A study measuring UE functional capacity and daily performance change during outpatient rehabilitation poststroke found inconsistencies between change in UE capacity and performance.
MeSH TERMS: accelerometry, activities of daily living, occupational therapy, paresis, stroke, upper extremity
Abstract
OBJECTIVE. This study explored how upper-extremity (UE) functional capacity and daily performance change during the course of outpatient rehabilitation in people with stroke.
METHOD. Fifteen participants receiving outpatient occupational therapy services for UE paresis poststroke were enrolled. UE motor capacity was measured with the Action Research Arm Test (ARAT), and UE performance was measured using bilateral, wrist-worn accelerometers. Measurements were taken at or near the start of therapy, at every 10th visit or every 30 days throughout the duration of services, and at discharge.
RESULTS. Three patterns were observed: (1) increase in ARAT scores and more normalized accelerometry profiles, (2) increase in ARAT scores but no change in accelerometry profiles, and (3) no change in ARAT scores or in accelerometry profiles.
CONCLUSION. UE performance in daily life was highly variable, with inconsistencies between change in UE capacity and change in UE performance. UE capacity and performance are important constructs to assess separately during rehabilitation.
After stroke, 65% of people cannot incorporate their paretic hand into daily activity (Dobkin, 2005) and will eventually discontinue 57% of their meaningful activities (Hartman-Maeir, Soroker, Ring, Avni, & Katz, 2007). Given the bilateral nature of most upper-extremity (UE) activities (Bailey & Lang, 2013), attributing decreased engagement in activities in part to UE paresis is reasonable. Rehabilitation services after stroke often address many deficits, including stroke-related motor impairment of the UE, and UE interventions have been shown to improve UE functional capacity (i.e., what a person can do) after stroke (Birkenmeier, Prager, & Lang, 2010; Harris, Eng, Miller, & Dawson, 2009; Page, Levine, & Leonard, 2007; Taub et al., 2006; Wolf et al., 2006). However, despite improved UE functional capacity, UE performance (i.e., what a person actually does) may remain severely limited after stroke (Dobkin, 2005; Nakayama, Jørgensen, Raaschou, & Olsen, 1994; Rand & Eng, 2012; Wilkinson et al., 1997).
During poststroke rehabilitation, occupational therapists rely on in-clinic assessments to measure change in UE functional capacity (e.g., Action Research Arm Test [ARAT; Lyle, 1981], Wolf Motor Function Test; Wolf et al., 2001]). The assumption is that improved UE functional capacity translates to increased UE performance in daily life. This assumption, however, is not supported by data (Rand & Eng, 2012; Rand, Eng, Tang, Jeng, & Hung, 2009), thus emphasizing the importance of assessing UE functional capacity and UE performance separately (Bailey, Klaesner, & Lang, 2015; Young, Williams, Yoshida, Bombardier, & Wright, 1996).
Currently, self-report measures (e.g., Stroke Impact Scale, Motor Activity Log) may be used in the clinical setting to quantify UE performance in daily life (Duncan et al., 1999; Uswatte, Taub, Morris, Vignolo, & McCulloch, 2005; van der Lee, Beckerman, Knol, de Vet, & Bouter, 2004). These measures are subject to bias, particularly with respect to physical activity (Adams et al., 2005) and because of cognitive impairments after stroke (Bradburn, Rips, & Shevell, 1987; Tatemichi et al., 1994). Given that a key goal of rehabilitation is to increase UE performance in daily life, assessments should effectively measure this construct.
Accelerometry is a technique to capture and quantify movement using an acclerometer, a device that constantly measures physical acceleration. Accelerometry has been established as an objective, unbiased tool to measure real-world UE performance in daily life (Bailey, Klaesner, & Lang, 2014; Uswatte, Foo, et al., 2005; Uswatte et al., 2006). Accelerometers have been used to record UE performance of people in the inpatient rehabilitation setting very early after stroke (Lang, Wagner, Edwards, & Dromerick, 2007) and to determine hours of total UE activity in healthy control participants and people who have had a stroke (Bailey, Klaesner, & Lang, 2015; Bailey & Lang, 2013; de Niet, Bussmann, Ribbers, & Stam, 2007; Uswatte, Foo, et al., 2005; Uswatte et al., 2006). More recently, accelerometry has been used to quantify the intensity of UE movement and further define how the paretic and nonparetic UE compare with one another poststroke (de Niet et al., 2007; Gebruers et al., 2008; Michielsen, Selles, Stam, Ribbers, & Bussmann, 2012). Last, established accelerometry metrics have been shown to provide valid measurements of task-specific interventions and are sensitive to change (Bailey, Klaesner, & Lang, 2015; Urbin, Bailey, & Lang, 2015; Urbin, Waddell, & Lang, 2015).
Although the body of evidence supporting the use of accelerometry continues to grow, the data available on UE performance are limited, particularly on UE performance during outpatient stroke rehabilitation. This pilot study explored how UE functional capacity and daily performance change in people who are receiving routine outpatient occupational therapy services poststroke. We chose to assess UE functional capacity and performance in people receiving routine occupational therapy rather than in people receiving therapy services as part of a research intervention because the former better represent the general rehabilitation population. We questioned whether UE capacity, UE performance, or both would change in people with stroke. These observational data will provide occupational therapy practitioners and other providers with critical insight into the potential patterns of change in UE performance and UE functional capacity during outpatient stroke rehabilitation services.
Method
All participants in this pilot prospective observational cohort were receiving usual occupational therapy services in either day rehabilitation or outpatient settings at facilities operated within city limits by the Rehabilitation Institute of Chicago. All sites offer similar comprehensive occupational therapy services and receive access to continuing education opportunities. This research study involved only measurements (see “Study Measures” section) and did not influence the amount, content, or duration of occupational therapy intervention provided to the enrolled participants. This study was approved by the institutional review board of Northwestern University, and informed consent was obtained from all participants.
Participants
We recruited 15 participants with UE paresis resulting from stroke who were participating in outpatient occupational therapy as part of their routine rehabilitation management to address UE functional deficits. Before the study began, we determined that a sample size of 15 was appropriate for a first pilot project examining UE functional capacity and UE performance; this sample size is consistent with that of other UE projects (Birkenmeier et al., 2010; Combs, Kelly, Barton, Ivaska, & Nowak, 2010; Page, Levine, & Leonard, 2005; Page, Levine, Sisto, & Johnston, 2001). Participants were recruited by means of consecutive referrals from the treating occupational therapists to a research team member (Caitlin A. Doman).
Inclusion criteria were as follows: (1) history of stroke, according to International Classification of Diseases, Ninth Revision (World Health Organization, 1980), criteria, with resulting UE paresis; (2) age ≥18 yr; (3) receiving occupational therapy services in an outpatient or day rehabilitation setting; (4) the presence of some spared motor capacity, as indicated by a UE Motricity Index (Collin & Wade, 1990) score between 29 and 91 points on the paretic side; (4) the ability to follow a one-step command as indicated by a score of 0 or 1 on the command item of the National Institutes of Health Stroke Scale (Brott et al., 1989); and (5) the ability to provide signed, informed consent. Participants were excluded if they (1) had another diagnosed neurological condition; (2) had a history of a diagnosed psychiatric condition; or (3) were currently pregnant or trying to become pregnant. Medical records indicated that the participants received both one-on-one and group services with registered and licensed occupational therapists.
