Abstract
Quantifying exposure and dose to manganese (Mn) containing airborne particles in welding fume presents many challenges. Common biological markers such as Mn in blood or Mn in urine have not proven to be practical biomarkers even in studies where positive associations were observed. However, hair Mn (MnH) as a biomarker has the advantage over blood and urine that it is less influenced by short-term variability of Mn exposure levels because of its slow growth rate. The objective of this study was to determine whether hair can be used as a biomarker for welders exposed to manganese. Hair samples (1cm) were collected from 47 welding school students and individual air Mn (MnA) exposures were measured for each subject. MnA levels for all days were estimated with a linear mixed model using welding type as a predictor. A 30-day time-weighted average MnA (MnA30d) exposure level was calculated for each hair sample. The association between MnH and MnA30d levels was then assessed. A linear relationship was observed between log-transformed MnA30d and log-transformed MnH. Doubling MnA30d exposure levels yields a 20% (95% confidence interval: 11–29%) increase in MnH. The association was similar for hair washed following two different wash procedures designed to remove external contamination. Hair shows promise as a biomarker for inhaled Mn exposure given the presence of a significant linear association between MnH and MnA30d levels.
KEYWORDS: biomarker of exposure, exposure assessment, exposure science, hair, manganese, welding
INTRODUCTION
Manganese (Mn) is a naturally occurring transition metal (Agency for Toxic Substances and Disease Registry, 2012). It is most commonly used to harden steel, as a pigment, in welding rods, and in dry cell batteries. In low doses it is an essential nutrient and functions as a cofactor in enzymes that are important in detoxification of reactive oxygen species (United States Environmental Protection Agency, 2003). Above the estimated safe and adequate daily dietary intake of Mn of 2–5mg day−1 (Greger, 1998), inhaled Mn may induce a Parkinson-like neurodegenerative disease called manganism (Aschner and Aschner, 2005). The mechanisms for this disease are not yet fully understood, partly due to challenges associated with quantifying exposure and dose to Mn-containing airborne particles from sources such as welding fume.
During welding, fume containing Mn is released into the air, inhaled, and may be absorbed mostly through the lung and to a lesser degree through the gastrointestinal tract. Once absorbed, Mn may pass through the blood and deposit in the brain and other tissues (Aschner et al., 2007).
Welding fume exposure varies widely due to the type of welding, the specific welding filler metal, and the base metal composition. For instance, Mn exposure from welding fume may vary from <10 µg m−3 in gas tungsten arc welding (GTAW) to >580 µg m−3 in flux core arc welding (FCAW) (Pesch et al., 2012). In addition, exposure levels may vary widely due to the welding environment, use of ventilation, and use of respiratory protective equipment (Flynn and Susi, 2009; Liu, 2010; Hobson et al., 2011). For instance, welders may work in open environments such as construction sites, or in small confined spaces with poor ventilation, especially in ship-building. The variability of welding fume constituents and exposure makes accurate estimation of welders’ exposures challenging. Consequently, biomarkers that reflect exposure integrated over time and multiple uptake routes are an attractive alternative to traditional exposure monitoring using personal air samples.
Typical biomarkers for Mn include its concentration in blood (or blood components) and in urine, fluids which are relatively easily accessible and commonly collected. Studies have examined relationships between ambient airborne concentrations of Mn and the level of Mn in whole blood, red blood cells, plasma, serum, and urine of exposed workers (Roels et al., 1987, 1992; Järvisalo et al., 1992; Apostoli et al., 2000; Myers et al., 2003). Some of these studies found associations between airborne particles containing Mn and Mn in blood or urine (or both) at the group level, but not at the individual subject level. A recent meta-analysis showed these associations are in general only observed at higher exposure levels above ~10 µg m−3 (Baker et al., 2014). However, even in studies with positive associations, blood and urine have not proven to be practical biomarkers due to little variability in biomarker concentrations over a wide range of air exposures (Smith et al., 2007). In addition, associations between Mn in blood or urine and inhaled Mn at an individual level may be masked by dietary intake, because the daily dietary intake of Mn is on average higher than the daily airborne Mn intake of welders (Greger, 1998; Agency for Toxic Substances and Disease Registry, 2012).
Hair as a moiety for biomarkers has the advantage over blood and urine, because it is less influenced by short-term variability of Mn exposure levels because of its slow growth rate. Estimates for hair growth rate range from 0.35mm per day (~1cm per month) (Tobin, 2005) to ~0.5mm per day (~2cm per month) (Chamberlain and Dawber, 2003). Thus, hair levels of a contaminant may be more representative of an integrated average exposure.
However, a major problem with the use of hair is its direct contamination from the external environment, which may be enhanced by electrical charge and residual oil on the hair (Eastman et al., 2013). In addition, individual exposure can influence the extent of external hair contamination. Detergents used to remove external contamination may also release Mn from welding fume particles attached to the hair surface. Different research groups have used different hair washing procedures commonly using either a water-based detergent (i.e. mixed with Triton X-100) or acetone for washing, but only a few methods have been thoroughly tested (Eastman et al., 2013; Wołowiec et al. 2013) and an accepted standard method has not been identified (Kempson and Henry, 2010; Wołowiec et al., 2013).
