Abstract
Several problems limit the application of gene transfer to correct the cystic fibrosis (CF) Cl– transport defect in airway epithelia. These include inefficient transduction with vectors applied to the apical surface, a low rate of division by airway epithelial cells, failure of transgene expression to persist, and immune responses to vectors or vector-encoded proteins. To address these issues, we used a feline immunodeficiency virus–based (FIV-based) vector. FIV vector formulated with a calcium chelator transduced fully differentiated, nondividing human airway epithelia when applied to the apical surface. FIV-based vector encoding the cystic fibrosis transmembrane conductance regulator cDNA corrected the Cl– transport defect in differentiated CF airway epithelia for the life of the culture (>3 months). When this approach was applied in vivo, FIV vector expressing β-galactosidase transduced 1–14% of adult rabbit airway epithelia. Transduced cells were present in the conducting airways, bronchioles, and alveoli. Importantly, gene expression persisted, and cells with progenitor capacity were targeted. FIV-based lentiviral vectors may be useful for the treatment of genetic lung diseases such as CF.
This article may have been published online in advance of the print edition. The date of publication is available from the JCI website, http://www.jci.org. J. Clin. Invest. 104:R55–R62 (1999).
Introduction
Gene therapy is the most direct means to correct the Cl– transport defect responsible for cystic fibrosis (CF) lung disease (1–4). However, several problems limit the successful in vivo application of gene transfer to airway epithelia. These include inefficient transduction, immune responses to vectors and vector-encoded proteins, and lack of persistent transgene expression. Such limitations must be overcome if gene transfer is to advance as a treatment for CF and other lung diseases.
Recombinant adeno-associated virus (AAV) (5, 6), Moloney murine leu-kemia virus (MuLV) (7–11), and lentivirus (12) vectors address the problem of poor persistence due to their ability to integrate. Lentivirus-based vectors offer the advantage of infecting nondividing cells, a significant consideration in the airways where most cells are mitotically inactive (12). However, limited studies to date suggest that HIV-based lentivirus vectors inefficiently transduce differentiated airway epithelia (13).
A first-generation lentivirus vector derived from the feline immunodeficiency virus (FIV) was recently reported (14). Similar to HIV, FIV vectors transduce nondividing cells (14, 15). Wild-type FIV is antigenically and genetically distinct from HIV and does not infect human cells or cause disease in humans (16). Therefore, FIV-based vectors may offer additional safety features compared with HIV-based systems (15). We developed a second-generation FIV vector in which unnecessary trans-acting elements (vif, orf2) were deleted, further reducing the possibility of production of replication competent virus (15). Here, we use this FIV-based vector to efficiently transduce airway epithelia in vitro and in vivo. We present novel methods of vector formulation and delivery that facilitate gene transfer to the airways in vivo. FIV vectors may offer advantages over other vectors for airway gene transfer.
Methods
Culture of human airway epithelia.
Airway epithelia were isolated from nasal polyps, trachea, and bronchi and grown at the air-liquid interface as described previously (17). All preparations used were well differentiated (>2 weeks old; resistance >1,000 ohm × cm2) (17, 18). This study was approved by the Institutional Review Board at the University of Iowa.
Drugs and chemicals.
Aphidicolin (20 μg/mL) (Sigma Chemical Co., St. Louis, Missouri, USA) was applied to cells for 24 hours before retroviral transduction to arrest cell growth in G1/S phase (12, 14). To inhibit retroviral RT, 5 μM 3′-azido-3′-deoxythymidine (AZT; Glaxo Wellcome, Research Triangle Park, North Carolina, USA) was added at the time of viral transduction.
Vector production.
The second-generation FIV vector system was reported previously (15). Plasmid constructs consist of an FIV packaging construct with a deletion in the env gene and mutations in vif and orf2, an FIV vector construct expressing either cytoplasmic Escherichia coli β-galactosidase or cystic fibrosis transmembrane conductance regulator (CFTR) genes, and an envelope plasmid in which the human cyto-megalovirus (CMV) early gene promoter directs transcription of the vesicular stomatitis virus G protein (VSV-G). In the vector constructs, the CMV promoter directs β-galactosidase expression (FIV-βgal), whereas the MuLV long terminal repeat promoter directs CFTR expression (FIV-CFTR) (19).
VSV-G–pseudotyped FIV vector particles were generated by transient transfection of plasmid DNA into 293T cells as described previously (15). Each FIV-βgal preparation was titered on NIH 3T3 cells by limiting dilutions; final titers of approximately 5 × 107 to 5 × 109 CFU/mL were obtained. To titer the FIV-CFTR vector, a PCR-based assay system was developed. HT-1080 target cells were transduced with serial dilutions of crude or processed FIV vector preparations in the presence of 4 μg/mL hexadimethrine bromide (Sigma Chemical Co.). Twenty-four hours later, media were changed and cells were cultured for an additional 24–48 hours. The samples were washed with 1X PBS, and incubated with 2.5 mL of lysis buffer (100 mM Tris [pH 8], 5 mM EDTA, 0.2% SDS, 100 mM NaCl, and 100 μg/mL proteinase K [QIAGEN Inc., Valencia, California, USA]) at 37°C for 2 hours, and the DNA was precipitated. DNA pellets were washed with 70% ethanol and resuspended in 500 μL TE buffer, and total genomic DNA was quantified by staining with Hoechst dye H33258 and compared directly against calf thymus DNA standards using the CytoFluor II fluorometer (PerSeptive Biosystems, Framingham, Massachusetts, USA). A total of 100 ng of each genomic DNA sample was subjected to automated PCR (50 μL volume) using a PE ABI Prism 7700 system (Perkin-Elmer Corp., Norwalk, Connecticut, USA) and a synthetic oligonucleotide primer set directed against FIV packaging signal sequences, yielding an 80-bp product. The resulting fluorescence was detected, and provector copy number titer was expressed as transduction units per milliliter (TU/mL). Titers of 9.7 × 108 to 4.6 × 109 TU/mL were obtained in 2 preparations.