Study Measures
Measurements of UE functional capacity and UE performance in daily life were taken at or near the start of therapy, every 10th visit or 30 days (whichever came first) throughout occupational therapy services, and at discharge. UE functional capacity was measured using the ARAT, a 19-item, activity-based assessment scored on a scale ranging from 0 to 57 points, on which a score of 57 indicates normal UE movement (Lyle, 1981). The psychometric properties of the ARAT have been established; it has excellent interrater reliability (r = .95), test–retest reliability (r = .99), and construct validity (rs = .92–.95; Lang, Bland, Bailey, Schaefer, & Birkenmeier, 2013; Lang, Edwards, Birkenmeier, & Dromerick, 2008; Lin et al., 2009; Platz et al., 2005).
UE performance was recorded over 24-hr wearing periods, at the times indicated previously, using two accelerometers (ActiGraph, wGT3X-BT; ActiGraph LLC, Pensacola, FL), one worn on each wrist. The wear period was chosen as a practical compromise to obtain the data but not overburden the participants with measures and decrease wearing adherence (Bailey, Klaesner, & Lang, 2015; Barak, Wu, Dai, Duncan, & Behrman, 2014). Accelerometers are a well-established valid and reliable tool used to objectively measure UE use in daily life (Bailey et al., 2014; Lang et al., 2013; Uswatte, Foo, et al., 2005). They are the size of a wristwatch and measure UE activity in raw accelerations along three axes. Accelerations were recorded at 30 Hz and binned into 1-s epochs; values were recorded as activity counts, where 1 count equaled 0.016318 gravitational units (1 g = 9.8 m/s2). Data were uploaded using ActiLife 6 software (ActiGraph LLC, Pensacola, FL) and processed via custom-written algorithms in MATLAB.
Each participant’s accelerometry profile included the primary variables of interest for UE performance: bilateral magnitude of arm accelerations, magnitude ratio, and use ratio. The bilateral magnitude measurement quantifies the intensity of movement across both UEs. (Note that bilateral magnitude is a different construct from intensity as an index of cardiovascular stimulus or stress during exercise.) We calculated bilateral magnitude by combining accelerations along the three axes into a single value called the vector magnitude (√[x2 + y2 + z2]; Bailey et al., 2014) and then summing the vector magnitude from each limb for each second of activity (Bailey et al., 2014). A bilateral magnitude value of 0 indicates that no activity occurred in either UE at that second. An increase in the bilateral magnitude indicates that the UEs moved with increasing intensity. For example, lower bilateral magnitude values would be expected during tasks such as typing or writing, and higher values would be expected during a task such as placing an item on a shelf with both hands (Bailey et al., 2014).
The magnitude ratio quantifies the contribution of each UE to activity (Bailey et al., 2014) and is calculated for each second of data by dividing the vector magnitude of the paretic UE by the vector magnitude of the nonparetic UE. To prevent skewness, the natural log of this ratio is then used as the magnitude ratio (Bailey et al., 2014). For seconds when unilateral activity occurred, the magnitude ratio is indeterminate; thus, a constant value is assigned as follows: a value of −7 for unilateral nonparetic UE activity and a value of 7 for unilateral paretic UE activity (Bailey et al., 2014). When each UE contributes equally to an activity, the magnitude ratio is approximately 0. Negative magnitude ratio values indicate that the nonparetic UE contributed more; positive magnitude ratio values indicate that the paretic UE contributed more (Bailey et al., 2014). Later in this article, the figures display 24 hr of data for the bilateral magnitude and magnitude ratio, and the table displays median values across the 24 hrs.
The use ratio quantifies the length of time that the paretic UE was active relative to the nonparetic UE (Bailey & Lang, 2013; Uswatte, Foo, et al., 2005). Using the vector magnitudes described earlier, seconds in which the vector magnitude value was ≥2 were categorized as “movement” and seconds in which it was <2 were categorized as “nonmovement.” Seconds of movement were then summed for each side to determine duration of activity. The use ratio was calculated as duration of paretic activity divided by duration of nonparetic activity. Use ratio values closer to 1 indicate that the total time both the paretic and the nonparetic UEs were active was relatively equal. Lower values indicate that the total time the paretic UE was used in daily tasks was less than the total time the nonparetic UE was used. Community-dwelling adults have a relatively constant mean use ratio of 0.95 (standard deviation [SD] = 0.06; Bailey & Lang, 2013), and adults with chronic stroke typically have lower use ratios (Bailey, Birkenmeier, & Lang, 2015). The use ratio and magnitude ratio capture conceptually similar constructs. The use ratio (simpler calculation) is more of a summary statistic across the recording period, and the magnitude ratio (more complex calculation) provides a higher temporal resolution view of bilateral UE performance. Both are included here because which is most clinically relevant has not yet been determined.
A bivariate histogram (i.e., density plot), shown in Figures 1–3, was used to visually depict the bilateral magnitude and magnitude ratio for each participant at each assessment (Supplemental Figures 1–3, available with this article online, are color versions of the figures and are referred to in the discussion that follows; navigate to this article at http://otjournal.net, and click on “Supplemental”). The intensity of activity (y-axis; bilateral magnitude) and the contribution of each UE (x-axis; magnitude ratio, with 0 indicating that both limbs contributed the same amount) are plotted for every second of data during the 24-hr wear period. The color represents the frequency of each combination of values, with cooler (i.e., blue) colors representing lower frequencies (less time) and warmer (i.e., red) colors representing higher frequencies (more time). The two bars on either side (i.e., magnitude ratio of ±7) show the amount of isolated UE activity, with the nonparetic UE on the left and the paretic UE on the right. The shape in the middle shows the activity when the two UEs are used together. For reference, density plots of healthy, community-dwelling middle-aged and older adults have a highly consistent characteristic shape and color scheme (Bailey, Klaesner, & Lang, 2015). An example of a normal plot can be seen in Supplemental Figure 4 (available online). Other examples are shown in Figure 1 of Bailey, Klaesner, and Lang (2015) and Figure 3 of Hayward et al. (2015).
