Abstract
BACKGROUND
Collagenous colitis (CC) and lymphocytic colitis (LC) are chronic inflammatory disorders of the colon. There is a paucity of data on differences in etiology, natural history, and treatment response between CC and LC.
METHODS
Between 2002 to 2013, we identified new diagnoses of CC and LC using the Research Patient Data Registry in a tertiary referral center. We used Chi Square or Fischer exact test and Wilcoxon rank-sum tests to compare the differences in clinical characteristics, treatment types, and response rates between LC and CC.
RESULTS
Through 2013, we confirmed 131 patients with a new diagnosis of MC (55 LC, 76 CC). Compared to cases of LC, patients with a diagnosis of CC were more likely to be women (86% vs. 69%, P = 0.03), have elevated erythrocyte sedimentation rate (mean 28 vs. 13 mm/hr, P = 0.04), and less likely to be diabetic (5% vs. 18%, P = 0.02). Budesonide was the most effective treatment for both CC and LC (94% and 80%, respectively). However, there were no statistically significant differences in response to various treatments according to the type of microscopic colitis (All P > 0.10). Older age at the time of diagnosis was associated with better response to bismuth subsalicylate (OR 1.76; 95% CI, 1.21–2.56 for every 5-year increase) for both CC and LC.
CONCLUSION
Despite differences in the clinical characteristics, response rates to available treatments appeared to be similar in both LC and CC. Older patients may have a better response to bismuth subsalicylate therapy.
Keywords: Microscopic colitis, Collagenous colitis, Lymphocytic colitis, Loperamide, Budesonide, Cholestyramine, Bismuth Subsalicylate, Treatment Patterns and Response
Introduction
Microscopic colitis (MC) is a chronic relapsing disease of the colon, characterized by a triad of watery non-bloody diarrhea, usually normal colonoscopic findings, and typical histologic findings. It is frequently accompanied by abdominal pain, nocturnal diarrhea, and mild weight loss 1. Diarrhea may range from mild to severe, with a considerable impact on quality of life 2. MC comprises two major histological subtypes: collagenous colitis (CC) characterized by a distinctive thickened band of subepithelial collagen (≥10–20 μm), and lymphocytic colitis (LC), with an increased number of intraepithelial lymphocytes (≥20 lymphocytes per 100 epithelial cells)1.
Although the exact etiology of MC remains largely unknown, a few observational studies have suggested associations with autoimmune disorders (e.g. celiac disease, and thyroid disorders), cigarette smoking status, and medications such as non-steroidal anti-inflammatory drugs (NSAIDs), proton pump inhibitors (PPIs), selective serotonin reuptake inhibitors (SSRI), and statins 1,3–7. Previous studies have mainly focused on epidemiology and treatment of CC 8–20 with limited data available on differences in clinical characteristics and treatment response between CC and LC. We therefore sought to examine clinical characteristics, treatment patterns, and predictors of response to treatment of CC and LC in a tertiary referral center.