The objective of this project was to determine the relationship between quantitatively assessed exposure to Mn in welding fume and Mn levels in scalp hair.
METHODS
The samples for this study were derived from a longitudinal inception cohort study of welding trainees; details have been presented elsewhere (Baker et al., 2014). Briefly, we recruited 53 students enrolled in a welding training program between April 2011 and June 2013. Most students enter the program without prior welding experience and then progress through a 5-quarter training schedule. They learn and practice different welding techniques typically in the order: oxyacetylene, shielded metal arc welding (SMAW), FCAW encompassing both dual shield (DS) and inner shield (IS), gas metal arc welding (GMAW), and GTAW. We recruited students into the study during the first week of their program or at study inception, and followed them through the end of their enrollment or until the end of the study. Participants were asked to provide blood and urine samples on the Monday and Friday of the first and last week of each school quarter and were monitored for exposure to welding fume on the same days. At the end of each sampling day, students completed a daily questionnaire to assess their workplace and personal characteristics such as smoking habits, respirator usage, and welding duration. At the beginning of the first quarter and at the end of each quarter including the first quarter, subjects were asked to give a scalp hair sample. All study protocols were reviewed and approved by the University of Washington Institutional Review Board and study subjects provided written informed consent.
Forty-seven subjects provided a total of 154 hair samples in ~3-month intervals. Six subjects did not submit hair samples, because they were bald or for personal reasons. A hair sample bundle was cut from the occipital region of the head with ceramic scissors as close as possible to the scalp. The cut hair bundles were attached with tape inside a new Ziploc bag for storage and transport to the laboratory. In the laboratory hair bundles were cut into first and second centimeter segments from the proximal end. The 1-cm hair samples were electrically discharged (Mettler Ionizer Antistatic System Model 11238–354) and weighed with an analytical balance (Mettler Toledo model AG285; limit of quantification: 0.1mg). Only the first centimeter of the samples was used for this analysis.
The first set of 56 hair samples was washed with a solution of 0.5ml 1% Triton X-100 mixed with 930mg EDTA in 50ml ultrapure water. Hair samples were vortexed for 30min and rinsed with ultra-distilled water until hair samples demonstrated no residual detergent, and were dried for 24h at room temperature (Triton X-100, Vortex, Water; TVW procedure). When washing hair with TVW we noticed that due to static effects occasionally hair strands were not suspended into the washing solution and some hair stands were lost, even though we increased the time to electrically discharge the hair and test tubes. Thus further testing of alternative washing procedures was indicated.
In order to identify a washing procedure with less static charge effects and adequately efficient washing, we evaluated four washing procedures to test the effectiveness of detergent (acetone and Triton X-100), mechanical agitation (vortex and sonication), and rinse solvent (ultrapure water, acetone, or water followed by acetone). Details of the experiment and results are provided in the Supplementary Data.
Briefly, a sample of human hair was obtained from a local barbershop and collected into two bundles. One bundle was put aside for an ‘uncontaminated’ hair, and the other was exposed to high concentrations of SMAW welding fume in the study training facility by mechanically rotating the sample in the welding plume for ~10min. The contaminated bundle was subsequently divided and cut into samples for analysis and weighed on an analytical balance (Mettler Toledo, Model AG285; limit of quantification: 0.1mg). Five contaminated samples were used for the positive control, and triplicate samples of contaminated and uncontaminated hair were prepared for each of four wash procedures.
Washed hair samples were dried in a vacuum oven, then digested with nitric acid using open vessel microwave assisted digestion (Puchyr et al., 1998), and analyzed following EPA method 6020a Rev.1, using an Agilent 7500-CE ICP-MS (inductively coupled plasma-mass spectrometry) operated in He collision mode to eliminate polyatomic interferences (United States Environmental Protection Agency, 1998). Details of the quality control measures for the analytic method are provided in the Supplementary Data.
We used ordinary least squares regression with robust standard errors to determine whether the washed contaminated hair was significantly cleaner than the unwashed contaminated hair and analysis of variance was used to test whether the four washing procedures differed from each other in residual Mn content. Washing contaminated hair produced on average 80% lower MnH levels than unwashed contaminated hair (P < 0.01). There was no significant difference between the MnH levels associated with the four washing procedures (P = 0.239), and there were substantially fewer problems with static charge when washing hair with acetone. Thus the more efficient acetone wash procedure was adopted for subsequent samples (n = 98).
Subsequent to the results of the hair washing evaluation study, the second set of 98 hair samples was washed and rinsed with acetone (Fischer scientific: Optima A929-4 ultragrade and Alfa Aesar Acetone HPLC Grade 99.5%). Hair samples were covered with 25ml of acetone, shaken for 15 s, and sonicated for 30min. Acetone was removed and the procedure was repeated. Samples were dried in a vacuum-drying oven at ~80°C for ~1h. All dried hair samples were prepared for the analysis and analyzed as described above.