Gene transfer
In vitro.
To transduce differentiated human epithelia, the FIV vector was mixed with cell culture medium to a final volume of 100 μL (∼10 moi). This mixture was applied to either the apical surface or the basal surface as described previously (9). To enhance transduction from the apical surface, vector was mixed at a 1:1 (vol/vol) ratio with 12 mM EGTA HEPES/saline solution (pH 7.3) and applied apically for 4 hours as reported previously for MuLV vectors (9). Polybrene (8 μg/mL) was included in the transduction solutions.
To study the persistence of recombinant FIV-mediated correction of CFTR Cl– current, results were compared with recombinant adenovirus. We previously reported that adenovirus infects human airway epithelia through the basolateral side by a fiber-dependent mechanism (20). Ad2/CFTR-16 (50 moi) (21) was applied in a 25-μL volume to the bottom of the epithelia. After 30 minutes, the epithelia were rinsed and returned to the culture dish. Epithelia were studied at intervals for the life of the culture (90–180 days).
A control experiment was performed to rule out protein transfer or pseudotransduction as reported for concentrated AAV and retroviral vectors (22, 23). When applying the FIV-βgal vector/EGTA solution to the apical surface, cells were treated with AZT to inhibit the retroviral RT. AZT-treated cells showed no significant expression of vector-encoded product (not shown), confirming that FIV-vector transduction under these experimental conditions was not due to protein transfer.
In vivo.
For tracheal gene transfer, adult New Zealand white rabbits were anesthetized with 32 mg/kg ketamine, 5.1 mg/kg xylazine, and 0.8 mg/kg acepromazine intramuscularly; a ventral midline incision was made; and a tracheotomy was performed. An approximately 1.5-cm tracheal segment cephalad to the tracheotomy was isolated and cannulated on each end with PE 330 tubing (Becton Dickinson, Parsippany, New Jersey, USA). The tracheal segment was rinsed and then filled with a solution of 12 mM EGTA in 10 mM HEPES buffer (pH 7.4, “hypotonic buffer”) for 30–60 minutes. The EGTA solution was then replaced with 300 μL of FIV–βgal vector (titer 1 × 108 to 5 × 108 CFU/mL). The vector solution was left in place for 45 minutes, and then the cannulae were removed and the incisions closed. Five days or 6 weeks later, the tissues studied for β-galactosidase expression. For lower airway gene transfer, a PE50 catheter passed via the trachea until it lodged in a subsegmental bronchus. A total of 200–600 μL of FIV-βgal formulated in hypotonic buffer with 6 mm EGTA was instilled. Five days later, the tissues were studied for β-galactosidase expression.
Tissue histochemistry
β-galactosidase expression.
Epithelial cells were fixed and X-gal stained as reported elsewhere (9, 24). Epithelia counterstained with DAPI were examined microscopically en face for β-galactosidase expression. The percentage of β-galactosidase–positive cells was determined by counting a minimum of 1,000 cells from representative en face views of each treated epithelia.
Rabbit tissues were fixed in 2% paraformaldehyde/PBS overnight, X-gal stained, and embedded in paraffin, and sections were cut for histological examination (24). Sections were counterstained with nuclear fast red or hematoxylin and eosin. To determine the percentage of β-galactosidase–expressing cells in each 1.5- to 2-cm tracheal specimen, serial sections were cut every 20 μm, and ≥ 50 slides were examined. To quantify gene transfer to lower airways, the blue tissue areas of the X-gal–stained lungs (see Figure 4a) were dissected and embedded in paraffin, and serial sections cut at 40-μm intervals. The percentage of β-galactosidase–positive cells in lower airway tissues was quantified by cell counting. β-galactosidase–expressing cells were categorized by the size of the airway in which expression was noted (>750 μm, 500–750 μm, 250–500 μm, 0–250 μm) using a calibrated eyepiece reticle. To identify the cell types expressing β-galactosidase, standard morphologic criteria were used. Transgene-expressing cells were identified by their physical characteristics: (a) ciliated cells are columnar cells with cilia; (b) goblet cells are columnar cells containing secretory granules; (c) basal cells are basally located cuboidal cells having no contact with the mucosal surface; (d) intermediate cells are columnar cells in the lower half of the epithelium having no contact with the lumen; (e) Clara cells are nonciliated, columnar to cuboidal surface cells that are more prevalent in the distal airways; and (f) alveolar type II cells are cuboidal, “corner” cells of the alveolar epithelium.
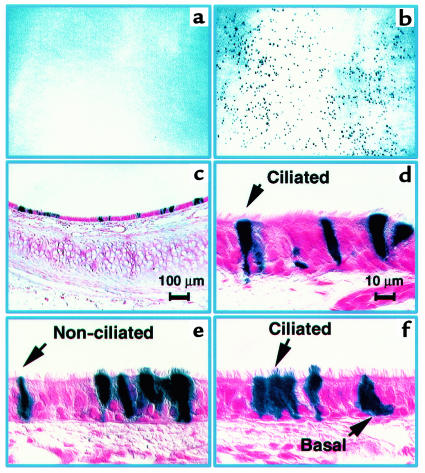
Figure 4.