METHODS
Study Population
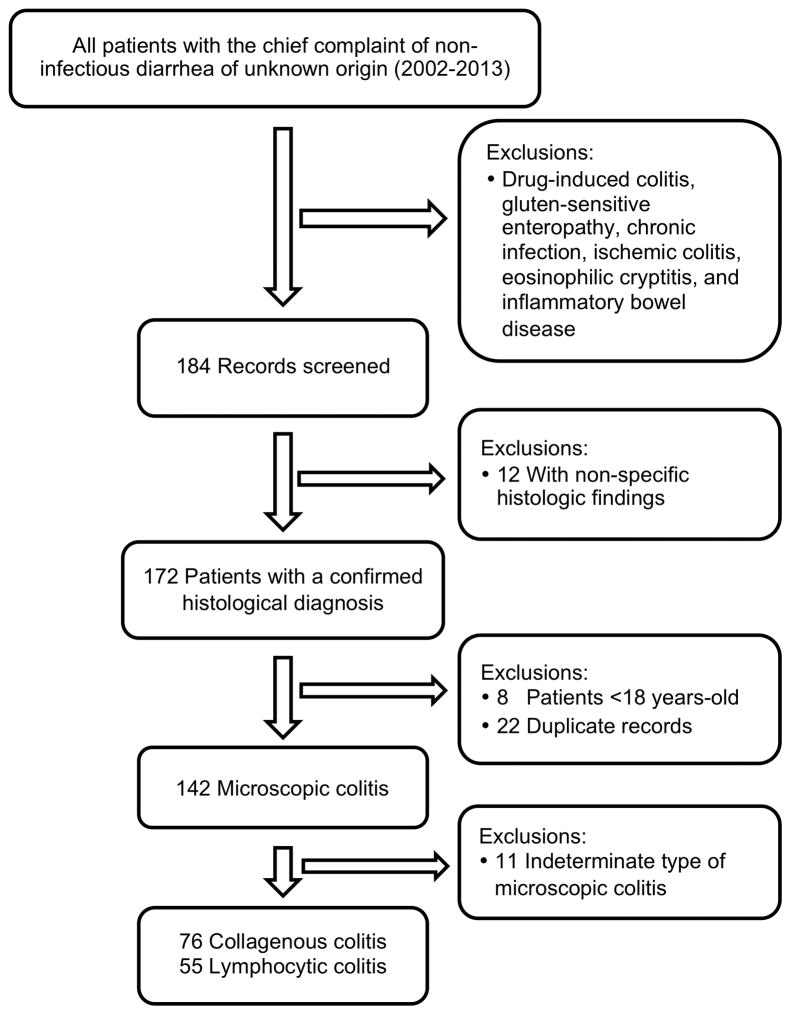
From 2002 to 2013, we obtained data on all participants over age 18 undergoing colonoscopy for non-infectious diarrhea (ICD9 = ‘558.9’ and ICD10 = ‘K52.89’) through Research Patient Data Registry (RPDR) at Massachusetts General Hospital(n=2,788). Briefly, Research Patient Data Registry allows for simultaneous search for various diagnoses and procedures codes within our inpatient and outpatient electronic medical records. We then screened the pathology records of potential participants using the key words “microscopic colitis”, “collagenous colitis”, and “lymphocytic colitis”. Two gastroenterologists then reviewed the medical records of eligible patients and confirmed diagnoses of MC based on previously defined clinical and histologic criteria 2,19,21. Any discrepancies were resolved through consensus among the reviewers. We excluded patients with diagnoses of drug-associated colitis, diarrhea related to celiac disease, chronic infections, ischemic colitis, eosinophilic cryptitis, and inflammatory bowel disease. We excluded 12 patients for histologic diagnosis of non-specific colitis, 8 patients under the age of 18 (adult only), and 22 patients with flares during follow-up (duplicate records). Eleven patients were considered to have indeterminate MC because they did not meet the classical histologic findings. This group included patients with early CC, minimally active microscopic colitis, overlapping forms between CC and LC, extensive diffuses non-distorted colitis with neutrophilic cryptitis, CC or LC with superimposed infection, and CC or LC with increased eosinophils. After all exclusions, our study population included 131 patients with MC that could be definitely defined as CC or LC by histopathology (55 CC and 76 LC). All cases of MC had at least two follow up appointments following their diagnosis.