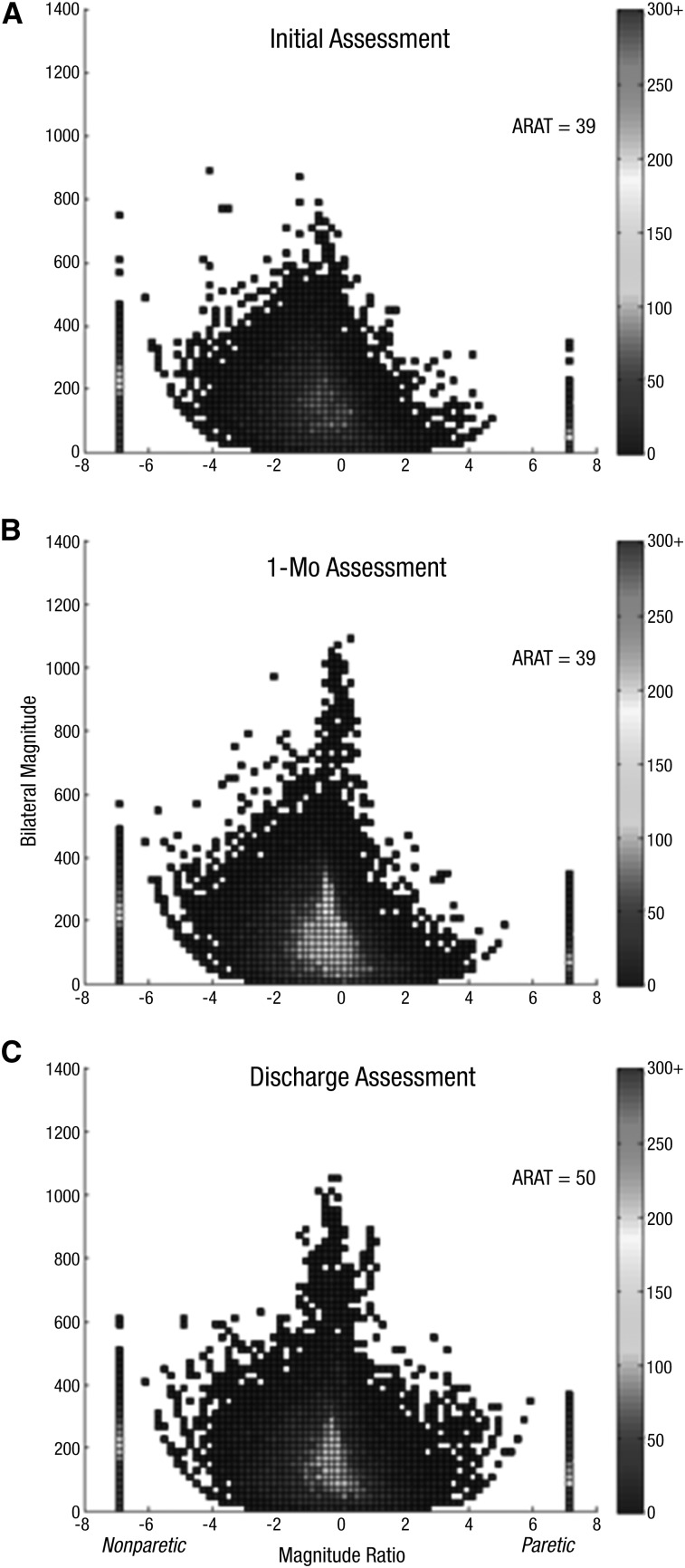
Figure 1.
Density plot showing 24 hr of UE performance in daily life for Participant 1, who showed a change in UE capacity and a change in UE performance: Initial assessment (A), 1-mo assessment (B), discharge assessment (C).
Note. On the x-axis is the magnitude ratio (the contribution of each UE to an activity); on the y-axis is the bilateral magnitude (intensity of movement across both UEs). The color represents the frequency of movement; cooler colors represent lower frequencies (less time), and warmer colors represent higher frequencies (more time). ARAT = Action Research Arm Test; UE = upper extremity.
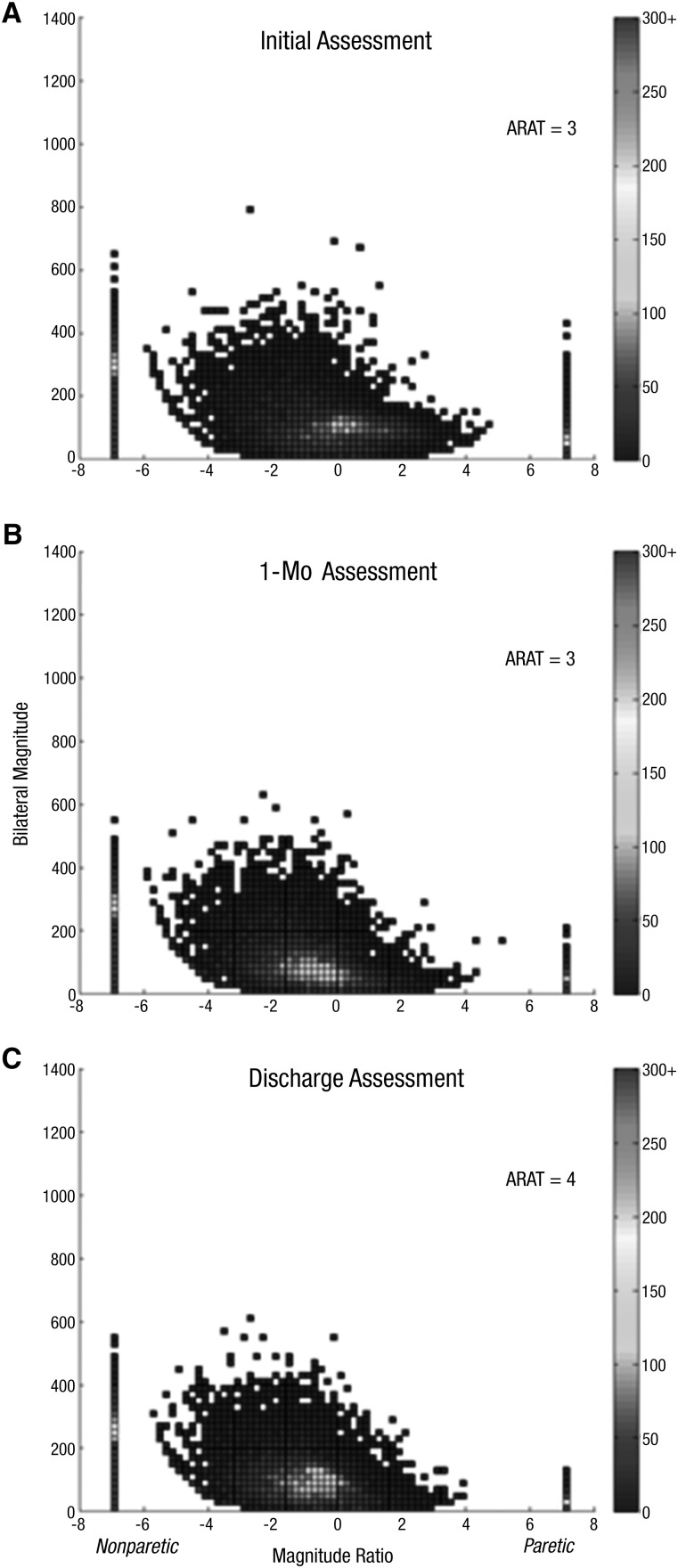
Figure 3.
Density plot showing 24 hr of UE performance in daily life for Participant 13, who showed no change in UE capacity and no change in UE performance: Initial assessment (A), 1-mo assessment (B), discharge assessment (C).
Note. On the x-axis is the magnitude ratio (the contribution of each UE to an activity); on the y-axis is the bilateral magnitude (intensity of movement across both UEs). The color represents the frequency of movement; cooler colors represent lower frequencies (less time), and warmer colors represent higher frequencies (more time). ARAT = Action Research Arm Test; UE = upper extremity.
