Abstract
Objective
To evaluate the variation in all-cause attrition (mortality and loss to follow-up (LTFU)) among HIV-infected individuals in Botswana by health district during the rapid and massive scale-up of the National Treatment Program.
Methods
Analysis of routinely collected longitudinal data from 226,030 patients who received ART through the Botswana National HIV/AIDS Treatment Program across all 24 health districts from 2002 to 2013. A time-to-event analysis was used to measure crude mortality and loss to follow-up rates (LTFU). A marginal structural model was used to evaluate mortality and LTFU rates by district over time, adjusted for individual-level risk factors (e.g., age, gender, baseline CD4, year of treatment initiation, and antiretroviral regimen).
Results
Mortality rates in the districts ranged from the lowest 1.0 (95% CI 0.9–1.1) in Selibe-Phikwe, to the highest 5.0 (95% CI 4.0–6.1), in Mabutsane. There was a wide range of overall LTFU across districts, including rates as low as 4.6 (95% CI 4.4–4.9) losses per 100 person-years in Ngamiland, and 5.9 (95% CI 5.6–6.2) losses per 100 person-years in South East, to rates as high as 25.4 (95% CI 23.08–27.89) losses per 100 person-years in Mabutsane and 46.3 (95% CI 43.48–49.23) losses per 100 person-years in Okavango. Even when known risk factors for mortality and LTFU were adjusted for, district was a significant predictor of both mortality and LTFU rates
Conclusion
We found statistically significant variation in attrition (mortality and LTFU) and data quality among districts. These findings suggest that district-level contextual factors affect retention in treatment. Further research needs to investigate factors that can potentially cause this variation.
Keywords: HIV, attrition, ART, marginal structural model, multilevel, Botswana
Introduction
Rapid expansion of HIV testing and counseling, and the scale-up of antiretroviral therapy (ART) has significantly changed the course of the HIV epidemic. Health systems, however, have had a hard time coping with the scale and speed of this rapid expansion, mainly due to limited resources, particularly health professionals, poorly-designed service delivery, supply chain management and under-developed monitoring and evaluation capacity[1].
In 2002, Botswana was one of the first countries in sub-Saharan Africa to provide free antiretroviral therapy (ART) for eligible citizens, and has been a leader in spurring an effective regional and global response to the epidemic. The Botswana National ART Program, called Masa (the Setswana word for “new dawn”), has achieved universal coverage of ART by providing care to more than 95% of individuals who have tested positive and qualify for treatment in the country[2], serving more than 250,000 patients [3]. Through proactive policies and programs around HIV/AIDS, Botswana has made major gains and has laid the foundation for continued progress against the epidemic. However, the overall effectiveness of the program has been adversely affected by high levels of attrition across the HIV care continuum [4, 5].
Evaluation of the Masa Program shows improved survival [4] and quality of life of HIV-infected patients enrolled in the program [6]. However, there is scarce information about the performance of the Program at sub-national level. There is ample evidence indicating substantial geographical differences in health inequality both between and within countries [7, 8]. People living in places with access to better care consequently have better health outcomes [9, 10]. For example, in the United States, despite considerable overall mortality decline among HIV-infected patients over the last two decades, studies show growing socioeconomic and racial inequality in HIV mortality [11, 12]. Similar patterns of mortality among the HIV-infected population were found in sub-Saharan Africa. One study in South Africa found elevated risk of mortality in rural areas as compared to urban areas [13].
Examining geographic inequality is important as it may help policy makers reduce contextual barriers to healthcare for all patients, particularly for HIV-infected individuals. Moreover, this kind of study can shed light on variation in access and resources available to healthcare services in different locations. There is a need for a comprehensive analysis measuring the impact of an ART program while simultaneously accounting for individual predictors of disease progression and the context in which these programs operate. Given inequality in health and mortality has long been an important area of public health research, this study aimed to examine whether individuals receiving ART in different health districts1 in Botswana experience different mortality risk once demographic, immunologic and medical variables have been taken into account. Unlike previous studies [7, 14], rather than relying on aggregate level data, this analysis used a rich, longitudinal national registry of HIV-infected patients collected at the individual level to measure the effect of the place-based contextual factors on individual outcomes, highlighting how place of residence can influence an individual’s health.
Methods
Data source and study population
We used routinely collected data from 226,030 HIV-infected adults aged 18 to 68 enrolled in the Masa program to assess inequality in all-cause mortality and loss to follow-up (LTFU) among HIV-infected individuals. During the data cleaning process, we removed 620 individuals’ observations for having clinical records over 90 days after documented death. These individuals received ART in 176 healthcare facilities in all 24 health districts across Botswana from January 2002 to October 2013. The details of the database have been previously described [15].
Primary outcome measures for this analysis were mortality and LTFU. Although active tracing for patients late for clinic appointments is recommended, resource limitations constrain tracing activities and most documented deaths are passively reported. Those considered LTFU could still be in treatment, but because of the failure to enter the data from paper forms into electronic database at facilities or because of silent transfers of the patients to new facilities the patients could not be tracked down in the electronic database. For this reason, it is also necessary to determine the number of patients who are LTFU as they may have been patients who died, but the death was not recorded in the system. For this analysis, LTFU will be defined as a patient who had not been seen at the clinic 90 days after a missed scheduled visit.
Physical access to health facilities is one of the important factors that can influence adherence and retention in care. Traditionally, access has been measured by distance or travel time to nearest health facility. As we did not have the actual distance travelled to access healthcare, we used population density as a proxy for ease of access to care. There is a wide variation in population density in Botswana, from 13.8 persons/km2 in the South Eastern region to fewer than 1 person/km2 in the Western region [16]. Over the 2001 and 2011 intercensal period, population density in the South Eastern Region significantly increased while in the Northern and Western Region remained stagnant [16]. According to Botswana 2011 Census Analytical Report, districts with lower population density have fewer health facilities and poor road conditions compared to the districts with higher population density, which can restrict residents’ easy access to health facilities [16]. We used HIV prevalence in each district in each year as proxy for the potential level of patient volume seen at facilities in the district, which can be translated into the level of workload for health workers. We used Gaborone, the capital of the country as the reference group.
Statistical analysis
We used a person-years (P-Y) approach to analyze event rates. We calculated survival from the date of ART initiation until death or the date of the last follow-up. The data were censored on the date of the patient’s last follow-up visit. Districts were ranked based on unadjusted mortality and LTFU rates in 2004, 2008 and 2012.