Study Covariates
All covariates were collected by review of medical records independently by two physicians. Any discrepancies were resolved through consensus. Age at diagnosis was calculated from date of colonoscopy and date of birth. Body mass index (BMI) was derived from the medical records and represented BMI around the time of MC diagnosis (up to 1 month prior to diagnosis). We also collected information on presence of abdominal pain and weight loss (>5 kg) at the time of diagnosis, current cigarette smoking, medication history up to one month prior to diagnosis of MC including selective serotonin receptor inhibitors (SSRIs), non-steroidal anti-inflammatory drugs (NSAIDs), proton pump inhibitors (PPIs), statins, and antibiotics use, erythrocyte sedimentation rate (ESR), and other comorbidities including history of diabetes mellitus (DM) type 1 or 2, gastro esophageal reflux (GERD), and presence of autoimmune diseases including Hashimoto’s thyroiditis, Grave’s disease, celiac disease, psoriasis, rheumatoid arthritis (RA), and Sjögren’s disease. Therapeutic choices of MC for each patient were recorded. Successful treatment response was collected from medical records, and was defined as complete resolution of diarrhea (passage of two or fewer stools per day), achieved within 8 weeks of treatment. To avoid concerns for reporting efficacy based on the effect of multiple medications used in successive order, we only considered the response to the first treatment choice, which was defined as budesonide, mesalamine, or bismuth subsalicylate. Only combination therapies included loperamide or cholestyrmaine in combination with mesalamines or budesonide.
Statistical Analysis
All statistical analyses were carried out using SAS version 9.3 (Cary, NC). The data for each variable were expressed in frequency, percentage, and median or mean value ± standard deviation (SD). We used Wilcoxon rank-sum and Chi-square tests or Fisher exact test (for absolute numbers smaller than 5) for pairwise comparisons of continuous and categorical variables, respectively. To identify predictors of response to therapy from variables listed in Table 1, we used logistic regression modeling with an automated forward selection with a stepwise value threshold for removal of 0.1. Logistic regression results were reported as odd ratio (OR) with 95% confidence intervals (95% CIs). Data on treatment type and response were 100% complete. There were less than 2% missing values for other covariates. Missing observations were excluded from analyses. All P values were two-sided and values < 0.05 were considered statistically significant.
Table 1.
Baseline characteristics of the patients in two subtypes of microscopic colitis a
| Histological subtype | P value | |||
|---|---|---|---|---|
| Lymphocytic colitis (n=55) | Collagenous colitis (n=76) | |||
| Age (years) | Mean (SD) | 59.9 (20.8) | 61.3 (15.1) | 0.72 |
| BMI (Kg/m2) | Mean (SD) | 25.8 (5.2) | 24.7 (4.6) | 0.38 |
| Sex | ||||
| Female | %(n) | 69.1 (38) | 85.5 (65) | 0.03 |
| Race | ||||
| White | %(n) | 90.9 (50) | 94.7 (71) | 0.49 |
| Abdominal Pain | %(n) | 41.8 (23) | 32.0 (24) | 0.27 |
| Weight Loss | %(n) | 27.3 (15) | 30.7 (23) | 0.70 |
| Type 2 Diabetes | ||||
| Mellitus | %(n) | 18.2 (10) | 5.3 (4) | 0.02 |
| Autoimmune disease | %(n) | 41.8 (23) | 42.7 (32) | 1.00 |
| Hashimoto’s disease | 47.8 (11) | 37.5 (12) | 0.58 | |
| Celiac disease | 17.4 (4) | 28.1 (9) | 0.52 | |
| Othersb | 60.9 (14) | 59.4 (19) | 1.00 | |
| GERD | %(n) | 38.2 (21) | 41.3 (31) | 0.86 |
| Smoke | %(n) | 23.6 (13) | 15.8 (12) | 0.27 |
| ESR (mm/h) | Mean (SD) | 13.3 (19.3) | 28.2 (18.6) | 0.04 |
| SSRIsc | %(n) | 36.4 (20) | 27.8 (20) | 0.34 |
| NSAIDsc | %(n) | 38.2 (21) | 54.9 (39) | 0.07 |
| Antibioticsc | %(n) | 16.4 (9) | 20.8 (15) | 0.65 |
| PPIsc | %(n) | 38.2 (21) | 34.7 (25) | 0.71 |
| Statinsc | %(n) | 21.8 (12) | 23.6 (17) | 0.83 |
Abbreviations: SD = Standard deviation; GERD = Gastro esophageal reflux; ESR = Erythrocyte sedimentation rate; SSRIs = Selective serotonin reuptake inhibitors; NSAIDs = Non-steroidal anti-inflammatory drugs; PPIs = Proton pump inhibitors.
