Abstract
Objectives:
To review the evidence on the association between age and limited health literacy, overall and by health literacy test, and to investigate the mediating role of cognitive function.
Method:
The Embase, MEDLINE, and PsycINFO databases were searched. Eligible studies were conducted in any country or language, included participants aged ≥50 years, presented a measure of association between age and health literacy, and were published through September 2013.
Results:
Seventy analyses in 60 studies were included in the systematic review; 29 of these were included in the meta-analysis. Older age was strongly associated with limited health literacy in analyses that measured health literacy as reading comprehension, reasoning, and numeracy skills (random-effects odds ratio [OR] = 4.20; 95% confidence interval [CI]: 3.13–5.64). By contrast, older age was weakly associated with limited health literacy in studies that measured health literacy as medical vocabulary (random-effects OR = 1.19; 95% CI: 1.03–1.37). Evidence on the mediating role of cognitive function was limited.
Discussion:
Health literacy tests that utilize a range of fluid cognitive abilities and mirror everyday health tasks frequently observe skill limitations among older adults. Vocabulary-based health literacy skills appear more stable with age. Researchers should select measurement tests wisely when assessing health literacy of older adults.
Key Words: Adults, Aging, Cognition, Health literacy, Measurement
Limited functional health literacy among adults is a major public health problem. Functional health literacy is defined as an individual’s capacity to obtain, process, and understand basic health information and services sufficiently to make appropriate health decisions and will be used interchangeably with the term “health literacy” in this review (Institute of Medicine, 2004). Limited health literacy is of particular concern among older adults, who often have increased needs for health information and services to maintain their health and well-being. National literacy surveys indicate that more than 70% of adults aged older than 65 years in North America lack the basic health literacy skills required for successful interactions with health systems (Canadian Council on Learning, 2008; Kutner, Greenberg, Jin, & Paulsen, 2006). Outcomes of limited health literacy among older adults include incorrect taking of prescription medication, poor chronic disease management, low use of preventive health services, and increased risk of overall mortality (Berkman, Sheridan, Donahue, Halpern, & Crotty, 2011; Sudore et al., 2006b).
The nature of the association between aging and health literacy is unclear. Functional health literacy skills reflect, at least in part, the cognitive abilities used to manage health (Federman, Sano, Wolf, Siu, & Halm, 2009; Reeve & Basalik, 2014; Wolf et al., 2012). “Fluid” cognitive abilities such as verbal fluency, working memory, and reasoning are essential to health literacy skills and undergo mild decline during aging in the absence of dementia as early as mid-adulthood, whereas “crystallized” abilities such as generalized knowledge and vocabulary are more stable with age (O’Carroll, 1995; Singh-Manoux et al., 2012). Therefore, performance on health literacy tests that require the use of fluid cognitive abilities in the context of medical and health-related information may decline with age (e.g., the Test of Functional Health Literacy in Adults [TOFHLA] or the Newest Vital Sign [NVS]; Parker, Baker, Williams, & Nurss, 1995; Weiss et al., 2005), whereas tests that assess health literacy as medical vocabulary may show little decline in performance with age (e.g., the Rapid Estimate of Adult Literacy in Medicine [REALM]; Davis et al., 1993).
If health literacy skills represent the functional use of cognitive abilities in health contexts, then certain health literacy skill sets, but not necessarily others, would be expected to decline with age. Furthermore, any association between age and health literacy would be expected to be at least partly explained by cognitive aging. We hypothesized that the functional health literacy skills assessed by TOFHLA and similar tests (representing fluid cognitive abilities) would be more likely to show an inverse association with age than health literacy skills assessed by the REALM and similar tests (representing crystallized cognitive abilities). We aimed to review the evidence on the association between age and health literacy, overall and by health literacy test, and to investigate the mediating role of cognitive function.
Method
Identification of Studies
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement recommendations (Moher, Liberati, Tetzlaff, & Altman, 2009). The search strategy was developed by two reviewers following instruction from University College London librarians. The Embase, MEDLINE, and PsycINFO databases were used to search for relevant articles conducted in any country or language and published in English through September 30, 2013 (see the Supplementary Table 1 for the complete electronic search strategy). Studies were included in the narrative synthesis if health literacy was measured using an objective instrument (the TOFHLA, REALM, or NVS; see Table 1) and if a measure of association between age and health literacy was presented with an associated statistical significance level. Studies were excluded if the study population did not include adults aged ≥50 years or if the study population entirely comprised individuals with diagnosed cognitive or mental health impairments, as these studies would be restricted in participant variance of cognitive ability. Studies were included in the meta-analysis if odds ratio (OR) and 95% confidence interval (CI) for limited health literacy by age (≥65 vs <65 years; if this comparison was not available, then similar age cutoffs were acceptable) were computable from the presented results.
Table 1.
Characteristics of the Health Literacy (HL) Tests: The “Test of Functional Health Literacy in Adults” (TOFHLA), the “Newest Vital Sign” (NVS), and the “Rapid Estimate of Adult Literacy in Medicine” (REALM)
| Test | Year | Measure | Skills assessed | Scoring | Short form | Adaptations |
|---|---|---|---|---|---|---|
| TOFHLA | 1995 | Common medical materials (e.g., prescription labels) followed by comprehension questions using the Cloze procedure—a technique that omits every 5–7 words in a sentence 22min to administer |
Reading comprehension (50 items) Numeracy (17 items) |
0–59: inadequate HL 60–74: marginal HL 75–100: adequate HL <75: limited HL |
S-TOFHLA | UK-TOFHLA translations: Korean Serbian French Italian German Portuguese |
| NVS | 2005 | A 6-item test based on the ability to read and apply information from an ice cream nutrition label 3min to administer |
Reading comprehension Numeracy |
<2: greater than a 50% chance of having marginal or inadequate HL 2–4: possibility of limited HL >4: adequate HL |
Translation: Turkish |
|
| REALM | 1991 | 66 medical words ranging from “fat,” “flu”, and “pill” to “obesity,” “osteoporosis,” and “impetigo,” which the participant is instructed to read out loud 2–3min to administer |
Word recognition Pronunciation |
Reading level according to score: 0–18: ≤3rd grade 19–44: 4–6th grade 45–60: 7–8th grade 61–66: ≥9th grade <61: limited HL |
REALM-SF REALM-R |
Translation: Turkish |
Article Screening and Data Abstraction
Returned article titles and abstracts were screened and those that did not meet the exclusion criteria were downloaded in full text. The methods and results sections of the downloaded articles were then screened for final inclusion. When multiple articles on the same study population were eligible, either the article that was published first or the one that presented a multivariable-adjusted measure of association was selected. Reference lists of included articles were hand-searched for additional references. One reviewer (L. C. Kobayashi) performed the initial search, screening, and data extraction. The second (C. von Wagner) checked all included articles and extracted data. The two authors were in 100% agreement over the articles included and data extracted. The data items extracted were: (a) study design, country, and language of conduct, source of the study population, inclusion and exclusion criteria, sample size, and participation rates; (b) age statistics of the study population, including mean, median, and range; (c) the health literacy instrument used and health literacy score/level of the study population by age in categories defined by the study authors, if given; and (d) the measure of association between health literacy and age with corresponding p values, the statistical test(s) used, and confounding variables adjusted for, including cognitive function (if applicable).
