Supplemental Digital Content is Available in the Text.
The Pain in Neuropathy Study (PiNS), an observational cross-sectional multicentre study, demonstrated a positive correlation between greater diabetic neuropathy severity and the presence and severity of neuropathic pain.
Keywords: Diabetes, Diabetic neuropathy, Neuropathic pain, Quantitative sensory testing, Chronic pain
Abstract
Disabling neuropathic pain (NeuP) is a common sequel of diabetic peripheral neuropathy (DPN). We aimed to characterise the sensory phenotype of patients with and without NeuP, assess screening tools for NeuP, and relate DPN severity to NeuP. The Pain in Neuropathy Study (PiNS) is an observational cross-sectional multicentre study. A total of 191 patients with DPN underwent neurological examination, quantitative sensory testing, nerve conduction studies, and skin biopsy for intraepidermal nerve fibre density assessment. A set of questionnaires assessed the presence of pain, pain intensity, pain distribution, and the psychological and functional impact of pain. Patients were divided according to the presence of DPN, and thereafter according to the presence and severity of NeuP. The DN4 questionnaire demonstrated excellent sensitivity (88%) and specificity (93%) in screening for NeuP. There was a positive correlation between greater neuropathy severity (r = 0.39, P < 0.01), higher HbA1c (r = 0.21, P < 0.01), and the presence (and severity) of NeuP. Diabetic peripheral neuropathy sensory phenotype is characterised by hyposensitivity to applied stimuli that was more marked in the moderate/severe NeuP group than in the mild NeuP or no NeuP groups. Brush-evoked allodynia was present in only those with NeuP (15%); the paradoxical heat sensation did not discriminate between those with (40%) and without (41.3%) NeuP. The “irritable nociceptor” subgroup could only be applied to a minority of patients (6.3%) with NeuP. This study provides a firm basis to rationalise further phenotyping of painful DPN, for instance, stratification of patients with DPN for analgesic drug trials.
1. Introduction
Diabetic peripheral neuropathy (DPN) is one of the most frequent complications of diabetes mellitus46 and affects between 28% and 49% of patients.1,53 Between 25% and 50% of patients with DPN develop neuropathic pain (NeuP)1,8,50—defined as pain arising as a consequence of a lesion or disease of the somatosensory nervous system.26,48 This pain is typically localised to the feet but can spread proximally with more advanced disease.47 Although analgesic agents exist for the symptomatic treatment of NeuP (including tricyclic antidepressants, serotonin–norepinephrine reuptake inhibitors and gabapentinoids),12,21 treatment is often inadequate and NeuP has a subsequent major deleterious impact on quality of life.17,24,50 Diabetic peripheral neuropathy is one of the commonest causes of NeuP in the community, and given the increasing prevalence of type 2 diabetes mellitus, it will become even more frequent in the future.55
The pathophysiology of NeuP in DPN is complex and not fully understood. The initial inciting event is a “dying back” axonopathy principally affecting sensory neurons.12 It is believed that these neurons become hyperexcitable as a consequence of altered gene expression and posttranslational modification of key ion channels.30,51 There are also changes with the central nervous system, both at the level of the spinal cord and brain at an anatomical and functional level leading to amplification of nociceptive processing.45
In the last decade, significant advances in techniques to define somatosensory phenotype in the context of NeuP have been developed. These include questionnaires, such as DN4,6 painDETECT,22 and LANSS,4 designed to distinguish NeuP from other types of pain using patient-reported pain descriptors (and in some cases examination findings) and standardisation of quantitative sensory testing (QST) (for instance, the standardised protocol of the German Neuropathic Pain Network).38 Quantitative sensory testing assesses evoked sensory perception in response to a defined sensory stimulus. This can be used to test a range of sensory modalities including small-fibre sensory function (eg, thermal and pain thresholds) and large-fibre modalities (eg, vibration threshold).38
Application of these techniques has shown that NeuP is a multidimensional entity, and there are distinct subgroups of patients who have particular patterns of sensory symptoms and signs. Such sensory phenotypes may reflect particular pathophysiological mechanisms.51 For example, certain patients have principally deafferentation with loss of sensory function, whereas others have evidence of preserved small-fibre function and associated hypersensitivity, a pattern termed the irritable nociceptor.16,20 An aspiration is to use patient stratification in regard to targeting therapy.2 Sensory phenotyping may aid in the design of randomised controlled trials of analgesics, for instance, by enrolling patients according to the sensory phenotype that may be most responsive to a particular drug.9,41
In the Pain in Neuropathy Study (PiNS), we aimed to characterise, in unprecedented depth, the somatosensory phenotype of patients with DPN. Our objectives were (1) to compare patients with and without NeuP to identify sensory abnormalities that were specific to NeuP, (2) to compare some of the existing commonly used screening tools for NeuP in these populations, and (3) to relate sensory phenotype to measures of DPN severity and HbA1c to investigate the relationship between NeuP and the underlying disease process.
2. Material and methods
2.1. Study design and patients
The PiNS is an observational cross-sectional multicentre study approved by the National Research Ethics Service of the United Kingdom (No.: 10/H07056/35). Most of the study participants were recruited from primary care practices in London and Oxford. Study participants were also recruited from diabetes clinics at Chelsea and Westminster Hospital (London), Sheffield Teaching Hospitals and Oxford University Teaching Hospitals; neurology clinics at King's College Hospital (London), and through advertisements. All study participants signed written consent before participating.
Patients with diabetes mellitus aged above 18 years with diagnosed DPN, or patients with symptoms and signs suggestive of DPN were included. Exclusion criteria were pregnancy, coincident major psychiatric disorders, poor or no English language skills, severe pain at recruitment from a cause other than DPN (to prevent potential confounding influence on pain reporting as well as psychological and quality-of-life reported outcomes), patients with documented central nervous system lesions, or patients with insufficient mental capacity to provide informed consent or to complete questionnaires.
The sensory phenotyping paradigm was aligned with a previous study of HIV polyneuropathy.36 The study design consisted of a single clinical assessment appointment with questionnaires that were completed either before or after the appointment and then returned to the study centre by post. During the clinical assessment appointment, participants had detailed medical and drug histories documented that included sex, age, ethnicity, medical history, date of diabetes mellitus diagnosis, presence of a family history of neuropathy, presence of other potential causes of neuropathy (hypothyroidism, HIV, alcohol misuse, vitamin B12 deficiency, and neurotoxic drug exposure); smoking and alcohol consumption were assessed using UK Department of Health methodology.23 Basic clinical parameters were then measured for each participant (weight, height, and lying/standing blood pressures). Participants then underwent a structured neurological examination, a detailed QST assessment, nerve conduction studies, and skin biopsy. After the clinical assessment, the study investigators collected further drug, laboratory, and clinical investigation data from the clinical records. The data, where available, included detailed drug histories, nerve conduction study data, and the most recent routine haematological and biochemical parameters.
2.2. Structured neurological examination
A comprehensive structured upper and lower limb neurological examination was performed to detect clinical signs of a peripheral neuropathy.27,34 The examination included assessment of temperature, light touch and pinprick sensation, joint position proprioception, vibration perception, deep-tendon reflexes, muscle bulk, and motor power. Orthostatic hypotension, as a marker of autonomic neuropathy, was assessed by measuring lying and standing blood pressure in accordance with established protocols.14 Orthostatic hypotension was defined as either a 20 mm Hg reduction in systolic or a 10 mm Hg reduction in diastolic blood pressure assessed two minutes after standing.
2.3. Nerve conduction tests
Nerve conduction tests were performed with an Advance system (NeuroMetrix, Waltham, MA) and used conventional reusable electrodes. Sural sensory and peroneal motor nerve conduction studies were performed in one lower extremity. If both studies were normal,11 no further tests were performed. If either test was abnormal, additional nerve conductions were performed that included ipsilateral tibial motor nerve; contralateral sural sensory nerve, peroneal motor or tibial motor nerves; or ulnar sensory, median sensory, and ulnar motor nerves in one upper extremity. The minimum case definition criterion for electrodiagnostic confirmation of DPN was an abnormality of any attribute of nerve conduction in 2 separate nerves, one of which was the sural nerve. Variables such as skin temperature, age, height, sex, and weight were measured and accounted for when interpreting nerve conduction tests. Our protocol was in line with those recommended by the American Academy of Neurology and American Association of Electrodiagnostic Medicine.19 Nerve conduction tests were not repeated if study participants had previous results.
2.4. Skin biopsy for intraepidermal nerve fibre density assessment
The determination of intraepidermal nerve fibre density (IENFD) from skin biopsy samples is a validated and sensitive diagnostic tool for the assessment of small-fibre neuropathies, including diabetic neuropathy.29 Punch biopsies of the skin were performed immediately after the completion of QST. Skin biopsies were not taken from participants on warfarin or those who had other contraindications to skin biopsy. Biopsy samples were taken in accordance with the consensus document produced by the European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the utilisation of skin biopsy samples in the diagnosis of peripheral neuropathies.29 Subcutaneous anaesthesia was achieved with lidocaine (1%, 1-1.2 mL) before the biopsy was taken under sterile conditions. Skin biopsies were taken 10 cm proximal to the lateral malleolus with a disposable 3-mm punch biopsy circular blade (Stiefel Laboratories Inc, GSK Plc, Brentford, United Kingdom). The biopsy was fixed in fresh periodate-lysine-paraformaldehyde (2%) for 12 to 24 hours. Tissue was then washed in 0.1 M phosphate buffer and stored for 2 to 3 days in 15% sucrose in 0.1 M phosphate buffer. After embedding in O.C.T. (Fisher Scientific UK Ltd, Loughborough, United Kingdom), the tissue was snap-frozen and stored at −80°C.