Throughout the study, welding students wore personal air sampling pumps during their work day on the Monday and Friday of the first and last week of each school quarter. For each student, on average 3.7±1.6 air samples were taken per quarter and 10.7±5.5 throughout the study, respectively. Total particulate matter was collected on 37mm mixed cellulose ester (MCE) filters in a closed face filter cassette connected to a personal sampling pump and worn outside of the welding helmet, which is not expected to introduce an overall bias (Harris et al., 2005). Pumps were pre- and post-calibrated to ~2 l per minute. At the end of each sampling day, samples were transported to the Environmental Health Laboratory at the University of Washington for analysis. At least two field blank MCE filters were collected on each sampling day.
Filters were analyzed for Mn by using ICP-MS based on a modified EPA 6020a Rev.1 procedure using an Agilent 7500-CE ICP-MS operated in He collision mode to eliminate polyatomic interferences (United States Environmental Protection Agency, 1998). Filters and deposited fume were digested with 10ml of a 1:1 mixture of concentrated nitric acid and deionized water, using open vessel microwave assisted digestion (MarsXpress, CEM Corp., Matthews, NC, USA). Quality control samples including field blank and spike filters were included with each batch of field samples. Assay accuracy and precision based on the spike recovery samples were 103±6%. Reporting limits for Mn ranged from 0.01 to 0.02 µg depending on analysis-batch-specific field blanks, and were based on three times the standard deviation of the blanks, which were treated the same as the samples in the field. Values below the reporting limit were replaced with the analytical-batch-specific reporting limit divided by square root of two (Hornung and Reed, 1990). MnA concentrations were calculated using the mass of Mn determined and divided by the volume of air sampled. The resulting air concentrations were standardized to an 8-h time-weighted average.
Mn air concentrations were log-transformed to normalize their distribution and used in a linear mixed model to estimate daily Mn exposure levels by welding type (fixed effect), adjusted for individual subject (random effect) (Stata Version 11, xtmixed, College Park, TX, USA). The model estimates were then used to predict daily individual and welding-type-specific Mn exposures as the maximum likelihood estimate of the arithmetic mean, using the within-subject variance. The estimated exposure level was then assigned for each subject-day depending on the subjects’ welding activity, attendance, and duration of welding activity, as reported by the welding school and the individual. For each hair sample, a 30-day time-weighted average Mn exposure was calculated for the 30 days prior to the sample collection date using the individual’s daily estimated exposures.
Each subject was classified as a consistent respirator user or nonuser based on whether they self-reported respirator use for more than 90% of welding days. Although crude and stringent, this classification was designed to address the lack of systematic respiratory protection fit testing, and the inconsistent reporting of respiratory protective equipment observed at the location.
MnH and predicted MnA30d concentrations were log-transformed to normalize their distributions and used in a multivariate linear mixed model to estimate the effects of predictors on log MnH levels. In addition to the log-transformed MnA30d, which was forced into all models, age, body weight, gender, race, ethnicity, smoking status, pack-years, self-reported drinking status (yes/no), respirator user (yes/no), time in welding program, and washing procedure were tested for contributions to the model using the Akaike information criterion (AIC). We also evaluated the interaction between log-transformed MnA30d and washing procedure to determine whether differences in the washing procedure modified the effect of exposure. The model also estimated the between- and within-subject variance components. Analysis was conducted using the R3.1.1 (32-bit) platform with the lme-function from the nlme-package (version: nlme_3.1–117). The resulting coefficients and 95% confidence intervals were exponentiated to the base 2 in order to determine the percentage increase of MnH levels for doubling the exposure (MnA30d).
RESULTS
The demographic and exposure characteristics of the welder trainees who provided hair samples are presented in Table 1. The forty-seven trainee welders had a mean age of 26.7±9.1 years, ranging between 18 and 56 years. Seventy-five percent were white, 13% black, and 4% each were Asian, American Indian, and other or mixed race. The group offering hair samples was similar to the entire apprentice cohort (n = 53) except for race, because fewer hair samples were collected from black subjects. Approximately 43% of subjects who provided a hair sample never smoked and 34% were current smokers.
Table 1.