We followed the recommendations in the Cochrane handbook to develop risk criteria based on existing guidelines (The Cochrane Collaboration, 2011; von Elm et al., 2007; Wells et al., n.d.): (a) study designs – with prospective studies ranked as having lower risk of bias; (b) participation rate – those with higher participation rates ranked as having lower risk of bias; and (c) adjustment for confounding. All eligible analyses were cross-sectional and only half reported participation rates; we therefore categorized risk of bias according to the third criterion only.
Statistical Analysis
As a scoping summary to aid the narrative review, the percentage of studies detecting a statistically significant association between age and health literacy was calculated for all studies combined by health literacy test, by participation rate, and by whether they reported an adjusted measure of association. For the meta-analysis, the outcome was a pooled OR and 95% CI for the association between age and limited health literacy. The age cutoff of 65 years was chosen as it is useful in terms of policy purposes (e.g., it is the age of retirement in several Western countries) and it was a common cutoff used in studies in an early literature scan. “Limited” health literacy was the outcome (Table 1). It represents a clinically significant cut point where individuals begin to have difficulty with everyday health tasks and where the risks of several adverse health outcomes begin to increase (Davis et al., 2006; Lee, Arozullah, Cho, Crittenden, & Vicencio, 2009; Peterson, Dwyer, Mulvaney, Dietrich, & Rothman, 2007; Scott, Gazmararian, Williams, & Baker, 2002; Sudore et al., 2006a).
Standardized effect size measures (e.g., Cohen’s d or Hedge’s g) were not used for this analysis. Meta-analytic techniques pooling these measures would assume that between-study variations in the standard deviations for mean health literacy scores are due to scale differences, rather than to any true variability in health literacy test performance between study populations (Greenland & O’Rourke, 2008; The Cochrane Collaboration, 2011). This assumption cannot be made in the context of our meta-analysis, given that the varying sociodemographic compositions of individual study populations including varying age ranges, countries, and languages would likely give rise to variability in health literacy performance between study populations.
When effect estimates were not reported as ORs for limited health literacy, but data were sufficient to compute these values (i.e., in cross-tabular format), they were transformed into ORs using Comprehensive Meta-Analysis (CMA) software (Version 2.2.064). As raw ORs cannot be meaningfully aggregated, all ORs were transformed to the natural log (lnOR) for analyses, then transformed back to OR and 95% CI for interpretation. In cases where ORs for limited health literacy could not be computed, study authors were contacted to retrieve the data in an appropriate format for data synthesis. Studies reporting mean age by categories of health literacy score (n = 20) were excluded from the meta-analysis as these studies treat age as the dependent variable and thus cannot produce an OR predicting health literacy. These studies were summarized narratively.
The meta-analysis was performed using fixed- and random-effects models. The random-effects model is likely to be more valid, as the true association between age and health literacy cannot be assumed equal between studies for the same reason that variability in health literacy performance across study populations must be assumed. Heterogeneity in the fixed-effects models was assessed using the Q value and Higgins and Thompson’s I 2 statistics. The Q value tests whether the observed variance in effects is not greater than that would be generated by sampling error; a Q value with a corresponding p < .05 indicates the presence of heterogeneity and that a random-effects model is appropriate. The I 2 statistic is an estimate of the proportion of total variation in study estimates due to heterogeneity (The Cochrane Collaboration, 2011). Fixed-effects models with corresponding heterogeneity tests were ran first, followed by random-effects models. The meta-analysis was performed for all studies together and stratified by health literacy test, to test our hypothesis that study results would differ by test.
A sensitivity analysis removing one study at a time from the pooled analysis examined for influential individual studies on the overall pooled result. This technique allows for identification of particular aspects of an individual study that may skew the overall combined result. A second sensitivity analysis was performed, removing from the meta-analysis all studies using age cutoffs other than age 65. A random-effects meta-regression was performed to assess the extent to which heterogeneity in the pooled result can be related to each of the following individual study characteristics: health literacy test, health status of study population, country of study, language of study, and participation rate. Publication bias was assessed using the classic fail-safe N method, which is the theoretical number of unpublished studies with a null result that would be required to render the calculated pooled result null. A funnel plot of standard error by lnOR was also generated, and Duval and Tweedie’s “trim and fill” method used to estimate the number of studies missing due to publication bias. This method provides an imputed estimate of the pooled effect size after publication bias is taken into account (Greenland & O’Rourke, 2008).
Statistical analyses were conducted using CMA (Biostat, Englewood, NJ) and StataSE 13.1 (StataCorp, College Station, TX).
Results
Search Results
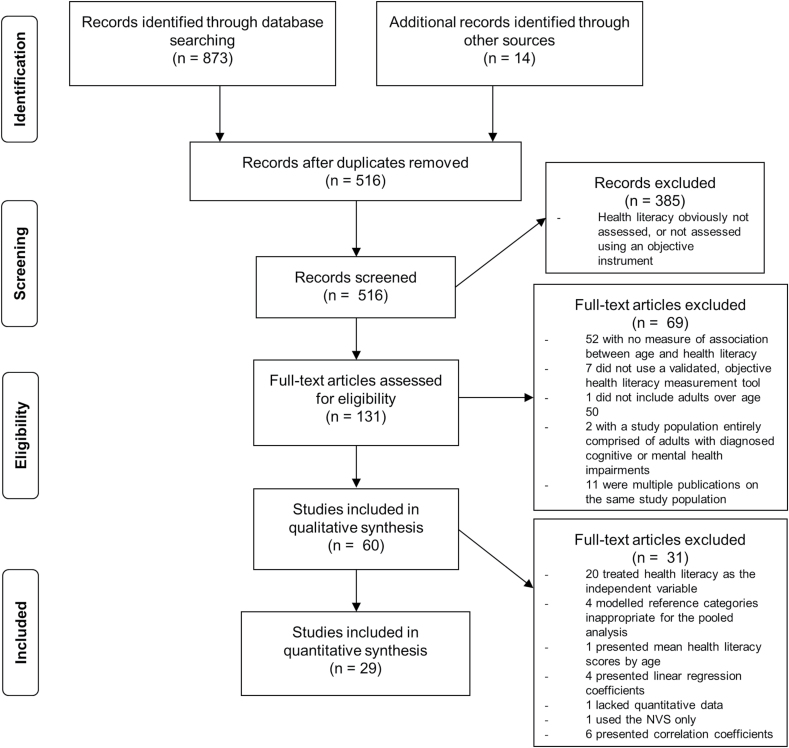
A total of 70 analyses in 60 studies with data on 33,379 participants were included in the narrative review. A total of 29 analyses with data on 18,492 participants were included in the meta-analysis (Figure 1; Supplementary Table 2).
Figure 1.
PRISMA flow diagram.
Overview of Included Studies
Countries of origin.
Studies were conducted in nine different countries and languages (Supplementary Table 2). The predominant country of study was the United States and the predominant language English. All manuscripts were written in English.
Study designs and populations.