This study used a multilevel model to assess the extent that the district where healthcare is provided explains mortality among HIV patients controlling for individual-level risk factors. We compared odds of death in HIV-infected population among districts using marginal structural modeling. We used this technique to address the complex issue of LTFU and prevent bias in interpreting the results [17, 18]. Using observed variables, this approach allows us to derive a weight for each individual in each time period, i.e., the inverse probability of remaining in the study in that interval [19]. Following the literature, the stabilized version of inverse probability of LTFU was estimated as a ratio of two predicted probabilities from two pooled logistic regression models. The model for the numerator of this ratio includes only the baseline covariates, while the denominator includes baseline and time-varying covariates [20]. Because the dataset was formatted as one observation per person per quarter, we used a pooled logistic regression, which is close to a time-dependent covariate Cox regression model [21], to model the probability that each individual was treated in each quarter.
Results
Baseline characteristics
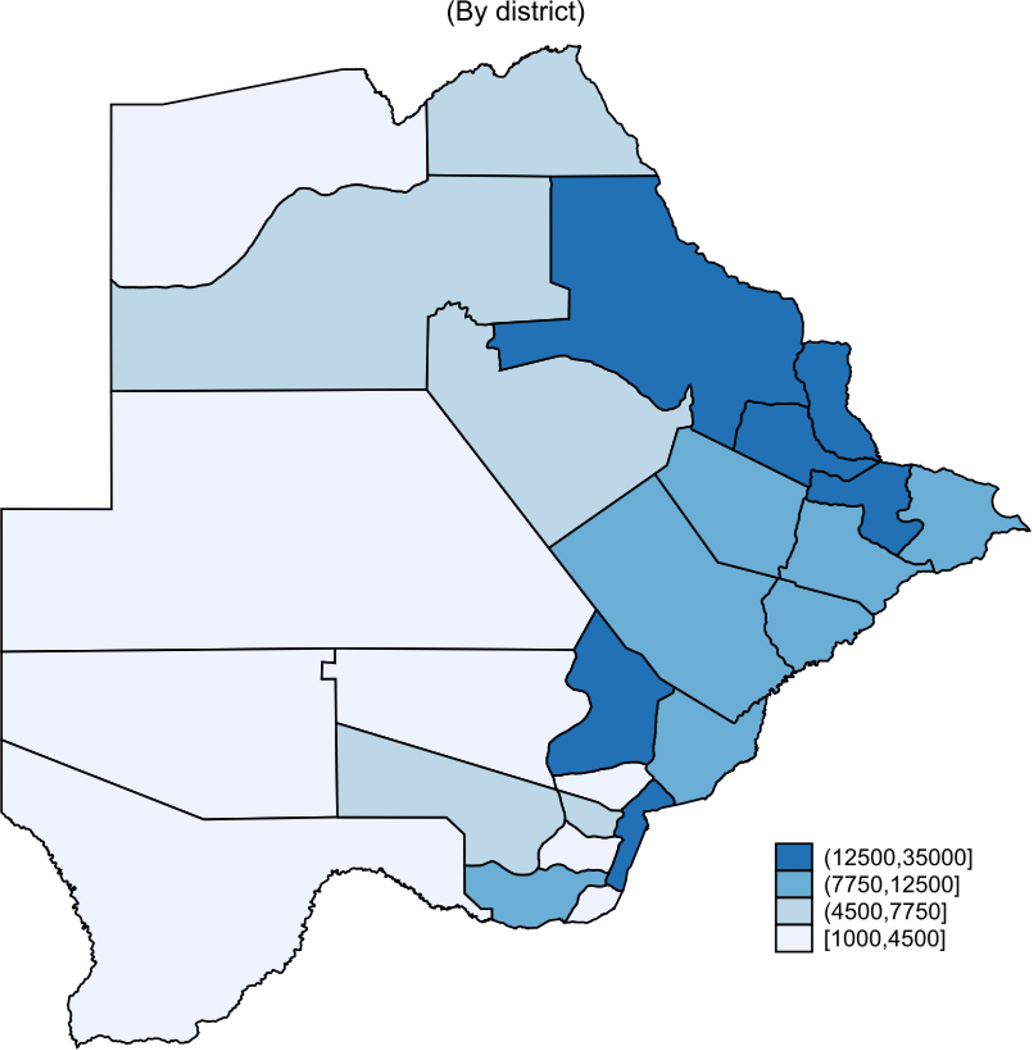
Table 1 shows summary statistics for the patient population in the Masa Program. The analysis included 687,759 person-years of follow-up time during 2002–2013 from all the 24 health districts in Botswana. Gaborone has the largest patient population with over 35,000 individuals or 16% of the patient population. Other districts with high patient populations include Francistown, Kweneng East, Tutume, and Selibe-Phikwe. Some districts, namely Mabutsane, Okavango, Kgalagadi North and South, and Kweneng West have comparatively smaller patient populations, with each representing less than 2% of the total patient population (Figure 1). The patient population is 62% female, although within districts it ranges from 59% in Francistown, to 79% in Good Hope. The median age is 35 years (inter-quartile range [IQR] 29–42) overall, with very little variation across districts. The median baseline CD4 cell count is 147 cells/mm3 (IQR 79–200). The highest median baseline CD4 cell count was in Serowe/Palapye district (221 cells/mm3 (IQR 127–410) and the lowest was in Tutume (113 cells/mm3 (IQR 58–170).
Table 1.