Characteristics of welders who contributed hair samples.
| Characteristic | K (%) | N | mean ± SD (range) |
|---|---|---|---|
| Age (years) | ≥47 (100) | 26.7±9.1 (18.0–56.4) | |
| Body weight (kg) | ≥47 (100) | 83.8±19.2 (56.7–156.5) | |
| Male | 43 (91.5) | ||
| Race | |||
| White | 35 (74.5) | ||
| Black | 6 (12.8) | ||
| Asian | 2 (4.3) | ||
| American Indian | 2 (4.3) | ||
| Other | 2 (4.3) | ||
| Ethnicity | |||
| Non-Hispanic | 43 (91.5) | ||
| Pack-years | 2.8±6.0 (0–36) | ||
| Smoker | |||
| Never | 20 (42.6) | ||
| Current | 16 (34.0) | ||
| Past | 11 (23.4) | ||
| Drinks alcohol | 31 (66.0) | ||
| Time welding/day (min) | 47 | 527 | 312.6±52.9 (66–405) |
| Respirator user | 11 (23.4) | ||
| MnA30d (µg m−3) | 47 | 154 | 13.1±10.2 (0.2–44.7) |
| Acetone wash only | 33 | 98 | 13.8±10.2 (0.2–44.7) |
| Triton X wash only | 27 | 56 | 11.9±10.1 (0.2–32.8) |
| Manganese concentration in hair (µg g−1) | 47 | 154 | 3.9±7.2 (0.1–51.5) |
| Acetone wash only | 33 | 98 | 5.2±8.6 (0.1–51.5) |
| Triton X wash only | 27 | 56 | 1.7±2.1 (0.1–10.6) |
K, number of subjects; N, number of samples.
Over the ~2-year study duration, we collected a total of 600 personal MnA and 154 hair samples. Table 2 shows MnA exposure concentrations by type of welding. The highest mean concentration and variability was found in FCAW-DS (40.7 µg m−3) followed by SMAW (34.7 µg m−3). Lower levels were found among oxyacetylene (5.2 µg m−3), and GTAW (5.5 µg m−3).
Table 2.
Eight-hour TWA measured air Mn concentration by welding type (µg m−3).
| Welding type | N | AM | GM | GSD |
|---|---|---|---|---|
| All | 600 | 29.1 | 16.5 | 3.4 |
| Flux core arc welding-dual shield (FCAW-DS) | 75 | 40.7 | 25.5 | 3.6 |
| Flux core arc welding-inner shield (FCAW-IS) | 32 | 34.5 | 23.6 | 3.0 |
| Gas metal arc welding (GMAW) | 62 | 28.6 | 21.0 | 2.3 |
| Oxyacetylene (Oxy) | 80 | 5.2 | 4.2 | 2.0 |
| Shielded metal arc welding (SMAW) | 315 | 34.7 | 22.8 | 3.0 |
| Gas tungsten arc welding (GTAW) | 36 | 5.5 | 4.0 | 2.3 |
N, number of samples; AM, arithmetic mean; GM, geometric mean; GSD, geometric standard deviation.
Table 1 shows a summary of the predicted MnA30d air concentrations and the observed Mn in hair for the 47 subjects who provided 154 hair samples. The average hair sample mass was 12.1±7.3mg (range: 1.4–47.6mg). Those with samples washed with acetone only had slightly higher MnA concentrations, though the distributions were widely overlapping. MnH levels were more widely separated, with the acetone wash samples demonstrating a higher average concentration of Mn, and a wider distribution of concentrations.
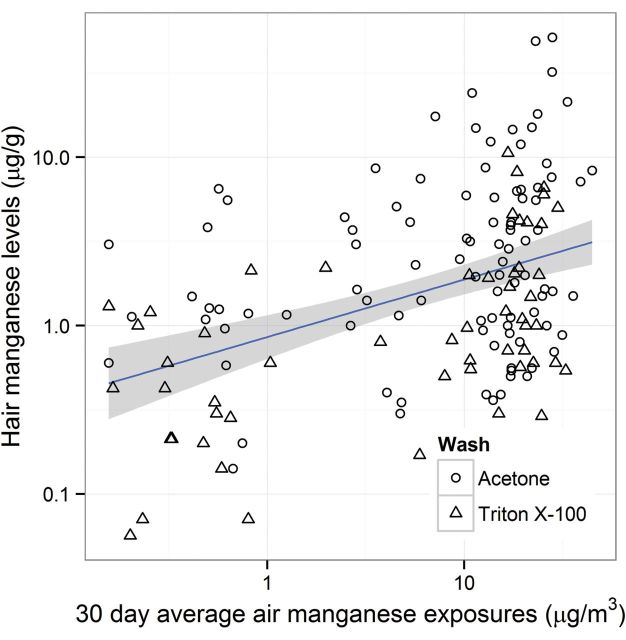
Figure 1 shows measured MnH levels as a function of predicted MnA30d air concentrations. A simple linear regression line and its 95% confidence interval are overlaid. MnH levels increase with increasing MnA30d levels.
Figure 1.
Relationship between 30-day average air Mn (MnA30d) exposure and hair Mn (MnH) level.
Table 3 shows the results of the mixed model analysis. The only variables included in the final model were the log-transformed Mn30d exposure and the wash method. Thirty-day MnA levels were significantly associated with an increase in MnH, yielding a 20% [95% confidence interval (CI): 11–29%] increase in MnH with doubling of the MnA30d exposure levels. More of the remaining variance is within individuals (77.1%) than between subjects (22.9%). When washing procedure was not included in the model, the effect estimate increased to a 25% (95% CI: 16–36%) increase in MnH for each doubling of MnA30d levels.
