Abstract
Background:
The aim of this study was to compare specimen adequacy of SAP-1 provided for cytology with that of dilation and curettage (D & C) or hysteroscopy for histology, and evaluate the accuracy of combining endometrium sampling by SAP-1 and liquid-based cytology using SurePath preparation for screening endometrial carcinoma and its precursor.
Methods:
Endometrial specimens from women (n = 1514) with risk factors were obtained using an SAP-1 device for cytological analysis; histological samples were obtained from 375 of these women who underwent D & C or hysteroscopy. Cytological specimens were prepared to liquid-based smear using SurePath technology and stained by Papanicolaou. Histological samples were processed in routine pathology and stained by hematoxylin and eosin.
Results:
Adequate specimens for cytology were obtained from 1458/1541 patients (96.3%), while adequate samples for pathology were obtained from 285/375 patients (76%). However, for postmenopausal women, 1006 of 1045 cytology (86.3%) were adequate, 153 of 238 histology (64.3%) were adequate, it was easier to collect cytological specimens than histological specimens (P < 0.05). The accuracy of endometrial cytology for detecting endometrial carcinoma and its precursor was 92.4% (sensitivity, 73%; specificity, 95.8%; positive predictive value, 75%; and negative predictive value, 95.3%).
Conclusions:
Endometrial cytology using SAP-1 sampling and SurePath preparation may be a reliable approach for screening patients with endometrial carcinoma and its precursor.
Keywords: Diagnostic Accuracy, Endometrial Carcinoma, Liquid-based Cytology, Specimen Adequacy
INTRODUCTION
In developed countries, endometrial cancer morbidity has exceeded that of cervical cancer to become the most common invasive malignancy of the female genital tract.[1] Early screening methods cervical cancer have made major progress because of the improvement of cytologic preparation methods and unified evaluation system in the 1950s.[2] Drawing from the successful experience of cervical carcinoma screening, we are pressed for an effective approach to screening endometrial carcinoma and its precursor to reduce new cases and deaths.
Endometrial complex hyperplasia with atypia and endometrial intraepithelial neoplasia (EIN) are considered as precursors of Type I endometrioid adenocarcinoma, and endometrial glandular dysplasia is the precursor of Type II carcinoma.[3,4] A certain period of time for these lesions developing to carcinoma provide a chance for screening them.
Traditionally, endometrial samples for histologic analysis can be obtained from D & C or under hysteroscope, and these methods are considered to be reliable for evaluating the endometrial condition. To date, the adequacy of specimens obtained using the SAP-1 device compared to specimens obtained by D & C or hysteroscopic biopsy, which is better for evaluating the condition of the endometrium, is unknown. This is the first objective of the current study.
The aim of this study was to investigate whether liquid-based SurePath preparation for endometrial cytology test (ECT) can maintain the three-dimensional structure of the endometrium. Histology is the gold standard for evaluating the accuracy, sensitivity, specificity, positive prediction value (PPV), and negative prediction value (NPV) of ECT.
METHODS
This study was performed from November 2011 to May 2014 at the Obstetrics and Gynecology Department of Peking University First Hospital and it had been approved by the hospital ethics committee. Women had at least only one risk factors were enrolled into this study. The risk factors included: (1) Age ≥ 40 years; (2) intrinsically high estrogen including polycystic ovarian syndrome and ovarian tumors with an abnormal level of estrogen such as granulosa cell tumors; (3) extrinsically high estrogen including hormone replacement therapy and postoperative breast cancer patients taking tamoxifen; (4) high body mass index (BMI) ≥25 kg/m2; (5) hypertension, hyperlipidemia and diabetes; (6) family history of cancer including hereditary nonpolyposis colon cancer, Lynch syndrome, and first-degree relatives with gynecologic tumor; (7) history of radiation and smoking; (8) abnormal uterus bleeding (AUB), especially postmenopausal vaginal bleeding; (9) abnormal endometrium assessed by ultrasound as follows: Thickness (postmenopausal women ≥4 mm or menopausal women ≥20 mm), occupation disease of the uterine cavity or heterogeneous. While patients with following conditions were excluded out of this study: (1) Ultrasound scanning suggested uterus cavity was distorted by multiple uterine myomas or adenomyoma; (2) patients with intra-uterine device. 1514 patients were consulted to entry into this study. They provided written informed consent and underwent an ECT using the direct sampler SAP-1 device (Saipujiuzhou Corporation, Beijing, China) [Figure 1]. This device was patented and received permission to use it in our clinic in China.