Sections with 50-μm thickness were cut on a cryostat, and immunohistochemistry for protein gene product 9.5 was performed on free-floating sections using the immunoperoxidase method. Samples were selected at random. Staining was performed on 24-well plates allowing reagents complete penetration of floating samples. For the bright-field method, samples were washed with TBS and placed on a 5% hydrogen peroxide solution on ethanol, followed by blockade of nonspecific protein binding with 4% normal donkey serum, 0.5% milk, and 0.1% Triton X in TBS. Primary antibody rabbit anti–protein gene product 9.5 Ab (1:15,000; Ultraclone Ltd, Yarmouth, Isle of Wight, United Kingdom) was incubated overnight at room temperature. After rinsing the samples with TBS, secondary Biotinylated Goat Anti-Rabbit IgG Antibody (1:400; Vector Laboratories, Burlingame, CA) was used followed by addition of VECTASTAIN ABC Kit (Vector Laboratories) for 1 hour. Samples were washed and transferred to the DAB Peroxidase Substrate Kit, 3,3′-diaminobenzidine (Vector Laboratories) until a visible stain emerged. Samples were rinsed with distilled water and progressively dehydrated with ethanol. Samples were mounted using DPX mounting media. Protein gene product 9.5-immunoreactive nerve fibres crossing the basal membrane of the epidermis were counted under a ×40 objective, and a measurement of the length of the sample was also obtained. The IENFD was assessed using a double bright-field microscope at ×40 magnification using established counting rules and expressed as fibres per millimetre of epidermal length. IENFD was considered abnormal if it was below the fifth centile for age- and sex-matched healthy controls.28
2.5. Quantitative sensory testing
Somatosensory phenotypes were determined according to a previously published protocol of the German research network of neuropathic pain (DFNS).38 The investigators (A.C.T., S.R.P., and J.D.R.) underwent a formal training in conducting the DFNS QST protocol at Mannheim University using healthy volunteers. Cold and warm detection thresholds as well as cold and heat pain thresholds and thermal sensory limen (including paradoxical heat sensations) were established using a Thermotest (Somedic, Hörby, Sweden). We also tested mechanical detection and pain thresholds and mechanical pain sensitivity, allodynia, pressure pain thresholds (PPTs), wind-up ratio (WUR), and vibration detection thresholds. Participants were familiarized with the testing procedure on the dorsum of the forearm before all parameters were measured over the dorsum of both feet (S1 dermatome). Pressure pain thresholds were recorded over the arch of the foot, and vibration detection thresholds were tested over the medial malleolus.
Quantitative sensory testing data were entered into the data analysis system Equista provided by the DFNS. Equista transformed the raw QST data into z-scores thus normalising for age, sex, and the body location of testing.31,39 Positive z-scores denote gain of function, whereas negative z-scores denote loss of function. The sample size was determined according to the warm detection threshold data for patients with diabetes.40 This calculation revealed that a minimum sample size of 34 was required per group for a power of >0.8 (difference in means 2.0; SD 4.3; α = 0.05).
We calculated warm and cold sensibility indices to determine the pain sensitivity range in which thermal sensations were felt.25 The warm sensibility index (WSI) was defined as: (warm pain detection threshold − warm threshold)/(warm pain detection threshold − reference temperature). The cold sensibility index (CSI) was defined as: (cold pain detection threshold − cold threshold)/(cold pain detection threshold − reference temperature).
2.6. Neuropathy screening tools, symptom, and function questionnaires
A set of questionnaires assessing the presence of pain, pain intensity, pain distribution, and the psychological and functional impact of pain were either completed before the appointment or after the appointment and then returned to the study centre by post. We briefly describe the tools used. Detailed descriptions of the tools can be found in supplementary methods (available online as Supplemental Digital Content at http://links.lww.com/PAIN/A226).
Patients were asked to keep a pain intensity diary for 7 days, recording pain at 9 am and 9 pm daily on an 11-point scale, with 0 being no pain and 10 the worst pain imaginable. Study participants also completed a body map highlighting the distribution of any pain experienced. The surface area of NeuP was calculated from the body areas highlighted on the body map. Areas of pain were highlighted on a computer-generated image of a standardised body map, and the surface area of NeuP was subsequently quantified. The pain diary was used to assess the presence and severity of painful diabetic neuropathy.
The Toronto Clinical Scoring System (TCSS)10 was used as a screening tool for DPN and correlates with diabetic neuropathy severity. The Douleur Neuropathique en 4 Questions (DN4)6 was used as a screening tool for NeuP. The PainDETECT22 was used as a self-administered screening tool for NeuP. The Brief Pain Inventory (BPI)44 includes pain severity and pain interference scores that were used to assess the severity any type of pain (nonneuropathic and neuropathic) experienced and the impact of the pain on activities of daily living. The Neuropathic Pain Symptom Inventory (NPSI),7 a self-administered questionnaire, was used to evaluate NeuP symptoms including evoked pain, spontaneous pain, paroxysmal pain, and dysesthesias. The Pain Catastrophizing Scale (PCS)35 was used to assess the cognitive process by which pain is appraised. The Depression Anxiety Positive Outlook instrument (DAPOS)37 was used to measure mood and anxiety. The Pain Anxiety Symptom Scale 20 (PASS-20)33 was used to assess pain-related anxiety. Survey of Autonomic Symptoms (SAS)54 was used to measure the presence and impact of autonomic symptoms. The Insomnia Severity Index (ISI)3 was used to measure the study participant's perception, subjective symptoms, and the consequences of any sleep disturbances. The Short-Form 36 of the MOS Outcomes Study (SF-36)52 was used for the assessment of health-related quality of life.
2.7. Definition of painful diabetic neuropathy
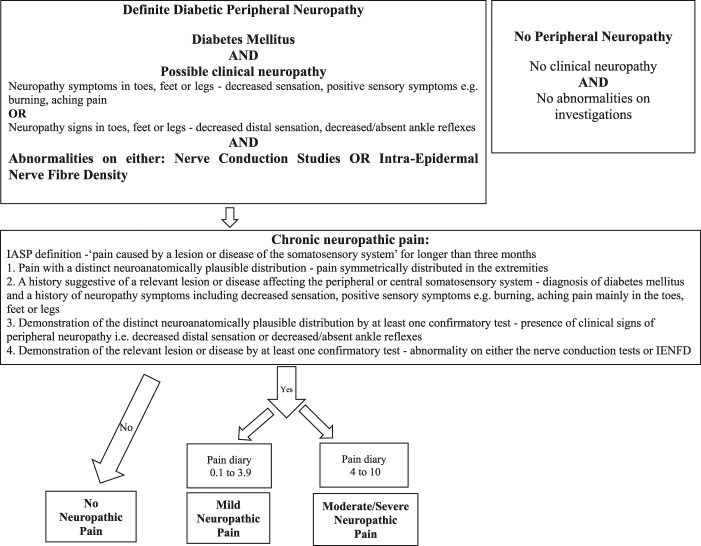
All study participants enrolled had diabetes mellitus. Study participants were screened for a “possible” clinical neuropathy defined as a combination of symptoms or signs of neuropathy including any one or more of the following: neuropathic symptoms (decreased sensation, positive sensory symptoms, eg, burning, aching pain) mainly in the toes, feet, or legs; decreased distal sensation; decreased/absent ankle reflexes, and the DPN was then confirmed by abnormalities on either the nerve conduction studies or IENFD46 (Figure 1). Only study participants with a confirmed DPN proceeded to NeuP subtyping.
Figure 1.
Flow diagram of study participant recruitment and the criteria used to subdivide the study participants into the different subgroups.
The presence of chronic NeuP caused by peripheral DPN was determined at the time of the clinical assessment and was in line with the IASP definition of NeuP, ie, “pain caused by a lesion or disease of the somatosensory system” (Figure 1) and the IASP/NeuPSIG grading system was used.48
The assessment of each study participant therefore satisfied the following criteria:
Pain with a distinct neuroanatomically plausible distribution, ie, pain symmetrically distributed in the extremities—completion of body map and clinical history.
A history suggestive of a relevant lesion or disease affecting the peripheral or central somatosensory system—diagnosis of diabetes mellitus and a history of neuropathic symptoms including decreased sensation, positive sensory symptoms, eg, burning, aching pain mainly in the toes, feet, or legs
Demonstration of the distinct neuroanatomically plausible distribution by at least 1 confirmatory test—presence of clinical signs of peripheral neuropathy, ie, decreased distal sensation or decreased/absent ankle reflexes
Demonstration of the relevant lesion or disease by at least 1 confirmatory test—abnormality on either the nerve conduction tests or IENFD.