Table 3.
Determinants of MnH levels (log–log).
| Fixed effects | ln MnH estimate (95% CI) | SE | P-value |
|---|---|---|---|
| Intercept | 0.35 (−0.72 to −0.01) | 0.18 | 0.055 |
| ln MnA30d | 0.26 (0.16 to 0.37) | 0.05 | <0.001 |
| Wash procedurea | −1.06 (−1.47 to −0.65) | 0.21 | <0.001 |
| Variance components | |||
| ln MnH | % | ||
| Within-subject variance | 1.027 | 77.1 | |
| Between-subject variance | 0.305 | 22.9 | |
| Total variance | 1.332 | ||
a0: acetone washed, 1: Triton X-100 washed.
Although the results of our hair washing method evaluation sub-study suggested that differences between the washing procedures should not influence MnH, the acetone washing procedure did yield higher estimated MnH levels than detergent washing in the model. However, the association between exposure and MnH was unaffected by the different wash procedure, as evidenced by a small and nonstatistically significant interaction of the washing procedure on the exposure related increase. The interaction produces a doubling effect of 7.2% (95% CI: −7.9 to 24.8%, P = 0.362).
The association between MnA30d and MnH did not change with the inclusion of the variables age, body weight, gender, race, ethnicity, smoking status, pack-years, self-reported drinking status, respirator user, and time in welding program. The P-values of these variables were not significant (P > 0.05) and the inclusion of these variables did not substantially change the AIC.
DISCUSSION
In this study, welders with higher air exposure to Mn in welding fume had higher Mn levels expressed in scalp hair. These relationships remain significant and consistent, despite using two different washing procedures to prepare the samples. These relationships indicate that MnH may be a useful biomarker for Mn exposure, even at the relatively low exposure levels observed in this study.
The MnA concentrations of our study (Table 2) are substantially lower than MnA concentrations found in other studies. For example, an average SMAW MnA concentration of 160±190 µg m−3 was reported by (Hobson et al., 2011) and 543±1530 µg m−3 by (Liu et al., 2011) while our average was 34.7±32.2 µg m−3. Thus, the levels observed in this setting are useful for evaluating hair as biomarker of Mn exposure in moderately well controlled, or short duration occupational exposures.
The MnA levels observed in this study exceed the recently lowered current and very stringent American Conference of Governmental Industrial Hygienists (ACGIH) Threshold Limit Value (TLV) of 0.02mg m−3 for respirable Mn 52% of the time. The ACGIH TLV of 0.1mg m−3 for inhalable Mn was exceeded 4% of the time. None of the samples exceeded either the National Institute for Occupational Safety and Health (NIOSH) Recommended Exposure Limit (REL) of 1.0mg m−3 or the prevailing Occupational Safety and Health Administration (OSHA) regulatory standard Permissible Exposure Limit (PEL) of 5mg m−3 as a ceiling value.
MnH levels have been used previously as a biomarker for exposure in environmental and occupational studies. MnH was used as a biomarker of exposure in a comparison study of low and high Mn water level exposures in rural Quebec where no other source of Mn was known. MnH levels were significantly higher in children who consumed water with high Mn levels (Bouchard et al., 2007). Because the source of Mn was drinking water, the hair samples were not washed in this study, although the potential for external contamination from bathing in the water cannot be ruled out.
We investigated Mn levels in drinking water in our study area, because MnH levels could be influenced by Mn in water (Bouchard et al., 2007). In the study region, Mn in drinking water is monitored and removed with green sand filters by the local water utility company. Manganese levels are measured daily and are estimated to be on average 0.08±0.026mg l−1 pre- and 0.01±0.0mg l−1 post-treatment (personal communication Thomas Malphrus, City of Renton). These treated water levels are sufficiently low to have little impact on the observed levels in our study. Further, in order to have influenced our results, they would have to have been associated with duration in the welding program.
MnH in children’s hair was also used as biomarker of Mn exposure downwind of a ferro-manganese alloy production plant. Hair samples were prepared similarly to our study and washed with a Triton X-100 solution. The authors reported an increase of MnH with time of mother’s exposure before child birth and a decrease of MnH with increasing distance to plant (Menezes-Filho et al., 2011).
MnH has also been used in occupational settings as a biomarker for air exposures. MnH was associated with MnA levels on a group level, but not on an individual level in studies with manufacturing workers in dry cell battery facilities (Bader et al., 1999). Workplace MnA concentrations ranged from 1 to ~800 µg m−3 in three different job sites with averages of 4, 40, and 400 µg m−3, and hair levels of 4.6±5.8, 5.2±4.5, and 8.2±6.7 µg g−1, respectively.