Figure 1.
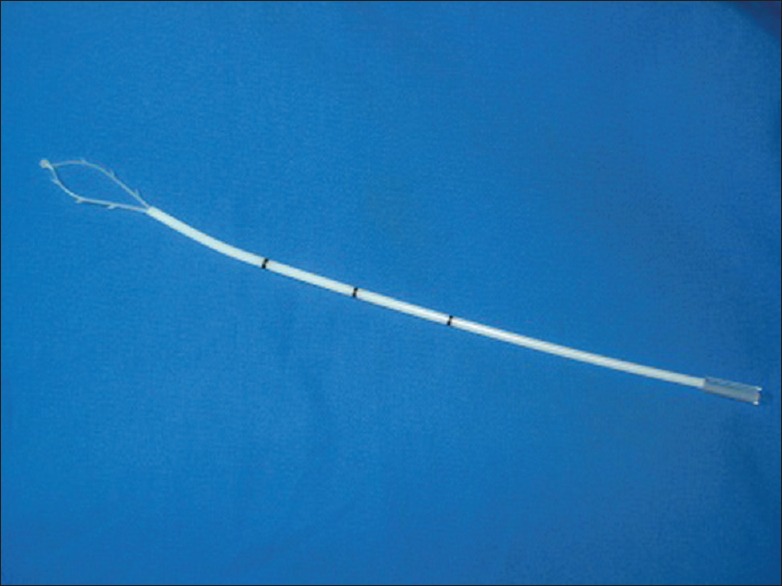
The SAP-1 sampling device.
The SAP-1 sampler measures approximately 3 mm in diameter and 25 cm in length. It consists of a flexible latex loop with spines on the side and a smooth tip to prevent injury to the myometrium. There is an outer protective sheath outside the loop to prevent contamination from cervical and vaginal cells. It is easy to operate and can be used in an outpatient setting, at health examination centers and in community hospitals. The operating steps are described in Figure 2.
Figure 2.
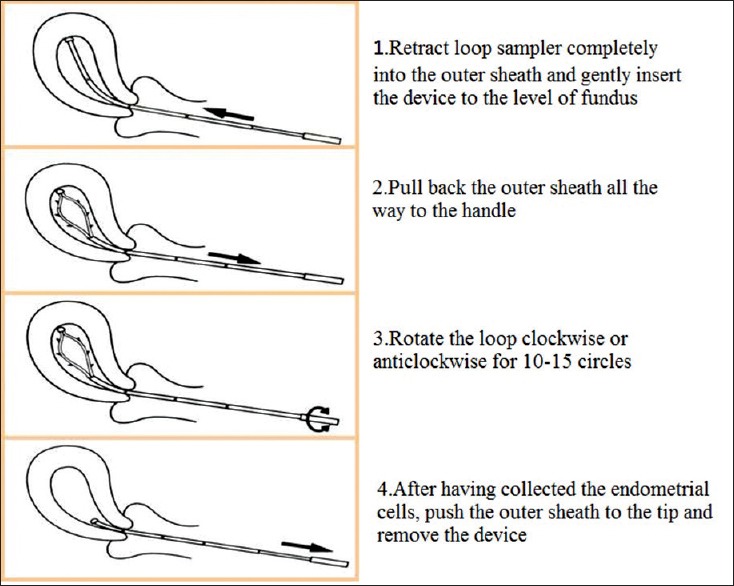
Steps for obtaining samples using the SAP-1 device
The loop with the specimen was then immersed in the SurePath cell preservation container (BD Diagnostic, Burlington, NC, USA) to release the cells. The 10 ml specimen was transferred into centrifuge tubes with a density reagent (BD Diagnostic, Burlington, NC, USA) to remove blood and mucus. After a two-stage centrifugation at 1000 rpm for 2 min 15 s and then 2000 rpm for 10 min 15 s (Rotina 46S, Hettich Corporation, German), the centrifuge tube was put into the SurePath semi-automated slide processor and stained using Papanicolaou.