Psoriasis, Type 1 Diabetes Mellitus, Rheumatoid arthritis, Grave’s disease, Sjögren’s disease, and unspecified immune disease disorder.
Defined as use up to one month prior to onset of symptoms.
RESULTS
Baseline characteristics of participants with CC and LC
Through 2013, we confirmed 131 patients with new of diagnosis of microscopic colitis (76 CC and 55 LC) (Figure 1). The mean age at the time of diagnosis was 61 years (SD = 17.5) with the majority of cases occurring in women (78%)(Table 1). There was no statistically significant difference in age of diagnosis comparing LC and CC (61.3 vs 59.9 years, P = 0.72). However, compared to LC, CC patients were more likely to be female (85.5% vs. 69.1%, P = 0.03), less likely to have diabetes mellitus (5.3 vs. 18.2%, P = 0.02), and were more likely to have higher ESR values (mean: 28.2 vs 13.3 mm/hr, P = 0.04). In addition, compared to LC, CC patients were more likely to have taken NSAIDs prior to diagnosis (54.9% vs. 38.2%, P = 0.07), although this did not reach statistical significance. Over 40% of MC patients also were diagnosed with an autoimmune disease and this was not significantly different comparing CC to LC (42.7% vs. 41.8%, P = 1.00). There were also no significant differences in rates of antibiotics, PPI, SSRIs, or statin use comparing LC to CC (All Pcomparisons > 0.10).
Figure 1.
Flowchart of the study
Treatment patterns and response rates
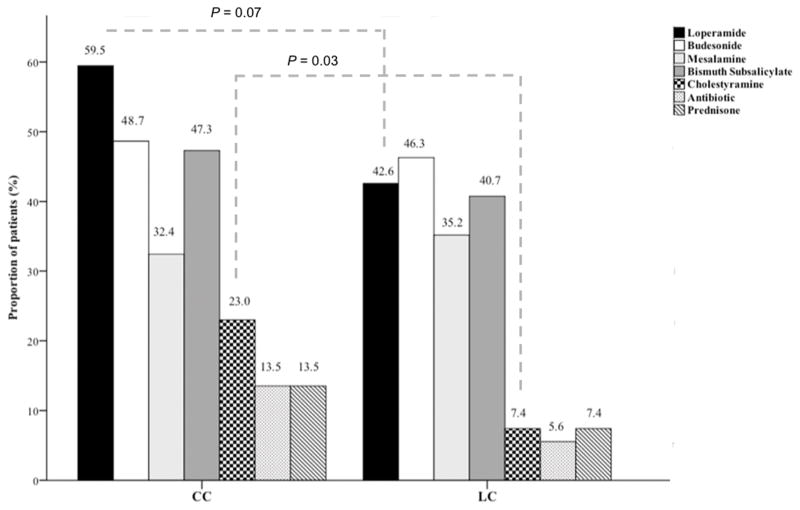
We examined the patterns of treatment for LC and CC. Over half of the patients with MC (54%) were treated with loperamide as needed to improve diarrheal symptoms, with CC patients being somewhat more likely to take loperamide compared to LC patients (59.5% vs. 42.6%, P = 0.07) (Figure 2). Budesonide (46%) and bismuth subsalicylate (46%) were the most common treatments for MC with both medications being used with similar frequencies for CC (48.6% and 47.3%, respectively) and LC (46.3% and 40.7%, respectively). Compared to LC, CC patients were more likely to receive cholestyramine (23.0% vs. 7.4%, P = 0.03). Nearly a third of all MC patients were treated with mesalamine compounds with similar frequencies in LC and CC (32.4% vs. 35.2%). Only four patients (7.4%) with LC and ten patients with CC (13.5%) required systemic steroid therapy with oral prednisone. Combination therapy was only used with loperamide or cholestyramine.