There are a few studies available reporting a relationship between Mn exposure among welders and Mn in hair (Xie et al., 1995; Ramakrishna et al., 1996; Zhang et al., 1996; Lin, 2002; Huang and Cao, 2003). However, each of these studies has important limitations. All of these studies show higher MnH among welders in comparison to unexposed controls, but they lack a quantitative assessment of subject-specific Mn exposure levels. In only one of these five papers the washing procedure was described (Ramakrishna et al., 1996). They followed the procedure of the International Atomic Energy Agency (IAEA) (International Atomic Energy Agency, 1985), which is an acetone, a detergent, water, and acetone rinse procedure. The lack of exposure assessment and details of the hair washing procedures used makes it difficult to evaluate the findings.
Additionally, two of the papers demonstrate a relationship with years of exposure as a welder, which suggests a relationship with cumulative exposure, rather than exposure occurring during the time period expressed in the length of the hair sample (Xie et al., 1995; Lin, 2002). Given the growth of hair of ~1–2cm per month, years of welding would only be associated with MnH levels if it reflects a long-term reservoir of Mn in other body tissues. Consequently, these papers do not provide compelling evidence of a quantitative relationship between exposure and MnH.
In contrast to the previous discussed welding studies, a significant strength of our study was the large number of individual air exposure measurements, and our ability to model these data to estimate individual 30-day MnA subject-specific exposures, the time window directly relevant to the hair samples collected. To the best of our knowledge, no other Mn biomarker study has had such a rich exposure dataset with which to quantitatively estimate air exposures over an etiologically appropriate time scale. Furthermore, matching the segment of hair to the integration period of exposure as we have done is an important component of biomarker evaluation, but heretofore has frequently been overlooked.
The bioavailability of the manganese in airborne exposures is of potential consequence to this analysis, and is related to the particle size distribution (PSD) of the metal fume. We measured the PSD of fume in the training facility using a 10 stage Micro-Ofrice Uniform Deposit Impactor. The mass median aerodynamic diameters (MMADs) ranged from 0.88 to 1.25 μm, depending on welding type. Except for GTAW, the average GSD ranged from 3.5 to 4. The average GSD for GTAW was 6.21 (Warner, 2014). While the MMADs are somewhat higher than those observed in other studies (Taube, 2013), they indicate primarily alveolar deposition, and potential uptake through both pulmonary and gastrointestinal routes.
In this study, we found the majority of variability in MnH to be within subjects as opposed to between subjects, indicating substantial remaining variability in the MnH measurement that was unaccounted for by either individual differences in expression of Mn in hair, or by our estimated exposure levels. Uncertainty in both the quantification of MnH due to imprecision in cutting the first cm from the scalp, transfer of the weighed sample of hair to the digestion tube, and residual external contamination of the sample could contribute to this error. In addition, our estimated 30-day air exposure for each subject contains error due to inter-individual variability in exposure for a specified welding type, and the individual ascertainment of welding duration, effects of respirator use, etc. These errors necessarily contribute to the residual error in our model despite our large exposure sample size and individually assessed welding parameters.
A challenging element of our study was the hair washing procedure. While the importance of hair washing has been previously discussed (Eastman et al., 2013), its importance is often overlooked in hair biomarker studies. Due to issues with static electrical charge when washing our initial hair samples we conducted a sub-study to evaluated four washing procedures. Ultimately two wash procedures were used with the hair samples in this analysis. We first used a Triton X-100 procedure and then changed to an acetone based wash procedure because the two approaches did not show any statistical difference in the sub-study. However, our final results indicated that hair washed with the Triton X-100 procedure had MnH levels that were consistently lower than samples washed with acetone, potentially due to more complete washing. Nevertheless, the relationship between air exposure and hair levels on the log scale was not significantly modified by the use of the two methods.
Various hair washing procedures have been proposed since the early attempts of measuring chemicals in hair as an exposure biomarker (Bate, 1965). The need for standardization of the procedures has also been recognized (Bencko, 1995). Although the IAEA proposed a standard hair washing procedure in 1985 (International Atomic Energy Agency, 1985), it has still not been widely adopted. Very little consensus exists on which hair washing procedures should be used (Kempson and Henry, 2010). Very thorough work on wash procedures for using hair as a biomarker for Mn was also done earlier (Salmela et al., 1981). However, that analysis was limited to bulk hair samples and had higher limits of detection than are available today. Most recently, as an alternative analytical approach, laser-ablation ICP-MS has also been proposed to verify the effectiveness of a washing procedure (Eastman et al., 2013).
We quantified four different washing procedures on purposely contaminated hair (see the Supplementary Data for details). The four washing procedures produced on average 80% lower MnH levels than what was measured in unwashed contaminated hair. However, we likely experienced residual contamination, because the Mn content in contaminated hair after washing (0.8±0.5 µg g−1) was higher than the Mn content of washed not contaminated hair (0.6±0.7 µg g−1).
Given the apparent difference in the MnH between the two wash procedures, it is important to consider if the observed relationship between MnA and MnH could have been due to residual contamination. Given the prior evidence of Mn accumulation in the hair from environmental sources including water supplies, the demonstration of good washing efficiency in our washing study (80%), the use of two wash procedures with different types of solvents, and an essentially parallel association of MnA and MnH in the log–log space, we think it is unlikely that residual external contamination could explain the observed results.