The cytological smears were evaluated by two independent gynecological cytologists who were blinded to the study procedures. Based on a previously-published diagnostic system,[5] the cytological results were subdivided into four categories as follows: Negative for epithelial lesions, benign endometrium, atypical endometrial cell, and suspected for malignant [Figures 3a–d and 4a–d]. 375 cases were performed D & C or hysteroscopic biopsy or hysterectomy. Endometrial tissue samples were fixed in neutral buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin. Two gynecological pathologists independently assessed the slides, based on the World Health Organization diagnostic criteria[6] and EIN.[7] If a normal or benign endometrium was given, D & C or hysteroscopic pathology was regarded as a final result, we occasionally encountered a situation as cell features fall short of the criteria of simple/complex hyperplasia with atypia, we should carefully evaluated these lesions to determine whether or not a diagnosis of EIN could be made.
Figure 3.
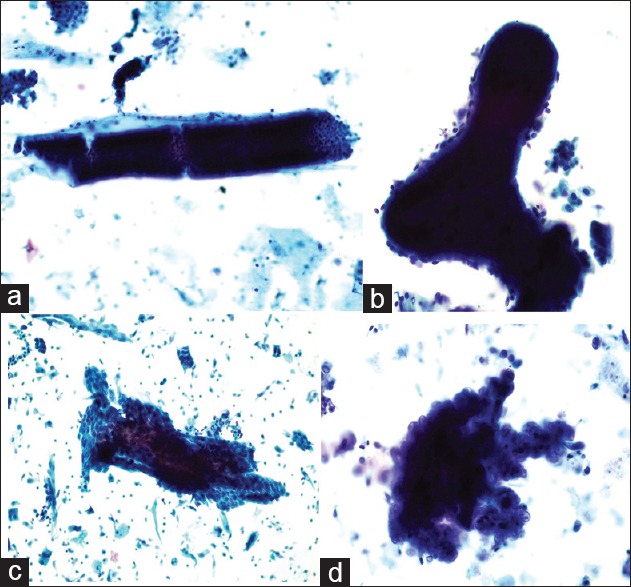
(a) Negative for endometrial lesions: Long, straight tube-shaped cell clumps with a small amount of stromal cells on the margin is the most common type of cell clumps in the proliferative endometrium, observed using a low-power microscope (Papanicolaou stain, ×20); (b) Benign endometrium: Dilated and branched cell clumps are always seen. The contour of the cell clumps is smooth and occasionally a few stromal cells can be observed (Papanicolaou stain, ×40); (c) Atypical endometrial cell: Double-layer or folded irregular cell clumps are observed (Papanicolaou stain, ×20); (d) Suspected endometrial carcinoma: Papillo-shaped bordered cell clumps with atypical cells can be observed (Papanicolaou stain, ×100).
Figure 4.