To facilitate interpretation of the data in this article, a brief description of “normal” is provided here (see Supplemental Figure 4). First, normal plots are always symmetrical, indicating that the dominant and nondominant upper UEs are used about the same amount of time and mostly together. Second, the plots are wider on the bottom, indicating that most UE performance involves low-intensity movements. The rims of the bowl-like shape occur during activities in which one limb is accelerating and the other is relatively still, such as placing objects in a container with one hand and holding the container with the other (Bailey et al., 2014). Third, there is typically a “warm glow” in the middle, which represents frequent lower intensity movements in which both limbs are working together, such as cutting food with a knife and fork (Bailey et al., 2014). Last, the middle peak occurs with higher intensity activities involving both limbs, such as stacking boxes on a shelf (Bailey et al., 2014).
Descriptive Statistics
Because this was a pilot observational study with a small, heterogeneous sample, data from each participant were examined separately across time. Demographic data, ARAT scores, and accelerometry profiles for each participant were visually inspected to see how closely they resembled normal UE functional capacity, as determined by an ARAT score of 57, and UE performance. Individual changes in ARAT scores were examined and compared with the estimated minimal clinically important difference values of at least 5.7 points in people with longstanding stroke (van der Lee, Beckerman, Lankhorst, & Bouter, 2001). Accelerometer data from each participant in this study were compared with previously published referent values (group mean and SD) obtained from nondisabled adults for the bilateral magnitude (median of second-by-second values = 135, SD = 36), magnitude ratio (median of second-by-second values = −0.1, SD = 0.16), and use ratio (mean = 0.95, SD = 0.06; Bailey, Klaesner, & Lang, 2015; Bailey & Lang, 2013).
We examined each participant’s data for three potential patterns of change. The first possible pattern was an increase in ARAT scores and more normalized accelerometry profiles, which would indicate improved UE functional capacity and improved UE performance in daily life over the course of outpatient occupational therapy services. The second possible pattern was an increase in ARAT scores but no change in the accelerometry profiles, which would indicate improved UE functional capacity but no change in UE performance. The third potential pattern was one in which participants showed no change in ARAT scores and no change in accelerometry profiles, indicating no improvement in UE functional capacity or UE performance.
When stratifying each participant into one pattern, we considered a notable positive change across all three accelerometer metrics from baseline to discharge to be an improved accelerometry profile. A marked visual change in the individual density plots from baseline to discharge also contributed to the classification. We did not complete a group-level statistical analysis because each participant had a different number of assessment sessions, and length of time between initial and discharge assessments varied because of the heterogeneity of routine clinical rehabilitation services. Completing a group-level statistical analysis could have masked or dismissed individual patterns of response to occupational therapy services, which were of particular interest in this pilot project.
Results
Of the 15 participants enrolled, 13 completed more than one assessment, had available discharge data, or both. Of the 2 participants with only one assessment, 1 was admitted to acute care and did not return to therapy, and the other was unable to complete therapy because of insurance limitations. The results presented here represent the 13 participants with more than one assessment. Individual descriptive data and assessment scores are reported in Table 1.
Table 1.
Participant Characteristics, ARAT, and Accelerometer Variables
| Participant | Age | Sex | Days Since Stroke | Total OP Visits | Paretic Side | ARATa | Bilateral Magnitudeb | Magnitude Ratioc | Use Ratiod | ||||
| Initial | Discharge | Initial | Discharge | Initial | Discharge | Initial | Discharge | ||||||
| 1 | 47 | M | 47 | 30 | D | 39 | 50 | 97.35 | 111.49 | −1.96 | −0.76 | .63 | .83 |
| 3 | 80 | F | 31 | 32 | D | 34 | 48 | 79.97 | 80.51 | −2.70 | −2.05 | .58 | .62 |
| 4 | 69 | M | 60 | 78 | D | 37 | 52 | 91.18 | 90.16 | −0.67 | −0.42 | .80 | .85 |
| 6 | 85 | M | 310 | 17 | ND | 8 | 6 | 82.42 | 74.25 | −4.58 | −7 | .51 | .49 |
| 7 | 62 | M | 497 | 29 | ND | 38 | 45 | 66.42 | 76.21 | −1.34 | −1.20 | .74 | .73 |
| 8 | 82 | M | 236 | 14 | ND | 29 | 30 | 59.71 | 53.50 | −7 | −7 | .46 | .53 |
| 9 | 54 | F | 22 | 12 | ND | 51 | 57 | 114.81 | 110.98 | −1.39 | −1.30 | .71 | .68 |
| 10 | 89 | F | 38 | 21 | ND | 36 | 37 | 57.31 | 65 | −7 | −7 | .44 | .39 |
| 11 | 31 | F | 142 | 38 | ND | 5 | 7 | 73.35 | 77.48 | −7 | −7 | .44 | .38 |
| 12 | 64 | M | 79 | 39 | D | 22 | 21 | 58.80 | 57.23 | −7 | −7 | .42 | .51 |
| 13 | 48 | F | 59 | 26 | D | 3 | 4 | 81.37 | 68.1 | −7 | −7 | .42 | .38 |
| 14 | 70 | M | 35 | 10 | D | 36 | 39 | 88.04 | 74.82 | −1.04 | −0.82 | .76 | .77 |
| 15 | 84 | F | 216 | 17 | D | 12 | 19 | 78.81 | 90.44 | −3.85 | −2.03 | .53 | .64 |
Note. ARAT = Action Research Arm Test; D = dominant; F = female; M = male; ND = nondominant; OP = outpatient; UE = upper extremity.
Scored on a scale ranging from 0 to 57 points, where higher scores indicate better UE function (clinically important change = 5.7 points).
Values are the median of each participant’s second-by-second values. Normative value are as follows: mean = 135, standard deviation = 36, where higher values equal greater intensity of movement.
Values are the median of each participant’s second-by-second values. Normative values are as follows: mean = −0.1, standard deviation = 0.16, where 0 indicates equal contribution from each UE to an activity.
Normative values are as follows: mean = 0.95, standard deviation = 0.06, where 1 indicates equal duration of activity for both UEs.
Participants had a wide range in age (31–89 yr), days poststroke (22–497), and number of occupational therapy visits (10–78). Their initial level of UE functional capacity varied, as indicated by ARAT scores (range = 3–51 points). The initial use ratio and median magnitude ratio values for each participant were at least 2 SDs below the normative mean at the initial assessment, indicating that the majority of UE activity was unilateral, favoring the nonparetic UE. The median number of participant assessments was three, with a range from two (Participants 6, 8, 9, 14, and 15) to eight (Participant 4). The variation in number of assessments across participants likely reflects variation in the duration of services, where duration of services may be determined by multiple factors (e.g., therapist prescription, payer limits).
The first pattern observed was a change in ARAT score and a change in the accelerometry profile, indicating improved UE motor capacity and improved UE performance. This pattern was observed in Participants 1 and 15. Figure 1 shows a representative example of this pattern. Participant 1 had three assessments and 30 visits. He increased his paretic-side ARAT score and had a more normalized accelerometry profile by discharge (see Table 1), indicating both improved UE motor capacity and improved UE performance. The initial assessment picture (Figure 1A) was asymmetrical (negative magnitude ratios), with fewer data points from paretic UE activity (positive, right side of figure). Most of the figure is displayed in cooler, blue colors, indicating less frequent movement, and the overall bilateral magnitude was low. At the approximately 1-mo assessment (Figure 1B), the ARAT score remained the same, but the picture became more symmetrical, had higher bilateral magnitude values, and had brighter colors in the center, indicating more bilateral, intense, and frequent activity. By the discharge assessment (Figure 1C), the participant’s ARAT score had increased (11-point change), and the figure more closely resembles that of neurologically intact adults. Figure 1C is nearly symmetrical with a more normal height and width, indicating increased and more normal UE performance in daily activities. The increased UE performance was also reflected in duration of use, as indicated by the progression of use ratio values from .63 to .78 to .83 over the three assessments.