Figure 2.
Treatment patterns in collagenous colitis and lymphocytic colitis
Additionally, we examined the response rate to each treatment according to histologic subtype (Table 2). The most effective therapy appeared to be budesonide with over 80% of cases responding to this treatment. There was no difference in response to budesonide according to histologic subtype (80.0% for LC and 94.4% for CC, P = 0.11). The response rate to mesalamine was slightly over 50% with no difference in response according to histologic subtype (57.9% for LC vs. 52.2% for CC, P = 0.76). We also did not observe any significant difference in response to therapy according to histologic subtype with bismuth subsalicylate, cholestyramine, or prednisone (all Pcomparisons > 0.20). In exploratory analyses, we looked for clinical predictors of response to various treatment options and identified older age to be a predictor of response to bismuth subsalicylate (OR 1.76; 95% CI, 1.21–2.56 for every 5-year increase in age ). Conversely, there were no clinical predictors of response to budesonide or mesalamine compounds.
Table 2.
Treatment response according to subtypes of microscopic colitis.
| Response to treatment | Histological subtype
|
P value | ||
|---|---|---|---|---|
| Lymphocytic colitis (n=55) | Collagenous colitis (n=76) | |||
| Budesonide | %(n) | 80.0 (20) | 94.4 (34) | 0.11 |
| Mesalamine | %(n) | 57.9 (11) | 52.2 (12) | 0.76 |
| Bismuth subsalicylate | %(n) | 45.5 (10) | 63.6 (21) | 0.27 |
| Cholestyramine | %(n) | 75.0 (3) | 41.2 (7) | 0.31 |
| Prednisone | %(n) | 100.0 (4) | 70.0 (7) | 0.51 |
Discussion
In a retrospective cohort of CC and LC patients, we show that despite differences in clinical characteristics between the two diseases, the response rates to different treatment modalities are very similar. Our data shows that budesonide is the most effective treatment for CC and LC, and older patients may have a better response to bismuth subsalicylate therapy. In addition, there seems to be a greater utilization of antidiarrheal medications such as cholestyramine and loperamide for CC compared to LC.
Our results are supported by several other studies. Our cohort included primarily women in their seventh decade which appears consistent with previously described populations.22,23 Similar to other studies, our cohort included a high proportion of patients with type 2 diabetes mellitus, autoimmune disorders, and NSAIDs users. 5–7,24,25 Previous randomized controlled trials have shown that budesonide is effective in treatment of CC and LC with response rates consistently exceeding 80% similar to our findings. 8–15, 26 In addition, a recent randomized controlled trial showed budesonide to be more effective than mesalamine in treatment of CC with response rates of 80% and 44%, respectively, comparable to response rates in our cohort. 27
Despite the rising incidence of MC, little is known about the etiology of the disease. The presence of differing risk factors for both diseases points to potentially diverging biological pathways involved in the etiopathogenesis of the two diseases.7 Nonetheless, CC and LC appear to have identical clinical presentation with LC typically having a shorter disease course.22 In addition, a recent study, failed to identify differences in the characteristics of inflammatory cells involved in mucosal inflammation in CC and LC patients.28 Therefore, our findings that both CC and LC have high response rates to budesonide may be explained by presence of similar patterns of inflammation in both diseases.
Our study has several strengths. First, we confirmed all cases of CC and LC by medical records reviews using standardized criteria which is a significant advantage over prior studies that rely on self-report or clinic/hospital discharged codes, which may not accurately reflect true diagnoses. Second, we collected detailed information on other important life style factors, medications, and comorbidities in nearly all of our cases and were therefore able to account for their potential role in our analyses. Third, we collected information on treatment course for each patient and therefore were able to identify response rate for each treatment.