Summary statistics of ART enrollees in Botswana’s national antiretroviral therapy program during 2002–2013 by district
| Follow-up days after ART initiated |
Median Age | Median Baseline CD4 Cell Count |
HIV Prevalence | Population Density |
|
|---|---|---|---|---|---|
| District | Median (IQR) | Median (IQR) | Median (IQR) | % of HIV infected Population 18 months and older |
Per km2 |
| Bobirwa | 1061 (273–1937) | 36 (30–44) | 170 (96–234) | 17.8 | 5.3 |
| Boteti | 531 (72–1269) | 35 (30–42) | 132 (65–172) | 15.8 | 1.9 |
| Chobe | 723 (83–1680) | 35 (30–41) | 130 (71–176) | 23.3 | 1 |
| Francistown | 832 (256–1658) | 35 (30–43) | 123 (63–179) | 23.5 | 1211.5 |
| Gaborone | 1458 (370–2490) | 35(29–41) | 121 (60–175) | 17.1 | 1285.6 |
| Gantsi | 882 (267–1696) | 35 (30–42) | 149 (81–194) | 14.4 | 0.3 |
| Goodhope | 531 (8–1440.5) | 33(28–39) | 121 (66–173) | 14.4 | 3.9 |
| Jwaneng Town | 668 (75–1742) | 33 (27–38) | 126 (73–185) | 16.2 | 172.3 |
| Kgalagadi | 881 (182–1813) | 33 (27–40) | 134 (88–184) | 15.5 | 0.9 |
| Kgalagadi North | 816 (37–1839) | 35 (31–44) | 134 (79–184) | 13.3 | 0.3 |
| Kgatleng | 716 (83–1546) | 35 (29–42) | 134 (73–178) | 16.6 | 10.9 |
| Kweneng East | 476 (27–1177) | 35 (30–42) | 134 (77–178) | 16.9 | 28.6 |
| Kweneng West | 503 (54.5–1411) | 36 (31–43) | 124 (84–197) | 10.4 | 1.9 |
| Lobatse | 591 (189–1527) | 35 (30–43) | 154 (82–215) | 16.6 | 767.4 |
| Mabutsane Sub | 0 (0–629) | 37(26–42) | 164 (53–217) | 16.5 | 1.4 |
| Mahalapye | 707 (227–1188) | 36 (30–43) | 139 (79–187) | 18.1 | 7.3 |
| Masunga | 752 (135–1581) | 36 (30–44) | 141 (77–185) | 20 | 11.2 |
| Ngamiland | 1630 (501–2812) | 35 (30–42) | 135 (78–184) | 17.3 | 1 |
| Okavango | 324 (113–599) | 35 (29–42) | 131 (81–171) | 14.5 | 2.5 |
| Selibe-Phikwe | 860 (281–1822) | 34 (29–41) | 165 (95–231) | 25.4 | 1061.7 |
| Serowe/Palapye | 292 (81–1204) | 35(30–42) | 221 (127–410) | 17 | 5.8 |
| South East | 1020 (330–1731) | 35(30–42) | 157 (83–218) | 14.2 | 43.5 |
| Southern | 792 (118–1671) | 36 (31–44) | 125 (63–177) | 12.7 | 9 |
| Tutume | 532 (28–1441) | 37 (30–44) | 113 (58–170) | 19.1 | 3.1 |
| National | 798(161–1750) | 35(29–42) | 147(79–200) | 16.9 | 193.3 |
95% confidence interval in parenthesis
HIV prevalence and population density is averaged over the study period (2002–2013)
Figure 1.
Distribution of HIV-Infected People on ART in Botswana(2013)
Table 2 reports cumulative number of death and LTFU among ART enrollees in each district. The proportion of the patients died in the program ranges from 12% in Jwaneng Town to 3% in Selebe-Phikwe. There is also wide variation with regards to LTFU. South East with 23% has the lowest proportion of LTFU, while Mabutsane Sub with 72% has the highest proportion of LTFU.The median follow-up time was 26 months across districts (IQR 5–57). Ngamiland, Gaborone, Bobirwa and South East have the longest median patient follow-up, each above 33 months, while Kweneng East, Okavango, Serowe/Palapye and Mabutsane each had a median follow-up time of 15 months or less.
Table 2.
District variations in cumulative incidence of death and LTFU among ART enrollees in Botswana’s national antiretroviral therapy program during 2002–2013
| Number of Patients | Female | Deaths | LTFU | Follow Up Time (days) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Districts | N | % | % | N | % | N | % | Median | IQR |
| Bobirwa | 11356 | 5% | 64% | 964 | 8% | 3060 | 27% | 1061 | (273–1937) |
| Boteti | 6934 | 3% | 64% | 418 | 6% | 3512 | 51% | 531 | (72–1269) |
| Chobe | 4953 | 2% | 63% | 247 | 5% | 2719 | 55% | 723 | (83–1680) |
| Francistown | 27399 | 12% | 59% | 2030 | 7% | 16368 | 60% | 832 | (256–1658) |
| Gaborone | 35132 | 16% | 62% | 2351 | 7% | 14027 | 40% | 1458 | (370–2490) |
| Gantsi | 4747 | 2% | 61% | 331 | 7% | 2525 | 53% | 882 | (267–1696) |
| Goodhope | 4276 | 2% | 79% | 444 | 10% | 2226 | 52% | 531 | (8–1440.5) |
| Jwaneng Town | 4188 | 2% | 65% | 494 | 12% | 2087 | 50% | 668 | (75–1742) |
| Kgalagadi | 3260 | 1% | 69% | 334 | 10% | 1808 | 55% | 881 | (182–1813) |
| Kgalagadi North | 2780 | 1% | 59% | 214 | 8% | 1287 | 46% | 816 | (37–1839) |
| Kgatleng | 9149 | 4% | 64% | 607 | 7% | 5810 | 64% | 716 | (83–1546) |
| Kweneng East | 20073 | 9% | 61% | 1060 | 5% | 12043 | 60% | 476 | (27–1177) |
| Kweneng West | 3492 | 2% | 71% | 324 | 9% | 1859 | 53% | 503 | (54–1411) |
| Lobatse | 7303 | 3% | 63% | 393 | 5% | 2888 | 40% | 591 | (189–1527) |
| Mabutsane Sub | 1193 | 1% | 67% | 82 | 7% | 861 | 72% | 0 | (0–629) |
| Mahalapye | 12438 | 6% | 63% | 704 | 6% | 7710 | 62% | 707 | (227–1188) |
| Masunga | 6519 | 3% | 68% | 419 | 6% | 3378 | 52% | 752 | (135–1581) |
| Ngamiland | 6434 | 3% | 61% | 738 | 11% | 1593 | 25% | 1630 | (501–2812) |
| Okavango | 1701 | 1% | 71% | 123 | 7% | 1186 | 70% | 324 | (113–599) |
| Selibe-Phikwe | 13598 | 6% | 63% | 465 | 3% | 5179 | 38% | 860 | (281–1822) |
| Serowe/Palapye | 9007 | 4% | 64% | 473 | 5% | 3800 | 42% | 292 | (81–1204) |
| South East | 7335 | 3% | 63% | 540 | 7% | 1687 | 23% | 1020 | (330–1731) |
| Southern | 7919 | 4% | 60% | 608 | 8% | 2882 | 36% | 792 | (118–1671) |
| Tutume | 14844 | 7% | 68% | 787 | 5% | 8768 | 59% | 532 | (28–1441) |
| Total | 226030 | 100% | **62% | 15150 | **7% | 109263 | **49% | 798 | (161–1750) |
- 95% confidence interval in parenthesis
Averaged over districts
Mortality
Overall, the crude mortality rate was 2.06 per 100 person-years (95% CI 2.03–2.10). Table 3 reports the mortality and LTFU rates at different intervals in all Botswana districts. Mortality in the first year of treatment was 5.3 deaths per 100 person-years (95% CI 5.2–5.4), with a large range in the districts. South East and Selibe-Phikwe had the lowest one-year mortality rates at 1.8 deaths per 100 person-years (95% CI 1.5–2.2) and 2.0 per 100 person-years (95% CI 1.8–2.3), respectively. Mabutsane, Jwaneng, and Good Hope all have one-year mortality rates over 10 deaths per 100 person-years. Death in the first three months after treatment initiation remains high. Overall, the mortality rate in this time period is 11.8 deaths per 100 person-years (95% CI 11.5–12.1). South East, Selibe-Phikwe, Lobatse, Bobirwa, and Chobe have first-quarter mortality rates of less than 10 deaths per 100 person-years, while Kweneng West, Kgalagadi South, Jwaneng and Good Hope have mortality rates over 20 deaths per 100 person-years in the same period.
