coli, is a significant predictor of Bd susceptibility in this host species (Figs 3 and 4). However, the importance of complement for Bd susceptibility in other species and the precise role of complement activity in limiting Bd damage to the host remain to be tested (Figs 3 and 4). An equally important finding is that uninfected individuals showed a broad range of BKA, suggesting that baseline levels of circulating complement proteins vary widely among individuals and might be a useful metric for predicting Bd susceptibility in individuals or populations. Our interpretation of these findings is complicated, however, by the fact that complement proteins can be produced locally in the epidermis (i.e. the Bd infection site) or imported from the bloodstream (Dovezenski et al., 1992). Given that we measured only BKA of blood-based complement, we do not know how these values correspond to those at the site of infection. Thus, it is difficult to know whether the observed relationship between blood BKA and Bd tolerance is correlative or causative.
When used with fresh whole blood, the BKA is a broad-spectrum challenge assay testing both humoral and cell-mediated killing mechanisms present in circulating blood, including antimicrobial peptides, lysozyme, complement, monocytes, neutrophils and natural killer cells (Janeway et al., 2001). In contrast, when plasma or serum is used the white bloods cells have been removed, and therefore, only humoral immune mechanisms are assessed. Given that we used the Gram-negative bacteria E. coli as our pathogen, and lysozyme is primarily effective against Gram-positive bacteria, the BKA in our study was likely to be an assessment of complement activity. Our study provides an interesting comparison to the whole-blood BKA results in the only other study investigating Bd-induced immune parameters in a North American ranid frog, R. cascadae (Gervasi et al., 2013). Although we found no significant increase in plasma BKA in infected surviving frogs, infected surviving R. cascadae individuals had significantly stronger blood bactericidal activity than control frogs (Gervasi et al., 2013). This difference suggests that effective immune responses against Bd may involve humoral mechanisms other than complement, that there are species- or strain-specific differences in immune response to Bd, that environmental differences between our studies dictate frog immune function, or some combination thereof.
Compared with BKA values, baseline levels of blood leucocytes were more consistent. Among Bd-infected frogs, we observed an increase in the ratios of circulating neutrophils to lymphocytes (N/L), consistent with an infection and/or a stress response. Interestingly, this response was not sustained among individuals that survived infection (Figs 3 and 4). Thus, blood N/L ratios may also be an important hallmark of severe chytridiomycosis, with persistently high values indicating susceptibility and a return to baseline values predicting survival. Elevated N/L ratios may result from the loss of lymphocytes in circulation, as these cells migrate from the blood into the tissues. Given the evidence for Bd-induced lymphocyte apoptosis (Fites et al., 2013), this cellular response may be ineffective in frogs with chytridiomycosis. Whether resistant frogs are able to protect their lymphocytes from destruction by Bd is an intriguing question for future research. Elevated N/L ratios may also arise from a greater input of neutrophils into the blood from the bone marrow, where a large reserve of mature neutrophils is maintained (Furze and Rankin, 2008). Neutrophil mobilization is a rapid and crucial component of the inflammatory response. However, a sustained inflammatory response can be highly damaging to the host (Sears et al., 2011) and may directly contribute to death in Bd-susceptible amphibians. Consistent with this idea, expression of pro-inflammatory genes is elevated during Bd infection in four frog species, and expression levels correlate positively with susceptibility (Ellison et al., 2015). Likewise, infected R. cascadae individuals exhibit elevated N/L ratios but no significant change in lymphocyte counts (Gervasi et al., 2013), implying that the ratio change was attributable to an increase in pro-inflammatory neutrophils. Given the small volumes of blood available, we did not perform total (i.e. complete) blood counts in L. yavapaiensis and therefore cannot resolve whether changes in N/L ratios were driven by neutrophil or lymphocyte counts (or both). Here, we can only conclude that cell-mediated processes play some role in determining chytridiomycosis outcomes, meriting further investigation across a range of host amphibians.