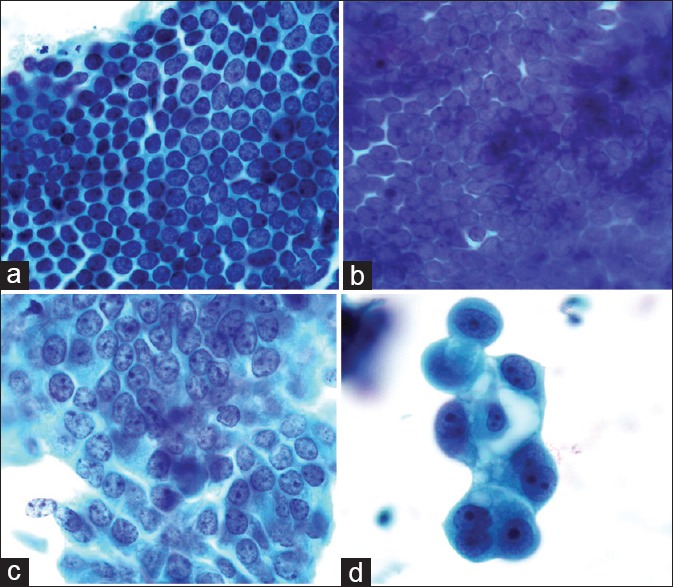
(a) Negative for endometrial lesion: Regularly arranged and mono-layer endometrial cells with an oval or round nucleus. The spaces between nuclei are regular, and the chromatin in endometrial cells is delicate (Papanicolaou stain, ×100); (b) Benign endometrium: Crowded cells arranged into a single layer with delicate chromatin and a small nucleolus (Papanicolaou stain, ×100); (c) Atypical endometrial cell: The spaces in atypical cells are heterogeneous. Some areas are sparse, while others are crowded or even overlapping. The chromatin is coarse (Papanicolaou stain, ×100); (d) Suspected carcinoma: Variable size cells with obviously round nucleoli. The nucleus to cytoplasm ratio is increasing. Varying and large vacuoles appear inside the cytoplasm (Papanicolaou stain, ×100).
Statistical analysis
Statistical analysis was performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). The contingency table Chi-square test was used to compare the adequacy of specimens collected using SAP-1 with those collected via D & C and hysteroscopic biopsy. P < 0.05 was considered statistically significant. Negative for epithelial lesions and benign endometrium were considered negative, while atypical endometrial cells and suspected for malignant were considered positive. Histopathologic results were the gold standard. A four-fold table was created to calculate the accuracy, sensitive, specificity, PPV, and NPV.
RESULTS
The current study comprised of 1514 patients with endometrial carcinoma risk factors underwent SAP-1 sampling on an outpatient basis, and 375 of these women also underwent D & C or hysteroscopy. Characteristics including age distribution, menstrual status, and patients’ symptoms and signs are presented in Table 1. Most of the patients were over 40 years of age in both the cytology and histopathology groups (91.2% and 90.7%, respectively). The percentage of postmenopausal women was 69% and 63.5%, respectively, in the two groups. Among 1514 cases with cytological specimens, 576 (38%) patients had AUB, and 910 (60.1%) had ≥ 4 mm thickness endometrium measured by ultrasound. 169 (45.1%) out of 375 patients with histopathology had AUB, and 245 patients (65.3%) had ≥ 4 mm thickness endometrium.
Table 1.
Patient characteristics
| Charateristics | Cytological specimens n = 1514 (%) | Histopathological specimens n = 375 (%) |
|---|---|---|
| Age | ||
| <40 year | 133 (8.8) | 35 (9.3) |
| ≥40 year | 1381 (91.2) | 340 (90.7) |
| Menstrual status | ||
| Premenopausal | 469 (31) | 137 (36.5) |
| Postmenopausal | 1045 (69) | 238 (63.5) |
| AUBa | 576 (38) | 169 (45.1) |
| Endometrial thicknessb | ||
| <4 mm | 330 (21.9) | 50 (13.3) |
| ≥4 mm | 910 (60.1) | 245 (65.3) |
aAUB: Abnormal uterus bleeding; bSome patients were not examined by ultrasound, and their endometrial thickness is missing.
As presented in Table 2, 1458 patients (96.3%) had adequate specimens for cytology out of the 1514 patients sampled using SAP-1, while 285 cases (76%) had adequate specimens for pathology out of the 375 patients who underwent D & C or hysteroscopic biopsy. There were 56 (3.7%) cytology and 90 (24%) biopsies that were inadequate. SAP-1 can provide more sufficient materials for cytology than D & C or hysteroscopic biopsy for histology (P < 0.01).