Only study participants with chronic NeuP present for at least 3 months were included in the NeuP groups. Study participants with non-NeuP in the extremities, such as musculoskeletal pain of the ankle, were included in the no NeuP group. The severity of NeuP was calculated as the mean of the pain scores obtained from the 7-day pain intensity diary. The study participants were thereafter allocated into 3 groups based on the mean pain intensity score: 0: no NeuP, 0.1 to 3.9: mild NeuP, and 4 to 10: moderate/severe NeuP.18
2.8. Statistical analysis
SPSS Statistics Version 21 (IBM) and GraphPad Prism were used for statistical analysis. The QST z-scores were compared across the 3 groups with 1-way ANOVA (LSD post hoc test), or between 2 groups with unpaired t-tests. The QST z-score data were expressed as mean ± 95% CI. All other data were tested for normality with the D'Agostino-Pearson normality test and by visual inspection of their distribution. All other data were not normally distributed and reported as median with interquartile range (IQR). Data were compared across the 3 groups with the Kruskal–Wallis test (Dunn pots hoc test), or between 2 groups with the Mann–Whitney U test. Spearman correlation analyses were performed to explore associations between histological parameters, QST findings, neurophysiological data, and symptom and functional outcomes. Categorical data were analysed with χ2 test of association. Significance was set at P = 0.05.
3. Results
3.1. Study participants
A total of 209 patients were assessed. We recruited 191 study participants with DPN (9 study participants were excluded because they did not complete their 7-day pain intensity dairies). A smaller group, as a result of targeted recruitment for DPN, of 18 study participants were found not to have DPN according to our criteria. All study participants were clinically assessed by one of the study investigators (A.C.T., J.D.R., and P.R.S.). Study participants with DPN were divided into groups according to the severity of their NeuP: 80 participants had no NeuP, 41 had mild NeuP, and 70 had moderate/severe NeuP.
3.2. Demographics and pharmacotherapy use
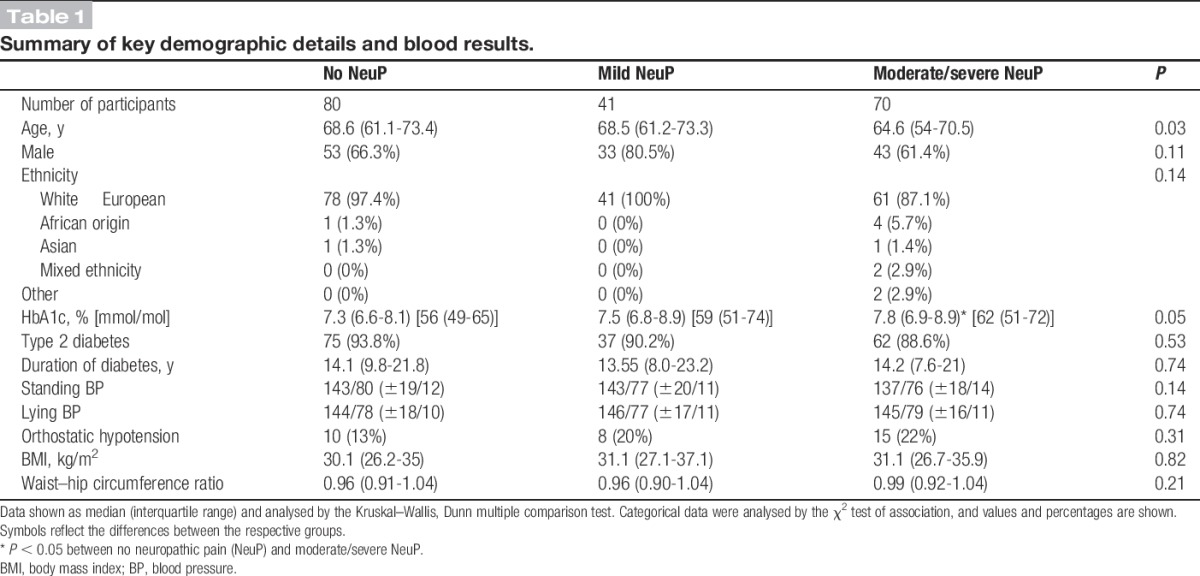
All study participants had a diagnosis of diabetic mellitus. Most of the study participants were aged above 60 years and were white, and two thirds of the participants were men (Table 1). There were no significant differences between the different groups in terms of sex, ethnicity, body mass index, waist–hip circumference. Most of the study participants (91.1%) had type 2 diabetes mellitus, in line with population prevalence. Study participants across the 3 groups were diagnosed with diabetes for a similar duration. There were 17 study participants who had type 1 diabetes mellitus and 12 participants with DPN NeuP. It is therefore not possible to comment on variations of somatosensory phenotype between type 1 and type 2 diabetic participants, as the size of the type 1 cohort is too small to make a meaningful comparison. However, a significant finding is that the median (IQR) duration of diabetic treatment was different between the 2 groups: 31.8 (28.4) years for type 1 diabetes mellitus and 13.3 (12.4) years for type 2 diabetes mellitus (Mann–Whitney U test, P < 0.01). The participants with moderate/severe NeuP were slightly younger and had poorer diabetic control (demonstrated by a significantly higher HbA1c, results available in 199 (95%) of study participants) compared with those with DPN with no NeuP (Table 1). HbA1c correlated with NeuP severity (r = 0.21, P < 0.01), and although the association is not strong, it is statistically significant.
Table 1.
Summary of key demographic details and blood results.
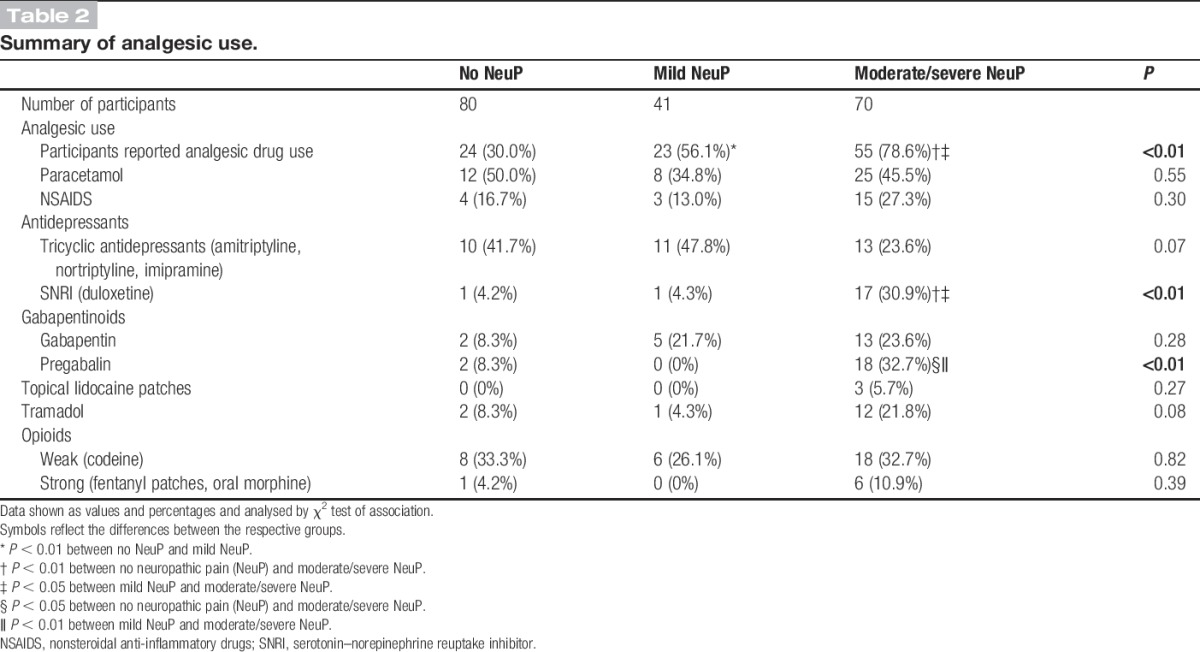
There was increased reported analgesic use in study participants with NeuP (Table 2). Those with the moderate/severe NeuP reported greater use of the serotonin–norepinephrine reuptake inhibitors (SNRIs) duloxetine and pregabalin. Although the mild NeuP reported greater use of analgesics, the choice of analgesic did not differ compared with the study participants with no NeuP. Study participants with DPN with no NeuP were prescribed antidepressants or antiepileptics classically used for NeuP. The reasons for the use of amitriptyline were either as a night time sedative for sleeping difficulties or for pain (not necessarily neuropathic) that was unrelated to their DPN. Gabapentinoids were prescribed for suspected NeuP unrelated to their DPN, and it should be noted that these patients did not have a history of painful DPN that was relieved completely by their treatment. We have included a summary of all reported drug use (Supplementary Table 1, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A226).
Table 2.
Summary of analgesic use.
3.3. Pain questionnaires, pain diary, and body maps
Study participants with painful DPN scored higher on screening tools, the DN4 and painDETECT, designed to detect pain with neuropathic characteristics (Table 3). Our gold standard for the definition of NeuP was the IASP/NeuPSIG grading system.48 Using this as a reference, the sensitivity of DN4 and painDETECT was 88.3% and 61.3%, respectively, and the specificity of DN4 and painDETECT was 92.5% and 91.7%, respectively. The DN4 and painDETECT, correlated well with the mean score obtained from the 7-day pain intensity diary (Fig. 2). Some study participants with DPN and no NeuP still experienced pain in their feet or legs, such as musculoskeletal pain of the ankle or leg cramps, that was unrelated to their DPN. The DN4 and painDETECT quantified the NeuP characteristics of this nonneuropathic lower limb pain (Table 3)
Table 3.