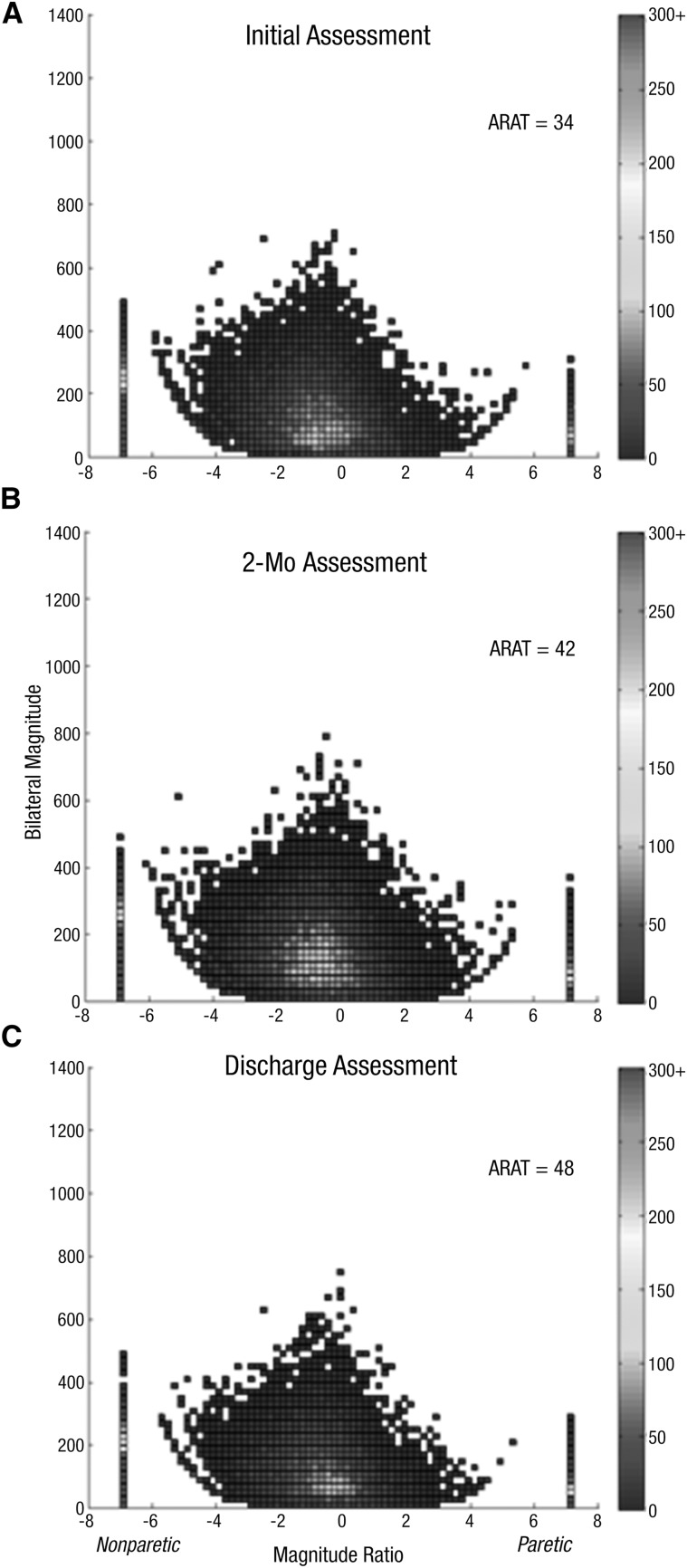
The second pattern observed was an increase in ARAT score but no change in the accelerometry profile, indicating improved UE motor capacity and no improvement in UE performance. This pattern was observed in Participants 3, 4, 7, and 9. Figure 2 shows a representative example of this second pattern. Participant 3 had five assessments over 32 visits. She increased her ARAT score but did not make any substantial or sustainable changes in her accelerometry profile (Table 1), indicating an improvement in UE functional capacity, but not in UE performance. Figure 2A shows the initial assessment; Figure 2B, the approximately 2-mo assessment; and Figure 2C, the discharge assessment. ARAT score increased across the time points, but Figure 2C was largely unchanged from Figure 2A. Even by the discharge assessment, the height (bilateral magnitude) was relatively low, and the asymmetrical shape (magnitude ratio) of the figure had not changed. Although the medians for these values shifted slightly (see initial and discharge values for Participant 3 in Table 1), UE performance remained far from normal at discharge (e.g., the discharge median magnitude ratio of −2.05 is 12 SDs below the referent mean). The pattern of UE activity did not change, as depicted by the unchanging colors in the center of the figure. Note the small, vertical color bar on both sides of the figure. The presence of red on the left vertical bar indicates that majority of activity was completed with the nonparetic UE throughout the recording period. The use ratio (Table 1) fluctuated over the course of therapy and remained more than 5 SDs below the referent mean.
Figure 2.
Density plot showing 24 hr of UE performance in daily life for Participant 3, who showed a change in UE capacity and no change in UE performance: Initial assessment (A), 2-mo assessment (B), discharge assessment (C).
Note. On the x-axis is the magnitude ratio (the contribution of each UE to an activity); on the y-axis is the bilateral magnitude (intensity of movement across both UEs). The color represents the frequency of movement; cooler colors represent lower frequencies (less time), and warmer colors represent higher frequencies (more time). ARAT = Action Research Arm Test; UE = upper extremity.
The third pattern observed was no change in ARAT score and no change in the accelerometry profile, indicating no improvement in UE motor capacity and no improvement in UE performance. This pattern was observed in Participants 6, 8, 10, 11, 12, 13, and 14. These participants had initial ARAT scores between 3 and 36 points, but their change scores only ranged from –1 to 3 points. Across the assessments for each of these participants, there were no regular, sustained changes in accelerometry values, indicating neither a change in UE functional capacity nor a change in UE performance. Figure 3 shows a representative example of this third pattern. Participant 13 had three assessments over 26 visits. The initial (Figure 3A), approximately 1-mo (Figure 3B), and discharge (Figure 3C) assessments were markedly different from normal and markedly similar to each other. Figures 3A−3C are short in height (bilateral magnitude) and less symmetrical (magnitude ratio) than the corresponding parts of Figures 1 and 2. The short height indicates low-intensity activity, and the asymmetry indicates a lack of activity with the paretic UE. Note the red bar on the left, indicating frequent unilateral nonparetic activity. The initial and final median magnitude ratios were the lowest possible value, −7, indicating that more than 50% of total UE activity was completed with the nonparetic UE alone. The use ratio values were unchanged or perhaps even decreased.