Although study designs varied, age and health literacy were analyzed cross-sectionally in all studies. Study populations were healthy, community-dwelling adults (11/60; 18%) (Adams et al., 2009; Connor et al., 2013; Federman et al., 2009; Gazmararian et al., 1999; Jackson & Eckert, 2008; Kim, 2009; McDougall et al., 2012; Roth & Ivey, 2005; Stewart et al., 2013; Sudore et al., 2006a; von Wagner et al., 2007), community-dwelling outpatients recruited in health care settings (18/60; 30%) (Aguirre, Ebrahim, & Shea, 2005; Backes & Kuo, 2012; Bains & Egede, 2011; Carthery-Goulart et al., 2009; Chew et al., 2004; Davis et al., 2006; Ferguson, Lowman, & DeWalt, 2011; Haun, Luther, & Dodd, 2012; Jovic-Vranes et al., 2009, 2011; Jovic-Vranes & Bjegovic-Mikanovic, 2012; Lindau et al., 2002; Miller et al., 2007; Mosher et al., 2012; Ozdemir, Alper, Uncu, & Bilgel, 2010; Peterson et al., 2007; Shah, West, Bremmeyr, & Savoy-Moore, 2010; Shea, Beers, McDonald, Quistberg, Ravenell, & Asch, 2004), chronic disease patients (23/60; 38%) (Armistead-Jehle et al., 2010; Cavanaugh et al., 2010; Colbert, Sereika, & Erlen, 2013; Cox, Bowmer, & Ring, 2011; Gordon et al., 2002; Green et al., 2011; Ibrahim et al., 2008; Juzych et al., 2008; Kalichman et al., 2000; Kirk et al., 2012; Laramee et al., 2007; Levinthal et al., 2008; Mancuso & Rincon, 2006; Mbaezue et al., 2010; Morrow et al., 2006; Nokes et al., 2007; Osborn et al., 2010; Robinson et al., 2011; Rowlands et al., 2013; Schillinger et al., 2002; Swearingen et al., 2010; Williams, Baker, Parker, & Nurss, 1998; Zhang, Li, Fong, & Thumboo, 2009), emergency department/acute care inpatients (5/60; 8%); Baker et al., 1998; Ginde et al., 2008; McNaughton et al., 2011; Morris et al., 2011; Olives et al., 2011, and a refugee population (1/60; 2%; Supplementary Table 2). Two studies had samples comprising hospital inpatients and outpatients (Downey & Zun, 2008; Walker, Pepa, & Gerard, 2010).
All studies used “convenience” samples except for two that aimed to recruit samples representative of the general population (Adams et al., 2009; von Wagner, Knight, Steptoe, & Wardle, 2007). Studies sampling from emergency room patients and acute care hospital inpatients excluded those who were too ill or distressed to participate. Half reported participation rates (median reported rate = 87%; range: 26%–98%). Sample sizes ranged from 44 to 3,260; eight studies had <100 participants.
Health literacy measurements.
Thirty-six studies (60%) assessed health literacy using the TOFHLA or S-TOFHLA in the original or a translated or culturally adapted version (Supplementary Table 2). Two of these (Aguirre et al., 2005; Connor, Mantwill, & Schulz, 2013) stratified their study populations by ethnicity and language (Aguirre et al., 2005) or by language (Connor et al., 2013) to give three analyses each, for a total of 40 analyses using the TOFHLA or S-TOFHLA. Twenty-six studies (43%) assessed health literacy using the REALM or one of its short forms (Supplementary Table 2). Four studies used the NVS (Adams et al., 2009; Kirk et al., 2012; Ozdemir et al., 2010; Shah et al., 2010). Three used both the TOFHLA and the REALM (Haun et al., 2012; McNaughton, Wallston, Rothman, Marcovitz, & Storrow, 2011; Walker et al., 2010), one used the REALM and the NVS (Ozdemir et al., 2010), and one used all three instruments (Kirk et al., 2012). Therefore, a total of 70 analyses were performed in the 60 studies included in the narrative review. The three separate analyses in Connor et al.’s (2013) study were collapsed by the authors for the meta-analysis, to give a total of 29 analyses in 31 studies in the meta-analysis.
Overview of Study Results
Narrative review.
Overall, 41/70 analyses (59%) observed a statistically significant inverse association between age and health literacy. This association was more frequently observed in analyses using the S-TOFHLA/TOFHLA (32/40; 80%) and NVS (3/4; 75%) than in those using the REALM (6/26; 23%); a statistically significant difference (χ2(2) = 21.51, p < .001). Twenty-two out of 28 (79%) analyses presenting a multivariable-adjusted measure of association between age and health literacy compared with 19/42 (45%) analyses presenting an unadjusted association observed a statistically significant inverse relationship (χ2(1) = 7.69, p = .006). This finding may be in part a methodological artifact, as several studies that observed a nonsignificant result in unadjusted analysis did not include age in multivariable modeling. The likelihood of observing a statistically significant result did not differ by participation rate (Kruskal–Wallis χ2(1) = 0.067, p = .80) or by whether a participation rate was reported (χ2(1) = 0.11, p = .74). Among studies that compared mean age across health literacy score categories, 6/8 (75%) that used the TOFHLA or S-TOFHLA observed that adults in lower health literacy categories had a higher mean age, compared with 3/12 (25%) studies that used the REALM (χ2(1) = 4.85, p = .028).
Meta-analysis.
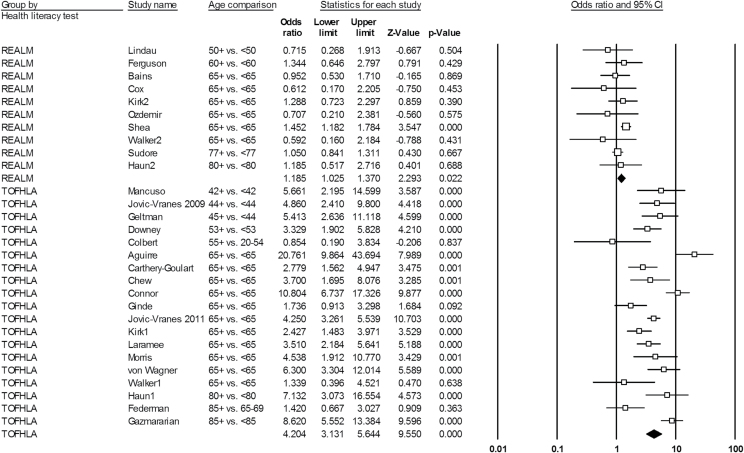
The meta-analysis of 29 individual analyses gave an overall fixed-effects OR of 2.33 (95% CI: 2.12–2.56) for the association between older age and limited health literacy. The Q value was 274.68 (df = 28; p < .0001) and I 2 statistic was 89.81, indicating that significant heterogeneity within the fixed-effects results and that results from the random-effects model (OR = 2.56; 95% CI: 1.85–3.53) are appropriate for interpretation. Within studies using the S-TOFHLA/TOFHLA, the fixed-effects OR was 4.44 (95% CI: 3.89–5.06). The Q value was 77.70 (df = 18; p < .0001) and I 2 statistic was 76.83, indicating significant heterogeneity and that results from the random-effects model (OR = 4.20; 95% CI: 3.13–5.64) are again appropriate for interpretation. Within studies using the REALM, the fixed-effects OR was 1.20 (95% CI: 1.05–1.37), with a Q value of 9.40 (df = 9; p = .40) and I 2 statistic of 4.26, indicating that heterogeneity may not be important. The random-effects OR was 1.19 (95% CI: 1.03–1.37). In this instance, the fixed- and random-effects ORs were negligibly different; we select the random-effects OR for interpretation to be conservative and consistent with reporting. Figure 2 shows a forest plot and individual study statistics for the random-effects meta-analyses.
Figure 2.
Forest plot of random-effects pooled odds ratios for the association between older age and limited health literacy, stratified by health literacy test.