We found that Bd infection intensity significantly reduced growth rate, supporting the well-established trade-off between vertebrate immune responses and fitness that occurs as the host redistributes nutritional and energy resources from anabolic to disease-fighting processes (Lochmiller and Deerenberg, 2000). Several non-exclusive processes may explain this trade-off in our study. Immune cells consume substantial amounts of energy (Newsholme and Newsholme, 1989), thus fitness trade-offs are most pronounced for the acquired (and primarily cell-mediated) immune system (Fearon and Locksley, 1996). However, the extent to which acquired immune responses are mobilized in response to Bd infection is unknown. One possibility is that acquired immune responses play a larger role in amphibian responses to infection than previously appreciated and that the negative relationship between infection load and body mass was driven by proliferation of immune cells. Alternately, Bd infection might primarily activate the innate immune system, but these responses might be more energetically expensive than previously appreciated. Furthermore, infected animals might reduce food intake (Kyriazakis et al., 1998) or absorb nutrients less efficiently, either of which might contribute to declines in growth or body condition (Lochmiller and Deerenberg, 2000). In the present study, we monitored food intake qualitatively and observed cessation of eating among dying frogs, which was one of the criteria for diagnosing chytridiomycosis. However, infected surviving frogs continued to eat normally throughout the experiment, suggesting that their lower gain in mass compared with control animals was attributable to energetic costs of infection, not a reduction in food intake. Finally, the skin shedding we observed among all infected frogs might have contributed substantially to the observed energetic costs of infection. Other studies of amphibian chytridiomycosis have found correlations between decreased mass and increased skin shedding among infected animals (Murphy et al., 2011; Peterson et al., 2013), and the rate of skin shedding is positively correlated with Bd infection intensity in the tree frog L. caerulea (Ohmer et al., 2015). Discriminating among which immunological or other physiological parameters drive the metabolic changes associated with chytridiomycosis is therefore an important avenue of continued research.
Given that diseased animals are unlikely to survive in natural habitats, it has been suggested that the rapid, innate immune response is the most important determinant of survival and fitness (Lochmiller and Deerenberg, 2000). This is considered particularly true for short-lived species facing a high risk of predation (Lee, 2006). Here, we found that some frogs were able to maintain fair body condition with no signs of disease, despite high Bd loads, for at least 55 days after infection. This finding suggests that, even in the case of a highly infectious pathogen and a relatively short-lived host, certain individuals might survive infection long enough for adaptive immune responses to be effective. However, precise characterization of innate vs. adaptive immune activation in amphibians infected with Bd is needed before we can draw broad conclusions about the relative roles of different immune pathways in combating chytridiomycosis. Interestingly, some natural populations of L. yavapaiensis maintain similarly high Bd loads [millions of genome equivalents (GE)] with no indication of morbidity or mortality from chytridiomycosis, whereas other populations exhibit chytridiomycosis die-offs at significantly lower infection intensities, and climatic factors predict these differences (Savage et al., 2015). This pattern holds across a range of amphibian species and geographical regions; temperature largely explains the variation in Bd infection intensity among individuals in a number of recent field- and laboratory-based studies (Murray et al., 2013; Fernández-Beaskoetxea et al., 2015; Tinsley et al., 2015).
Experimental Bd infections also find that amphibians can survive and recover from signs of chytridiomycosis, but maintain Bd infection indefinitely (Carver et al., 2010). Likewise, our experimental study provides further evidence that infection intensity is not a straightforward predictor of chytridioymycosis susceptibility and suggests that Bd tolerance (i.e. limiting pathogen damage rather than pathogen burden) might be an important survival mechanism.
Our study has important implications for understanding the functional immune parameters that influence chytridiomycosis outcomes after Bd exposure. We demonstrated that Bd infection significantly altered the distribution of circulating host leucocyte types and that this effect was temporary in individuals that could survive and tolerate Bd infection, but persisted in susceptible frogs. Although positively associated with Bd survival, BKA in uninfected frogs varied widely and did not differ between tolerant or susceptible individuals. We therefore suggest that innate immune function measured by BKA is an important predictor of future susceptibility to chytridiomycosis if exposed to Bd. Elevated N/L ratio is also a key biomarker for Bd susceptibility among infected animals, and differentiating the relative contribution of increased neutrophils vs. decreased lymphocytes in driving this pattern might be a crucial next step in understanding immune responses against Bd. Our study contributes to the growing body of evidence that host immunity is critical for surviving Bd infection (Carey et al., 1999; Savage and Zamudio, 2011; McMahon et al., 2014) and suggests that variation observed across the amphibian world in host tolerance or resistance to Bd arises from variation in host immune competence. Here, we find preliminary evidence to suggest that complement-based BKA promotes Bd tolerance and enables some individuals to survive despite high pathogen burdens. Whether such tolerance is attributable to genetics, environmental conditions or gene-by-environment interactions remains an unanswered question. Using functional genomic approaches to understand the precise conditions, time points and molecular pathways that can promote effective host immunity and reduce Bd pathogenicity is a crucial next step in mitigating amphibian declines.