A review of the assessment and treatment clinical records for all participants indicated that the occupational therapy services included both therapeutic activities and therapeutic exercise to address UE-related goal areas and improve UE function for each participant. No obvious differences could be ascertained from these records with respect to the clinical management of participants grouped into the three patterns. Further review of the variables in Table 1 also did not reveal any distinguishing characteristics that might have separated the participants into these three patterns. Across the three patterns, participants had overlapping ranges in age, days since stroke, total outpatient occupational therapy visits, and initial ARAT scores. For example, the range in number of visits for the first pattern was 17–30; for the second pattern, 12–78; and the third pattern, 14–39. Finally, gender and paretic sides were mixed across patterns.
Discussion
To our knowledge, this study is the first of its kind to quantify UE performance in daily life during routine outpatient occupational therapy without using self-report measures. The data showed a high degree of variability in UE functional capacity and UE performance in people receiving outpatient services, as might be expected. Of more interest, however, is the variability in change over the course of therapy. Of 13 participants, 2 improved in both UE functional capacity and performance, 4 improved in UE functional capacity but not in performance, and 7 improved in neither UE functional capacity nor performance.
These results provide preliminary evidence to challenge the common assumption that improvements in functional capacity seen in the clinic will translate to increased performance in daily life. Although this assumption was true for some participants, it was not true for all of them. Indeed, several participants had large, impressive changes in UE functional capacity but no change in performance. In previous research studies, the Motor Activity Log and Stroke Impact Scale have been used to measure perceived changes in UE performance. Available data on self-reported performance have largely come from constraint-induced movement therapy (CIMT) studies in which a highly selective population received a substantially controlled intervention (Wolf et al., 2006). Thus, it is difficult to draw comparisons with our participants, who received routine outpatient occupational therapy services. In addition, because CIMT study participants did not wear accelerometers, comparison of our daily UE performance results with the published data is not possible.
The Canadian Occupational Performance Measure (COPM) is another tool used to measure perceived performance and satisfaction with daily activities (Pollock, 1993). Data on the use of the COPM in routine occupational therapy regarding specific UE performance are limited, however. Self-identified goals gathered using the COPM are often related to activities of daily living or instrumental activities of daily living; only a small percentage are related to isolated UE movement (Waddell, Birkenmeier, Bland, & Lang, 2015). Our data support the idea that assessment of UE capacity and UE performance as separate constructs is important for the delivery of outpatient occupational therapy services. Without a separate assessment of UE performance, it is not possible to determine whether gains seen during therapy sessions carry over to real life. Using accelerometers may be an efficient, unbiased, and clinically feasible way to capture this important construct.
Of 13 participants, 4 demonstrated increased UE functional capacity but did not increase UE performance. People who demonstrate such a pattern present a unique opportunity for occupational therapists. Our data indicate that this pattern may be more common than previously thought and may require an alternative approach to facilitate translation of capacity gains into performance gains. Discovering the reasons why the overall UE performance of these participants did not increase is beyond the scope of this study because the interventions provided were not controlled or monitored. Review of treatment notes suggests that these 4 participants received care that was similar to that of the other participants and likely had a sufficient number of occupational therapy visits (range = 12–78) for at least some detectable changes in UE performance to have occurred.
The science and practice of occupational therapy are well suited to develop, refine, and test approaches to translate therapeutic gains into improved performance. Routine occupational therapy practice, however, may need to be accompanied by emerging health behavior approaches, such as the transfer package used in CIMT (Taub et al., 2013) and the Graded Repetitive Arm Supplementary Program (Harris et al., 2009), to guide translation of UE capacity acquired during treatment into daily UE performance. This type of skill transfer may be ideal for people who, as with some participants observed in this study, demonstrate an increase in UE capacity but not in UE performance.
Two primary limitations should be considered when interpreting these data. First, this was a pilot observational study. Thus, our results are best viewed as opening a discussion regarding the important constructs of UE capacity versus performance and how to evaluate outcomes of occupational therapy services. Second, we did not control or accurately measure any aspects of how therapy was delivered, so no causal inferences about the changes over time or lack thereof can be made. On the basis of prior observation studies, one would expect a great deal of variability in how the interventions were delivered, the method in which they were delivered, and the doses delivered (Lang et al., 2007, 2009). Larger, well-controlled studies are needed to see whether specific interventions, methods, and doses change UE performance, as objectively quantified here with accelerometry.
Implications for Occupational Therapy Practice
The results of this study provide unique insight into UE capacity and performance changes during routine outpatient occupational therapy and have the following key implications for occupational therapy practice:
Changes in UE capacity measured in the clinic may or may not result in increased UE performance in daily life. UE capacity and performance are different constructs and need to be measured separately.
Accelerometry is one option to measure and track change in UE performance during rehabilitation.
The results from UE capacity and performance assessments, taken throughout the duration of outpatient occupational therapy services, can guide clinical decision making regarding the provision of services and selection of specific interventions.
Conclusion
Our data indicate that a high degree of variability occurred in UE functional capacity and UE performance changes during outpatient occupational therapy poststroke. Increased UE functional capacity measured in the clinic may not result in increased UE performance in daily life. This pilot study opens the door for dialogue among clinicians and researchers about the importance of measuring both constructs and the option to use accelerometry in the clinic as an unbiased alternative to self-reported measures of daily UE performance. Indeed, our results give rise to more questions than they answer regarding the assessment and delivery of outpatient occupational therapy poststroke. For example, how widespread are the results observed here? Why did some people improve and others not? How might occupational therapy services be modified to increase UE performance and not just capacity? Although this study focused on therapy services provided by one professional discipline to one patient population, we speculate that the conceptual issues regarding capacity and performance extend to all disciplines and populations involved in neurorehabilitation.
Supplementary Material
Acknowledgments
This study was partially supported by the Buchanan Family Fellowship to C. A. Doman and National Institutes of Health Grant R01HD068290 to C. E. Lang.