Summary of neuropathic pain (NeuP) screening scores.
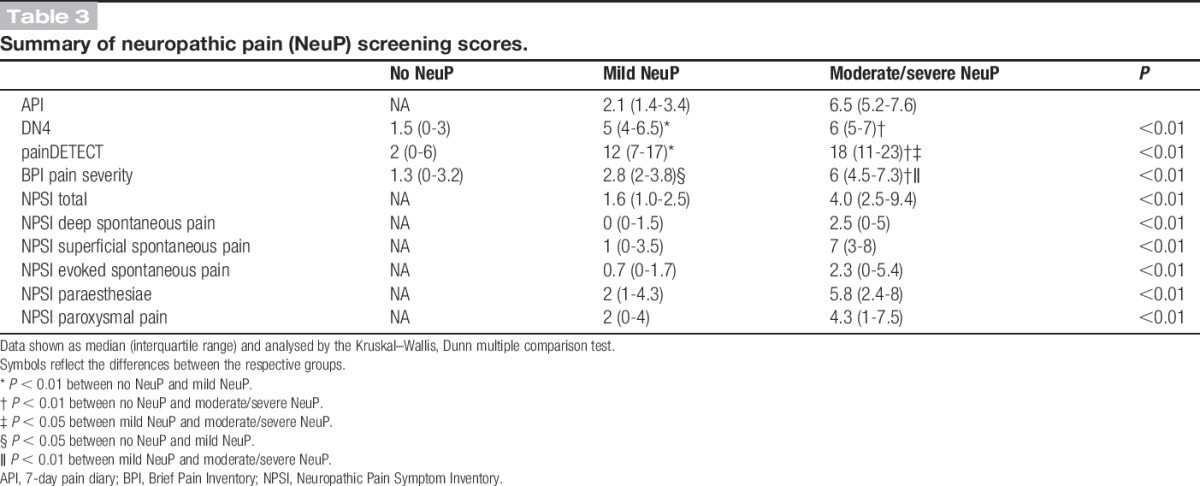
Figure 2.
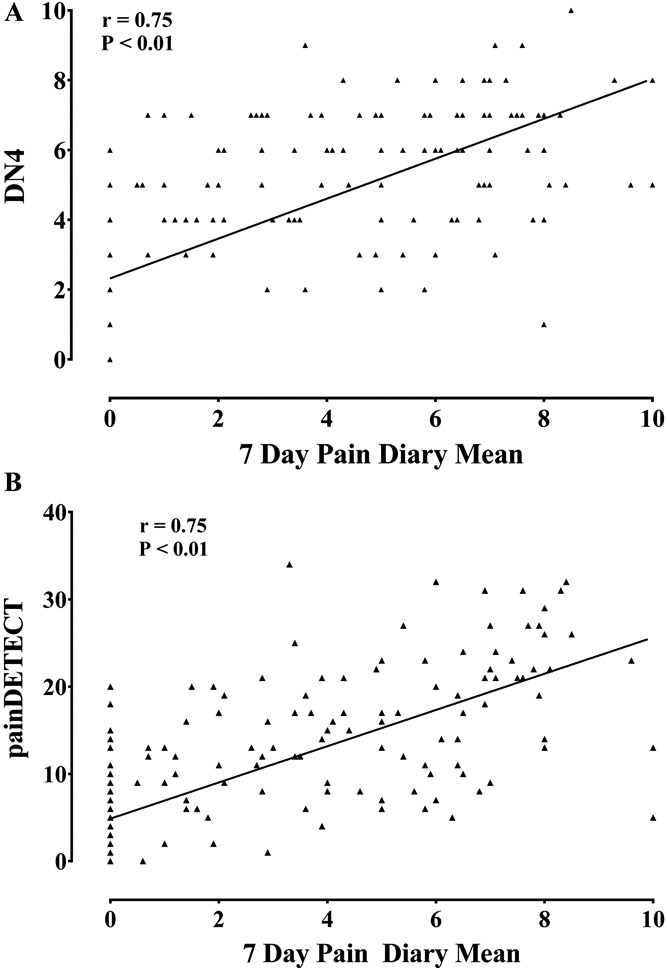
Scatter plot of (A) DN4 scores, (B) painDETECT scores, against 7-day pain intensity diary mean. All screening scores were highly correlated with the 7-day pain dairy mean (Spearman correlation).
A similar proportion of study participants with mild NeuP and moderate/severe NeuP reported NeuP in their hands; however, those with moderate/severe NeuP experienced NeuP over a greater surface area of their hands (P < 0.05, Mann–Whitney U test) (Fig. 3C). There were no differences in either the frequency or surface area distribution of NeuP over the leg between study participants with mild NeuP and moderate/severe NeuP (Fig. 3C). Using the NPSI, study participants with moderate/severe NeuP reported greater severity of symptoms across all parameters of the NPSI compared with those with mild NeuP (Table 3).
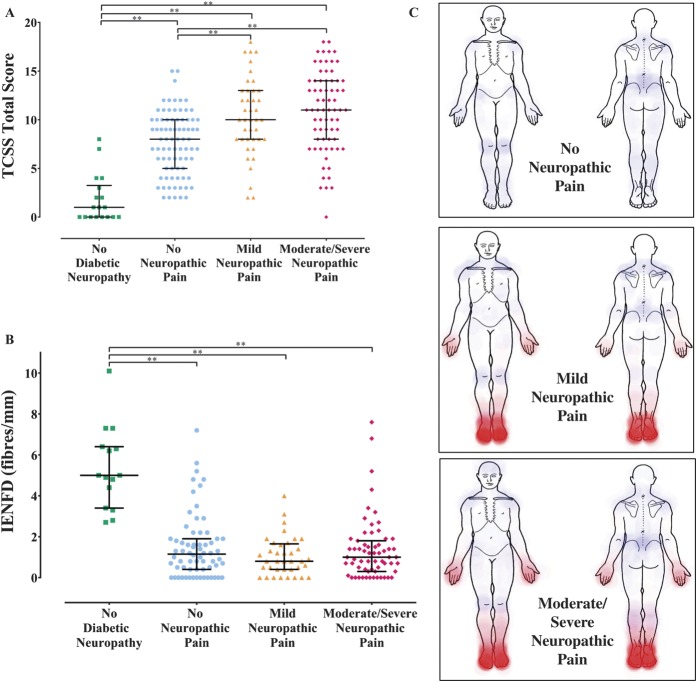
Figure 3.
(A) Scatter plot and median (interquartile range [IQR]) of Toronto Clinical Scoring System (TCSS) scores for study participants with no peripheral diabetic neuropathy, and diabetic neuropathy with no neuropathic pain (NeuP), mild NeuP, moderate/severe NeuP. Kruskal–Wallis, Dunn multiple comparison test: **P < 0.01. (B) Scatter plot and median (IQR) of intraepidermal nerve fibre density (IENFD) from the distal leg for study participants with no peripheral diabetic neuropathy, and diabetic neuropathy with no NeuP, mild NeuP, moderate/severe NeuP. Intraepidermal nerve fibre densities were determined for 182 (87%) study participants. Kruskal–Wallis, Dunn multiple comparison test: **P < 0.01. (C) Heat maps obtained from the 7-day pain diary demonstrating the areas of NeuP (in red) and non-NeuP (in blue) within the study participants with diabetic neuropathy and no NeuP, mild NeuP, and moderate/severe NeuP.
Study participants in all groups did report pain of nonneuropathic origin, and the distribution of the pain is shown as blue areas in Figure 3C. In the majority of cases, the non-NeuP related to nonspecific lower back pain and musculoskeletal pain of the hip, knee, or ankle. The high frequency of such non-NeuP highlights the importance of detailed pain assessment in clinical trials of analgesic agents designed to ameliorate NeuP in diabetes mellitus.
3.4. Structured neurological examination, nerve conduction studies, and intraepidermal nerve fibre density
The TCSS total score was significantly higher in study participants with NeuP compared with no NeuP (Fig. 3A and Supplementary Table, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A226). The study participants with a NeuP reported more frequent sensory symptoms such as paraesthesiae and numbness, which was also of greater intensity. Patients with painful neuropathy also had a more severe DPN on clinical examination. There was greater proximal spread of clinical signs as evidenced by a higher Medical Research Council (MRC) sensory sum score (Supplementary Table 2, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A226). The TCSS and MRC sensory sum scores correlated with NeuP intensity (Fig. 4). The nerve conduction studies (Supplementary Table 2, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A226) and IENFD (Fig. 3 and Supplementary Table 2, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A226) did not discriminate between study participants with and without a NeuP. Sural sensory nerve responses were absent in 54% of study participants with diabetic neuropathy; and in study participants with preserved responses, the amplitudes were reduced with slowing of conduction. Peroneal motor response was absent in 23% of study participants with diabetic neuropathy; and in study participants with preserved responses, the amplitudes were reduced with slowing. There were no electrophysiological differences between participants with and without NeuP (Supplementary Table 2, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A226). The IENFD measured in a biopsy taken at 10 cm above the lateral malleolus was markedly reduced among all study participants with a peripheral neuropathy and did not correlate with the mean pain scores and was not significantly different between groups (Fig. 3 and Supplementary Table 2, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A226).