FIV-βgal transduction of lower airway epithelia 5 days after gene transfer. (a) En face view of pleural surface of lung after fixation and X-gal staining showing βgalactosidase–expressing cells. All treated animals had similar segments of β-galactosidase–expressing cells extending to the pleural surface. (b–f) Higher magnification views of tissue sections showing lower airway and parenchymal cells transduced. (b) Low-magnification view of a large bronchus (>750 μm diameter) demonstrating patches of β-galactosidase–expressing cells extending around the circumference of the epithelium. (c) High-magnification view of expression in a large bronchus (>750 μm diameter) showing expression in ciliated cells and basal cells. (d) High-magnification view of expression in a medium sized airway (500–750 μm diameter) demonstrating expression in nonciliated surface cells (Clara cells). (e) β-galactosidase expression in a small bronchus (250–500 μm diameter) showing expression in nonciliated surface cells (Clara cells). (f) Distal lung sample (airways 0–250 μm diameter) showing expression in cuboidal cells consistent with alveolar type II cells. (g) Gene transfer expressed as a function of airway size. Numbers above each bar represent the number of animals with transduced cells in the corresponding region. Tissues from 12 animals were studied. Scale bar in d also applies to e and f.
Measurement of transepithelial CFTR Cl– current.
To measure transepithelial bioelectric properties, epithelia were mounted in Ussing chambers and studied 3, 13, 30, 60, 90, and 180 days after gene transfer as described previously (17). The cAMP-stimulated Isc (Isc(IBMX/Forsk)) is the increase in current after basolateral addition of cAMP agonists (10 μM forskolin plus 100 μM 3-isobutyl 1-methylxanthine [IBMX]) in the presence of 10 μM amiloride. Data from each experiment were normalized to the mean Isc(IBMX/Forsk) seen 3 days after transduction. CF airway epithelia were genotyped and were compound heterozygotes for the ΔF508 mutation (ΔF508/-, ΔF508/1717-1G→A).
Results
FIV vectors transduce nondividing airway epithelia in vitro.
On the basis of previous literature (25, 26) as well as our own studies with MuLV (9, 11), we suspected that the receptors for VSV-G–pseudotyped FIV vectors were only expressed on the basolateral surface of airway epithelia. Indeed, when VSV-G–pseudotyped FIV-βgal was applied to the apical surface, no gene transfer occurred (Figure 1a). In contrast, FIV- βgal applied to the basolateral surface transduced the epithelia (Figure 1b). We hypothesized that if epithelial junctions were opened, FIV vector particles would have a better chance to interact with cell-surface receptors and gain entry when applied apically. Scratching the epithelial sheet with a pipette tip before applying vector to the apical surface enhanced gene transfer only in areas where the cells were mechanically disrupted (Figure 1c). Thus, if receptors were made accessible, gene transfer was achieved with VSV-G–pseudotyped FIV vectors.
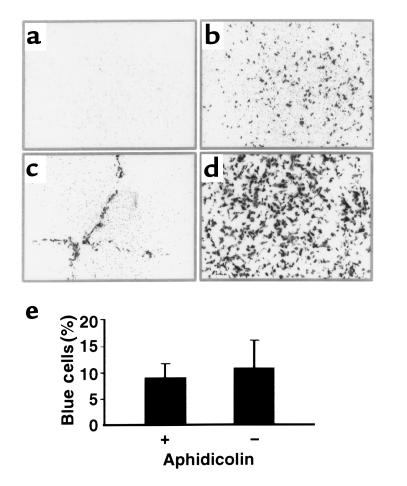
Figure 1.
FIV vectors transduce nondividing airway epithelia in vitro. (a) Application of FIV-βgal (moi 10) to the apical surface of the epithelial sheet resulted in no gene transfer. Representative en face view of X-gal stained epithelia 3 days after vector application. (b) FIV-βgal vector applied to airway epithelia from the basolateral surface in vitro (moi ∼10) resulted in gene transfer. Representative en face view is shown. (c) Gene transfer from the apical surface with VSV-G FIV is enhanced by physical disruption of epithelia. The epithelial sheet was scratched with a pipette tip prior to apical vector application. En face view shows gene transfer (gray cells) only along the area of epithelial disruption. (d) FIV transduces aphidicolin growth-arrested cells (en face view). Vector was applied to apical surface in the presence of 6 mM EGTA in hypotonic buffer. Approximately 17% of epithelia were transgene positive (range 12–22%; n = 5 epithelia from 2 different preparations). For cells in control media without aphidicolin, approximately 20% of cells expressed the transgene (data not shown; range 8–30%; n = 5 epithelia from 3 different preparations). (e) Quantification of gene transfer results under conditions shown in d (mean ± SEM; n = 5 epithelia; 3 different preparations).
To demonstrate that FIV vectors transduce nondividing epithelia, we performed experiments in the presence or absence of aphidicolin-induced growth arrest (12, 14). As we found previously that calcium chelation with EGTA and hypotonic solutions disrupted epithelial junctions and facilitated gene transfer with apically applied MuLV vectors (9), a similar approach was used with the FIV vector. Formulation of FIV-βgal with 6 mM EGTA in a hypotonic buffer (∼10 moi) greatly increased gene transfer from the apical surface (Figure 1d). Approximately 17% of epithelia growth-arrested with aphidicolin were transgene positive 3 days after transduction, whereas approximately 20% of epithelia in control media were transduced (Figure 1, d and e). Previous studies showed that approximately 7% of cells are proliferating in this model as assayed by BrdU histochemistry (9). The EGTA solution alone had no effect on cell proliferation as assayed by BrdU histochemistry (data not shown). Thus, when allowed access to receptors, FIV vectors effectively transduced nondividing epithelia.