Table 2.
Adequate and inadequate specimens obtained using cytology and biopsy
| Items | Cytology n = 1514 (%) | Biopsy n = 375 (%) |
|---|---|---|
| Specimens | ||
| Adequate | 1458 (96.3) | 285 (76.0) |
| Inadequate | 56 (3.7) | 90 (24) |
| P value | <0.01 | |
Of the 469 cytology specimens from premenopausal women, 452 patients (96.4%) had an adequate specimen, and 17 patients (3.6%) had an inadequate specimen. While in 137 cases of premenopausal women obtained biopsy specimens, 132 patients (96.4%) had an adequate biopsy, and only 5 patients (3.6%) had an inadequate biopsy. There was no difference (P = 0.919) between cytology and biopsy in premenopausal women.
However, 1006 specimens (86.3%) were adequate, and 37 specimens (3.7%) were inadequate out of the 1045 cytology samples obtained using SAP-1 in postmenopausal women. In 238 biopsy specimens obtained from postmenopausal women, 153 biopsy specimens (64.3%) were adequate, and 85 specimens (35.7%) were inadequate. It was easier to collect cytology specimens than histology specimens (P < 0.05). The results are shown in Table 3.
Table 3.
Patientsa with both cytologic and histologic results
| Cytology | Histology (gold standard) | ||||
|---|---|---|---|---|---|
| Negativeb | Benignb | Atypiac | Carcinomac | Total | |
| Negativeb | 55 | 90 | 6 | 2 | 153 |
| Benignb | 25 | 35 | 2 | 0 | 62 |
| Atypiac | 3 | 7 | 3 | 5 | 18 |
| Carcinomac | 1 | 0 | 1 | 19 | 21 |
| Total | 84 | 132 | 12 | 26 | 254 |
an = 254 patients; bNegative and benign were served as negative; cAtypia and carcinoma were as positive.
Of the 254 patients who had both of cytology and histology, 205 (80.7%) patients were diagnosed as negative and benign using cytology and histology. 11 (4.3%) patients were evaluated as positive by cytology, but they were confirmed as negative or benign lesions using histology. There were 28 patients who were positive for atypical cells, as determined by both cytology and histology. There were 10 (3.9%) patients who were diagnosed as negative or benign using cytology, while their occult lesions were discovered upon subsequent hysterectomy. The accuracy of cytology for detecting endometrial precursor and carcinoma was estimated at 91.7%, sensitivity at 73.6%, specificity at 94.9%, PPV at 71.9%, and NPV at 95.3%. The results are presented in Table 3.
DISCUSSION
In past decades, endometrial carcinoma has become the most prevalent gynecologic malignancy in developed cities such as Beijing, Shanghai, and Guangzhou in China.[1,8] The increased morbidity of endometrial carcinoma is attributed to changes in lifestyle, such as a lack of exercise and increasing fat intake lead to obesity and high BMI. Obesity triggered several pathways including hormonal imbalance and hyperactive proliferative pathways to involve in pathogenesis of endometrial carcinoma.[9] The effective procedure for reducing morbidity and mortality in endometrial carcinoma is to screen certain groups with risk factors and design a reliable tool suitable for a mass screening program.
Dilation and curettage have been used clinically for many years, and originally it was intended to serve as a screening tool for endometrial carcinoma, but researchers realized that specimen adequacy and diagnosis accuracy were unsatisfactory. Yarandi et al.[10] reported that the accuracy of D & C was 40.5%, sensitivity 40.5%, specificity 72.3%, PPV 77.1% and NPV 25.1%, and in 52.7% of the patients, D & C failed to detect intrauterine disorders, especially focal lesions of the endometrium. Moreover, most patients complained of pain and severe discomfort during the operation, and they had persistent bleeding for several days after the procedure. In Mossa et al.'s study, the patients undergoing D & C had higher pain scores compared with those who underwent brush cytology.[11] For these reasons, D & C is not an ideal screening method.