Contributor Information
Caitlin A. Doman, Caitlin A. Doman, MS, OTR/L, is Occupational Therapist, Rehabilitation Institute of Chicago, Chicago, IL
Kimberly J. Waddell, Kimberly J. Waddell, MS, OTR/L, is Graduate Student, Movement Science and Program in Physical Therapy, Washington University School of Medicine, St. Louis, MO
Ryan R. Bailey, Ryan R. Bailey, MSOT, MSCI, PhD, was Graduate Student, Movement Science Program and Program in Physical Therapy, Washington University School of Medicine, St. Louis, MO, at the time of the study
Jennifer L. Moore, Jennifer L. Moore, PT, DHS, NCS, is Physical Therapist, Rehabilitation Institute of Chicago, Chicago, IL
Catherine E. Lang, Catherine E. Lang, PT, PhD, is Professor, Program in Physical Therapy, Program in Occupational Therapy, and Department of Neurology, Washington University School of Medicine, St. Louis, MO; langc@wustl.edu
References
- Adams S. A., Matthews C. E., Ebbeling C. B., Moore C. G., Cunningham J. E., Fulton J., & Hebert J. R. (2005). The effect of social desirability and social approval on self-reports of physical activity. American Journal of Epidemiology, 161, 389–398. http://dx.doi.org/10.1093/aje/kwi054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R. R., Birkenmeier R. L., & Lang C. E. (2015). Real-world affected upper limb activity in chronic stroke: An examination of potential modifying factors. Topics in Stroke Rehabilitation, 22, 26–33. http://dx.doi.org/10.1179/1074935714Z.0000000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R. R., Klaesner J. W., & Lang C. E. (2014). An accelerometry-based methodology for assessment of real-world bilateral upper extremity activity. PLoS One, 9, e103135 http://dx.doi.org/10.1371/journal.pone.0103135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R. R., Klaesner J. W., & Lang C. E. (2015). Quantifying real-world upper-limb activity in nondisabled adults and adults with chronic stroke. Neurorehabilitation and Neural Repair, 29, 969–978. http://dx.doi.org/10.1177/1545968315583720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R. R., & Lang C. E. (2013). Upper-limb activity in adults: Referent values using accelerometry. Journal of Rehabilitation Research and Development, 50, 1213–1222. http://dx.doi.org/10.1682/JRRD.2012.12.0222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S., Wu S. S., Dai Y., Duncan P. W., & Behrman A. L.; Locomotor Experience Applied Post-Stroke (LEAPS) Investigative Team. (2014). Adherence to accelerometry measurement of community ambulation poststroke. Physical Therapy, 94, 101–110. http://dx.doi.org/10.2522/ptj.20120473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier R. L., Prager E. M., & Lang C. E. (2010). Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: A proof-of-concept study. Neurorehabilitation and Neural Repair, 24, 620–635. http://dx.doi.org/10.1177/1545968310361957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradburn N. M., Rips L. J., & Shevell S. K. (1987). Answering autobiographical questions: The impact of memory and inference on surveys. Science, 236, 157–161. http://dx.doi.org/10.1126/science.3563494 [DOI] [PubMed] [Google Scholar]
- Brott T., Adams H. P. Jr., Olinger C. P., Marler J. R., Barsan W. G., Biller J., . . . Walker M. (1989). Measurements of acute cerebral infarction: A clinical examination scale. Stroke, 20, 864–870. http://dx.doi.org/10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- Collin C., & Wade D. (1990). Assessing motor impairment after stroke: A pilot reliability study. Journal of Neurology, Neurosurgery, and Psychiatry, 53, 576–579. http://dx.doi.org/10.1136/jnnp.53.7.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs S. A., Kelly S. P., Barton R., Ivaska M., & Nowak K. (2010). Effects of an intensive, task-specific rehabilitation program for individuals with chronic stroke: A case series. Disability and Rehabilitation, 32, 669–678. http://dx.doi.org/10.3109/09638280903242716 [DOI] [PubMed] [Google Scholar]
- de Niet M., Bussmann J. B., Ribbers G. M., & Stam H. J. (2007). The stroke upper-limb activity monitor: Its sensitivity to measure hemiplegic upper-limb activity during daily life. Archives of Physical Medicine and Rehabilitation, 88, 1121–1126. http://dx.doi.org/10.1016/j.apmr.2007.06.005 [DOI] [PubMed] [Google Scholar]
- Dobkin B. H. (2005). Clinical practice: Rehabilitation after stroke. New England Journal of Medicine, 352, 1677–1684. http://dx.doi.org/10.1056/NEJMcp043511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan P. W., Wallace D., Lai S. M., Johnson D., Embretson S., & Laster L. J. (1999). The Stroke Impact Scale Version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke, 30, 2131–2140. http://dx.doi.org/10.1161/01.STR.30.10.2131 [DOI] [PubMed] [Google Scholar]
- Gebruers N., Truijen S., Engelborghs S., Nagels G., Brouns R., & De Deyn P. P. (2008). Actigraphic measurement of motor deficits in acute ischemic stroke. Cerebrovascular Diseases, 26, 533–540. http://dx.doi.org/10.1159/000160210 [DOI] [PubMed] [Google Scholar]
- Harris J. E., Eng J. J., Miller W. C., & Dawson A. S. (2009). A self-administered Graded Repetitive Arm Supplementary Program (GRASP) improves arm function during inpatient stroke rehabilitation: A multi-site randomized controlled trial. Stroke, 40, 2123–2128. http://dx.doi.org/10.1161/STROKEAHA.108.544585 [DOI] [PubMed] [Google Scholar]
- Hartman-Maeir A., Soroker N., Ring H., Avni N., & Katz N. (2007). Activities, participation and satisfaction one-year post stroke. Disability and Rehabilitation, 29, 559–566. http://dx.doi.org/10.1080/09638280600924996 [DOI] [PubMed] [Google Scholar]
- Hayward K. S., Eng J. J., Boyd L. A., Lakhani B., Bernhardt J., & Lang C. E. (2015). Exploring the role of accelerometers in the measurement of real-world upper limb use after stroke. Brain Impairment. Advance online publication. http://dx.doi.org/10.1017/BrImp.2015.21 [Google Scholar]
- Lang C. E., Bland M. D., Bailey R. R., Schaefer S. Y., & Birkenmeier R. L. (2013). Assessment of upper extremity impairment, function, and activity after stroke: Foundations for clinical decision making. Journal of Hand Therapy, 26, 104–114, quiz 115. http://dx.doi.org/10.1016/j.jht.2012.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C. E., Edwards D. F., Birkenmeier R. L., & Dromerick A. W. (2008). Estimating minimal clinically important differences of upper-extremity measures early after stroke. Archives of Physical Medicine and Rehabilitation, 89, 1693–1700. http://dx.doi.org/10.1016/j.apmr.2008.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C. E., Macdonald J. R., Reisman D. S., Boyd L., Jacobson Kimberley T., Schindler-Ivens S. M., . . . Scheets P. L. (2009). Observation of amounts of movement practice provided during stroke rehabilitation. Archives of Physical Medicine and Rehabilitation, 90, 1692–1698. http://dx.doi.org/10.1016/j.apmr.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C. E., Wagner J. M., Edwards D. F., & Dromerick A. W. (2007). Upper extremity use in people with hemiparesis in the first few weeks after stroke. Journal of Neurologic Physical Therapy, 31, 56–63. http://dx.doi.org/10.1097/NPT.0b013e31806748bd [DOI] [PubMed] [Google Scholar]
- Lin J. H., Hsu M. J., Sheu C. F., Wu T. S., Lin R. T., Chen C. H., & Hsieh C. L. (2009). Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Physical Therapy, 89, 840–850. http://dx.doi.org/10.2522/ptj.20080285 [DOI] [PubMed] [Google Scholar]