Supplementary material
Supplementary material is available at Conservation Physiology online.
Funding
This work was supported by a Smithsonian Institution Competitive Grants Program for Science grant to A.E.S., B.G. and R.C.F., the Smithsonian’s Center for Conservation and Evolutionary Genetics, a Smithsonian Institution Molecular Evolution Postdoctoral Fellowship to A.E.S., and a Smith Postdoctoral Fellowship to K.A.T.
Supplementary Material
Acknowledgements
We thank the National Zoological Park (NZP) Reptile Discovery Center staff and the Smithsonian Conservation Biology Institute veterinary staff for animal care support, the Genetics Laboratory for valuable feedback on this project, Tim Walsh for pathology training, and Scott Martin for assistance with animal husbandry. This work was conducted under NZP Institutional Animal Care and Use Committee permit #13-15 and Arizona Game and Fish Scientific Collecting Permit #SP550487.
References
- Bennett MF, Alspaugh JK (1964) Some changes in the blood of frogs following administration of hydrocortisone. Virginia J Sci 15: 76–79. [Google Scholar]
- Bennett MF, Gaudio CA, Johnson AO, Spisso JH (1972) Changes in the blood of newts, Notophthalmus viridescens, following administration of hydrocortisone. J Comp Physiol A 80: 233–237. [Google Scholar]
- Berger L, Speare R, Daszak P, Greene DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyatt AD, McDonald KR et al. (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA 95: 9031–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger L, Speare R, Kent A (2000) Diagnosis of chytridiomycosis in amphibians by histologic examination. Zoo Print J 15: 184–190. [Google Scholar]
- Berger L, Speare R, Skerratt LF (2005) Distribution of Batrachochytrium dendrobatidis and pathology in the skin of green tree frogs Litoria caerulea with severe chytridiomycosis. Dis Aquat Organ 68: 65–70. [DOI] [PubMed] [Google Scholar]
- Blaustein AR, Gervasi SS, Johnson PT, Hoverman JT, Belden LK, Bradley PW, Xie GY (2012) Ecophysiology meets conservation: understanding the role of disease in amphibian population declines. Philos Trans R Soc B Biol Sci 367: 1688–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle DG, Boyle DB, Olsen V, Morgan JA, Hyatt AD (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Organ 60: 141–148. [DOI] [PubMed] [Google Scholar]
- Bradley G, Rosen P, Sredl M, Jones T, Longcore J (2002) Chytridiomycosis in native Arizona frogs. J Wild Dis 38: 206–212. [DOI] [PubMed] [Google Scholar]
- Campbell TW. (2015) Peripheral Blood of Amphibians. Wiley-Blackwell, Malden. [Google Scholar]
- Carey C. (1993) Hypothesis concerning the causes of the disappearance of boreal toads from the mountains of Colorado. Conserv Biol 7: 355–362. [Google Scholar]
- Carey C, Cohen N, Rollins-Smith L (1999) Amphibian declines: an immunological perspective. Dev Comp Immunol 23: 459–472. [DOI] [PubMed] [Google Scholar]
- Carver S, Bell BD, Waldman B (2010) Does chytridiomycosis disrupt amphibian skin function? Copeia 2010: 487–495. [Google Scholar]
- Cathers T, Lewbart GA, Correa M, Stevens JB (1997) Serum chemistry and hematology values for anesthetized American bullfrogs (Rana catesbeiana). J Zoo Wild Med 28: 171–174. [PubMed] [Google Scholar]
- Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22: 760–772. [Google Scholar]
- Davis AK, Keel MK, Ferreira A, Maerz JC (2010) Effects of chytridiomycosis on circulating white blood cell distributions of bullfrog larvae (Rana catesbeiana). Comp Clin Pathol 19: 49–55. [Google Scholar]
- Densmore CL, Green DE (2007) Diseases of amphibians. Inst Lab Anim Res J 48: 235–254. [DOI] [PubMed] [Google Scholar]