Currently, several sampling devices were used to take endometrium cytology samples from patients; samplers include Endoflower, Tao Brush, and Endocyte. For Tao Brush sampling,[12] only 1% of all specimens were shown to be nondiagnostic. Buccoliero et al.[13] estimated that out of 519 patients, the samples of biopsy obtained using the Tao Brush was inadequate in 361 patients (39%), and samples obtained using the Endoflower were inadequate in 15 patients (2%). In addition, Buccoliero et al. showed the same results in another study[14] that compared the Endoflower to biopsy in patients receiving tamoxifen.
SAP-1, a direct endometrial sampler device, is especially designed for minimal pain during sampling, it meets the requirements for endometrial samplers, which include: (1) Avoiding contamination from the endocervix and vagina; (2) procuring an adequate representative sample of the entire endometrium to detect focal lesions; and (3) the procedure should be safe, easy to use, and well-tolerated by the patients. The device has to be noninvasive or minimally invasive, cost-effective, and user-friendly to be accepted by primary care physicians and patients for repeated tests.[15] A soft loop can be flexible within the endometrial cavity, and the spindles can easily obtain adequate endometrial cells with minimal chance of injuring myometrium and bleeding. We are the first to evaluate the adequacy of its specimens compared with D & C and hysteroscopic biopsy.
In the current study, adequate specimens obtained using the SAP-1 sampler (96.3%) are superior to those obtained using D & C (76%). In addition, most patients experienced no pain during SAP-1 sampling, claiming that they had the same feeling as the insertion of a cytobrush for cervical sampling.
Our previously-proposed screening strategy involved women of 40 years of age or older, or postmenopausal women as the target screening population.[16] In Buccoliero et al.'s study, of 107 asymptomatic postmenopausal women with a thin endometrium (<4 mm), a biopsy obtained sufficient material for the diagnosis in only 24% of the cases.[17] Our study also shows that there are no differences in specimen adequacy between the SAP-1 sampler and D & C in premenopausal women. However, for postmenopausal women, the SAP-1 sampler provides more adequate specimens than does D & C or hysteroscopic biopsy.
Conventional smears have not been widely applied in endometrial carcinoma screening attributes of overlapping cells and a heavy background.[18,19] Endometrial cells degenerate more easily than other cells. In addition, the three-dimensional structure is an important ECT reference to evaluate the condition of the endometrium. Requirements for a stable fixation system and preparation technique include: (1) Preserving the morphology of endometrial epithelial and stromal cells and the endometrial glandular structure; and (2) eliminating obscuring factors such as excess red blood cells, mucus, overlapping cells, and inflammatory cells.[15] The 95% alcohol fixation and conventional cytology cannot earn a place in endometrial cancer screen due to regression cells and high background levels, such as excess blood, mucus, overlapping cells, and inflammatory cells. According to previous Japanese publications, conventional cytology was initially used to examine the endometrial lesion, with a sensitivity about 78%, specificity of 95%, PPV of 56%, and NPV of 98%. Transvaginal ultrasonography was previously thought to be an efficient tool because of conventional cytology's low accuracy.[20] Compared with a conventional smear, thin-layer cytology provided more cell clumps and a clearer background, as shown by Norimatsu et al.[21]
To improve the diagnostic accuracy of endometrial cytology, a thin-layer method was proposed for endometrial carcinoma and precursor screening. Remondi et al.[22] used the Endoflower sampler and the Thinprep preparation to evaluate 768 postmenopausal women, and found an accuracy of 93.6%, sensitivity of 92%, specificity of 95%, PPV of 73%, and NPV of 99%. The sensitivity, specificity, PPV, and NPV for the Uterobrush were 88.9%, 100%, 100% and 98.9%, respectively.[23] Direct uterine sampling with the Tao Brush sampler using a liquid-based preparation method for detection of endometrial cancer and atypical hyperplasia, the sensitivity and specificity were 95% and 66%.[24] The results of the current study demonstrate that the SAP-1 sampler combined with the liquid-based SurePath preparation method is a reliable diagnostic method. The overall accuracy for detecting endometrial cancer, complex hyperplasia with atypia and EIN was 92.4%, with a sensitivity of 73%, a specificity of 95.8%, a PPV of 75%, and a NPV of 95.3%.