FIV vectors coding for CFTR persistently correct the CF Cl– transport defect.
On the basis of the results in normal human airway epithelia, we hypothesized that recombinant FIV vectors expressing CFTR would complement the Cl– transport defect in well-differentiated CF epithelia. For these studies, we used primary organotypic cultures of human airway epithelia from patients with CF, for the following reasons. Primary cultures of differentiated human CF airway epithelia recapitulate several important aspects of in vivo airway epithelial biology and CF disease. Cells cultured in this fashion morphologically resemble the human airways in vivo (17, 18). Similar to the in vivo human airways, they are relatively resistant to transduction by both viral and nonviral vectors applied to the apical surface (9, 17, 27, 28). They manifest the electrolyte and liquid transport defects characteristic of CF (18, 29). Importantly, unlike the long-term survival and minimal evidence of lung disease reported for CFTR-null mice or mice with specific human CFTR mutations (30–35), cultured human CF epithelia show an increased susceptibility to bacterial infection (36).
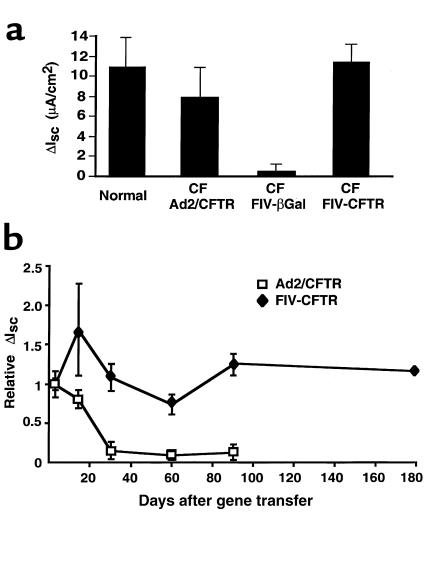
Tracheal epithelia were transduced in vitro from the apical surface with FIV-CFTR. Correction of the CFTR Cl– transport defect was assayed by measuring the change in short-circuit current in response to cAMP agonists (ΔIsc(IBMX/Forsk)), from 3 days to 6 months after gene transfer (Figure 2). CF epithelia transduced with FIV-CFTR or adenovirus expressing CFTR uniformly demonstrated Cl– secretion in response to cAMP agonists, whereas control cells treated with FIV-βgal showed no response (Figure 2a). The Cl– secretory responses in the corrected cells (Figure 2, a and b) were similar to those measured in normal airway epithelia (Figure 2, a and b). In cells transduced with adenovirus, ΔIsc(IBMX/Forsk) gradually declined over time. In contrast, the net ΔIsc(IBMX/Forsk) in FIV transduced cells remained stable (Figure 2b). In 1 FIV-CFTR–transduced culture that remained viable for 6 months, cAMP-activated Cl– current persisted at similar levels as day 3 (Figure 2b).
Figure 2.
FIV-CFTR corrects the CF Cl– transport defect. CF epithelia were transduced from the apical surface in the presence of EGTA with ∼10 moi of FIV vector expressing either β-galactosidase or human CFTR. For comparison, another group of CF cells received Ad2/CFTR (moi 50, basolateral application). At time points of 3, 13, 30, 60, 90, and 180 days later, epithelia were mounted in Ussing chambers, and the change in short circuit current was measured in response to cAMP agonists (ΔIsc(IBMX/Forsk)). (a) Comparison of ΔIsc(IBMX/Forsk) in response to cAMP agonists in CF epithelia transduced with adenovirus or FIV vectors, 3 days after gene transfer. Both Ad- and FIV-transduced epithelia express cAMP-activated Cl– currents similar to normal cells (n = 5 CF epithelia; n = 5 normal epithelia, for each time point). (b) CFTR expression persists in FIV-transduced epithelia. CF epithelia were transduced with FIV-βgal, FIV-CFTR, or Ad2/CFTR and ΔIsc(IBMX/Forsk) measured at the indicated time points after gene transfer (n = 5 CF epithelia; n = 5 normal epithelia, for each time point). Data from each experiment were normalized to the mean Isc(IBMX/Forsk) seen 3 days after infection. One CF preparation was viable 6 months after gene transfer.
FIV vectors transduce airway epithelia in vivo.
FIV vectors might also effectively transduce airway epithelia in vivo if VSV-G receptors were accessible. We used a similar protocol of epithelial junction disruption to test FIV vectors in vivo. After EGTA pretreatment, FIV-βgal vector was applied to the luminal surface of the trachea in adult rabbits. Five days later, the tissues were removed and studied for β-galactosidase expression. As shown in Figure 3, a–e, cells throughout the epithelium expressed the transgene. The transduction efficiency was 4.8 ± 5.6% (mean ± SEM; range: 1–14%; n = 4). The treated epithelia appeared intact, without evidence of injury or inflammatory cell infiltrates. Of note, basal cells, intermediate cells, and both ciliated and nonciliated surface cells expressed β-galactosidase (Figure 3, b–e). Previous studies in several species showed that the mitotic labeling indices for cells other than basal cells are very low (<1%) in adult airway epithelia (37). While these data suggest that FIV vectors transduce both dividing and nondividing airway cells in vivo, we cannot rule out the possibility that EGTA/hypotonic treatment stimulated some stationary-phase cells to divide. In the absence of EGTA formulation, there was no gene transfer (data not shown).
Figure 3.