Figure 4.
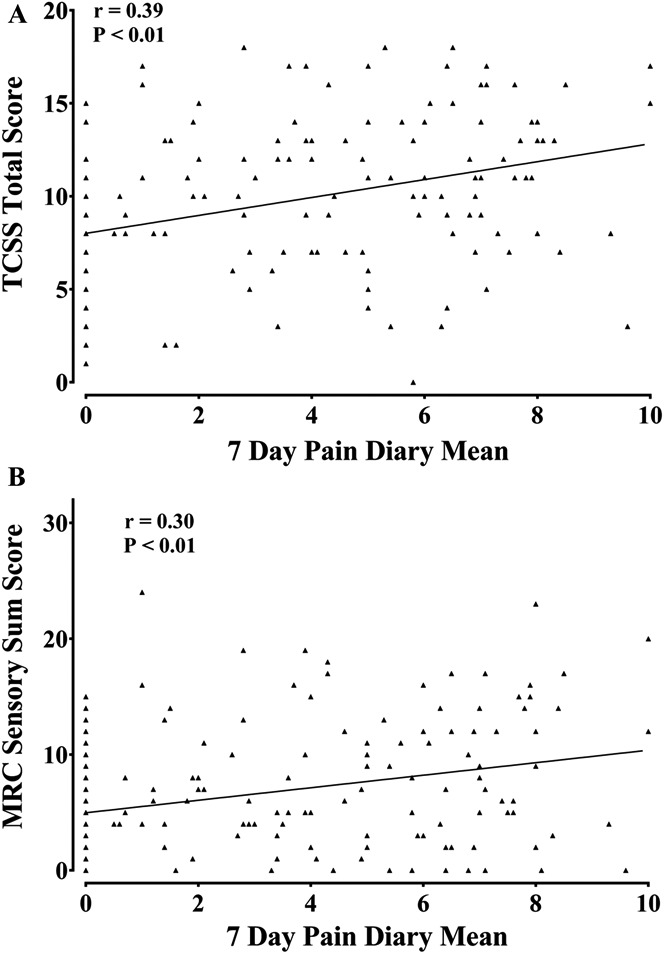
Scatter plot of (A) Toronto Clinical Scoring System (TCSS), and (B) MRC sensory sum score scores, against the 7-day pain intensity diary mean showing a correlation of clinical neuropathy severity and neuropathic pain intensity (Spearman correlations).
3.5. Quantitative sensory testing
Quantitative sensory testing is a means of assessing sensory phenotype, and differences in QST parameters may give insight into pathophysiological mechanisms. All study participants underwent QST of the feet using the protocol developed by the DFNS.
The data for thermal QST parameters for study participants with DPN are summarised in Figure 5A. The mean z-scores for all thermal parameters, apart from the cold detection threshold in participants with moderate/severe NeuP, fell within the normative range of the DFNS healthy control data, although data from individual participants did fall outside the normative range. Between-group comparisons revealed significant differences in the loss-of-function direction, and the shift towards thermal hypoaesthesia was greatest in the study participants with moderate/severe NeuP. The only thermal parameter that showed no differences across all 3 groups was cold pain thresholds as most study participants reached the cut-off temperature indicating a “flooring effect”. The mean z-scores for all thermal parameters for the study participants without DPN all fell within the normative range and were significantly higher across parameters when compared with study participants with DPN (Supplementary Figure 1, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A226).
Figure 5.
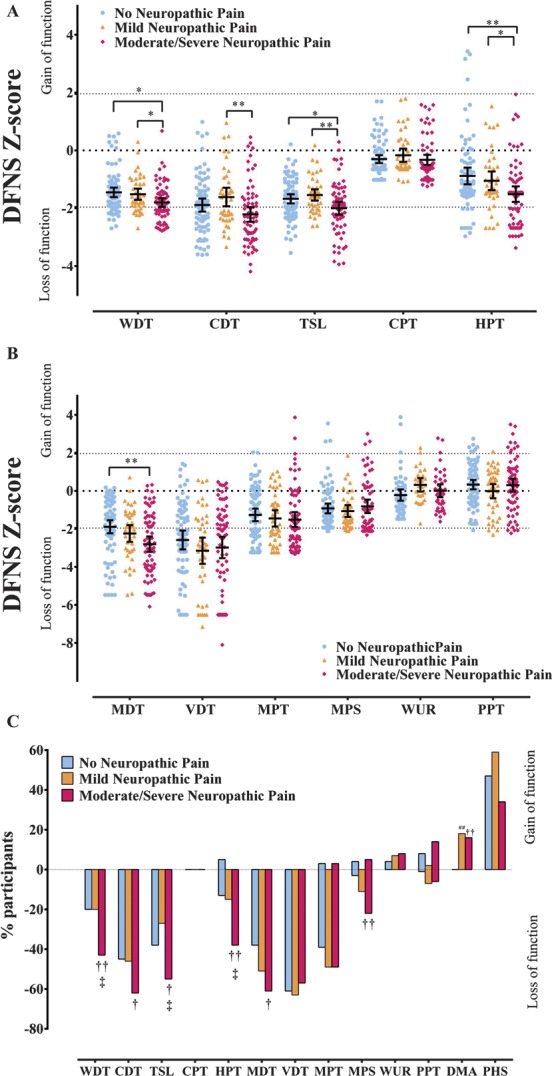
(A) Scatter plot and mean ± 95% CI of z-scores for thermal quantitative sensory testing (QST) parameters in study participants with no neuropathic pain (NeuP), mild NeuP, and moderate/severe NeuP. One-way analysis of variance (ANOVA), least significant difference (LSD) post hoc test: *P < 0.05; **P < 0.01. (B) Scatter plot and mean ± 95% confidence interval of z-scores for mechanical QST parameters in study participants with no NeuP, mild NeuP, and moderate/severe NeuP. One-way ANOVA, LSD post hoc test: **P < 0.01. (C) Loss and gain of sensory function. Comparison of study participants with no NeuP, mild NeuP, and moderate/severe NeuP who have QST values outside the 95% confidence interval of the German research network of neuropathic pain reference database. The y-axis shows the percentage of patients in each group with ‘gain’ of sensory function plotted upwards and “loss” of sensory function plotted downwards. Data analysed with χ2 test of association: †P < 0.05, ††P < 0.01 between no NeuP and moderate/severe NeuP, ##P < 0.01 between no NeuP and mild NeuP, ‡P < 0.05 between mild NeuP and moderate/severe NeuP. CDT, cold detection threshold; CPT, cold pain threshold; HPT, heat pain threshold; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; PPT, pressure pain threshold; TSL, thermal sensory limen; VDT, vibration detection threshold; WDT, warm detection threshold; WUR, wind-up ratio.
The WSI and CSI are measures for the range of thermal sensitivity. There were clear reductions in the WSI and CSI between study participants with no DPN and those with DPN (Supplementary Figure 2, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A226). However, WSI did not discriminate between patients with and without NeuP. In contrast, CSI showed small differences between the study participants with moderate/severe NeuP and the study participants with no or mild NeuP (Supplementary Figure 2, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A226).
The data for mechanical QST parameters for participants with DPN are summarised in Figure 5B. The mean z-score for mechanical detection threshold (MDT) in study participants with moderate/severe painful diabetic neuropathy was reduced (ie, indicating hyposensitivity) when compared with the DFNS healthy control cohort and was significantly lower than the study participants with no NeuP. Mean z-score values for the VDT when compared with the DFNS normative data revealed a loss of function for the VDT with no difference across the 3 groups. Mean z-scores of all other mechanical parameters fell within the normative range of the DFNS healthy control data. The mean z-scores of the mechanical parameters for the study participants without DPN all fell within the normative range, and apart from WUR and PPT, were significantly higher across parameters when compared with study participants with DPN (Supplementary Figure 1, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A226).
The frequency of abnormal QST values outside 2 SD of the mean for DFNS healthy control data is shown in Figure 5C. The overall pattern for thermal parameters is similar to the mean z-scores, with the highest frequency of loss of function in the study participants associated with moderate/severe painful diabetic neuropathy. For the mechanical parameters, all 3 groups showed a high frequency of loss of function across VDT, mechanical pain threshold, and MDT. Significant difference was observed for MDT, with a higher percentage of study participants showing a loss of function with moderate/severe NeuP compared with participants with no NeuP. Overall, a smaller frequency of study participants showed loss of function for mechanical pain sensitivity, with more study participants showing a loss of function with moderate/severe NeuP compared with no NeuP. Statistically significant gain of function was not observed across the thermal and mechanical parameters, apart from dynamic mechanical allodynia.
3.6. Allodynia, paradoxical heat sensation, and irritable nociceptor subtype
The presence of dynamic brush-evoked allodynia would suggest aberrant central processing contributing to NeuP in these patients. This was an example of a QST finding that was specific to NeuP as it was only observed in participants with NeuP, Figure 3C—7 (17%) in mild NeuP and 10 (14%) moderate/severe NeuP. The participants with allodynia did not differ from those in whom allodynia was not elicited across demographic data, clinical measurements, nerve conduction studies, IEFND and psychological problems, sleep disturbance, and health-related quality of life. A significantly higher number of study participants reported evoked pain, ie, allodynia, 39.8% on the NPSI than the 15% of study participants with NeuP who were found to have clinical evidence of dynamic brush-evoked allodynia (Fig. 6).