A sensitivity analysis removing one study at a time showed that no individual study exerted significant influence over the pooled result. The second sensitivity analysis removing all studies using age cutoffs other than age 65 showed similar results to the main analysis. In this analysis, the random-effects OR for limited health literacy was 4.23 (95% CI: 2.86–6.27) within studies using the S-TOFHLA/TOFHLA and was 1.31 (95% CI: 1.10–1.57) within studies using the REALM.
Meta-regression.
A random-effects meta-regression model showed that health literacy test was influential on the pooled estimate (Table 2). Studies using the S-TOFHLA/TOFHLA had, on average, an OR of 4.44 (95% CI: 1.75–5.53) higher than that of studies using the REALM to assess health literacy. The health status of study populations, whether socioeconomic status and/or cognitive impairment was adjusted for, country of study, language of study, and participation rate were not modifiers of the relationship between age and health literacy (Table 2). The τ2 statistic, which indicates the amount of residual between-study variance after accounting for these variables, was 0.22.
Table 2.
Random-Effects Meta-Regression for Influence of Study Characteristics on Pooled Result
| Study characteristic | Coefficient | Standard error | 95% CI | p Value |
|---|---|---|---|---|
| Health literacy test | ||||
| REALM | — | — | — | — |
| TOFHLA/S-TOFHLA | 4.44 | 1.34 | 1.75, 5.53 | <.0001 |
| Health status of study population | ||||
| Healthy, community dwelling | — | — | — | — |
| Chronic disease patients | 0.57 | 1.40 | 0.29, 1.13 | .11 |
| Community-dwelling outpatients | 0.92 | 1.32 | 0.53, 1.60 | .77 |
| Acute care patients | 0.46 | 1.54 | 0.20, 1.06 | .07 |
| Adjusted for socioeconomic or cognitive factors | ||||
| No | — | — | — | — |
| Yes | 1.13 | 0.79 | 0.64, 1.99 | .68 |
| Country of study | ||||
| United States | — | — | — | — |
| Other | 0.92 | 1.34 | 0.52, 1.65 | .84 |
| Language of study | ||||
| English | — | — | — | — |
| Other | 0.94 | 1.34 | 0.53, 1.67 | .84 |
| Participation rate reported | ||||
| No | — | — | — | — |
| Yes | 1.21 | 1.26 | 0.76, 1.92 | .42 |
Notes. CI = confidence interval; REALM = Rapid Estimate of Adult Literacy in Medicine; TOFHLA = Test of Functional Health Literacy in Adults. Regression coefficients indicate the estimated change in odds ratio for the variable category contrasted with the reference group, indicated by the dashed cells.
Publication bias.
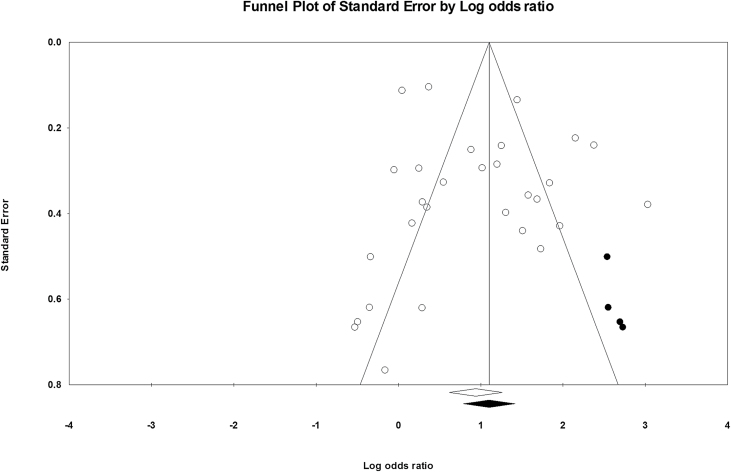
The classic fail-safe N was 2,080, indicating that this number of theoretically unpublished studies with null results would have to exist in order to attenuate the overall pooled effect estimate to the null. The funnel plot showed reasonable symmetry, although studies with larger lnOR values tended to have larger standard errors. Duval and Tweedle’s “fill and trim” method imputed four additional studies to produce symmetry in the graph (Figure 3). The imputed overall pooled random-effects OR that takes publication bias into account was 3.01 (95% CI: 2.19–4.13). This imputed OR is more extreme but not significantly different to the original overall random-effects OR of 2.56 (95% CI: 1.85–3.53).
Figure 3.
Funnel plot of standard error by log odds ratio to assess publication bias, with studies imputed using the “trim and fill” method shown in black.
The mediating role of cognition.
Only five studies adjusted for cognitive function when assessing the relationship between age and health literacy (Armistead-Jehle, Cifu, Wetzel, Carne, & Klanchar, 2010; Chew, Bradley, Flum, Cornia, & Koepsell, 2004; Gazmararian et al., 1999; Levinthal, Morrow, Tu, Wu, & Murray, 2008; Morrow et al., 2006). Two of these studies adjusted for the fluid cognitive abilities of speech comprehension, processing speed, working memory, and listening span, along with visual and auditory function (Levinthal et al., 2008; Morrow et al., 2006). Data from these studies were not available for inclusion in the meta-analysis but provide important insights into the influence of cognitive function on health literacy skills during aging. Levinthal and colleagues (2008) observed that age was no longer significantly associated with health literacy after accounting for cognitive and sensory variables in their linear regression model predicting S-TOFHLA score. By contrast, Morrow and colleagues (2006) observed that age differences in health literacy were explained by educational differences within their sample and not by cognitive or sensory ability in their regression model predicting S-TOFHLA score. However, each cognitive ability assessed was a strong predictor of S-TOFHLA score, regardless of age (βs ranged from 0.39 to 2.08, all with p < .001). The three other studies adjusted for age-related cognitive impairment as measured by the Mini-Mental State Examination (MMSE) and Mini-Cog but did not observe complete attenuation of the association between age and TOFHLA-assessed health literacy (Armistead-Jehle et al., 2010; Chew et al., 2004; Gazmararian et al., 1999). One study observed some attenuation of the age–health literacy association from OR = 5.9 (95% CI: 2.8–12.5) to OR = 3.7 (95% CI: 1.7–8.1) after adjustment for cognitive impairment, educational attainment, and employment status (Chew et al., 2004). The degree of attenuation of the age–health literacy relationship by cognitive impairment in the other two studies is unascertainable, as neither presented the crude measures of association prior to adjustment for cognitive impairment.
Discussion
Our findings are consistent with a 2005 review on the prevalence of limited health literacy in the United States, which observed older adults to be more likely to have limited health literacy (Paasche-Orlow, Parker, Gazmararian, Nielsen-Bohlman, & Rudd, 2005). In our review, older age was strongly associated with having limited health literacy in studies that assessed health literacy as reading comprehension, reasoning, and numeracy skills, using the TOFHLA or S-TOFHLA. Older age was weakly associated with limited health literacy in studies that assessed health literacy as medical vocabulary, using the REALM. These findings suggest that aging-related health literacy decline primarily occurs with skills requiring fluid, rather than crystallized cognitive abilities. However, evidence on the cognitive processes that may explain health literacy decline was limited, with one study but not another indicating that fluid cognitive abilities explain age differences in S-TOFHLA scores.
The Role of Cognition
The role of cognition in the apparent aging-related health literacy decline is not yet well understood. Although MMSE score and S-TOFHLA-assessed health literacy have been strongly positively associated among older adults, independent of age (Baker et al., 2002; Dahlke, Curtis, Federman, & Wolf, 2014; Federman et al., 2009), age-related cognitive impairment according to the MMSE did not explain why health literacy tended to decrease with increasing age in the few studies reviewed here. It may be that milder degrees of cognitive impairment than those detectable by the MMSE affect functional health literacy skills.