- Dovezenski N, Billetta R, Gigli I (1992) Expression and localization of proteins of the complement system in human skin. J Clin Invest 90: 2000–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier L, Schwager J, Flajnik MF (1989) The immune system of Xenopus. Ann Rev Immunol 7: 251–275. [DOI] [PubMed] [Google Scholar]
- Ellison AR, Tunstall T, DiRenzo GV, Hughey MC, Rebollar EA, Belden LK, Harris RN, Ibanez R, Lips KR, Zamudio KR (2015) More than skin deep: functional genomic basis for resistance to amphibian chytridiomycosis. Genome Biol Evol 7: 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon DT, Locksley RM (1996) The instructive role of innate immunity in the acquired immune response. Science 272: 50–55. [DOI] [PubMed] [Google Scholar]
- Fernández-Beaskoetxea S, Carrascal LM, Fernández-Loras A, Fisher MC, Bosch J (2015) Short term minimum water temperatures determine levels of infection by the amphibian chytrid fungus in Alytes obstetricans tadpoles. PLoS ONE 10: e0120237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fites JS, Ramsey JP, Holden WM, Collier SP, Sutherland DM, Reinert LK, Gayek AS, Dermody TS, Aune TM, Oswald-Richter K et al. (2013) The invasive chytrid fungus of amphibians paralyzes lymphocyte responses. Science 342: 366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik MF, Kasahara M (2001) Comparative genomics of the MHC: glimpses into the evolution of the adaptive immune system. Immunity 2001: 351–362. [DOI] [PubMed] [Google Scholar]
- Forrest MJ, Schlaepfer MA (2011) Nothing a hot bath won’t cure: infection rates of amphibian chytrid fungus correlate negatively with water temperature under natural field settings. PLoS ONE 6: e28444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furze RC, Rankin SM (2008) Neutrophil mobilization and clearance in the bone marrow. Immunology 125: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi SS, Hunt EG, Lowry M, Blaustein AR (2013) Temporal patterns in immunity, infection load and disease susceptibility: understanding the drivers of host responses in the amphibian-chytrid fungus system. Funct Ecol 28: 569–578. [Google Scholar]
- Hayman J, Bly J, Levine R, Lobb C (1992) Complement deficiencies in channel catfish (Ictalurus punctatus) associated with temperature and seasonal mortality. Fish Shellfish Immunol 2: 183–192. [Google Scholar]
- Heatley JJ, Johnson M (2009) Clinical technique: amphibian hematology: a practitioner’s guide. J Exot Pet Med 18: 14–19. [Google Scholar]
- Hedrick MS, Hillman SS, Drewes RC, Withers PC (2013) Lymphatic regulation in nonmammalian vertebrates. J Appl Physiol 115: 297–308. [DOI] [PubMed] [Google Scholar]
- Hyatt AD, Boyle DG, Olsen V, Boyle DB, Berger L, Obendorf D, Dalton A, Kriger K, Heros M, Hines H et al. (2007) Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis Aquat Organ 73: 175–192. [DOI] [PubMed] [Google Scholar]
- Jain NC. (1993) Essentials of Veterinary Hematology. Blackwell Publishing, Philadelphia. [Google Scholar]
- Janeway CAJ, Travers P, Walport M, Shlomchik MJ (2001) Immunobiology. Garland Science, New York. [Google Scholar]
- Jiang C, Zhang J, Yao J, Liu S, Li Y, Song L, Li C, Wang X, Liu Z (2015) Complement regulatory protein genes in channel catfish and their involvement in disease defense response. Dev Comp Immunol 53: 33–41. [DOI] [PubMed] [Google Scholar]
- Kaiser HF. (1960) The application of electronic computers to factor analysis. Ed Psych Measurement 20: 141–151. [Google Scholar]
- Koppenheffer TL. (1987) Serum complement-systems of ectothermic vertebrates. Dev Comp Immunol 11: 279–286. [DOI] [PubMed] [Google Scholar]
- Kyriazakis I, Tolkamp BJ, Hutchings MR (1998) Towards a functional explanation for the occurrence of anorexia during parasitic infections. Anim Behav 56: 265–274. [DOI] [PubMed] [Google Scholar]
- Lee KA. (2006) Linking immune defenses and life history at the levels of the individual and the species. Int Comp Biol 46: 1000–1015. [DOI] [PubMed] [Google Scholar]