Table 3.
Mortality and LTFU Rates over Time in Botswana Masa Program
| Mortality rate per 100 P-Y | LTFU rate per 100 P-Y | |||||
|---|---|---|---|---|---|---|
| District | 3 month | 12 months | overall | 3 month | 12 months | overall |
| Bobirwa | 8.9 (7.8–10.1) | 4.1 (3.7–4.5) | 2.2 (2.1–2.4) | 11.5 (10.2–12.9) | 8.6 (8.0–9.3) | 5.9 (5.7–6.2) |
| Boteti | 11.9 (10.2–13.9) | 6.3 (5.6–7.0) | 2.7 (2.4–3.0) | 20.1 (17.9–22.6) | 18.1 (16.9–19.3) | 17.5 (16.8–18.1) |
| Chobe | 9.2 (7.4–11.3) | 4.0 (3.4–4.7) | 1.6 (1.4–1.9) | 13.2 (11.1–15.7) | 13.9 (12.7–15.2) | 14.9 (14.2–15.5) |
| Francistown | 10.9 (10.1–11.8) | 5.3 (5.0–5.6) | 2.2 (2.1–2.3) | 13.1 (12.2–14.1) | 23.7 (23.1–24.4) | 15.1 (14.9–15.4) |
| Gaborone | 11.0 (10.3–11.8) | 4.7 (4. 5–5.0) | 1.4 (1.3–1.5) | 16.1 (15.2–17.0) | 10.8 (10.4–11.2) | 7.1 (7–7.27) |
| Gantsi | 11.2 (9.4–13.5) | 5.2 (4.5–6.0) | 2.6 (2.0–2.5) | 11.3 (9. 5–13.6) | 14.2 (13.0–15.4) | 15.4 (14.7–16.0) |
| Goodhope | 27.5 (24.0–31.4) | 11.4 (10.2–12.7) | 4.3 (3.9–4.7) | 11.9 (9.7–14.7 | 12.5 (11.2–13.9) | 15.9 (15.2–16.7) |
| Jwaneng | 22.7 (19.6–26.2) | 10.7 (9.5–11.9) | 4.2 (3.8–4.6) | 9.8 (7.9–12.2) | 13.6 (12.3–15.0) | 13.6 (13.0–14.4) |
| Kgalagadi | 20.2 (17.1–23.8) | 8.3 (7.2–9.5) | 3.2 (2.9–3.6) | 9.1 (7.1–11.6) | 10.9 (9.7–12.3) | 15.6 (14.8–16.4) |
| Kgalagadi North | 16.6 (13.4–20.5) | 6.8 (5.7–8.1) | 2.5 (2.2–2.9) | 7.2 (5.3–10.0) | 8.2 (7.0–9.6) | 10.3 (9.6–11.0) |
| Kgatleng | 15.6 (13.9–17.6) | 6.5 (5.9–7.1) | 2.4 (2.2–2.6) | 10.1 (8.7–11.7) | 12.9 (12.0–13.8) | 18.3 (17. 8–18.9) |
| Kweneng East | 13.1 (11.9–14.3) | 5.6 (5.2–6.1) | 2.3 (2.1–2.4) | 18.2 (16.8–19.6) | 20.2 (19.4–20.9) | 19.0 (18.6–19.4) |
| Kweneng West | 20.1 (17.0–23.9) | 9.4 (8.2–10.7) | 3.6 (3.2–4.0) | 7.6 (5.8–10.0) | 15.3 (13.8–17.0) | 16.8 (15.9–17.6) |
| Lobatse | 8.0 (6.8–9.5) | 3.8 (3.3–4.4) | 1.6 (1.4–1.8) | 11.4 (9.8–13.1) | 14.7 (13.8–15.8) | 13.9 (13.4–14.5) |
| Mabutsane | 18.4 (12.3–27.4) | 10.3 (7.7–13.7) | 5.0 (4–6.14) | 19.9 (13.5–29.2) | 28.1 (23.6–33.46) | 25.4 (23.1–27.9) |
| Mahalapye | 11.9 (10.7–13.3) | 5.4 (5–5.9) | 2.1 (2.0–2.3) | 22.7 (21.0–24.5) | 21.5 (20.6–22.4) | 21.8 (21.2–22.3) |
| Masunga | 11.5 (9.8–13.5) | 5.4 (4.7–6.1) | 2.2 (2.0–2.4) | 11.6 (9.9–13.5) | 12.2 (11.3–13.3) | 15.3 (14.7–15.8) |
| Ngamiland | 15.6 (13.7–17.7) | 7.4 (6.7–8.1) | 2.1 (2.0–2.3) | 6.9 (5.7–8.4) | 5.6 (5.0–6.3) | 4.6 (4.4–4.85) |
| Okavango | 14.8 (11.3–19.3) | 7.2 (5.8–8.9) | 4.6 (3.8–5.6) | 22.7 (18.3–28.2) | 40.1 (36.7–44.0) | 46.2 (43.5–49.2) |
| Selibe-Phikwe | 4.7 (4.0–5.6) | 2.0 (1.8–2.3) | 1.0 (0.9–1.1) | 17.1 (15.7–18.6) | 15.3 (14.6–16.1) | 11.0 (10.7–11.3) |
| Serowe/Palapye | 12.2 (10.6–13.9) | 5.4 (4.8–6.0) | 2.4 (2.2–2.6) | 6.1 (5.1–7.4) | 8.8 (8.1–9.6) | 14.2 (13.6–14.7) |
| South East | 2.9 (2.1–3.8) | 1.8 (1.5–2.2) | 2.1 (1.9–2.3) | 7.75 (6.5–9.2) | 6.2 (5.6–6.9) | 5.9 (5.6–6.2) |
| Southern | 16.3 (14. 5–18.4) | 6.9 (6.3–7.7) | 2.6 (2.4–2.8) | 12.8 (11.2–14.6) | 11.6 (10.8–12.5) | 9.6 (9.2–10.0) |
| Tutume | 12.7 (11.5–14.2) | 5.7 (5.2–6.2) | 2.2 (2.0–2.3) | 19.0 (17.4–20.7) | 16.7 (15.9–17.6) | 18.1 (17.7–18.6) |
- 95% confidence interval in parenthesis
Loss to Follow-up
The overall rate of LTFU is 12.47 per 100 person-years (95% CI 12.38–12.55). Like mortality the rates of LTFU are higher in the first year and the first three months after treatment initiation (Table 3). For LTFU this rate was 14.2 losses per 100 person-years (95% CI 13.8–14.5) in the first three months, and 14.9 losses per 100 person years (95% CI 14.7–15.1) in the first year. There was a great range of overall LTFU across districts, including rates as low as 4.6 losses per 100 person-years in Kgalagadi North, and 5.9 losses per 100 person-years in Kgalagadi South, to rates as high as 25.4 losses per 100 person-years in Kweneng East and 46.3 losses per 100 person-years in Ngamiland. Nine districts had LTFU rates over 15 losses per 100 person-years in the first year, while 5 districts had LTFU rates of less than 10 losses per 100 person-years. Similarly, eight districts had LTFU rates higher than the overall rate of 14.2 losses per 100 person-years in the first three months, while 7 districts had LTFU rates under 10 losses per 100 person-years.