The two studies that assessed the mediating roles of fluid cognitive and sensory abilities in the relationship between age and health literacy showed conflicting results (Levinthal et al., 2008; Morrow et al., 2006). However, based on the bodies of evidence showing strong relationships between fluid cognitive ability and functional health literacy (Murray, Johnson, Wolf, & Deary, 2011; Reeve & Basalik, 2014; Wolf et al., 2012), and longitudinal declines in fluid cognitive ability during aging (O’Carroll, 1995; Salthouse, 2009; Singh-Manoux et al., 2012), it seems probable that cognitive aging plays a role in aging-related functional health literacy decline. It may also be that other factors related to cognition such as cognitive reserve also affect health literacy skills during aging. Practices that can help to improve or maintain cognition during aging such as social engagement and physical activity may help with the maintenance of health literacy skills (Sofi et al., 2011; Thomas, 2011). Longitudinal research is needed to determine the extent to which cognitive aging may explain health literacy decline, in addition to other likely processes.
Limitations
All analyses were cross-sectional and therefore could not assess the temporality of the association between aging and health literacy. The majority of studies were judged to be of a higher risk of bias, as most studies in the meta-analysis did not adjust for potential confounders. Selection bias may be present in individual studies if the reasons for nonresponse are related to age or health literacy, although the degree to which any cumulative selection bias across studies has influenced the results of this review is difficult to ascertain. Several studies excluded adults with cognitive impairment, which limited our ability to assess the role of cognitive impairment in age-related health literacy differences. Studies that analyzed health literacy score using continuous measures of association could not be included. Standardized mean difference statistics that would be used to pool continuous effect measures assume that variability in health literacy scores is due to scale differences, rather than due to true variation in health literacy across study populations, an assumption that is unlikely to be true. In these instances, study authors were contacted to obtain the data in a format useable for the purpose of this meta-analysis, with reasonable success (response from 8/16 authors). We were limited to studies published in English, although the included studies were conducted in nine different countries and languages. Finally, few studies investigated the role of cognitive abilities in age-related health literacy decline, which prevented us from drawing firm conclusions about this complex relationship. However, this meta-analysis may provide as guidance for future research into this emerging area of inquiry.
Strengths
This review adhered to the PRISMA guidelines for systematic review reporting (Moher et al., 2009) and closely followed guidelines in the Cochrane Handbook (The Cochrane Collaboration, 2011). It is the first quantitative synthesis of data on aging and health literacy that we are aware of. We excluded studies solely comprising individuals with diagnosed mental health and cognitive impairments. However, studies that included some adults with cognitive impairments were eligible, as we aimed to capture variability in cognitive ability in order to assess its mediating role in the age–health literacy relationship. We used the well-defined and clinically relevant outcome of “limited health literacy,” which allowed us to examine not only whether older adults had lower health literacy than younger adults, but also how much more likely they were to be below this threshold. Although the majority of studies were judged to be of a higher risk of bias, our meta-regression analysis did not identify adjustment for socioeconomic factors or cognitive impairment, or low/nonreporting of participation rates as influential factors over the pooled point estimate.
Conclusions
Limited functional health literacy is common among older adults and may lead to disenfranchisement from access to health care services due to limitations in navigation, comprehension, and decision making. However, the theoretical understanding of health literacy and aging has been hampered by the use of instruments assessing a variable range of constructs as “health literacy,” inconsistent measures of cognitive ability across the few studies examining cognitive processes, and a lack of longitudinal research.
Careful methodological decisions must be made in future research. Even though the TOFHLA, NVS, and REALM all assess functional skills, performance on these tests shows differential associations with age, highlighting variability within the research construct of functional health literacy. Scoring levels of these tests require psychometric testing for comparison against one another. A common definition of functional health literacy and a comprehensive instrument for its measurement should be agreed upon by researchers so that evidence in the field may become more comparable. In terms of the basic functional health literacy skills required for management of health and well-being in Western systems, the TOFHLA, S-TOFHLA, and potentially the NVS appear to be sensitive to detecting age-related functional limitations. The REALM is less sensitive in detecting age-related limitations in the broad skills required to manage health. The NVS appears promising but remains less tested in terms of its ability to perform well across age groups. Presently, the most appropriate instrument appears to be the TOFHLA or S-TOFHLA due to their relatively common usage, representativeness of daily health-related tasks, and correlation with a range of fluid cognitive abilities.
Longitudinal research that includes adults at age ranges older than 65 years is required to refine our understanding of the dynamics of health literacy decline during aging and its causal processes, cognitive or otherwise. Although direct evidence was limited, existing research suggests that decline in fluid cognitive ability may play a role in aging-related functional health literacy decline. Future research using the TOFHLA, S-TOFHLA, or similar instruments should determine whether the cross-sectional association between age and health literacy holds up in longitudinal studies; the age(s) at which older adults become at risk of health literacy skill loss; the threshold where cognitive aging may begin to affect functional health literacy skills; and the risk and protective factors that predict health literacy skill changes over time among older adults. In the context of these research questions and others, researchers should carefully select which test is most appropriate for their purposes when assessing the functional health literacy skills of older adults.
Supplementary Material
Supplementary material can be found at: http://psychsocgerontology.oxfordjournals.org/
Funding
This work was supported by a Doctoral Foreign Study Award (DFSA 201012) from the Canadian Institutes of Health Research and an Overseas Research Scholarship from University College London to L. C. Kobayashi and by a Cancer Research UK program grant (C1418/A14134) to J. Wardle. Neither sponsor had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supplementary Material
Acknowledgements
We thank Dr. Benjamin Gardner for statistical advice regarding the meta-analysis.