Gene transfer to rabbit tracheal epithelia in vivo using FIV-βgal vector. Panels show results 5 days after gene transfer. Low magnification en face view of X-gal–stained trachea from control (a) or FIV vector–treated trachea (b). Blue cells were only seen in the trachea transduced with the FIV vector (b). (c) Low-magnification view of X-gal–stained tracheal section. β-galactosidase–expressing cells are noted at both the surface and basal cell levels of the transduced epithelium. (d–f) Higher-magnification views of tracheal epithelium showing cell types expressing β-galactosidase. No inflammatory cells were noted in control or transduced specimens (n = 4 animals). Scale bar in d also applies to e and f.
CF lung disease begins in the small airways. To target the intrapulmonary airways, a small catheter was passed transtracheally into the peripheral airways, and hypotonic/EGTA formulated FIV-βgal vector was instilled. This approach allowed the vector solution to be concentrated within a relatively small area. As shown in Figure 4, a–e, we uniformly noted focal areas of gene transfer in the lung 5 days later. When serial sections of tissue were studied, epithelia expressing β-galactosidase were noted throughout the segment where virus was instilled, from cartilaginous bronchi with diameters greater than 750 μm out to the alveoli (Figure 4, b–e). The percentage of transgene-expressing cells across all airway sizes was 4.9 ± 2.2 % (mean ± SEM; range: 2.6–10.3%; n = 12). β-galactosidase expression occurred more frequently in the smaller airways than the larger airways, as might be expected with the method of vector introduction (Figure 4g). Importantly, the morphology of the transduced airway epithelia appeared normal, and all cell types of the lower airways were transduced, including proposed progenitors such as basal cells, nonciliated surface cells (Clara cells), and alveolar type II cells. Vector instillation without the EGTA formulation resulted in no significant gene transfer (not shown).
FIV gene transfer to airway epithelia persists in vivo.
Animals treated with FIV-βgal vector intratracheally were evaluated 6 weeks after gene transfer for persistence of gene expression. In contrast to the results at day 5 (Figure 3, a–e), larger clusters of β-galactosidase–positive cells were noted on both the en face views and the cross sections of the trachea (Figure 5), suggesting that targeted cells clonally expanded over time. As shown in Figure 5, we noted β-galactosidase–expressing cells throughout the epithelium. The percentage of βgalactosidase–expressing cells was 2.5 ± 2% (mean ± SEM; range: 0.4–5.4%; n = 4). When compared with the level of expression at 5 days (Figure 3), this change was not significant (P = 0.5 by t test). Transduced cell types included basal cells, nonciliated surface cells, ciliated surface cells, cells containing mucus granules, intermediate cells, and rarely, epithelia of submucosal glands. As noted at the 5-day time point, the epithelial morphology appeared normal.
Figure 5.
β-galactosidase expression persists in vivo 6 weeks after gene transfer. (a) En face view demonstrating β-galactosidase–positive cells in the trachea. Larger clusters of blue cells (arrows) were noted at 6 weeks than at the earlier time point, suggesting clonal expansion of transduced cells. (b–d) Cross sections of tissue shown in a. Multiples cell types were targeted as indicated by the arrows. Clusters of β-galactosidase–positive cells were noted, suggesting clonal expansion of targeted cells (n = 4 animals). Epithelial morphology appeared normal as determined by examination of hematoxylin and eosin stained sections. Scale bar in d also applies to b and c. SMG = submucosal gland.
Discussion
For CF lung disease to be treated by gene therapy, there must be lasting correction of defective Cl– transport. In these studies, we make significant progress in addressing several fundamental limitations for gene transfer to airway epithelia. A shortcoming of most current vectors is their inability to effectively transduce airway epithelia when applied to the apical surface. The native receptors for many recombinant viruses are distributed on the basolateral cell surface of polarized cells. Previous investigators noted that VSV-G pseudotyped HIV lentivirus (13) and MuLV (10) inefficiently transduced differentiated airway epithelia in vivo. We (9, 11) and others (38) reported this limitation with MuLV retrovirus envelopes, and there is precedence for such a polarity of gene transfer to airway epithelia with other delivery systems including AAV (28, 39) and adenovirus vectors (20, 40) as well as cationic lipids (41). Although VSV-G–pseudotyped FIV transduced cells poorly from the apical surface, this limitation was overcome using hypotonic/EGTA vector formulation to transiently open epithelial junctions (42–44). Under these conditions, cells throughout the epithelium were transduced in vitro and in vivo, and the CFTR Cl– transport defect was corrected in vitro.
The transduction efficiency of FIV vectors formulated with EGTA is within the range of 6–10% believed sufficient to correct the CF defect (45). Although this work focused on a single vector dose administration, it is possible that lentiviral vectors may be readministered with minimal immune responses and further increase the number of permanently corrected cells (46). This vector formulation method represents a technical advance for vector administration to polarized epithelia in vivo. To translate such a result to patients, epithelial junctions in the human lung could be transiently opened pharmacologically to facilitate vector access to receptors. Aerosol studies in humans show that it is technically feasible to transiently expose the airway epithelium to hypotonic conditions (H2O aerosol) (47) or calcium chelators (EDTA aerosol) (48). In addition, pulmonary lavage under anesthesia might be developed to deliver vector solutions to the human airways. Such whole lung lavage procedures are currently used clinically to treat patients with alveolar proteinosis (49). With current integrating vectors, a lavage approach may be required to facilitate access to receptors on airway progenitor cells, as some of these cell types reside below the mucosal surface (e.g., basal cells, intermediate cells) (50–54).