Table 4 provides crude mortality and LTFU rates, with corresponding rankings for each district in 2004, 2008 and 2012, with lower numbers corresponding to higher mortality (or LTFU) rates and higher numbers corresponding to lower mortality (or LTFU) rates. In terms of mortality, the ranks of certain districts changed substantially over time. Some districts maintained their rank, while others had steady increases or decreases in ranks. However the largest proportion of districts had rates that decreased or increased between 2004 and 2008, but in 2012 returned to ranks more similar as those seen in 2004. For example, Kgalagadi North’s and Kweneng East’s mortality rate rank increased over time, while Kgatleng mortality rate ranks decreased over time. Serowe/Palapye and Good Hope, at the two ends of the mortality rate range, stay steady across these years. Southern is an example of a district whose rank greatly increased during 2008, but was relatively low in both 2004 and 2012. Inversely, Gantsi is an example of a district that had higher ranks in 2004 and 2012 but a much lower rank in 2008.
Table 4.
District-level adult ART mortality rates in Botswana ranked in 2004, 2008 and 2012
| Mortality rates per 100 P-Y | LTFU rates per 100 P-Y | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| District | 2004 | rank | 2008 | rank | 2012 | rank | 2004 | rank | 2008 | rank | 2012 | rank |
| Bobirwa | 0.11 | 11 | 0.03 | 8 | 0.006 | 12 | - | - | 9.5 | 10 | 13.6 | 7 |
| Boteti | 0.10 | 12 | 0.03 | 9 | 0.007 | 11 | 14.0 | 16 | 17.5 | 18 | 9.0 | 5 |
| Chobe | 0.25 | 4 | 0.02 | 18 | 0.005 | 18 | 3.5 | 5 | 15.9 | 15 | 30.1 | 17 |
| Francistown | 0.09 | 13 | 0.02 | 14 | 0.005 | 17 | 7.9 | 10 | 8.5 | 5 | 16.0 | 10 |
| Gaborone | 0.03 | 19 | 0.01 | 19 | 0.005 | 16 | 6.4 | 7 | 6.1 | 1 | 18.7 | 12 |
| Gantsi | 0.05 | 18 | 0.04 | 4 | 0.006 | 14 | 3.1 | 3 | 16.9 | 16 | 4.6 | 1 |
| Good Hope | 0.36 | 1 | 0.05 | 2 | 0.012 | 2 | 11.0 | 13 | 12.7 | 14 | 51.1 | 23 |
| Jwaneng town | 0.09 | 14 | 0.04 | 5 | 0.004 | 21 | 13.7 | 15 | 11.5 | 12 | 32.1 | 18 |
| Kgalagadi North | - | - | 0.03 | 11 | 0.010 | 4 | - | - | 7.5 | 4 | 18.9 | 13 |
| Kgalagadi | 0.26 | 3 | 0.03 | 10 | 0.010 | 3 | 1.7 | 2 | 7.5 | 3 | 42.7 | 22 |
| Kgatleng | 0.29 | 2 | 0.02 | 15 | 0.004 | 20 | - | - | 6.6 | 2 | 38.7 | 21 |
| Kweneng East | 0.08 | 15 | 0.02 | 12 | 0.009 | 6 | 10.3 | 11 | 12.1 | 13 | 20.1 | 14 |
| Kweneng West | - | - | 0.06 | 1 | 0.020 | 1 | 10.6 | 12 | 17.2 | 17 | 29.4 | 15 |
| Lobatse | 0.13 | 9 | 0.02 | 16 | 0.007 | 9 | 0.6 | 1 | 39.3 | 20 | 4.8 | 2 |
| Mabutsane | - | - | 0.04 | 6 | 0.002 | 23 | - | - | 24.4 | 19 | 34.6 | 19 |
| Mahalapye | 0.13 | 7 | 0.01 | 22 | 0.007 | 10 | 7.0 | 8 | 57.8 | 21 | 6.0 | 3 |
| Masunga | 0.03 | 20 | 0.03 | 7 | 0.008 | 8 | - | - | 9.4 | 9 | 29.9 | 16 |
| Ngamiland | 0.06 | 17 | 0.01 | 21 | 0.005 | 19 | 7.2 | 9 | 9.4 | 8 | 8.5 | 4 |
| Okavango | 0.13 | 8 | - | - | - | - | 3.2 | 4 | - | - | - | - |
| Selibe-Phikwe | 0.07 | 16 | 0.01 | 20 | 0.006 | 15 | 12.0 | 14 | 61.5 | 22 | 17.0 | 11 |
| Serowe/Palapye | 0.02 | 21 | 0.01 | 23 | 0.004 | 22 | - | - | - | - | 15.5 | 9 |
| South East | 0.12 | 10 | 0.04 | 3 | 0.008 | 7 | - | - | 8.5 | 6 | 9.0 | 6 |
| Southern | 0.17 | 5 | 0.02 | 13 | 0.009 | 5 | 5.6 | 6 | 11.1 | 11 | 14.5 | 8 |
| Tutume | 0.17 | 6 | 0.02 | 17 | 0.006 | 13 | 14.7 | 17 | 8.8 | 7 | 36.6 | 20 |
- The missing data indicates lack of mortality data from the district.