References
- Adams R. J., Appleton S. L., Hill C. L., Dodd M., Findlay C., Wilson D. H. (2009). Risks associated with low functional health literacy in an Australian population. The Medical Journal of Australia, 191, 530–534. Retrieved from https://www.mja.com.au/journal/2009/191/10/risks-associated-low-functional-health-literacy-australian-population [DOI] [PubMed] [Google Scholar]
- Aguirre A. C., Ebrahim N., Shea J. A. (2005). Performance of the English and Spanish S-TOFHLA among publicly insured Medicaid and Medicare patients. Patient Education and Counseling, 56, 332–339. doi:10.1016/j.pec.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Armistead-Jehle P., Cifu D. X., Wetzel R., Carne W., Klanchar L. A. (2010). Health literacy among patients diagnosed with movement disorders: A pilot study. PM & R, 2, 43–47. doi:10.1016/j.pmrj.2009.05.008 [DOI] [PubMed] [Google Scholar]
- Backes A. C., Kuo G. M. (2012). The association between functional health literacy and patient-reported recall of medications at outpatient pharmacies. Research in Social & Administrative Pharmacy, 8, 349–354. doi:10.1016/j.sapharm.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Bains S. S., Egede L. E. (2011). Association of health literacy with complementary and alternative medicine use: A cross-sectional study in adult primary care patients. BMC Complementary and Alternative Medicine, 11, 138. doi:10.1186/1472-6882-11-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. W., Gazmararian J. A., Sudano J., Patterson M., Parker R. M., Williams M. V. (2002). Health literacy and performance on the Mini-Mental State Examination. Aging & Mental Health, 6, 22–29. doi:10.1080/13607860120101121 [DOI] [PubMed] [Google Scholar]
- Baker D. W., Parker R. M., Williams M. V., Clark W. S. (1998). Health literacy and the risk of hospital admission. Journal of General Internal Medicine, 13, 791–798. doi:10.1046/j.1525-1497.1998.00242.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman N. D., Sheridan S. L., Donahue K. E., Halpern D. J., Crotty K. (2011). Low health literacy and health outcomes: An updated systematic review. Annals of Internal Medicine, 155, 97–107. doi:10.7326/0003-4819-155-2-201107190-00005 [DOI] [PubMed] [Google Scholar]
- Canadian Council on Learning. (2008). Health literacy in Canada: A healthy understanding. Ottawa: Author. [Google Scholar]
- Carthery-Goulart M. T., Anghinah R., Areza-Fegyveres R., Santoro Bahia V., Dozzi Brucki S. M., Damin A, … Nitrini R. (2009). Performance of a Brazilian population on the test of functional health literacy in adults; Desempenho de uma população brasileira no teste de alfabetização funcional para adultos na área de saúde. Revista de Saúde Pública, 43, 631–638. doi:10.1590/s0034-89102009005000031 [DOI] [PubMed] [Google Scholar]
- Cavanaugh K. L., Wingard R. L., Hakim R. M., Eden S., Shintani A., Wallston K. A, … Ikizler T. A. (2010). Low health literacy associates with increased mortality in ESRD. Journal of the American Society of Nephrology, 21, 1979–1985. doi:10.1681/ASN.2009111163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew L. D., Bradley K. A., Flum D. R., Cornia P. B., Koepsell T. D. (2004). The impact of low health literacy on surgical practice. American Journal of Surgery, 188, 250–253. doi:10.1016/j.amjsurg.2004.04.005 [DOI] [PubMed] [Google Scholar]
- Colbert A. M., Sereika S. M., Erlen J. A. (2013). Functional health literacy, medication-taking self-efficacy and adherence to antiretroviral therapy. Journal of Advanced Nursing, 69, 295–304. doi:10.1111/j.1365-2648.2012.06007.x [DOI] [PubMed] [Google Scholar]
- Connor M., Mantwill S., Schulz P. J. (2013). Functional health literacy in Switzerland–validation of a German, Italian, and French health literacy test. Patient Education and Counseling, 90, 12–17. doi:10.1016/j.pec.2012.08.018 [DOI] [PubMed] [Google Scholar]
- Cox N., Bowmer C., Ring A. (2011). Health literacy and the provision of information to women with breast cancer. Clinical Oncology (Royal College of Radiologists (Great Britain)), 23, 223–227. doi:10.1016/j.clon.2010.11.010 [DOI] [PubMed] [Google Scholar]
- Dahlke A. R., Curtis L. M., Federman A. D., Wolf M. S. (2014). The Mini Mental Status Exam as a surrogate measure of health literacy. Journal of General Internal Medicine, 29, 615–620. doi:10.1007/s11606-013-2712-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T. C., Wolf M. S., Bass P. F., 3rd, Thompson J. A., Tilson H. H., Neuberger M., Parker R. M. (2006). Literacy and misunderstanding prescription drug labels. Annals of Internal Medicine, 145, 887–894. doi:10.7326/0003-4819-145-12-200612190-0014 [DOI] [PubMed] [Google Scholar]
- Davis T. C., Long S. W., Jackson R. H., Mayeaux E. J., George R. B, Murphy P. W., Crouch M. A. (1993). Rapid estimate of adult literacy in medicine: A shortened screening instrument. Family Medicine, 25, 391–395. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8349060 [PubMed] [Google Scholar]
- Downey L. V. A., Zun L. S. (2008). Assessing adult health literacy in urban healthcare settings. Journal of the National Medical Assocation, 100, 1304–1309. doi:10.1016/j.annemergmed.2008.01.239 [DOI] [PubMed] [Google Scholar]
- Federman A. D., Sano M., Wolf M. S., Siu A. L., Halm E. A. (2009). Health literacy and cognitive performance in older adults. Journal of the American Geriatrics Society, 57, 1475–1480. doi:10.1111/j.1532-5415.2009.02347.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B., Lowman S. G., DeWalt D. A. (2011). Assessing literacy in clinical and community settings: The patient perspective. Journal of Health Communication, 16, 124–134. doi:10.1080/10810730.2010.535113 [DOI] [PubMed] [Google Scholar]
- Gazmararian J. A., Baker D. W., Williams M. V., Parker R. M., Scott T. L., Green D. C, … Koplan J. P. (1999). Health literacy among Medicare enrollees in a managed care organization. JAMA, 281, 545–551. doi:10.1001/jama.281.6.545 [DOI] [PubMed] [Google Scholar]
- Geltman P. L., Adams J. H., Cochran J., Doros G., Rybin D., Henshaw M, … Paasche-Orlow M. (2013). The impact of functional health literacy and acculturation on the oral health status of Somali refugees living in Massachusetts. American Journal of Public Health, 103, 1516–1523. doi:10.2105/AJPH.2012.300885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginde A. A., Weiner S. G., Pallin D. J., Camargo C. A. (2008). Multicenter study of limited health literacy in emergency department patients. Academic Emergency Medicine, 15, 577–580. doi:10.1111/j.1553-2712.2008.00116.x [DOI] [PubMed] [Google Scholar]
- Gordon M. M., Hampson R., Capell H. A., Madhok R. (2002). Illiteracy in rheumatoid arthritis patients as determined by the Rapid Estimate of Adult Literacy in Medicine (REALM) score. Rheumatology (Oxford, England), 41, 750–754. doi:10.1093/rheumatology/41.7.750 [DOI] [PubMed] [Google Scholar]
- Green J. A., Mor M. K., Shields A. M., Sevick M. A., Palevsky P. M., Fine M. J, … Weisbord S. D. (2011). Prevalence and demographic and clinical associations of health literacy in patients on maintenance hemodialysis. Clinical Journal of the American Society of Nephrology, 6, 1354–1360. doi:10.2215/CJN.09761110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S., O’Rourke K. (2008). Meta-analysis. In Rothman K. J., Greenland S., Lash T. L. (Eds.), Modern epidemiology (3rd ed., pp. 652–682). Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Haun J., Luther S., Dodd V. (2012). Measurement variation across health literacy assessments: Implications for assessment selection in research and practice. Journal of Health Communication, 17(Suppl. 3), 141–159. doi:10.1080/10810730.2012.712615 [DOI] [PubMed] [Google Scholar]