We acknowledge several limitations. Firstly, It is possible that the selection of certain treatments over others may have been differentially associated with some patient characteristics or disease severity. However, our study does provide data on the use of these treatments and their comparative effectiveness in actual clinical practice. Secondly, although our samples size was similar, if not larger than most of the prior studies, we may have had limited power to detect more modest differences or associations.” Third, we had data on a limited time period before and after development of disease and therefore may have been unable to fully account for use of other medications that were discontinued earlier as well as potential relapse rates.
To our knowledge, this is the first comprehensive study that directly compare clinical characteristics, treatment patterns, and response to therapy between CC and LC patients. Despite clinical differences between CC and LC, both diseases seem to have similar responses to available treatment options. Similar to European guidelines as well as a prior published meta-analysis, our data supports use of budesonide as the first line treatment for CC and LC29,30. In addition, we identified older age to be a potential predictor of response to bismuth subsalicylate for both CC and LC, which if replicated in other larger studies may provide additional data to guide clinicians in their selection among the various treatment options. In addition, we identified older age to be a potential predictor of response to bismuth subsalicylate for both CC and LC. Considering the lack of standardized treatment recommendations for CC or LC, our findings may provide some data to guide clinicians in their selection among the various treatment options. In addition, future studies to better understand the interaction between various lifestyle factors and treatment response may further shed light on the pathophysiology of CC and LC.
Acknowledgments
Grant Support: Funded by R01 CA137178, R01 CA050385, P01 CA87969, CA49449, CA67262, P30 DK043351, K24 098311, and K23 DK091742. Dr. Chan is supported by a senior investigator grant from the Crohn’s and Colitis Foundation of America (CCFA). Dr. Khalili is supported by a career development award from the American Gastroenterological Association (AGA) and by National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK099681). Dr. Ricciardiello is supported by the Italian Association for Cancer Research (AIRC – Investigator Grant n. 14281) and by the European Community’s Seventh Framework Program (Pathway-27) under grant agreement n. 311876.
Footnotes
Financial Disclosures: Dr. Richter is a consultant for policy analysis. Dr. Chan has served as a consultant for Bayer Healthcare, Pfizer Inc., and Pozen Inc. Other authors have no financial disclosures. Dr. Ricciardiello has received an unrestricted research grant by SLA Pharma AG.
Ethical Approval: The institutional review board at the Partners Healthcare approved this study.
Data sharing: Requests for access to data, statistical code, questionnaires, and technical processes may be made by contacting the corresponding author at hkhalili@mgh.harvard.edu.
Authors Contributions
Hamed Khalili takes responsibility for the integrity of the work as a whole, from inception to published article.
BS- acquisition of data; drafting of the manuscript.
KS - acquisition of data; critical revision of the manuscript.
GYL – study concept and design; critical revision of the manuscript for important intellectual content.
JMR - study concept and design; critical revision of the manuscript.
ATC- study concept and design; critical revision of the manuscript.
LR - study concept and design; critical revision of the manuscript.
HK - DC - study concept and design; acquisition of data; statistical analysis; interpretation of data; drafting of the manuscript.