A further encouraging result of this work was the normal morphology of the airway tissues after gene transfer. There was no evidence of cellular infiltration with immune effector cells when the rabbit airways were examined at the level of light microscopy (Figures 3, 4, and 5). Although this does not eliminate the possibility of any immune response, it contrasts with the cellular responses noted with adenoviral vectors (24, 55–57). Studies with HIV-based lentiviral vectors to date show no evidence of cellular immune responses at the sites of administration in vivo (46). Furthermore, HIV-based vectors (46) and MuLV vectors (58) can be administered a second time in vivo, suggesting that humoral immune responses may not prevent repeated dosing. Thus, enveloped viruses such as lentivirus or MuLV may be less immunogenic when delivered to the lung.
FIV vectors corrected the CFTR defect in vitro for the life of the culture. The failure of adenoviral-mediated CFTR correction to persist reflects both the lack of integration and the gradual loss and dilution of expressing cells by cell division. The persistence of CFTR correction in the FIV vector–transduced cells indicates successful targeting of cells with progenitor capacity. FIV-transduced epithelia also persisted in the trachea in vivo. There are several possible explanations for the small decline in gene expression we noted in the trachea between 5 days and 6 weeks. These include immune responses to the transgene, transcriptional shut off of the CMV promoter, loss of terminally differentiated cells that are targeted (i.e., ciliated cells), and variability related to the effective moi achieved in each animal. Future studies will evaluate these possibilities.
To our knowledge, this is the first evidence of in vivo transduction of airway epithelia with a lentiviral vector. As reported with HIV-based lentiviral vectors (12, 13, 46) or equine infectious anemia virus (EIAV) vectors (59), the present studies show that recombinant FIV vectors transduce nondividing cells in vitro. This is an important finding, as the majority of airway epithelia are mitotically quiescent in the postnatal airways (60–62). FIV vectors transduced epithelial cells throughout the adult rabbit airways in vivo, and gene expression persisted for 6 weeks. Further studies are needed to document the proliferation status of cells at the time of transduction in vivo. Cell types believed to have progenitor capacity in the airway epithelium such as basal cells, intermediate cells, and nonciliated surface cells were transduced (50–52, 54). Integration of the proviral DNA into the host cell chromosome should allow the persistent expression of a therapeutic gene such as CFTR. These studies provide a strong rationale for a vector delivery approach that may be used for the treatment of genetic lung diseases.
Acknowledgments
We thank Phil Karp, Pary Weber, and Jan Launsbach for culturing the human epithelial cells; and Camille Deering, Royce Burns, Jeffrey Brannen, Kerry Wiles, and David Lewis for technical assistance. We thank Hoger Roehl for the development of the PCR titer assay, and Philip Sheridan for the determination of titers by PCR. We thank Michael Welsh, Stanley Perlman, and John Engelhardt for helpful discussions. This work was funded by the Cystic Fibrosis Foundation PO96 (P.B. McCray and B.L. Davidson), National Institute of Health (NIH) RO1HL61460 (P.B. McCray and B.L. Davidson), NIH PPG HL-51670 (P.B. McCray and B.L. Davidson), and the Children’s Miracle Network Telethon. We acknowledge the support of the Morphology Core, the Vector Core and Cell Culture Core, partially supported by the Cystic Fibrosis Foundation, NHLBI (PPG HL51670-05), the Carver Foundation, and the Center for Gene Therapy for Cystic Fibrosis (NIH P30 DK-97-010). P.B. McCray is a recipient of a Career Investigator Award from the American Lung Association. B.L. Davidson and J. Zabner are Fellows of the Roy J. Carver Charitable Trust.
References
- 1.Welsh, M.J., Boat, T.F., Tsui, L.-C., and Beaudet, A.L. 1995. Cystic fibrosis. In The metabolic and molecular basis of inherited disease. C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, editors. McGraw-Hill Inc. New York, NY. 3799–3876.
- 2.Ramsey BW. Drug therapy: management of pulmonary disease in patients with cystic fibrosis [review] N Engl J Med. 1996;335:179–188. doi: 10.1056/NEJM199607183350307. [DOI] [PubMed] [Google Scholar]
- 3.Rich DP, et al. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990;347:358–363. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- 4.Drumm ML, et al. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell. 1990;62:1227–1233. doi: 10.1016/0092-8674(90)90398-x. [DOI] [PubMed] [Google Scholar]
- 5.Miao CH, et al. The kinetics of rAAV integration in the liver. Nat Genet. 1998;19:13–15. doi: 10.1038/ng0598-13. [DOI] [PubMed] [Google Scholar]
- 6.Nakai H, Iwaki Y, Kay MA, Couto LB. Isolation of recombinant adeno-associated virus vector-cellular DNA junctions from mouse liver. J Virol. 1999;73:5438–5447. doi: 10.1128/jvi.73.7.5438-5447.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen JC, et al. Correction of the apical membrane chloride permeability defect in polarized cystic fibrosis airway epithelia following retroviral-mediated gene transfer. Hum Gene Ther. 1992;3:253–266. doi: 10.1089/hum.1992.3.3-253. [DOI] [PubMed] [Google Scholar]
- 8.Halbert CL, Aitken ML, Miller AD. Retroviral vectors efficiently transduce basal and secretory airway epithelial cells in vitro resulting in persistent gene expression in organotypic culture. Hum Gene Ther. 1996;7:1871–1881. doi: 10.1089/hum.1996.7.15-1871. [DOI] [PubMed] [Google Scholar]