- Lochmiller RL, Deerenberg C (2000) Tradeoffs in evolutionary immunology: just what is the cost of immunity? Oikos 88: 87–98. [Google Scholar]
- McMahon TA, Sears BF, Venesky MD, Bessler SM, Brown JM, Deutsch K, Halstead NT, Lentz G, Tenouri N, Young S et al. (2014) Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature 511: 224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniero G, Carey C (1997) Changes in selected aspects of immune function in the leopard frog, Rana pipiens, associated with exposure to cold. Comp Biochem Physiol B Biochem Mol Biol 167: 256–263. [DOI] [PubMed] [Google Scholar]
- Merchant M, Roche C, Elsey RM, Prudhomme J (2003) Antibacterial properties of serum from the American alligator (Alligator mississippiensis). Comp Biochem Physiol B Biochem Mol Biol 136: 505–513. [DOI] [PubMed] [Google Scholar]
- Murphy PJ, St-Hilaire S, Corn PS (2011) Temperature, hydric environment, and prior pathogen exposure alter the experimental severity of chytridiomycosis in boreal toads. Dis Aquat Organ 95: 31–42. [DOI] [PubMed] [Google Scholar]
- Murray KA, Skerratt LF, Garland S, Kriticos D, McCallum H (2013) Whether the weather drives patterns of endemic amphibian chytridiomycosis: a pathogen proliferation approach. PLoS ONE 8: e61061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P, Newsholme EA (1989) Rates of utilization of glucose, glutamine and oleate and formation of end-products by mouse peritoneal macrophages in culture. Biochem J 261: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen CH, Leslie RGQ (2002) Complement’s participation in acquired immunity. J Leukocyte Biol 72: 249–261. [PubMed] [Google Scholar]
- Nonaka M, Kimura A (2006) Genomic view of the evolution of the complement system. Immunogenetics 58: 701–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmer ME, Cramp RL, White CR, Franklin CE (2015) Skin sloughing rate increases with chytrid fungus infection load in a susceptible amphibian. Funct Ecol 29: 674–682. [Google Scholar]
- Ohta Y, Goetz W, Hossain MZ, Nonaka M, Flajnik MF (2006) Ancestral organization of the MHC revealed in the amphibian Xenopus. J Immunol 176: 3674–3685. [DOI] [PubMed] [Google Scholar]
- Pessier AP, Nichols DK, Longcore JE, Fuller MS (1999) Cutaneous chytridiomycosis in Poison Dart Frogs (Dendrobates spp.) and White’s Tree Frogs (Litoria caerulea). J Vet Diagnost Invest 11: 194–199. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Steffen JE, Reinert LK, Cobine PA, Appel A, Rollins-Smith L, Mendonça MT (2013) Host stress response is important for the pathogenesis of the deadly amphibian disease, chytridiomycosis, in Litoria caerulea. PLoS ONE 8: e62146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray TL, Wuepper KD (1978) Experimental cutaneous candidiasis in rodents: II. Role of the stratum corneum barrier and serum complement as a mediator of a protective inflammatory response. Archiv Dermatol 114: 539–543. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2008) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Ribas L, Li MS, Doddington BJ, Robert J, Seidel JA, Kroll JS, Zimmerman LB, Grassly NC, Garner TW, Fisher MC (2009) Expression profiling the temperature-dependent amphibian response to infection by Batrachochytrium dendrobatidis. PLoS ONE 4: e8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert J, Ohta Y (2009) Comparative and developmental study of the immune system in Xenopus. Develop Dynam 238: 1249–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum EB, Poorten TJ, Settles M, Murdoch GK (2012) Only skin deep: shared genetic response to the deadly chytrid fungus in susceptible frog species. Mol Ecol 21: 3110–3120. [DOI] [PubMed] [Google Scholar]