- Ibrahim S. Y., Reid F., Shaw A., Rowlands G., Gomez G. B., Chesnokov M., Ussher M. (2008). Validation of a health literacy screening tool (REALM) in a UK population with coronary heart disease. Journal of public health (Oxford, England), 30, 449–455. doi:10.1093/pubmed/fdn059 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. (2004). Nielsen-Bohlman L., Panzer A., Kindig D. (Ed.), Health literacy: A prescription to end confusion. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Jackson R. D., Eckert G. J. (2008). Health literacy in an adult dental research population: A pilot study. Journal of Public Health Dentistry, 68, 196–200. doi:10.1111/j.1752-7325.2007.00063.x [DOI] [PubMed] [Google Scholar]
- Jovic-Vranes A., Bjegovic-Mikanovic V. (2012). Which women patients have better health literacy in Serbia? Patient Education and Counseling, 89, 209–212. doi:10.1016/j.pec.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Jovic-Vranes A., Bjegovic-Mikanovic V., Marinkovic J. (2009). Functional health literacy among primary health-care patients: Data from the Belgrade pilot study. Journal of Public Health (Oxford, England), 31, 490–495. doi:10.1093/pubmed/fdp049 [DOI] [PubMed] [Google Scholar]
- Jovic-Vranes A., Bjegovic-Mikanovic V., Marinkovic J., Kocev N. (2011). Health literacy in a population of primary health-care patients in Belgrade, Serbia. International Journal of Public Health, 56, 201–207. doi:10.1007/s00038-010-0181-0 [DOI] [PubMed] [Google Scholar]
- Juzych M. S., Randhawa S., Shukairy A., Kaushal P., Gupta A., Shalauta N. (2008). Functional health literacy in patients with glaucoma in urban settings. Archives of Ophthalmology, 126, 718–724. doi:10.1001/archopht.126.5.718 [DOI] [PubMed] [Google Scholar]
- Kalichman S. C., Benotsch E., Suarez T., Catz S., Miller J. (2000). Health literacy and health-related knowledge among persons living with HIV/AIDS. American Journal of Preventive Medicine, 18, 325–331. doi:10.1016/s0749-3797(00)00121-5 [DOI] [PubMed] [Google Scholar]
- Kim S. H. (2009). Health literacy and functional health status in Korean older adults. Journal of Clinical Nursing, 18, 2337–2343. doi:10.1111/j.1365-2702.2008.02739.x [DOI] [PubMed] [Google Scholar]
- Kirk J. K., Grzywacz J. G., Arcury T. A., Ip E. H., Nguyen H. T., Bell R. A, … Quandt S. A. (2012). Performance of health literacy tests among older adults with diabetes. Journal of General Internal Medicine, 27, 534–540. doi:10.1007/s11606-011-1927-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner M., Greenberg E., Jin Y., Paulsen C. (2006). The health literacy of America’s adults: Results from the 2003 National Assessment of Adult Literacy (NCES 2006-483). Washington, DC: National Center for Education Statistics. [Google Scholar]
- Laramee A. S., Morris N., Littenberg B. (2007). Relationship of literacy and heart failure in adults with diabetes. BMC Health Services Research, 7, 98. doi:10.1186/1472-6963-7-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-Y., Arozullah A. M., Cho Y. I., Crittenden K., Vicencio D. (2009). Health literacy, social support, and health status among older adults. Educational Gerontology, 35, 191–201. doi:10.1080/03601270802466629 [Google Scholar]
- Levinthal B. R., Morrow D. G., Tu W., Wu J., Murray M. D. (2008). Cognition and health literacy in patients with hypertension. Journal of General Internal Medicine, 23, 1172–1176. doi:10.1007/s11606-008-0612-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau S., Tomori C., Lyons T., Langseth L., Bennett C., Garcia P. (2002). The association of health literacy with cervical cancer prevention knowledge and health behaviors in a multiethnic cohort of women. American Journal of Obstetrics and Gynecology, 186, 938–943. doi:10.1067/mob.2002.122091 [DOI] [PubMed] [Google Scholar]
- Mancuso C. A., Rincon M. (2006). Asthma patients’ assessments of health care and medical decision making: The role of health literacy. Journal of Asthma, 43, 41–44. doi:10.1080/02770900500447052 [DOI] [PubMed] [Google Scholar]
- Mbaezue N., Mayberry R., Gazmararian J., Quarshie A., Ivonye C., Heisler M. (2010). The impact of health literacy on self-monitoring of blood glucose in patients with diabetes receiving care in an inner-city hospital. Journal of the National Medical Association, 102, 5–9. doi:10.2337/diacare.28.6.1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall G. J., Jr., Mackert M., Becker H. (2012). Memory performance, health literacy, and instrumental activities of daily living of community residing older adults. Nursing Research, 61, 70–75. doi:10.1097/NNR.0b013e31823b18f4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton C., Wallston K. A., Rothman R. L., Marcovitz D. E., Storrow A. B. (2011). Short, subjective measures of numeracy and general health literacy in an adult emergency department. Academic Emergency Medicine, 18, 1148–1155. doi:10.1111/j.1553-2712.2011.01210.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. P., Jr., Brownlee C. D., McCoy T. P., Pignone M. P. (2007). The effect of health literacy on knowledge and receipt of colorectal cancer screening: A survey study. BMC Family Practice, 8, 16. doi:10.1186/1471-2296-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLOS Medicine, 6, 1–6. doi:10.1371/journal.pmed.1000097 [PMC free article] [PubMed] [Google Scholar]
- Morris N. S., Grant S., Repp A., Maclean C., Littenberg B. (2011). Prevalence of limited health literacy and compensatory strategies used by hospitalized patients. Nursing Research, 60, 361–366. doi:10.1097/NNR.0b013e31822c68a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow D., Clark D., Tu W., Wu J., Weiner M., Steinley D., Murray M. D. (2006). Correlates of health literacy in patients with chronic heart failure. The Gerontologist, 46, 669–676. doi:10.1093/geront/46.5.669 [DOI] [PubMed] [Google Scholar]
- Mosher H. J., Lund B. C., Kripalani S., Peter J. (2012). Association of health literacy with medication knowledge, adherence, and adverse drug events among elderly veterans. Journal of Health Communication, 17(Suppl. 3), 241–251. doi:10.1080/10810730.2012.712611 [DOI] [PubMed] [Google Scholar]
- Murray C., Johnson W., Wolf M. S., Deary I. J. (2011). The association between cognitive ability across the lifespan and health literacy in old age: The Lothian Birth Cohort 1936. Intelligence, 39, 178–187. doi:10.1016/j.intell.2011.04.001 [Google Scholar]
- Nokes K. M., Coleman C. L., Cashen M., Dole P., Sefcik E., Hamilton M. J, … Holzemer W. (2007). Health literacy and health outcomes in HIV seropositive persons. Research in Nursing & Health, 30, 620–627. doi:10.1002/nur.20219 [DOI] [PubMed] [Google Scholar]
- O’Carroll R. (1995). The Assessment of premorbid ability : A critical review. Neurocase, 1, 83–9. doi:10.1093/neucas/1.1.83 [Google Scholar]
- Olives T., Patel R., Patel S., Hottinger J., Miner J. R. (2011). Health literacy of adults presenting to an urban ED. The American Journal of Emergency Medicine, 29, 875–882. doi:10.1016/j.ajem.2010.03.031 [DOI] [PubMed] [Google Scholar]
- Osborn C. Y., Cavanaugh K., Wallston K. A., Rothman R. L. (2010). Self-efficacy links health literacy and numeracy to glycemic control. Journal Health Communication, 15(Suppl. 3), 146–58. doi:10.1080/10810730.2010.499980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir H., Alper Z., Uncu Y., Bilgel N. (2010). Health literacy among adults: A study from Turkey. Health Education Research, 25, 464–477. doi:10.1093/her/cyp068 [DOI] [PubMed] [Google Scholar]