- Lyle R. C. (1981). A performance test for assessment of upper limb function in physical rehabilitation treatment and research. International Journal of Rehabilitation Research/Internationale Zeitschrift fur Rehabilitationsforschung/Revue Internationale de Recherches de Readaptation, 4, 483–492. http://dx.doi.org/10.1097/00004356-198112000-00001 [DOI] [PubMed] [Google Scholar]
- Michielsen M. E., Selles R. W., Stam H. J., Ribbers G. M., & Bussmann J. B. (2012). Quantifying nonuse in chronic stroke patients: A study into paretic, nonparetic, and bimanual upper-limb use in daily life. Archives of Physical Medicine and Rehabilitation, 93, 1975–1981. http://dx.doi.org/10.1016/j.apmr.2012.03.016 [DOI] [PubMed] [Google Scholar]
- Nakayama H., Jørgensen H. S., Raaschou H. O., & Olsen T. S. (1994). Recovery of upper extremity function in stroke patients: The Copenhagen Stroke Study. Archives of Physical Medicine and Rehabilitation, 75, 394–398. http://dx.doi.org/10.1016/0003-9993(94)90161-9 [DOI] [PubMed] [Google Scholar]
- Page S. J., Levine P., & Leonard A. C. (2005). Modified constraint-induced therapy in acute stroke: A randomized controlled pilot study. Neurorehabilitation and Neural Repair, 19, 27–32. http://dx.doi.org/10.1177/1545968304272701 [DOI] [PubMed] [Google Scholar]
- Page S. J., Levine P., & Leonard A. (2007). Mental practice in chronic stroke: Results of a randomized, placebo-controlled trial. Stroke, 38, 1293–1297. http://dx.doi.org/10.1161/01.STR.0000260205.67348.2b [DOI] [PubMed] [Google Scholar]
- Page S. J., Levine P., Sisto S., & Johnston M. V. (2001). A randomized efficacy and feasibility study of imagery in acute stroke. Clinical Rehabilitation, 15, 233–240. http://dx.doi.org/10.1191/026921501672063235 [DOI] [PubMed] [Google Scholar]
- Platz T., Pinkowski C., van Wijck F., Kim I. H., di Bella P., & Johnson G. (2005). Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: A multicentre study. Clinical Rehabilitation, 19, 404–411. http://dx.doi.org/10.1191/0269215505cr832oa [DOI] [PubMed] [Google Scholar]
- Pollock N. (1993). Client-centered assessment. American Journal of Occupational Therapy, 47, 298–301. http://dx.doi.org/10.5014/ajot.47.4.298 [DOI] [PubMed] [Google Scholar]
- Rand D., & Eng J. J. (2012). Disparity between functional recovery and daily use of the upper and lower extremities during subacute stroke rehabilitation. Neurorehabilitation and Neural Repair, 26, 76–84. http://dx.doi.org/10.1177/1545968311408918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand D., Eng J. J., Tang P. F., Jeng J. S., & Hung C. (2009). How active are people with stroke? Use of accelerometers to assess physical activity. Stroke, 40, 163–168. http://dx.doi.org/10.1161/STROKEAHA.108.523621 [DOI] [PubMed] [Google Scholar]
- Tatemichi T. K., Desmond D. W., Stern Y., Paik M., Sano M., & Bagiella E. (1994). Cognitive impairment after stroke: Frequency, patterns, and relationship to functional abilities. Journal of Neurology, Neurosurgery, and Psychiatry, 57, 202–207. http://dx.doi.org/10.1136/jnnp.57.2.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E., Uswatte G., King D. K., Morris D., Crago J. E., & Chatterjee A. (2006). A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke, 37, 1045–1049. http://dx.doi.org/10.1161/01.STR.0000206463.66461.97 [DOI] [PubMed] [Google Scholar]
- Taub E., Uswatte G., Mark V. W., Morris D. M., Barman J., Bowman M. H., . . . Bishop-McKay S. (2013). Method for enhancing real-world use of a more affected arm in chronic stroke: Transfer package of constraint-induced movement therapy. Stroke, 44, 1383–1388. http://dx.doi.org/10.1161/STROKEAHA.111.000559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbin M. A., Bailey R. R., & Lang C. E. (2015). Validity of body-worn sensor acceleration metrics to index upper extremity function in hemiparetic stroke. Journal of Neurologic Physical Therapy, 39, 111–118. http://dx.doi.org/10.1097/NPT.0000000000000085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbin M. A., Waddell K. J., & Lang C. E. (2015). Acceleration metrics are responsive to change in upper extremity function of stroke survivors. Archives of Physical Medicine and Rehabilitation, 96, 854–861. http://dx.doi.org/10.1016/j.apmr.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uswatte G., Foo W. L., Olmstead H., Lopez K., Holand A., & Simms L. B. (2005). Ambulatory monitoring of arm movement using accelerometry: An objective measure of upper-extremity rehabilitation in persons with chronic stroke. Archives of Physical Medicine and Rehabilitation, 86, 1498–1501. http://dx.doi.org/10.1016/j.apmr.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Uswatte G., Giuliani C., Winstein C., Zeringue A., Hobbs L., & Wolf S. L. (2006). Validity of accelerometry for monitoring real-world arm activity in patients with subacute stroke: Evidence from the extremity constraint-induced therapy evaluation trial. Archives of Physical Medicine and Rehabilitation, 87, 1340–1345. http://dx.doi.org/10.1016/j.apmr.2006.06.006 [DOI] [PubMed] [Google Scholar]
- Uswatte G., Taub E., Morris D., Vignolo M., & McCulloch K. (2005). Reliability and validity of the upper-extremity Motor Activity Log–14 for measuring real-world arm use. Stroke, 36, 2493–2496. http://dx.doi.org/10.1161/01.STR.0000185928.90848.2e [DOI] [PubMed] [Google Scholar]
- van der Lee J. H., Beckerman H., Knol D. L., de Vet H. C., & Bouter L. M. (2004). Clinimetric properties of the Motor Activity Log for the assessment of arm use in hemiparetic patients. Stroke, 35, 1410–1414. http://dx.doi.org/10.1161/01.STR.0000126900.24964.7e [DOI] [PubMed] [Google Scholar]
- van der Lee J. H., Beckerman H., Lankhorst G. J., & Bouter L. M. (2001). The responsiveness of the Action Research Arm Test and the Fugl-Meyer Assessment scale in chronic stroke patients. Journal of Rehabilitation Medicine, 33, 110–113. http://dx.doi.org/10.1080/165019701750165916 [DOI] [PubMed] [Google Scholar]
- Waddell K. J., Birkenmeier R. L., Bland M. D., & Lang C. E. (2015). An exploratory analysis of the self-reported goals of individuals with chronic upper-extremity paresis following stroke. Disability and Rehabilitation. Advance online publication. http://dx.doi.org/10.3109/09638288.2015.1062926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. R., Wolfe C. D., Warburton F. G., Rudd A. G., Howard R. S., Ross-Russell R. W., & Beech R. R. (1997). A long-term follow-up of stroke patients. Stroke, 28, 507–512. http://dx.doi.org/10.1161/01.STR.28.3.507 [DOI] [PubMed] [Google Scholar]
- Wolf S. L., Catlin P. A., Ellis M., Archer A. L., Morgan B., & Piacentino A. (2001). Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke, 32, 1635–1639. http://dx.doi.org/10.1161/01.STR.32.7.1635http://dx.doi.org/%20 [DOI] [PubMed] [Google Scholar]
- Wolf S. L., Winstein C. J., Miller J. P., Taub E., Uswatte G., Morris D., . . . Nichols-Larsen D.; EXCITE Investigators. (2006). Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. JAMA, 296, 2095–2104. http://dx.doi.org/10.1001/jama.296.17.2095 [DOI] [PubMed] [Google Scholar]
- Young N. L., Williams J. I., Yoshida K. K., Bombardier C., & Wright J. G. (1996). The context of measuring disability: Does it matter whether capability or performance is measured? Journal of Clinical Epidemiology, 49, 1097–1101. http://dx.doi.org/10.1016/0895-4356(96)00214-4 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (1980). International classification of diseases, ninth revision. Geneva: Author.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.