- Savage AE, Zamudio KR (2011) MHC genotypes associate with resistance to a frog-killing fungus. Proc Natl Acad Sci USA 108: 16705–16710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage AE, Sredl MJ, Zamudio KR (2011) Disease dynamics vary spatially and temporally in a North American amphibian. Biol Conserv 144: 1910–1915. [Google Scholar]
- Savage AE, Becker CG, Zamudio KR (2015) Linking genetic and environmental factors in amphibian disease risk. Evol Appl 8: 560–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer MA, Sredl MJ, Rosen PC, Ryan MJ (2007) High prevalence of Batrachochytrium dendrobatidis in wild populations of lowland leopard frogs Rana yavapaiensis in Arizona. EcoHealth 4: 421–427. [Google Scholar]
- Sears BF, Rohr JR, Allen JE, Martin LB (2011) The economy of inflammation: when is less more? Trends Parasitol 27: 382–387. [DOI] [PubMed] [Google Scholar]
- Sparkmann AM, Palacio MG (2009) A test of life-history theories of immune defence in two ecotypes of the garter snake, Thamnophis elegans. J Anim Ecol 78: 1242–1248. [DOI] [PubMed] [Google Scholar]
- Sredl MJ, Jennings RD (2005) Rana chiricahuensis: Platz and Mecham, 1979, Chiricahua leopard frogs. In Lanoo MJ, eds, Amphibian Declines: the Conservation Status of United States Amphibians. University of California Press, Berkeley, pp. 546–549. [Google Scholar]
- Sunyer JO, Tort L, Lambris JD (1997) Diversity of the third form of complement, C3, in fish: functional characterization of five forms of C3 in the diploid fish Sparus aurata. Biochem J 326: 877–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer JO, Zarkadis IK, Lambris JD (1998) Complement diversity: a mechanism for generating immune diversity? Immunol Today 19: 519–523. [DOI] [PubMed] [Google Scholar]
- Tagami H. (1992) The role of complement-derived mediators in inflammatory skin diseases. Arch Dermatol Res 284: S2–S9. [DOI] [PubMed] [Google Scholar]
- Terrell KA, Quintero RP, Murray S, Kleopfer JD, Murphy JB, Evans MJ, Nissen BD, Gratwicke B (2013) Cryptic impacts of temperature variability on amphibian immune function. J Exp Biol 216: 4204–4211. [DOI] [PubMed] [Google Scholar]
- Tinsley RC, Coxhead PG, Stott LC, Tinsley MC, Piccinni MZ, Guille MJ (2015) Chytrid fungus infections in laboratory and introduced Xenopus laevis populations: assessing the risks for UK native amphibians. Biol Conserv 184: 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RJ. (1988) Amphibians. In Rawley AF, Ratcliffe NA, eds, Vertebrate Blood Cells. Cambridge University Press, Cambridge, pp 129–209. [Google Scholar]
- Venesky MD, Wilcoxen TE, Rensel MA, Rollins-Smith L, Kerby JL, Parris MJ (2012) Dietary protein restriction impairs growth, immunity, and disease resistance in southern leopard frog tadpoles. Oecologia 169: 23–31. [DOI] [PubMed] [Google Scholar]
- Woodhams DC, Ardipradja K, Alford RA, Marantelli G, Reinert LK, Rollins-Smith LA (2007) Resistance to chytridiomycosis varies among amphibian species and is correlated with skin peptide defenses. Anim Conserv 10: 409–417. [Google Scholar]
- Zhao F, Yan C, Wang X, Yang Y, Wang G, Lee W, Zhang Y (2014) Comprehensive transcriptome profiling and functional analysis of the frog (Bombina maxima) immune system. DNA Res 21: dst035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Thangamani S, How B, Ding LD (2005) The ancient origin of the complement system. EMBO J 24: 382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman LM, Vogel LA, Bowden RM (2010. a) Understanding the vertebrate immune system: insights from the reptilian perspective. J Exp Biol 213: 661–671. [DOI] [PubMed] [Google Scholar]
- Zimmerman LM, Paitz RT, Vogel LA, Bowden RM (2010. b) Variation in the seasonal patterns of innate and adaptive immunity in the red-eared slider (Trachemys scripta). J Exp Biol 213: 1477–1483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.