- Paasche-Orlow M. K., Parker R. M., Gazmararian J. A., Nielsen-Bohlman L. T., Rudd R. R. (2005). The prevalence of limited health literacy. Journal of General Internal Medicine, 20, 175–184. doi:10.1111/j.1525-1497.2005.40245.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R., Baker D., Williams M., Nurss J. (1995). The test of functional health literacy in adults: A new instrument for measuring patients’ literacy skills. Journal of General Internal Medicine, 10, 537–541. doi: 10.1007/bf02640361 [DOI] [PubMed] [Google Scholar]
- Peterson N. B., Dwyer K. A., Mulvaney S. A., Dietrich M. S., Rothman R. L. (2007). The influence of health literacy on colorectal cancer screening knowledge, beliefs and behavior. Journal of the National Medical Association, 99, 1105–1112. doi:10.1080/10810730500267720 [PMC free article] [PubMed] [Google Scholar]
- Reeve C. L., Basalik D. (2014). Is health literacy an example of construct proliferation? A conceptual and empirical evaluation of its redundancy with general cognitive ability. Intelligence, 44, 93–102. doi:10.1016/j.intell.2014.03.004 [Google Scholar]
- Robinson S., Moser D., Pelter M. M., Nesbitt T., Paul S. M., Dracup K. (2011). Assessing health literacy in heart failure patients. Journal of Cardiac Failure, 17, 887–892. doi:10.1016/j.cardfail.2011.06.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. T., Ivey J. L. (2005). Self-reported medication use in community-residing older adults: A pilot study. The American Journal of Geriatric Pharmacotherapy, 3, 196–204. doi:10.1016/s1543-5946(05)80026-1 [DOI] [PubMed] [Google Scholar]
- Rowlands G. P., Mehay A., Hampshire S., Phillips R., Williams P., Mann A, … Tylee A. T. (2013). Characteristics of people with low health literacy on coronary heart disease GP registers in South London: A cross-sectional study. BMJ Open, 3, e001503. doi:10.1136/bmjopen-2012-001503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T. A. (2009). When does age-related cognitive decline begin? Neurobiology of Aging, 30, 507–514. doi:10.1016/j.neurobiolaging.2008.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillinger D., Grumbach K., Piette J., Wang F., Osmond D., Daher C, … Bindman A. B. (2002). Association of health literacy with diabetes outcomes. JAMA, 288, 475–482. doi:10.1001/jama.288.4.475 [DOI] [PubMed] [Google Scholar]
- Scott T. L., Gazmararian J. A., Williams M. V., Baker D. W. (2002). Health literacy and preventive health care use among Medicare enrollees in a managed care organization. Medical Care, 40, 395–404. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11961474 [DOI] [PubMed] [Google Scholar]
- Shah L. C., West P., Bremmeyr K., Savoy-Moore R. T. (2010). Health literacy instrument in family medicine: The “newest vital sign” ease of use and correlates. Journal of the American Board of Family Medicine, 23, 195–203. doi:10.3122/jabfm.2010.02.070278 [DOI] [PubMed] [Google Scholar]
- Shea J. A., Beers B. B., McDonald V. J., Quistberg D. A., Ravenell K. L., Asch D. A. (2004). Assessing health literacy in African American and Caucasian adults: Disparities in rapid estimate of adult literacy in medicine (REALM) scores. Family Medicine, 36, 575–581. doi:10.1093/intqhc/mzl068 [PubMed] [Google Scholar]
- Singh-Manoux A., Kivimaki M., Glymour M., Elbaz A., Berr C., Ebmeier K, … Dugravot A. (2012). Timing of onset of cognitive decline: Results from Whitehall II prospective cohort study. BMJ, 344, d7622. doi:10.1136/bmj.d7622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi F., Valecchi D., Bacci D., Abbate R., Gensini G. F., Casini A., Macchi C. (2011). Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. Journal of Internal Medicine, 269, 107–117. doi:10.1111/j.1365-2796.2010.02281.x [DOI] [PubMed] [Google Scholar]
- Stewart D. W., Adams C. E., Cano M. A., Correa-Fernández V., Li Y., Waters A. J, … Vidrine J. I. (2013). Associations between health literacy and established predictors of smoking cessation. American Journal of Public Health, 103, e43–e49. doi:10.2105/AJPH.2012.301062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudore R. L., Mehta K. M., Simonsick E. M., Harris T. B., Newman A. B., Satterfield S, … Yaffe K. (2006a). Limited literacy in older people and disparities in health and healthcare access. Journal of the American Geriatrics Society, 54, 770–776. doi:10.1111/j.1532-5415.2006.00691.x [DOI] [PubMed] [Google Scholar]
- Sudore R. L., Yaffe K., Satterfield S., Harris T. B., Mehta K. M., Simonsick E. M, … Schillinger D. (2006b). Limited literacy and mortality in the elderly: The health, aging, and body composition study. Journal of General Internal Medicine, 21, 806–812. doi:10.1111/j.1525-1497.2006.00539.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swearingen C. J., McCollum L., Daltroy L. H., Pincus T., Dewalt D. A., Davis T. C. (2010). Screening for low literacy in a rheumatology setting: More than 10% of patients cannot read “cartilage,” “diagnosis,” “rheumatologist,” or “symptom.” Journal of Clinical Rheumatology, 16, 359–364. doi:10.1097/RHU.0b013e3181fe8ab1 [DOI] [PubMed] [Google Scholar]
- The Cochrane Collaboration. (2011). Cochrane Handbook for Systematic Reviews of Interventions (5.1.0 ed.) (Higgins J. P. T., Green S., Eds.). Retrieved from www.cochrane-handbook.org [Google Scholar]
- Thomas P. A. (2011). Trajectories of social engagement and limitations in late life. Journal of Health and Social Behavior, 52, 430–443. doi:10.1177/0022146511411922 [DOI] [PubMed] [Google Scholar]
- von Elm E., Altman D., Egger M., Pocock S., Gotzche P., Vandenbroucke J.& STROBE Initiative. (2007). Strengthening the reporting of observational studies epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ, 335, 806–808. doi:10.1136/bmj.39335.541782.ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wagner C., Knight K., Steptoe A., Wardle J. (2007). Functional health literacy and health-promoting behaviour in a national sample of British adults. Journal of Epidemiology and Community Health, 61, 1086–1090. doi:10.1136/jech.2006.053967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J., Pepa C., Gerard P. S. (2010). Assessing the health literacy levels of patients using selected hospital services. Clinical Nurse Specialist, 24, 31–37. doi:10.1097/NUR.0b013e3181c4abd0 [DOI] [PubMed] [Google Scholar]
- Weiss B., Mays M., Martz W., Castro K., DeWalt D., Pignone M, … Hale F. (2005). Quick assessment of literacy in primary care: The Newest Vital Sign. Annals of Family Medicine, 3, 514–522. doi:10.1370/afm.405.College [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. (n.d.). The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Retrieved February 14, 2013, from http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf
- Williams M., Baker D., Parker R., Nurss J. (1998). Relationship of functional health literacy to patients’ knowledge of their chronic disease. Archives of Internal Medicine, 158, 166–172. doi:10.1001/archinte.158.2.166 [DOI] [PubMed] [Google Scholar]
- Wolf M. S., Curtis L. M., Wilson E. A. H., Revelle W., Waite K. R., Smith S. G, … Baker D. W. (2012). Literacy, cognitive function, and health: Results of the LitCog study. Journal of General Internal Medicine, 27, 1300–1307. doi:10.1007/s11606-012-2079-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-H., Li S.-C., Fong K.-Y., Thumboo J. (2009). The impact of health literacy on health-related quality of life (HRQoL) and utility assessment among patients with rheumatic diseases. Value Health, 12(Suppl. 3), 106–109. doi:10.1111/j.1524-4733.2009.00640.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.