Although the findings of current studies were informative, there were some limitations. Almost all patients enrolled into this study received the ECT for screening, so women with positive cytologic results or persistent bleeding underwent hysteroscopic biopsy or D & C. In the current study, patients with carcinoma that was subsequently confirmed by histology were initially diagnosed with atypical endometrial cells or suspected carcinoma using cytology. The main reasons that the accuracy of this study was lower than that in previously studies are as follows: Patients with positive results including atypical cells underwent hysteroscopic biopsy and D & C, and some of them were subsequently diagnosed as normal or benign. In addition, other methods, including enlarging the sample quantity to collect more information to correctly distinguish focal atypical endometrial lesions from benign lesions, should be investigated using immunohistochemistry and biochemistry to improve the accuracy of endometrial cytology.
In conclusion, the SAP-1 sampler combined with SurePath preparation may become a reliable method for screening endometrial carcinoma and its precursor, especially in postmenopausal and asymptomatic women. If this screening procedure can be used in the high risk-factor women, some unnecessary D & C may be avoided, and asymptomatic women with the precursor may benefit from early detection and management.
Footnotes
Edited by: Jian Gao
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Committee on Practice Bulletins – Gynecology. ACOG practice bulletin number 131: Screening for cervical cancer. Obstet Gynecol. 2012;120:1222–38. doi: 10.1097/aog.0b013e318277c92a. [DOI] [PubMed] [Google Scholar]
- 3.Kurman RJ, McConnell TG. Precursors of endometrial and ovarian carcinoma. Virchows Arch. 2010;456:1–12. doi: 10.1007/s00428-009-0824-9. [DOI] [PubMed] [Google Scholar]
- 4.Jarboe EA, Mutter GL. Endometrial intraepithelial neoplasia. Semin Diagn Pathol. 2010;27:215–25. doi: 10.1053/j.semdp.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Zhao J. The diagnostic system of endometrial cytology. China J Reprod Health. 2006;17:6–8. [Google Scholar]
- 6.Silverberg SG, Mutter GL, Kurman RJ, Kubik-Huch RA, Nogales F, Crum CP, et al. Tumors of the uterine corpus: Epithelial tumors and related lesions. In: Tavassoli FA, Stratton MR, editors. WHO Classification of Tumors: Pathology and Genetics of Tumors of the Breast and Female Genital Organs. Lyon: IARC Press; 2003. pp. 221–32. [Google Scholar]
- 7.Mutter GL. Endometrial intraepithelial neoplasia (EIN): Will it bring order to chaos? The Endometrial Collaborative Group. Gynecol Oncol. 2000;76:287–90. doi: 10.1006/gyno.1999.5580. [DOI] [PubMed] [Google Scholar]
- 8.Hao J, Zhao P, Chen WQ. Beijing, China: Military Medical Science Press; 2012. Chines Cancer Registry Annual Report; pp. 100–1. [Google Scholar]
- 9.Schmandt RE, Iglesias DA, Co NN, Lu KH. Understanding obesity and endometrial cancer risk: Opportunities for prevention. Am J Obstet Gynecol. 2011;205:518–25. doi: 10.1016/j.ajog.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarandi F, Izadi-Mood N, Eftekhar Z, Shojaei H, Sarmadi S. Diagnostic accuracy of dilatation and curettage for abnormal uterine bleeding. J Obstet Gynaecol Res. 2010;36:1049–52. doi: 10.1111/j.1447-0756.2010.01288.x. [DOI] [PubMed] [Google Scholar]