However, the possibility that proportional washing efficiency and residual contamination left on the surface of the hair cannot be fully ruled out in this study. Thus additional work on efficient hair washing techniques is needed before a fully validated and quantitative biomarker for Mn exposure in hair can be developed.
CONCLUSION
At relatively low occupational levels of exposure to welding fume, on a log–log scale MnH was linearly related to exposure, and thus may prove useful as an exposure biomarker in similar settings. We demonstrated this relationship using a time integrated quantitative estimate of exposure over the previous 30-day period, associated with the first centimeter of hair proximal to the scalp—the hair nominally grown over this same period of time. Removal of surface contamination of the hair is clearly an important component of the procedure for use of hair as a biomarker for airborne environmental contaminants, and requires additional development before this technique can be widely accepted as a quantitative exposure biomarker.
SUPPLEMENTARY DATA
Supplementary data can be found at http://annhyg.oxfordjournals.org/.
FUNDING
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under award number R01ES017809. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional funding was provided by the Department of Environmental and Occupational Health Sciences, University of Washington.
The authors have no competing financial interests.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank the welders and staff at Renton Technical College, Renton, WA, without whom this study would not have been possible. The authors further acknowledge the University of Washington Environmental Health Laboratory for analysis of biological and air samples and Christopher Warner for their technical support with collecting samples and analyzing data.
REFERENCES
- Agency for Toxic Substances and Disease Registry. (2012) Toxicological profile for manganese (Internet). Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; p. 556 Report No.: tp151. Available at http://www.atsdr.cdc.gov/toxprofiles/tp151.pdf. Accessed 2 January 2014. [Google Scholar]
- Apostoli P, Lucchini R, Alessio L. (2000) Are current biomarkers suitable for the assessment of manganese exposure in individual workers? Am J Ind Med; 37: 283–90. [DOI] [PubMed] [Google Scholar]
- Aschner JL, Aschner M. (2005) Nutritional aspects of manganese homeostasis. Mol Aspects Med; 26: 353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Guilarte TR, Schneider JS, et al. (2007) Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol; 221: 131–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader M, Dietz MC, Ihrig A, et al. (1999) Biomonitoring of manganese in blood, urine and axillary hair following low-dose exposure during the manufacture of dry cell batteries. Int Arch Occup Environ Health; 72: 521–7. [DOI] [PubMed] [Google Scholar]
- Baker MG, Simpson CD, Stover B, et al. (2014) Blood manganese as an exposure biomarker: state of the evidence. J Occup Environ Hyg; 11: 210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate LC. (1965) The use of activation analysis in procedures for the removal and characterization of the surface contaminants of hair. J Forensic Sci; 10: 60–72. [PubMed] [Google Scholar]
- Bencko V. (1995) Use of human hair as a biomarker in the assessment of exposure to pollutants in occupational and environmental settings. Toxicology; 101: 29–39. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Laforest F, Vandelac L, et al. (2007) Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect; 115: 122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain AJ, Dawber RP. (2003) Methods of evaluating hair growth. Australas J Dermatol; 44: 10–8. [DOI] [PubMed] [Google Scholar]
- Eastman RR, Jursa TP, Benedetti C, et al. (2013) Hair as a biomarker of environmental manganese exposure. Environ Sci Technol; 47: 1629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn MR, Susi P. (2009) Neurological risks associated with manganese exposure from welding operations–a literature review. Int J Hyg Environ Health; 212: 459–69. [DOI] [PubMed] [Google Scholar]
- Greger JL. (1998) Dietary standards for manganese: overlap between nutritional and toxicological studies. J Nutr; 128(2 Suppl): 368S–71S. [DOI] [PubMed] [Google Scholar]
- Harris MK, Ewing WM, Longo W, et al. (2005) Manganese exposures during shielded metal arc welding (SMAW) in an enclosed space. J Occup Environ Hyg; 2: 375–82. [DOI] [PubMed] [Google Scholar]
- Hobson A, Seixas N, Sterling D, et al. (2011) Estimation of particulate mass and manganese exposure levels among welders. Ann Occup Hyg; 55: 113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. (1990) Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg; 5:46–51. [Google Scholar]
- Huang C, Cao C. (2003) Analysis of hair manganese concentration of 807 welders. J Henan Univ Sci Technol Med Sci; 21:278–9. [Google Scholar]