Table 5 shows results from regression models examining characteristics associated with odds of attrition, including mortality and LTFU among HIV patients. Overall, differences by district were statistically significant when adjusting for demographic, immunologic and medication variables, including gender, age, baseline CD4 cell count category (0–49;50–249; 250–499; 500+) ART regimen, in addition to the district level variables of population density, and HIV prevalence level. Compared with Gaborone, Good Hope district did not have significantly different odds of mortality. Additionally, Mabutsane was omitted due to a small number of individuals remaining in the mortality model. Only two districts, Francistown and Lobatse had a higher odds ratio of mortality compared with Gaborone (odds ratio (OR) 1.47 [95% CI 1.33–1.62] and 1.21 [95% CI 1.04–1.41], respectively). All other districts had statistically significantly lower OR of mortality compared with Gaborone. When examining the odds of LTFU across districts, Six districts have lower odds of LTFU compared with Gaborone ranging from 0.39 to 0.62. The remaining 15 districts each have greater odds of LTFU compared with Gaborone..
Table 5.
The Multilevel Logistic Regression Model Describing the Association between Districts and Attrition Rates.
| District | Mortality | 95% CI | LTFU | 95 % CI |
|---|---|---|---|---|
| Bobirwa | 0.28*** | (0.21– 0.39) | 0.53*** | (0.46– 0.61) |
| Boteti | 0.52*** | (0.37– 0.73) | 1.77*** | (1.54– 2.06) |
| Chobe | 0.20*** | (0.14– 0.29) | 1.60*** | (1.38– 1.88) |
| Francistown | 1.47*** | (1.31– 1.60) | 4.34*** | (4.09– 4.55) |
| Gaborone | - | – | ||
| Gantsi | 0.25*** | (0.17– 0.35) | 1.37*** | (1.18– 1.57) |
| GoodHope | 0.87 | (0.60– 1.30) | 1.47*** | (1.26– 1.76) |
| Jwaneng | 0.28* | (0.07– 1.16) | 1.1 | (0.77– 1.57) |
| Kgalagadi South | 0.23*** | (0.10– 0.51) | 0.77* | (0.58– 1.04) |
| Kgalagadi North | 0.28*** | (0.13– 0.62) | 0.53*** | (0.38– 0.76) |
| Kgatleng | 0.14*** | (0.08– 0.26) | 1.42*** | (1.24– 1.68) |
| Kweneng East | 0.30*** | (0.22– 0.41) | 1.89*** | (1.64– 2.17) |
| Kweneng West | 0.36*** | (0.20– 0.64) | 1.79*** | (1.46– 2.14) |
| Lobatse | 1.21** | (1.04– 1.40) | 1.51*** | (1.43– 1.61) |
| Mabutsane | (omitted) | 13.34*** | (6.85– 30.95) | |
| Mahalapye | 0.28*** | (0.20– 0.38) | 1.98*** | (1.72– 2.26) |
| Masunga | 0.19*** | (0.13– 0.26) | 2.37*** | (2.06– 2.75) |
| Ngamiland | 0.25*** | (0.18– 0.34) | 0.39*** | (0.34– 0.46) |
| Okavango | 0.50*** | (0.33– 0.77) | 3.25*** | (2.83– 3.78) |
| Selibe-Phikwe | 0.61*** | (0.53– 0.70) | 2.96*** | (2.79– 3.16) |
| Serowe/Palapye | 0.06*** | (0.03– 0.12) | 0.43*** | (0.34– 0.55) |
| South East | 0.38*** | (0.28– 0.51) | 0.46*** | (0.41– 0.52) |
| Southern District | 0.20*** | (0.14– 0.27) | 0.62*** | (0.54– 0.72) |
| Tutume | 0.38*** | (0.27– 0.53) | 3.28*** | (2.85– 3.79) |
- Level of statistical significance: *0.1 **0.05 ***0.001
-Adjusted for patient-level variables: gender, age, baseline CD4, follow-up time after treatment initiated, ARV medications at treatment initiation, year treatment was initiated and district level variables: HIV prevalence and population density in each year. Gaborone, the capital, is the reference.
- Models are weighted (IPW) to account for LTFU.
Discussion
Analysis of medical records from 262,030 patients from 2002 to 2013 revealed significant differences in mortality rate among HIV-infected individuals receiving ART in different districts, with odds of mortality in many districts higher than Gaborone, the capital and most populous district, even after adjusting for patient-level variables, e.g., gender, age, baseline CD4 cell categories, duration of treatment, year of initiation, and first-line ART. However, when district-level variables, namely population density as a proxy for physical access to care and HIV prevalence for facility workload were included, the odds of mortality in almost all districts (except for Kgatleng and Jwaneng2) reversed. It seems that contextual factors, both inside and outside the health system may play a more important role in the survival of patients than expected.