Figure 6.
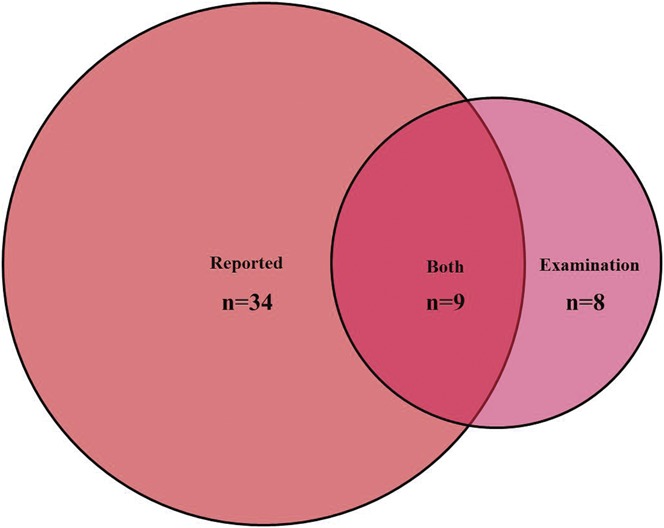
Venn diagram demonstrating the number of participants with brush-evoked allodynia reported only on questionnaires, dynamic mechanical allodynia only on examination, participants with both reported allodynia and allodynia on examination.
Paradoxical heat refers to the perception of heat in response to cooling and has been reported across a diverse range of peripheral neuropathies.32 Paradoxical heat sensations were observed across all groups with DPN, and the proportions were not statistically different, 41.3% without and with 40% with NeuP. Unlike allodynia, it was not discriminatory between patients with NeuP and those without NeuP. It can therefore not be seen as a “positive” sensory sign but likely represents loss of function. We assessed the presence of paradoxical heat sensation in only those who retained thermal perception; therefore, when compared with those who did not report paradoxical heat sensations, they retained thermal perception and had milder neuropathy. The concept of the “irritable nociceptor” is of a sensory phenotype with preserved small-fibre function (cold, warm, and pinprick sensitivity) together with hyperalgesia. Applying preexisting criteria16 for the definition of the irritable nociceptor, we found this to be a rare subtype in DPN. The irritable nociceptor subtype was observed in only 4.9% of participants with mild NeuP, and 7.1% of participants with moderate/severe NeuP. The participants with irritable nociceptor subtype did not differ from those without the irritable nociceptor subtype across demographic data, clinical measurements, NCS, IENFD, psychological morbidity, sleep disturbance, and health-related quality of life.
3.7. The relationship between clinical examination, quantitative sensory testing, and intraepidermal nerve fibre density
Quantitative sensory testing thermal and mechanical parameters, apart from WUR, correlated well with the clinical scores when considering all the study participants, ie, including those with and without DPN (Table 4). The clinical scores, TCSS and MRC sensory sum score, correlated negatively with QST z-scores. Therefore, the higher the clinical scores, ie, more severe the DPN, the greater the loss of sensation on QST parameters. The IENFD positively correlated with QST thermal and mechanical parameters, apart from WUR, among all the study participants, ie, including those with and without DPN. Therefore, preserved epidermal innervation correlates with preserved sensory function across the QST parameters.
Table 4.
Summary of correlations between quantitative sensory testing z-scores, and IENFD and clinical scores.
3.8. Psychological well-being and quality-of-life measures
Study participants with moderate/severe NeuP reported statistically significant higher scores for the ONLS, PCS, DAPOS, PASS-20, ISI, BPI Pain interference, and SF-36 when compared with study participants with no NeuP or mild NeuP (Table 5). Therefore, among the study participants with moderate/severe NeuP, the rates of physical disability, pain catastrophization, depression, anxiety, clinical insomnia, and poorer quality of life were significantly higher.
Table 5.
Summary of scores for quality life measures, depression, anxiety, insomnia, and autonomic symptom abnormalities.
4. Discussion
We have undertaken detailed sensory phenotyping using patient-reported pain descriptors, sensory examination, and QST in a large cohort of patients with diabetic neuropathy, predominantly in those with type 2 diabetes mellitus. This sensory phenotype has then been compared in patients with and without NeuP and related to detailed assessment of neuropathy severity. Key findings are that the DN4 questionnaire performed extremely well as a screening instrument for NeuP in this population, the presence (and severity) of NeuP was associated with more advanced DPN and higher HbA1c, and QST revealed hyposensitivity across a range of small-fibre and large-fibre mediated sensory modalities, and this sensory loss was most marked in patients with moderate/severe NeuP compared with patients with mild or no NeuP. Only a minority of patients had sensory gain signs, and very few patients with NeuP would be classified in the “irritable nociceptor” group.
Our diagnosis of DPN and NeuP was based on a combination of clinical probability followed by confirmatory investigations. We have used the NeuPSIG grading system48 as a gold standard to define patients with chronic NeuP. Such pain lasted longer than 3 months of duration, in a neuroanatomically plausible distribution (symmetrical pain in the extremities), accompanied by a history of a disorder affecting the somatosensory nervous system (confirmed DPN), associated with abnormal sensory signs and a confirmatory test showing a lesion of the somatosensory nervous system (reduced IENFD or abnormal nerve conduction studies). Compared with this gold standard, the patient-reported symptom-based approach of the DN4 questionnaire performed extremely well in identifying patients with NeuP and performed better than another commonly used screening tool for NeuP, the PainDETECT. One previous study has studied the DN4 in DPN43; however, there has never been a direct comparison between these 2 questionnaires in this population.
Patients with NeuP were grouped into mild (mean pain numerical rating scale >0 and <4) and moderate/severe (mean pain numerical rating scale ≥4). Patients with moderate/severe NeuP had greater spatial distribution of pain than those with mild NeuP. The use of analgesics paralleled the severity of NeuP with greater use of pregabalin and duloxetine in the moderate/severe group (reassuringly as these are drugs that are recommended for use in the NeuP).12,21 Study participants with more severe NeuP reported higher scores for anxiety, depression, poorer overall health, and poorer quality of sleep compared with the study participants with no NeuP and mild NeuP. It is unclear from our study whether these comorbidities are predisposing factors or complications of the NeuP.
The DFNS QST protocol has been increasingly used to categorise the somatosensory profile of patients with NeuP in a standardised manner. The PiNS is the first study to adopt the DFNS protocol to specifically assess DPN, and importantly, we have compared the sensory profile of subjects with and without NeuP. In our cohort, the QST pattern of diabetic neuropathy was consistent with loss of function in sensory modalities mediated by both large and small sensory fibres. Furthermore, the study participants with moderate/severe NeuP demonstrated the greatest loss of function compared with the study participants with mild NeuP or no NeuP. Diabetic NeuP would therefore be predominantly classified under the “deafferentation” phenotype. Interestingly, PPTs, as a measure of deep structure nociceptive innervation, were not impaired in DPN and did not correlate with NeuP suggesting that the deeper pain fibres are relatively intact.
Two broad sensory profiles that have emerged in the literature have been termed the “irritable nociceptor” profile with preserved small-fibre function (cold, warm, and pinprick sensitivity) together with hyperalgesia and a “deafferentation” profile that is dominated by sensory loss. Because very few DPN subjects have preserved small-fibre function (compared with postherpetic neuralgia), very few subjects would be classified within the “irritable nociceptor” group in our cohort. Some patients did however demonstrate signs of sensory gain (often in combination with hyposensitivity). Dynamic mechanical (brush-evoked) allodynia was the only evoked “gain of function” parameter that discriminated between study participants being present in 15% of DPN subjects with NeuP and absent from those without NeuP. The presence of dynamic brush-evoked allodynia would suggest aberrant central processing of sensory inputs in these patients. We could not find any demographic or clinical parameters that were specific to patients with brush-evoked allodynia (for instance, there was no evidence that these patients had less denervation)49; however, we were limited by the relatively small size of this group. The discrepancy between reported brush-evoked allodynia and clinical evidence of allodynia is an interesting paradox that will not be easily answered. Most of the study participants report allodynia in the evening, whereas we examined the study participants during the day. However, there is no evidence that allodynia varies during the day. There were a significant number of study participants who exhibited allodynia on clinical examination but did not report it, and questionnaires would not capture this group of patients. Paradoxical heat sensation (in which a cooling stimulus is perceived as being hot) was relatively common in all DPN groups but did not differ for those with and without NeuP, and this sign is probably as much a “negative” as a “positive” sensory sign.
A previous article studying peripheral neuropathy of multiple aetiologies also described a “deafferentation phenotype” in most of the subjects.32 Sensory profiling of NeuP has demonstrated distinct groups of patients with particular patterns of sensory dysfunction that can be seen across different aetiologies of NeuP. That is not to say however that sensory profile does not differ depending on aetiology. In contrast to DPN, patients with HIV-associated polyneuropathy, phenotyped using a protocol very similar to the PiNS, do not exhibit dynamic mechanical allodynia and do exhibit an increase in PPTs.36 Therefore, although HIV and diabetic neuropathy both fall under the “deafferentation” profile, there are clear differences between the different diseases.