- 9.Wang G, et al. Influence of cell polarity on retrovirus-mediated gene transfer to differentiated human airway epithelia. J Virol. 1998;72:9818–9826. doi: 10.1128/jvi.72.12.9818-9826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson LG, et al. Effect of host modification and age on airway epithelial gene transfer mediated by a murine leukemia virus-derived vector. J Virol. 1998;72:8861–8872. doi: 10.1128/jvi.72.11.8861-8872.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang G, et al. Keratinocyte growth factor induced epithelial proliferation facilitates retroviral-mediated gene transfer to pulmonary epithelia in vivo. J Gene Intern Med. 1999;1:22–30. doi: 10.1002/(sici)1521-2254(199901/02)1:1<22::aid-jgm1>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Naldini L, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 13.Goldman MJ, Lee P-S, Yang J-S, Wilson JM. Lentiviral vectors for gene therapy of cystic fibrosis. Hum Gene Ther. 1997;8:2261–2268. doi: 10.1089/hum.1997.8.18-2261. [DOI] [PubMed] [Google Scholar]
- 14.Poeschla EM, Staal FW, Looney DL. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat Med. 1998;4:354–357. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- 15.Johnston JC, et al. Minimum requirements for efficient transduction of dividing and nondividing cells by feline immunodeficiency virus vectors. J Virol. 1999;73:4991–5000. doi: 10.1128/jvi.73.6.4991-5000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartmann K. Feline immunodeficiency virus infection: an overview [review] Vet J. 1998;155:123–137. doi: 10.1016/S1090-0233(98)80008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zabner J, Zeiher BG, Friedman E, Welsh MJ. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time. J Virol. 1996;70:6994–7003. doi: 10.1128/jvi.70.10.6994-7003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 19.Rommens JM, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 20.Walters RW, et al. Basolateral localization of fiber receptors limits adenovirus infection of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 21.Scaria A, et al. Adenovirus-mediated persistent cystic fibrosis transmembrane conductance regulator expression in mouse airway epithelium. J Virol. 1998;72:7302–7309. doi: 10.1128/jvi.72.9.7302-7309.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu M-L, Winther BL, Kay MA. Pseudotransduction of hepatocytes by using concentrated pseudotyped vesicular stomatitis virus G glycoprotein (VSV-G)-moloney murine leukemia virus-derived retrovirus vectors: comparison of VSV-G and amphotropic vectors for hepatic gene transfer. J Virol. 1996;70:2497–2502. doi: 10.1128/jvi.70.4.2497-2502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander IE, Russell DW, Miller AD. Transfer of contaminants in adeno-associated virus vector stocks can mimic transduction and lead to artifactual results. Hum Gene Ther. 1997;8:1911–1920. doi: 10.1089/hum.1997.8.16-1911. [DOI] [PubMed] [Google Scholar]
- 24.McCray PB, Jr, et al. Adenoviral-mediated gene transfer to fetal pulmonary epithelia in vitro and in vivo. J Clin Invest. 1995;95:2620–2632. doi: 10.1172/JCI117964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuller S, von Bonsdorff CH, Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984;38:65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- 26.Thomas DC, Roth MG. The basolateral targeting signal in the cytoplasmic domain of glycoprotein G from vesicular stomatitis virus resembles a variety of intracellular targeting motifs related by primary sequence but having diverse targeting activities. J Biol Chem. 1994;269:15732–15739. [PubMed] [Google Scholar]
- 27.Zabner J, Fasbender AJ, Moninger T, Poellinger KA, Welsh MJ. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem. 1995;270:18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]
- 28.Duan D, Yue Y, McCray PB, Jr, Engelhardt JF. Polarity influences the efficiency of recombinant adeno-associated virus infection in differentiated airway epithelia. Hum Gene Ther. 1998;9:2761–2776. doi: 10.1089/hum.1998.9.18-2761. [DOI] [PubMed] [Google Scholar]
- 29.Zabner J, Smith JJ, Karp PH, Widdicombe JH, Welsh MJ. Loss of CFTR chloride channels alters salt absorption by cystic fibrosis airway epithelia in vitro. Mol Cell. 1998;2:397–403. doi: 10.1016/s1097-2765(00)80284-1. [DOI] [PubMed] [Google Scholar]
- 30.McCray PB, Jr, Zabner J, Jia HP, Welsh MJ, Thorne PS. Efficient killing of inhaled bacteria in ΔF508 mice: role of airway surface liquid composition. Am J Physiol. 1999;277:L183–L190. doi: 10.1152/ajplung.1999.277.1.L183. [DOI] [PubMed] [Google Scholar]
- 31.Zeiher BG, et al. A mouse model for the ΔF508 allele of cystic fibrosis. J Clin Invest. 1995;96:2051–2064. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Neal WK, et al. A severe phenotype in mice with a duplication of exon 3 in the cystic fibrosis locus. Hum Mol Genet. 1993;2:1561–1569. doi: 10.1093/hmg/2.10.1561. [DOI] [PubMed] [Google Scholar]
- 33.Colledge WH, et al. Generation and characterization of a ΔF508 cystic fibrosis mouse model. Nat Genet. 1995;10:445–452. doi: 10.1038/ng0895-445. [DOI] [PubMed] [Google Scholar]
- 34.Kent G, et al. Phenotypic abnormalities in long-term surviving cystic fibrosis mice. Pediatr Res. 1996;40:233–241. doi: 10.1203/00006450-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Snouwaert JN, et al. A murine model of cystic fibrosis. Am J Respir Crit Care Med. 1995;151:S59–S64. doi: 10.1164/ajrccm/151.3_Pt_2.S59. [DOI] [PubMed] [Google Scholar]
- 36.Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 37.Shami, S.G., and Evans, M.J. 1991. Kinetics of pulmonary cells. In Comparative biology of the normal lung. R.A. Parent, editor. CRC Press. Boca Raton, FL. 145–155.