- International Atomic Energy Agency. (1985) Health-related monitoring of trace element pollutants using nuclear techniques (Internet). Vienna, Austria: International Atomic Energy Agency; p. 224 Report No.: IAEA-TECDOC-300. Available at http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/16/049/16049200.pdf. Accessed 19 April 2014. [Google Scholar]
- Järvisalo J, Olkinuoral M, Kiilunen M, et al. (1992) Urinary and blood manganese in occupationally nonexposed populations and in manual metal are welders of mild steel. Int Arch Occup Environ Health; 63: 495–501. [DOI] [PubMed] [Google Scholar]
- Kempson IM, Henry DA. (2010) Determination of arsenic poisoning and metabolism in hair by synchrotron radiation: the case of Phar Lap. Angew Chem Int Ed Engl; 49: 4237–40. [DOI] [PubMed] [Google Scholar]
- Lin F. (2002) Significances of the analysis of hair manganese concentration of welders. Chinese Journal of Public Health Management. Chin J Public Health Manag; 18: 87. [Google Scholar]
- Liu S. (2010) Assessing exposures to particulate matter and manganese in welding fumes (Internet). Berkeley, CA: University of California; Available at http://escholarship.org/uc/item/2z91s6r1. Accessed 20 April 2014. [Google Scholar]
- Liu S, Hammond SK, Rappaport SM. (2011) Statistical modeling to determine sources of variability in exposures to welding fumes. Ann Occup Hyg; 55: 305–18. [DOI] [PubMed] [Google Scholar]
- Menezes-Filho JA, Novaes C, de O, et al. (2011) Elevated manganese and cognitive performance in school-aged children and their mothers. Environ Res; 111: 156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JE, teWaterNaude J, Fourie M, et al. (2003) Nervous system effects of occupational manganese exposure on South African manganese mineworkers. NeuroToxicol; 24: 649–56. [DOI] [PubMed] [Google Scholar]
- Pesch B, Weiss T, Kendzia B, et al. (2012) Levels and predictors of airborne and internal exposure to manganese and iron among welders. J Expo Sci Environ Epidemiol; 22: 291–8. [DOI] [PubMed] [Google Scholar]
- Puchyr RF, Bass DA, Gajewski R, et al. (1998) Preparation of hair for measurement of elements by inductively coupled plasma-mass spectrometry (ICP-MS). Biol Trace Elem Res; 62: 167–82. [DOI] [PubMed] [Google Scholar]
- Ramakrishna VV, Singh V, Garg AN. (1996) Occupational exposure amongst locomotive shed workers and welders using neutron activation analysis of scalp hair. Sci Total Environ; 192: 259–67. [DOI] [PubMed] [Google Scholar]
- Roels HA, Ghyselen P, Buchet JP, et al. (1992) Assessment of the permissible exposure level to manganese in workers exposed to manganese dioxide dust. Br J Ind Med; 49: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels HA, Lauwerys R, Genet P, et al. (1987) Relationship between external and internal parameters of exposure to manganese in workers from a manganese oxide and salt producing plant. Am J Ind Med; 11: 297–305. [DOI] [PubMed] [Google Scholar]
- Salmela S, Vuori E, Kilpiö JO. (1981) The effect of washing procedures on trace element content of human hair. Anal Chim Acta; 125: 131–7. [Google Scholar]
- Smith D, Gwiazda R, Bowler R, et al. (2007) Biomarkers of Mn exposure in humans. Am J Ind Med; 50: 801–11. [DOI] [PubMed] [Google Scholar]
- Taube F. (2013) Manganese in occupational arc welding fumes–aspects on physiochemical properties, with focus on solubility. Ann Occup Hyg; 57: 6–25. [DOI] [PubMed] [Google Scholar]
- Tobin DJ, editor. (2005) The biogenesis and growth of human hair. Hair in toxicology: an important bio-monitor; London: Royal Society of Chemistry; pp. 3–33. [Google Scholar]
- United States Environmental Protection Agency. (1998) Method 6020A (SW-846): Inductively coupled plasma-Mass Spectrometry, Revision 1. Washington, DC; Report No.: 6020A. Available at http://www2.epa.gov/homeland-security-research/epa-method-6020a-sw-846-inductively-coupled-plasma-mass-spectrometry. Accessed 19 September 2015 [Google Scholar]
- United States Environmental Protection Agency. (2003) Health effects support document for manganese (Internet). Washington, DC; p. 164 Report No.: EPA 822-R-03-003. Available at http://water.epa.gov/action/advisories/drinking/upload/2003_03_07_support_cc1_magnese_healtheffects.pdf. Accessed 24 April 2014. [Google Scholar]
- Warner C. (2014) Lung bioaccessibility of manganese in arc welding fume (Internet) (Master of Science). Seattle, WA: University of Washington; Available at https://digital.lib.washington.edu/researchworks/handle/1773/26345. Accessed 10 October 2014. [Google Scholar]
- Wołowiec P, Michalak I, Chojnacka K, et al. (2013) Hair analysis in health assessment. Clin Chim Acta; 419: 139–71. [DOI] [PubMed] [Google Scholar]
- Xie P, Lei Z, Yin X, et al. (1995) The investigation of the relationship between occupational manganese exposure and urine and hair manganese concentrations. Chin J Ind Med; 8: 202–5. [Google Scholar]
- Zhang Y, Zhang H, Wan G, et al. (1996) The investigation of the relationship between hair manganese concentration and occupational intoxication. Stud Trace Elem Health; 13: 45–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.