Many studies have recorded assessment of NeuP in DPN, but there are limited studies on the relationship between NeuP and DPN severity and none in a cohort of this size. Using 2 clinical measures of neuropathy severity, TCSS and MRC sensory sum scores were both higher in the mild and moderate/severe NeuP groups and correlated with the severity of the NeuP. The clinical scores were consistent with the greater sensory loss in these groups revealed by QST. Therefore, DPN severity correlates with NeuP consistent with other NeuP conditions in which the more severe the injury to the somatosensory system, the higher the likelihood of NeuP. The IENFD is known to be reduced in DPN40 and was used as a measure of loss of small fibres from the skin. We did not find a correlation between IENFD and the severity of NeuP in our cohort. Published studies on this point have been conflicting with some groups reporting an inverse correlation between IENFD and pain in DPN42 and others reporting no significant relationship.15 It is something of a paradox that on using QST to assess the functional integrity of small fibres, we saw greater hyposensitivity in small-fibre modalities in the DPN inversely correlating with NeuP; however, the measure of structural integrity of small fibres did not differ. The most likely reason for this is the flooring effect of IENFD, which was measured at a distal site and was found to be markedly reduced both in NeuP and no NeuP groups.
The presence and severity of NeuP correlates with poorer diabetic control in our patient cohort as reflected by higher HbA1c in the NeuP group. It has long been established that poorer diabetic control is a strong predictor for the development of diabetic neuropathy, and a number of putative mechanisms have been shown to contribute to hyperglycaemic induced nerve injury.12,13 The higher HbA1c in the moderate/severe NeuP group is consistent with the more severe DPN in this cohort. Interestingly, metabolites such as methylglyoxal produced as a consequence of hyperglycaemia can lead posttranslational modifications of ion channels such as Nav1.85 directly resulting in hyperexcitability and NeuP.
Our findings have implications for the conduct of clinical trials of analgesic agents for the treatment of NeuP in DPN. The DN4 is an effective screening tool for NeuP in DPN. Many patients with DPN have pain of nonneuropathic origin (for instance musculoskeletal pain). This non-NeuP needs to be considered, and particular attention should be paid to the location of the pain and exactly what pain is being rated by patients. Distinct patterns of sensory dysfunction are noted on QST, although the most common finding is hyposensitivity to sensory stimuli. A minority of patients did show sensory gain signs; however, the term “irritable nociceptor” could only be applied to 6.3% of painful DPN. Given the rarity of this “irritable nociceptor” group, it is unlikely to be helpful in stratifying patients with painful DPN, in contrast to some recent clinical trials in peripheral neuropathy of diverse causes.16
In summary, the PiNS has provided evidence in a well-characterised DPN cohort that NeuP is related to neuropathy severity. The sensory profile of patients with DPN with NeuP was distinct from those patients without NeuP showing greater hyposensitivity to sensory stimuli across a range of sensory modalities. The sensory profile was not uniform in the NeuP group, and an appreciable minority of patients also demonstrated positive sensory signs such as dynamic allodynia; however, very few patients would meet the criteria for the “irritable nociceptor” group. This study provides us with a firm basis on which to rationalise further phenotyping of painful diabetic neuropathy, for instance to optimise clinical trial outcomes. Future prospective studies will be needed to evaluate how sensory phenotype evolves over time in relation to DPN and NeuP.
Conflict of interest statement
D. L. H. Bennett received consultancy fees, outside the remit of submitted work, from Abide paid to the University of Oxford. A. S. C. Rice has no directly relevant interests to declare for this study. He is an owner of Share options in Spinifex Pharmaceuticals (acquired by Novartis in July 2015). In the last 36 months, Imperial College has received finds from Pfizer and Astellas to support research in ASCR's laboratory. A. S. C. Rice undertakes consultancy and advisory board work for Imperial College Consultants—in the last 36 months, this has included remunerated work for: Spinifex, Abide, Astellas, Neusentis, Merck, Medivir, Mitsubishi, Aquilas, Asahi Kasei, Relmada, Novartis, and Orion. S. Tesfaye reports grants and personal fees from NeuroMetrix, personal fees from Astellas Pharma, personal fees from Worwag Pharma, nonfinancial support from Impeto Medical, during the conduct of the study; personal fees from Pfizer, personal fees from Eli Lilly, outside the submitted work. The other authors have no conflicts of interest to declare.
This work was supported by the Wellcome Trust through a Strategic Award to the London Pain Consortium (Ref. No. 083259); the European Union's Horizon 2020 research and innovation programme under grant agreement No 633491 (DOLORisk), and research reported in this publication was part of the International Diabetic Neuropathy Consortium (IDNC) research programme, which is supported by a Novo Nordisk Foundation Challenge programme grant (Grant number NNF14SA0006). This study was adopted by the National Institution for Health Research of the United Kingdom. D. L. H. Bennett is a Senior Wellcome Trust Clinical Scientist. J. D. Ramirez was supported by the Wellcome Trust (London Pain Consortium) and is currently supported by the Francisco Jose de Caldas Scholarship, from Colciencias, awarded through LASPAU, Harvard University. A. C. Themistocleous was supported by the Wellcome Trust and Novo Nordisk and is an Honorary Research Fellow of the Brain Function Research Group, School of Physiology, Faculty of Health Science, University of the Witwatersrand.
Supplementary Material
Acknowledgements
The authors thank all patients and healthy volunteers for their participation and the research support team at Chelsea and Westminster Hospital NHS Foundation Trust for assistance with recruitment (Laura Braidford, Fiona Clarke, Rhian Bull and Mark Terry). The authors thank the medical, clerical, and nursing staff (consultants) from the Chelsea and Westminster Hospital NHS Foundation Trust Beta Cell unit (Consultants Dr Michael Feher, Dr Daniel Morganstein, Dr Kevin Shotliff and Dr Alison Wren) for assistance with recruitment and Diabetes UK for advertising their study through newsletters. The authors thank the GP practices and staff of the Thames Valley Primary Care Research Network, the staff of the Oxford Centre for Diabetes, Endocrinology and Metabolism, and the NIHR Thames Valley Primary Care Research Network for assisting with recruitment.
Author contributors: A. C. Themistocleous and J. D. Ramirez were involved in data collection, data interpretation, data analysis, literature review, manuscript preparation including figures and writing, prepared the first draft of the manuscript and contributed equally to the article. P. R. Shillo and D. Selvarajah contributed to data collection. JGL created the PiNS database and contributed to data analysis and data interpretation. C. Orengo contributed to data analysis, data interpretation. S. Tesfaye contributed to study design, data analysis, data interpretation. A. S. C. Rice contributed to securing funding (London Pain Consortium), study design, data analysis, data interpretation, literature review, and oversaw the conduct of the study at the Chelsea and Westminster site. D. L. H. Bennett assumed overall responsibility for the study, secured funding, and contributed to study design, data analysis, data interpretation, literature review, manuscript preparation including figures, and writing, and prepared the first draft of the manuscript. Andreas C. Themistocleous and Juan D. Ramirez contributed equally to the work. All authors approved the final report.