- 11.Mossa B, Ebano V, Marziani R. Reliability of oupatient endometrial brush cytology vs biopsy in postmenopausal symptomatic women. Eur J Gynaecol Oncol. 2010;31:621–6. [PubMed] [Google Scholar]
- 12.Norimatsu Y, Kouda H, Kobayashi TK, Moriya T, Yanoh K, Tsukayama C, et al. Utility of thin-layer preparations in the endometrial cytology: Evaluation of benign endometrial lesions. Ann Diagn Pathol. 2008;12:103–11. doi: 10.1016/j.anndiagpath.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Buccoliero AM, Gheri CF, Castiglione F, Garbini F, Barbetti A, Fambrini M, et al. Liquid-based endometrial cytology: Cyto-histological correlation in a population of 917 women. Cytopathology. 2007;18:241–9. doi: 10.1111/j.1365-2303.2007.00463.x. [DOI] [PubMed] [Google Scholar]
- 14.Buccoliero AM, Fambrini M, Gheri CF, Castiglione F, Garbini F, Barbetti A, et al. Surveillance for endometrial cancer in women on tamoxifen: The role of liquid-based endometrial cytology - Cytohistological correlation in a population of 168 women. Gynecol Obstet Invest. 2008;65:240–6. doi: 10.1159/000113047. [DOI] [PubMed] [Google Scholar]
- 15.Tao LC. Direct intrauterine sampling: The IUMC endometrial sampler. Diagn Cytopathol. 1997;17:153–9. doi: 10.1002/(sici)1097-0339(199708)17:2<153::aid-dc13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 16.Wu C. The screening strategy of endometrial cancer. China J Fam Plann. 2012;20:717–9. [Google Scholar]
- 17.Buccoliero AM, Caldarella A, Noci I, Borri P, Giachi M, Borrani E, et al. Thin-layer cytology in endometrial diagnosis. Pathologica. 2003;95:179–84. [PubMed] [Google Scholar]
- 18.Gusberg SB, Milano C. Detection of endometrial cancer and its precursors. Cancer. 1981;47(5 Suppl):1173–5. doi: 10.1002/1097-0142(19810301)47:5+<1173::aid-cncr2820471320>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Hattori M, Kobayashi TK, Nishimura Y, Machida D, Toyonaga M, Tsunoda S, et al. Comparative image analysis of conventional and thin-layer preparations in endometrial cytology. Diagn Cytopathol. 2013;41:527–32. doi: 10.1002/dc.22891. [DOI] [PubMed] [Google Scholar]
- 20.Tsuda H, Kawabata M, Yamamoto K, Inoue T, Umesaki N. Prospective study to compare endometrial cytology and transvaginal ultrasonography for identification of endometrial malignancies. Gynecol Oncol. 1997;65:383–6. doi: 10.1006/gyno.1997.4699. [DOI] [PubMed] [Google Scholar]
- 21.Norimatsu Y, Shigematsu Y, Sakamoto S, Ohsaki H, Yanoh K, Kawanishi N, et al. Nuclear characteristics of the endometrial cytology: Liquid-based versus conventional preparation. Diagn Cytopathol. 2013;41:120–5. doi: 10.1002/dc.21784. [DOI] [PubMed] [Google Scholar]
- 22.Remondi C, Sesti F, Bonanno E, Pietropolli A, Piccione E. Diagnostic accuracy of liquid-based endometrial cytology in the evaluation of endometrial pathology in postmenopausal women. Cytopathology. 2013;24:365–71. doi: 10.1111/cyt.12013. [DOI] [PubMed] [Google Scholar]
- 23.Iavazzo C, Vorgias G, Mastorakos G, Stefanatou G, Panoussi A, Alexiadou A, et al. Uterobrush method in the detection of endometrial pathology. Anticancer Res. 2011;31:3469–74. [PubMed] [Google Scholar]
- 24.Kipp BR, Medeiros F, Campion MB, Distad TJ, Peterson LM, Keeney GL, et al. Direct uterine sampling with the Tao brush sampler using a liquid-based preparation method for the detection of endometrial cancer and atypical hyperplasia: A feasibility study. Cancer. 2008;114:228–35. doi: 10.1002/cncr.23636. [DOI] [PubMed] [Google Scholar]