References
- 1.Brown WR, Tayal S. Microscopic colitis. A review. Journal of digestive diseases. 2013 Jun;14(6):277–281. doi: 10.1111/1751-2980.12046. [DOI] [PubMed] [Google Scholar]
- 2.Pardi DS, Kelly CP. Microscopic colitis. Gastroenterology. 2011 Apr;140(4):1155–1165. doi: 10.1053/j.gastro.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Kao KT, Pedraza BA, McClune AC, et al. Microscopic colitis: a large retrospective analysis from a health maintenance organization experience. World journal of gastroenterology : WJG. 2009 Jul 7;15(25):3122–3127. doi: 10.3748/wjg.15.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Banares F, Esteve M, Espinos JC, et al. Drug consumption and the risk of microscopic colitis. Am J Gastroenterol. 2007 Feb;102(2):324–330. doi: 10.1111/j.1572-0241.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- 5.Fine KD, Do K, Schulte K, et al. High prevalence of celiac sprue-like HLA-DQ genes and enteropathy in patients with the microscopic colitis syndrome. Am J Gastroenterol. 2000 Aug;95(8):1974–1982. doi: 10.1111/j.1572-0241.2000.02255.x. [DOI] [PubMed] [Google Scholar]
- 6.Riddell RH, Tanaka M, Mazzoleni G. Non-steroidal anti-inflammatory drugs as a possible cause of collagenous colitis: a case-control study. Gut. 1992 May;33(5):683–686. doi: 10.1136/gut.33.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonnenberg A, Genta RM. Lymphocytic and collagenous colitis: epidemiologic differences and similarities. Dig Dis Sci. 2013 Oct;58(10):2970–2975. doi: 10.1007/s10620-013-2718-6. [DOI] [PubMed] [Google Scholar]
- 8.Baert F, Schmit A, D’Haens G, et al. Budesonide in collagenous colitis: a double-blind placebo-controlled trial with histologic follow-up. Gastroenterology. 2002 Jan;122(1):20–25. doi: 10.1053/gast.2002.30295. [DOI] [PubMed] [Google Scholar]
- 9.Bonderup OK, Hansen JB, Birket-Smith L, Vestergaard V, Teglbjaerg PS, Fallingborg J. Budesonide treatment of collagenous colitis: a randomised, double blind, placebo controlled trial with morphometric analysis. Gut. 2003 Feb;52(2):248–251. doi: 10.1136/gut.52.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonderup OK, Hansen JB, Teglbjaerg PS, Christensen LA, Fallingborg JF. Long-term budesonide treatment of collagenous colitis: a randomised, double-blind, placebo-controlled trial. Gut. 2009 Jan;58(1):68–72. doi: 10.1136/gut.2008.156513. [DOI] [PubMed] [Google Scholar]
- 11.Madisch A, Heymer P, Voss C, et al. Oral budesonide therapy improves quality of life in patients with collagenous colitis. International journal of colorectal disease. 2005 Jul;20(4):312–316. doi: 10.1007/s00384-004-0660-y. [DOI] [PubMed] [Google Scholar]
- 12.Miehlke S, Heymer P, Bethke B, et al. Budesonide treatment for collagenous colitis: a randomized, double-blind, placebo-controlled, multicenter trial. Gastroenterology. 2002 Oct;123(4):978–984. doi: 10.1053/gast.2002.36042. [DOI] [PubMed] [Google Scholar]
- 13.Miehlke S, Madisch A, Bethke B, et al. Oral budesonide for maintenance treatment of collagenous colitis: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2008 Nov;135(5):1510–1516. doi: 10.1053/j.gastro.2008.07.081. [DOI] [PubMed] [Google Scholar]
- 14.Miehlke S, Madisch A, Voss C, et al. Long-term follow-up of collagenous colitis after induction of clinical remission with budesonide. Alimentary pharmacology & therapeutics. 2005 Dec;22(11–12):1115–1119. doi: 10.1111/j.1365-2036.2005.02688.x. [DOI] [PubMed] [Google Scholar]
- 15.Tromm A, Griga T, Mollmann HW, May B, Muller KM, Fisseler-Eckhoff A. Budesonide for the treatment of collagenous colitis: first results of a pilot trial. The American journal of gastroenterology. 1999 Jul;94(7):1871–1875. doi: 10.1111/j.1572-0241.1999.01222.x. [DOI] [PubMed] [Google Scholar]