Appendix A. Supplemental Digital Content
Supplemental Digital Content associated with this article can be found online at http://links.lww.com/PAIN/A226.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Abbott CA, Malik RA, van Ross ERE, Kulkarni J, Boulton AJM. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011;34:2220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baron R, Förster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet Neurol 2012;11:999–1005. [DOI] [PubMed] [Google Scholar]
- [3].Bastien C, Vallières A, Morin C. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med 2001;2:297–307. [DOI] [PubMed] [Google Scholar]
- [4].Bennett M. The LANSS Pain Scale: the leeds assessment of neuropathic symptoms and signs. PAIN 2001;92:147–57. [DOI] [PubMed] [Google Scholar]
- [5].Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, Sauer SK, Eberhardt M, Schnölzer M, Lasitschka F, Lasischka F, Neuhuber WL, Kichko TI, Konrade I, Elvert R, Mier W, Pirags V, Lukic IK, Morcos M, Dehmer T, Rabbani N, Thornalley PJ, Edelstein D, Nau C, Forbes J, Humpert PM, Schwaninger M, Ziegler D, Stern DM, Cooper ME, Haberkorn U, Brownlee M, Reeh PW, Nawroth PP. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med 2012;18:926–33. [DOI] [PubMed] [Google Scholar]
- [6].Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lantéri-Minet M, Laurent B, Mick G, Serrie A, Valade D, Vicaut E. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). PAIN 2005;114:29–36. [DOI] [PubMed] [Google Scholar]
- [7].Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, Rostaing S, Lanteri-Minet M, Collin E, Grisart J, Boureau F. Development and validation of the Neuropathic Pain Symptom Inventory. PAIN 2004;108:248–57. [DOI] [PubMed] [Google Scholar]
- [8].Bouhassira D, Letanoux M, Hartemann A. Chronic pain with neuropathic characteristics in diabetic patients: a French cross-sectional study. PLoS One 2013;8:e74195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bouhassira D, Wilhelm S, Schacht A, Perrot S, Kosek E, Cruccu G, Freynhagen R, Tesfaye S, Lledó A, Choy E, Marchettini P, Micó JA, Spaeth M, Skljarevski V, Tölle T. Neuropathic pain phenotyping as a predictor of treatment response in painful diabetic neuropathy: data from the randomized, double-blind, COMBO-DN study. PAIN 2014;155:2171–9. [DOI] [PubMed] [Google Scholar]
- [10].Bril V, Perkins BA. Validation of the Toronto clinical scoring system for diabetic polyneuropathy. Diabetes Care 2002;25:2048–52. [DOI] [PubMed] [Google Scholar]
- [11].Buschbacher R, Orahlow N. Manual of nerve conduction studies: Demos Medical Publishing, New York, 2006. [Google Scholar]
- [12].Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol 2012;11:521–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cashman CR, Höke A. Mechanisms of distal axonal degeneration in peripheral neuropathies. Neurosci Lett 2015;596:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology 1996;46:1470. [DOI] [PubMed] [Google Scholar]
- [15].Cheng HT, Dauch JR, Porzio MT, Yanik BM, Hsieh W, Smith AG, Singleton JR, Feldman EL. Increased axonal regeneration and swellings in intraepidermal nerve fibers characterize painful phenotypes of diabetic neuropathy. J Pain 2013;14:941–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, Jensen TS, Sindrup SH. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. PAIN 2014;155:2263–73. [DOI] [PubMed] [Google Scholar]
- [17].Dermanovic Dobrota V, Hrabac P, Skegro D, Smiljanic R, Dobrota S, Prkacin I, Brkljacic N, Peros K, Tomic M, Lukinovic-Skudar V, Basic Kes V. The impact of neuropathic pain and other comorbidities on the quality of life in patients with diabetes. Health Qual Life Outcomes 2014;12:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dworkin RH, Turk DC, Peirce-Sandner S, Burke LB, Farrar JT, Gilron I, Jensen MP, Katz NP, Raja SN, Rappaport BA, Rowbotham MC, Backonja MM, Baron R, Bellamy N, Bhagwagar Z, Costello A, Cowan P, Fang WC, Hertz S, Jay GW, Junor R, Kerns RD, Kerwin R, Kopecky EA, Lissin D, Malamut R, Markman JD, McDermott MP, Munera C, Porter L, Rauschkolb C, Rice ASC, Sampaio C, Skljarevski V, Sommerville K, Stacey BR, Steigerwald I, Tobias J, Trentacosti AM, Wasan AD, Wells GA, Williams J, Witter J, Ziegler D. Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. PAIN 2012;153:1148–58. [DOI] [PubMed] [Google Scholar]
- [19].England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, Cohen JA, Fisher MA, Howard JF, Kinsella LJ, Latov N, Lewis RA, Low PA, Sumner AJ. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005;64:199–207. [DOI] [PubMed] [Google Scholar]
- [20].Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol Dis 1998;5:209–27. [DOI] [PubMed] [Google Scholar]
- [21].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice ASC, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911–20. [DOI] [PubMed] [Google Scholar]
- [23].Hedges B, di Salvo P. Alcohol consumption and smoking. In: Prescott-Clarke P, Primatesta P, editors. Joint Health Surveys Unit of Social and Community Planning Research and University College London. (2010). Health Survey for England, 1996. [data collection]. 4th Edition. UK Data Service. SN:3886. p. 305–20. [Google Scholar]
- [24].Jain R, Jain S, Raison CL, Maletic V. Painful diabetic neuropathy is more than pain alone: examining the role of anxiety and depression as mediators and complicators. Curr Diab Rep 2011;11:275–84. [DOI] [PubMed] [Google Scholar]
- [25].Jensen TS, Bach FW, Kastrup J, Deigaard A, Brennum J. Vibratory and thermal thresholds in diabetics with and without clinical neuropathy. Acta Neurol Scand 1991;84:326–33. [DOI] [PubMed] [Google Scholar]
- [26].Jensen TS, Baron R, Haanpää M, Kalso E, Loeser JD, Rice ASC, Treede RD. A new definition of neuropathic pain. PAIN 2011;152:2204–5. [DOI] [PubMed] [Google Scholar]
- [27].Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve 1991;14:1103–9. [DOI] [PubMed] [Google Scholar]
- [28].Lauria G, Bakkers M, Schmitz C, Lombardi R, Penza P, Devigili G, Smith A, Hsieh S, Mellgren S, Umapathi T, Ziegler D, Faber C, Merkies I. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst 2010;15:202–7. [DOI] [PubMed] [Google Scholar]
- [29].Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies ISJ, Polydefkis M, Smith AG, Sommer C, Valls-Solé J. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 2010;17:79–92. [DOI] [PubMed] [Google Scholar]
- [30].Lauria G, Ziegler D, Malik R, Merkies IS, Waxman SG, Faber CG. The role of sodium channels in painful diabetic and idiopathic neuropathy. Curr Diab Rep 2014;14:538. [DOI] [PubMed] [Google Scholar]
- [31].Magerl W, Krumova EK, Baron R, Tölle T, Treede R-D, Maier C. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. PAIN 2010;151:598–605. [DOI] [PubMed] [Google Scholar]
- [32].Maier C, Baron R, Tölle TR, Binder A, Birbaumer N, Birklein F, Gierthmühlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihöfner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uçeyler N, Valet M, Wasner G, Treede RDD. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. PAIN 2010;150:439–50. [DOI] [PubMed] [Google Scholar]
- [33].McCracken L, Dhingra L. A short version of the Pain Anxiety Symptoms Scale (PASS-20): preliminary development and validity. Pain Res Manag 2002;7:45–50. [DOI] [PubMed] [Google Scholar]
- [34].Medical Research Council—Nerve Injuries Research Committee. Aids to the examination of the peripheral nervous system: Saunders Elsevier, Philadelphia, PA, on behalf of Guarantors of Brain, 2010. [DOI] [PubMed] [Google Scholar]
- [35].Osman A, Barrios F, Kopper B, Hauptmann W, Jones J, O'Neill E. Factor structure, reliability, and validity of the pain catastrophizing scale. J Behav Med 1997;20:589–605. [DOI] [PubMed] [Google Scholar]
- [36].Phillips T, Brown M, Ramirez J, Perkins J, Woldeamanuel Y, Williams A, Orengo C, Bennett D, Bodi I, Cox S, Maier C, Krumova E, Rice A. Sensory, psychological, and metabolic dysfunction in HIV-associated peripheral neuropathy: a cross-sectional deep profiling study. PAIN 2014;155:1846–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pincus T, Williams A, Vogel S, Field A. The development and testing of the depression, anxiety, and positive outlook scale (DAPOS). PAIN 2004;109:181–8. [DOI] [PubMed] [Google Scholar]
- [38].Rolke R, Baron R, Maier C, Tölle TR, Treede RDD, Beyer A, Binder A, Birbaumer N, Birklein F, Bötefür IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihöfner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. PAIN 2006;123:231–43. [DOI] [PubMed] [Google Scholar]
- [39].Rolke R, Magerl W, Campbell K, Schalber C, Caspari S, Birklein F, Treede R. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain 2006;10:77–88. [DOI] [PubMed] [Google Scholar]
- [40].Shun CT, Chang YC, Wu HP, Hsieh SC, Lin WM, Lin YH, Tai TY, Hsieh ST. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain 2004;127(pt 7):1593–605. [DOI] [PubMed] [Google Scholar]
- [41].Simpson DM, Schifitto G, Clifford DB, Murphy TK, Durso-De Cruz E, Glue P, Whalen E, Emir B, Scott GN, Freeman R; Group ObotHNS. Pregabalin for painful HIV neuropathy: a randomized, double-blind, placebo-controlled trial. Neurology 2010;74:413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sorensen L, Molyneaux L, Yue DK. The relationship among pain, sensory loss, and small nerve fibers in diabetes. Diabetes Care 2006;29:883–7. [DOI] [PubMed] [Google Scholar]
- [43].Spallone V, Morganti R, D'Amato C, Greco C, Cacciotti L, Marfia GA. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabet Med 2012;29:578–85. [DOI] [PubMed] [Google Scholar]
- [44].Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain 2004;5:133–7. [DOI] [PubMed] [Google Scholar]
- [45].Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care 2013;36:2456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tesfaye S, Vileikyte L, Rayman G, Sindrup SH, Perkins BA, Baconja M, Vinik AI, Boulton AJM. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev 2011;27:629–38. [DOI] [PubMed] [Google Scholar]
- [48].Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008;70:1630–5. [DOI] [PubMed] [Google Scholar]
- [49].Truini A, Biasiotta A, Di Stefano G, Leone C, La Cesa S, Galosi E, Piroso S, Pepe A, Giordano C, Cruccu G. Does the epidermal nerve fibre density measured by skin biopsy in patients with peripheral neuropathies correlate with neuropathic pain? PAIN 2014;155:828–32. [DOI] [PubMed] [Google Scholar]
- [50].Van Acker K, Bouhassira D, De Bacquer D, Weiss S, Matthys K, Raemen H, Mathieu C, Colin IM. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab 2009;35:206–13. [DOI] [PubMed] [Google Scholar]
- [51].von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012;73:638–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ware J, Sherbourne C. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- [53].Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993;36:150–4. [DOI] [PubMed] [Google Scholar]
- [54].Zilliox L, Peltier AC, Wren PA, Anderson A, Smith AG, Singleton JR, Feldman EL, Alexander NB, Russell JW. Assessing autonomic dysfunction in early diabetic neuropathy: the Survey of Autonomic Symptoms. Neurology 2011;76:1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol 2014;2:56–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.