- 38.Zsengeller ZK, et al. Keratinocyte growth factor stimulates transduction of the respiratory epithelium by retroviral vectors. Hum Gene Ther. 1999;10:341–353. doi: 10.1089/10430349950018797. [DOI] [PubMed] [Google Scholar]
- 39.Teramoto S, et al. Factors influencing adeno-associated virus-mediated gene transfer to human cystic fibrosis airway epithelial cells: comparison with adenovirus vectors. J Virol. 1998;72:8904–8912. doi: 10.1128/jvi.72.11.8904-8912.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickles RJ, et al. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu Q, et al. Binding and uptake of cationic lipid: pDNA complexes by polarized airway epithelial cells. Hum Gene Ther. 1999;10:25–36. doi: 10.1089/10430349950019165. [DOI] [PubMed] [Google Scholar]
- 42.Widdicombe JH, Azizi F, Kang T, Pittet JF. Transient permeabilization of airway epithelium by mucosal water. J Appl Physiol. 1996;81:491–499. doi: 10.1152/jappl.1996.81.1.491. [DOI] [PubMed] [Google Scholar]
- 43.Anderson JM, Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 44.Denker BM, Nigam SK. Molecular structure and assembly of the tight junction [review] Am J Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 45.Johnson LG, et al. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat Genet. 1992;2:21–25. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- 46.Kafri T, Blomer U, Peterson DA, Gage FH, Verma IM. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 47.Chan H-K, et al. Regional deposition of nebulized hypodense nonisotonic solutions in the human respiratory tract. Eur Respir J. 1994;7:1483–1489. doi: 10.1183/09031936.94.07081483. [DOI] [PubMed] [Google Scholar]
- 48.Brown J, Mellis CM, Wood RE. Edetate sodium aerosol in pseudomonas lung infection in cystic fibrosis. Am J Dis Child. 1985;139:836–839. doi: 10.1001/archpedi.1985.02140100098043. [DOI] [PubMed] [Google Scholar]
- 49.Rodi G, et al. Whole lung lavage. Monaldi Arch Chest Dis. 1995;50:64–66. [PubMed] [Google Scholar]
- 50.Plopper CG, Nishio SJ, Kass AP, Hyde DM. The role of the nonciliated bronchiolar epithelial (Clara) cell as the progenitor cell during bronchiolar epithelial differentiation in the perinatal rabbit lung. Am J Respir Cell Mol Biol. 1992;7:606–613. doi: 10.1165/ajrcmb/7.6.606. [DOI] [PubMed] [Google Scholar]
- 51.Johnson NF, Hubbs AF. Epithelial progenitor cells in the rat trachea. Am J Respir Cell Mol Biol. 1990;3:579–585. doi: 10.1165/ajrcmb/3.6.579. [DOI] [PubMed] [Google Scholar]
- 52.Inayama Y, et al. The differentiation potential of tracheal basal cells. Lab Invest. 1988;58:706–717. [PubMed] [Google Scholar]
- 53.Randell SH. Progenitor-progeny relationships in airway epithelium. Chest. 1992;101(Suppl.):11S–16S. doi: 10.1378/chest.101.3_supplement.11s. [DOI] [PubMed] [Google Scholar]
- 54.Evans MJ, Johnson LV, Stephens RJ, Freeman G. Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest. 1976;35:246–257. [PubMed] [Google Scholar]
- 55.Simon RH, et al. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: toxicity study. Hum Gene Ther. 1993;4:771–780. doi: 10.1089/hum.1993.4.6-771. [DOI] [PubMed] [Google Scholar]
- 56.Zsengeller ZK, et al. Persistence of replication-deficient adenovirus-mediated gene transfer in lungs of immune-deficient (nu/nu) mice. Hum Gene Ther. 1995;6:457–467. doi: 10.1089/hum.1995.6.4-457. [DOI] [PubMed] [Google Scholar]
- 57.Kaplan JM, et al. Humoral and cellular immune responses of nonhuman primates to long-term repeated lung exposure to Ad2/CFTR-2. Gene Ther. 1996;3:117–127. [PubMed] [Google Scholar]
- 58.McCormack JE, et al. Anti-vector im-munoglobulin induced by retroviral vectors. Hum Gene Ther. 1997;8:1263–1273. doi: 10.1089/hum.1997.8.10-1263. [DOI] [PubMed] [Google Scholar]
- 59.Olsen JC. Gene transfer vectors derived from equine infectious anemia virus. Gene Ther. 1998;5:1481–1487. doi: 10.1038/sj.gt.3300768. [DOI] [PubMed] [Google Scholar]
- 60.McCray PB, Jr, Wang G, O’Brien L, Davidson BL, Thomas P. Proliferation indices of pulmonary epithelia during human and ovine lung development: gene transfer targets for integrating vectors. Cell Vis. 1997;4:1–8. [Google Scholar]
- 61.Ayers MM, Jeffery PK. Proliferation and differentiation in mammalian airway epithelium. Eur Respir J. 1988;1:58–80. [PubMed] [Google Scholar]
- 62.Leigh MW, Kylander JE, Yankaskas JR, Boucher RC. Cell proliferation in bronchial epithelium and submucosal glands of cystic fibrosis patients. Am J Respir Cell Mol Biol. 1995;12:605–612. doi: 10.1165/ajrcmb.12.6.7766425. [DOI] [PubMed] [Google Scholar]