- 16.Munck LK, Kjeldsen J, Philipsen E, Fischer Hansen B. Incomplete remission with short-term prednisolone treatment in collagenous colitis: a randomized study. Scandinavian journal of gastroenterology. 2003 Jun;38(6):606–610. doi: 10.1080/00365520310002210. [DOI] [PubMed] [Google Scholar]
- 17.Miehlke S, Madisch A, Kupcinskas L, et al. Budesonide is more effective than mesalamine or placebo in short-term treatment of collagenous colitis. Gastroenterology. 2014 May;146(5):1222–1230. e1221–1222. doi: 10.1053/j.gastro.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Fine KOF, Lee E, Lafon G, Tanzi M. Randomized, double-blind, placebo-controlled trial of bismuth subsalicylate for microscopic colitis. Gastroenterology. 1999;116:A880. [Google Scholar]
- 19.Munch A, Aust D, Bohr J, et al. Microscopic colitis: Current status, present and future challenges: statements of the European Microscopic Colitis Group. Journal of Crohn’s & colitis. 2012 Oct;6(9):932–945. doi: 10.1016/j.crohns.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Munch A, Ignatova S, Strom M. Adalimumab in budesonide and methotrexate refractory collagenous colitis. Scandinavian journal of gastroenterology. 2012 Jan;47(1):59–63. doi: 10.3109/00365521.2011.639079. [DOI] [PubMed] [Google Scholar]
- 21.Giardiello FM, Lazenby AJ, Bayless TM. The new colitides, Collagenous, lymphocytic, and diversion colitis. Gastroenterology clinics of North America. 1995 Sep;24(3):717–729. [PubMed] [Google Scholar]
- 22.Rasmussen MA, Munck LK. Systematic review: are lymphocytic colitis and collagenous colitis two subtypes of the same disease - microscopic colitis? Alimentary pharmacology & therapeutics. 2012 Jul;36(2):79–90. doi: 10.1111/j.1365-2036.2012.05166.x. [DOI] [PubMed] [Google Scholar]
- 23.Munch A, Langner C. Microscopic Colitis: Clinical and Pathologic Perspectives. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014 Jan 7; doi: 10.1016/j.cgh.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Banares F, Esteve M, Espinos JC, et al. Drug consumption and the risk of microscopic colitis. The American journal of gastroenterology. 2007 Feb;102(2):324–330. doi: 10.1111/j.1572-0241.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- 25.Veress B, Lofberg R, Bergman L. Microscopic colitis syndrome. Gut. 1995 Jun;36(6):880–886. doi: 10.1136/gut.36.6.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miehlke S, Madisch A, Karimi D, et al. Budesonide is effective in treating lymphocytic colitis: a randomized double-blind placebo-controlled study. Gastroenterology. 2009 Jun;136(7):2092–2100. doi: 10.1053/j.gastro.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 27.Miehlke S, Madisch A, Kupcinskas L, et al. Budesonide is more effective than mesalamine or placebo in short-term treatment of collagenous colitis. Gastroenterology. 2014 May;146(5):1222–1230. e1221–1222. doi: 10.1053/j.gastro.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Goranzon C, Kumawat AK, Hultgren-Hornqvist E, et al. Immunohistochemical characterization of lymphocytes in microscopic colitis. J Crohns Colitis. 2013 Nov 1;7(10):e434–442. doi: 10.1016/j.crohns.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Munch A, Aust D, Bohr J, et al. Microscopic colitis: Current status, present and future challenges: statements of the European Microscopic Colitis Group. J Crohns Colitis. 2012 Oct;6(9):932–945. doi: 10.1016/j.crohns.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Chande N, MacDonald JK, McDonald JW. Interventions for treating microscopic colitis: a Cochrane Inflammatory Bowel Disease and Functional Bowel Disorders Review Group systematic review of randomized trials. Am J Gastroenterol. 2009 Jan;104(1):235–241. doi: 10.1038/ajg.2008.16. quiz 234, 242. [DOI] [PubMed] [Google Scholar]