Service availability, specifically physical access to services, is a pivotal component of healthcare [22]. Limited physical access to healthcare facilities can partly explain substantial differences in mortality rates in the sparsely populated districts in the north and west of the country. For example, mortality rates in northern districts, Okavango, Boteti, Tutume, and Chobe were the highest whether looking at overall mortality or odds of death controlling for all patient-level variables. However, after adjusting for population density and HIV prevalence, the odds of mortality were less than that of Gaborone. The patients in these districts have to travel long distances to get to a primary hospital or, for those who need advanced care, to referral hospitals, namely Princess Marina Hospital in Gaborone and Nyangabgwe Referral Hospital in Francistown [23]. That may partially explain the significant reduction in odds of mortality in these districts, compared to Gaborone, when population density and HIV prevalence were added to model.
Part of this variation in mortality and LTFU across districts could be explained by shortages of health professionals, exacerbated by their misdistribution across districts. Botswana, like other sub-Saharan African countries, has the lowest doctor and nurse to population ratio in the world [24]. ART scale-up needed a multitude of experienced and trained health professional [25]. The critical short-age of health personnel was further complicated by inequality in geographic distribution [26]. There is a significant variation across health districts with regards to the distribution of health professionals[26]. After the influx of patients seeking ART during scale-up, districts with a low healthcare-professional-to-patient ratio probably experienced higher mortality and LTFU among HIV patients. Further research is needed to evaluate this hypothesis.
Large-scale patient enrollment in chronic-care services in a developing country like Botswana has significantly added to the burden on services and providers. Particularly, districts with higher HIV prevalence have to handle many more patients than those with lower prevalence, with no evidence of the resources being distributed according to HIV prevalence in a district. The quality of care in districts with higher prevalence could have been adversely affected with an ever-increasing number of patients using limited resources, such as trained personnel, diagnostic and therapeutic equipment, medication, and intensive care services. The increasing workload due to higher prevalence may explain differences in odds of mortality between Gaborone and other districts. ks of
While some of these mortality rate differences can be, at least partially, explained, some cases are quite baffling. For example, the mortality differentials between Gaborone and Francistown, the two largest urban centers in the country, each one housing one of the two tertiary hospitals in the country, are hard to explain. HIV prevalence in Francistown in 2004, 2008 and 2013 was 24.6, 23.1 and 23.1, respectively, while in the same years HIV prevalence in Gaborone was 18.3, 17.1 and 16.2. Given the fact that case fatality rate in both cities is 7%, controlling for the patient- and district-level variables, the odds of a patient dying in Francistown are 30% higher than in Gaborone. Conversely given that the median baseline CD4 over the last 10 years in Gaborone and Francistown were almost the same (121 and 123 cells/mm3, respectively), it seems that superior adherence to medical protocols, or better care in Gaborone, may better explain the differences rather than does earlier screening or entry into care. Another possible reason for the differences is that more patients in advanced-stages of the disease may have been referred from neighboring districts to Francistown than to Gaborone.
As for LTFU, we also found significant variation between districts. However, in the regression model adding district-level variables reduced the odds, but did not change the direction of the odds in most of the districts. We could not detect a correlation between the geographical location of the districts and LTFU rates. For instance, Ngamiland and Tutume are both in the northern part of the country, with LTFU rates at 4.6 and 18.3 per 100 P-Y, respectively; or Bobirwa and Mahalapye are both in the central-eastern part of the country with LTFU rate of 5.9 and 18.1 per 100 P-Y, respectively. It seems that LTFU rates have much to do with data quality. For example, in Okavango and Mabutsane patient’s records entry into the electronic database gradually dwindled after 2008, consequently, it seems that these districts have a high rate of LTFU. Most likely many of those patients are still in care, however, because of unclear reasons their medical records are not captured in the national database or they have been transferred to the clinics closer to their residence and their medical records could not be linked.
One of the limitations of this study was data quality. Variable quality of data across districts could explain part of the total variation in health outcome. There are some facilities equipped with the hardware and software whose data have not been captured in the electronic database. Some facilities have been reporting only demographic data and fail to report the laboratory results or prescription drug regimens of clients. Even the reporting, or lack thereof, was not consistent, and fluctuates over time.
Our study poses the question why the same guidelines and medication regimens for the patients across districts can result in different outcomes? Why do mortality rates vary among districts? The attrition rate (retention and survival) has been increasingly used as a yardstick to assess the long-term success of large scale ART programs [16]. The fact that this study found significant attrition in-equality across districts suggests that the expanding ART program might not have effective management and sufficient resources in some districts to adequately support adherence and retention. Policy makers need to consider investing in building effective health systems as the paradigm shifts from emergency response to chronic disease management [1].
Our findings support earlier work identifying inequality at subnational and cross-national levels [11, 14]. The elimination of inequality in health outcome, particularly among HIV-infected individuals should be a priority of the Ministry of Health. Health officials should focus on targeted support to districts with disproportionately high attrition rate. Place of residence and subsequently where individuals receive care should be considered as important components of any national analysis, as there are many contextual factors inside and outside the healthcare delivery system that can cause variation in outcomes. While part of the variation found in this study is due to data quality, part of it may be due to scarcity and management of resources and infrastructure at district- and facility-level, and part to factors beyond the healthcare system, e.g., poverty, infrastructure, etc. [29] Future studies need to examine the existing ART delivery system and human resources components to identify the underlying factors at district- and facility-level. The findings of this study can help both researchers and policy-makers by advising them consider contextual factors as well individual-level factors while taking steps to address the underlying causes of variation in mortality and LTFU outcomes of patients on ART.
Acknowledgments
Disclaimer
We would like to acknowledge Mr. Bud Bowen at the US Centers for Disease Control for his support on this project. We would also like to acknowledge the support of the Botswana Ministry of Health, specifically those at the Department of HIV/AIDS Prevention and Care, and the Department of Health Policy, Development, Monitoring and Evaluation, including the Monitoring and Evaluation Division. This research was supported by the President’s Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Botswana is divided into nine administrative districts, which is further divided into 15 councils, including the nine districts councils from the nine districts plus some councils from urban or town councils. Each of these 24 subdivisions is considered a health district. Henceforth, we will refer to health districts as “districts.”
In these two districts, after adding the district-level variables the odds differences became statistically significant.
References
- 1.Atun R, Bataringaya J. Building a durable response to HIV/AIDS: implications for health systems. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011;57:S91–S95. doi: 10.1097/QAI.0b013e3182218441. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Global update on HIV treatment 2013: results, impact and opportunities. Geneva: World Health Organization; 2013. [Google Scholar]
- 3.M & E Unit. Monitoring & Evaluation Unit Monthly Report Feb. 2015. Gaborone, Botswana: Ministry of Health; 2015. [Google Scholar]
- 4.Farahani M, Vable A, Lebelonyane R, Seipone K, Anderson M, Avalos A, et al. Outcomes of the Botswana national HIV/AIDS treatment programme from 2002 to 2010: a longitudinal analysis. The lancet global health. 2014;2:e44–e50. doi: 10.1016/S2214-109X(13)70149-9. [DOI] [PubMed] [Google Scholar]
- 5.Govindasamy D, Kranzer K, Ford N. Strengthening the HIV cascade to ensure an effective future ART response in sub-Saharan Africa. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2014;108:1–3. doi: 10.1093/trstmh/trt105. [DOI] [PubMed] [Google Scholar]
- 6.Phaladze NA, Human S, Dlamini SB, Hulela EB, Mahlubi Hadebe I, Sukati NA, et al. Quality of Life and the Concept of “Living Well” With HIV/AIDS in Sub-Saharan Africa. Journal of nursing scholarship. 2005;37:120–126. doi: 10.1111/j.1547-5069.2005.00023.x. [DOI] [PubMed] [Google Scholar]
- 7.Nash D, Wu Y, Elul B, Hoos D, El Sadr W. Program-level and contextual-level determinants of lowmedian CD4+ cell count in cohorts of persons initiating ART in eight sub-Saharan African countries. AIDS (London, England) 2011;25:1523. doi: 10.1097/QAD.0b013e32834811b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farahani M, Subramanian SV, Canning D. Effects of state-level public spending on health on the mortality probability in India. Health Economics. 2010;19:1361–1376. doi: 10.1002/hec.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makinen M, Waters H, Rauch M, Almagambetova N, Bitran R, Gilson L, et al. Inequalities in health care use and expenditures: empirical data from eight developing countries and countries in transition. Bulletin of the World Health Organization. 2000;78:55–65. [PMC free article] [PubMed] [Google Scholar]
- 10.Farahani M, Subramanian S, Canning D. The effect of changes in health sector resources on infant mortality in the short-run and the long-run: a longitudinal econometric analysis. Social Science & Medicine. 2009;68:1918–1925. doi: 10.1016/j.socscimed.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Singh GK, Azuine RE, Siahpush M. Widening socioeconomic, racial, and geographic disparities in HIV/AIDS mortality in the United States, 1987–2011. Advances in preventive medicine. 2013;2013 doi: 10.1155/2013/657961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simard EP, Fransua M, Naishadham D, Jemal A. The influence of sex, race/ethnicity, and educational attainment on human immunodeficiency virus death rates among adults, 1993–2007. Archives of internal medicine. 2012;172:1591–1598. doi: 10.1001/archinternmed.2012.4508. [DOI] [PubMed] [Google Scholar]
- 13.Otwombe KN, Petzold M, Modisenyane T, Martinson NA, Chirwa T. Factors associated with mortality in HIV-infected people in rural and urban South Africa. Global health action. 2014;7 doi: 10.3402/gha.v7.25488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanna DB, Selik RM, Tang T, Gange SJ. Disparities among states in HIV-related mortality in persons with hiv infection, 37 US States, 2001–2007. AIDS (London, England) 2012;26:95. doi: 10.1097/QAD.0b013e32834dcf87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farahani M, Price N, El-Halabi S, Mlaudzi N, Keapoletswe K, Lebelonyane R, et al. Trends and determinants of survival for over 200,000 patients on ARV treatment in the Botswana National Program: 2002–2013. AIDS (in Press) 2015 doi: 10.1097/QAD.0000000000000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Botswana S, editor. Statistics Botswana. 2011 Population and Housing Census Analytical Report. Gaborone, Botswana: 2014. [Google Scholar]
- 17.Hernán MA, Brumback BA, Robins JM. Estimating the causal effect of zidovudine on CD4 count with a marginal structural model for repeated measures. Statistics in medicine. 2002;21:1689–1709. doi: 10.1002/sim.1144. [DOI] [PubMed] [Google Scholar]
- 18.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. John Wiley & Sons; 2012. [Google Scholar]
- 19.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. American journal of epidemiology. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fewell Z, Hernán MA, Wolfe F, Tilling K, Choi H, Sterne JA. Controlling for time-dependent confounding using marginal structural models. Stata J. 2004;4:402–420. [Google Scholar]
- 21.D'Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Statistics in medicine. 1990;9:1501–1515. doi: 10.1002/sim.4780091214. [DOI] [PubMed] [Google Scholar]
- 22.Frost LJ, Reich M. Access: how do good health technologies get to poor people in poor countries? Bibliomotion, Inc.; 2014. [Google Scholar]
- 23.Central Statistics Office, editor. CSO. Health statistics Report 2009. Gaborone, Botswana: Statistics Botswana; 2012. [Google Scholar]
- 24.WHO. The world health report: 2006: working together for health. Geneva: World Health Organization; 2006. [Google Scholar]
- 25.Van Damme W, Kober K, Laga M. The real challenges for scaling up ART in sub-Saharan Africa. Aids. 2006;20:653–656. doi: 10.1097/01.aids.0000216364.44409.b1. [DOI] [PubMed] [Google Scholar]
- 26.Nkomazana O, Peersman W, Willcox M, Mash R, Phaladze N. Human resources for health in Botswana: the results of in-country database and reports analysis: original research. African Primary Health Care and Family Medicine. 2014;6:1–8. doi: 10.4102/phcfm.v6i1.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sæbø JI. A Flexible Approach to Integrating Health Information Systems–The Case of Data Warehouse As Integrator in Botswana. Norway: University of Oslo; 2013. p. 149. [Google Scholar]
- 28.Thorseng AA. Managing complexity through flexible scaling : a case study of the expansion of a health information system in Botswana. Oslo, Norway: University of Oslo; 2008. [Google Scholar]
- 29.Rascon EG. Maping Poverty in Botswana 2010. Gaborone, Botswana: Wolrd Bank; 2015. [Google Scholar]