Abstract
Background
Among those 9-26 years of age, vaccination can prevent specific types of genital human papillomavirus (HPV), the most common sexually transmitted infection and cause of cervical and other cancers. The objective of this study was to estimate the prevalence of and factors associated with HPV vaccine initiation and completion among females surviving childhood cancer.
Procedure
One-hundred fourteen young adults and 230 mothers with daughters surviving childhood cancer completed surveys querying HPV vaccination history along with medical and sociodemographic factors potentially associated with vaccination outcomes. Vaccination rate differences by age necessitated analysis of outcomes by age group: 9-13 years (preadolescents), 14-17 years (adolescents), and 18-26 years (young adults). Multivariable logistic regression was utilized to identify factors associated with HPV vaccination outcomes.
Results
Overall, 34.6% (119/344) of survivors initiated and 20.9% (72/344) completed HPV vaccination. Preadolescents were least likely to have initiated vaccination (P<0.001). Physician recommendation was associated with initiation across age groups (OR=6.81–11.96, Ps<0.001-.01), whereas older age at diagnosis (≥12 years of age) was associated with lower vaccination initiation among young adults only (OR=0.28; 95% CI, 0.10–0.76, P=0.012). Physician recommendation (OR=7.54; 95% CI, 1.19–47.69, P=0.032; adolescent group) and greater treatment intensity (OR=5.25; 95% CI, 1.00–27.61, P=0.050; young adult group) were associated with vaccine completion, whereas being non-White was associated with decreased vaccination completion (OR=0.17; 95% CI, 0.05–0.66, P=0.010; adolescent group).
Conclusions
A minority of youths surviving childhood cancer have initiated or completed HPV vaccination. Strategies to increase vaccination among survivors are discussed.
Keywords: Oncology, Adolescents, Young Adults, Human Papillomavirus, Vaccination
Introduction
Genital human papillomavirus (HPV) is the most common sexually transmitted infection (STI) in the US and has a causal role in the occurrence of cervical and other cancers [1,2]. Unlike some STIs, HPV is transmitted skin-to-skin and spread via genital/genital, oral/genital, or digital/genital contact. The point prevalence of HPV is 30-49% among sexually active adolescents and young adults, with lifetime prevalence approaching 80% among sexually active females [3,4]. Cancer prevention efforts have led to the development of vaccines protecting against HPV and these vaccines are clinically available, safe, and effective [5-7]. Quadrivalent HPV vaccination, approved in 2006 for females aged 9-26 years, protects against HPV types 16 and 18, which account for 70% of cervical cancers, and 6 and 11, which account for 90% of genital warts [8,9]. Routine HPV vaccination is recommended for boys and girls aged 11-12 years, with catch-up vaccination for women through age 26 [10]. It is recommended that the vaccine be administered prior to sexual debut due to the mechanism of HPV transmission [11]. With appropriate vaccine utilization, the American Cancer Society estimates that cervical cancer risk will be reduced by over 70% within the next decade [12].
Relative to their peers, survivors of childhood cancer have an increased risk of developing later HPV-associated malignancies. Female survivors, for example, experience an excess risk of 40% overall, but this escalates to 760% for tonsillar cancers among those treated with radiation therapy [13]. Survivors at highest risk for cervical dysplasia and/or HPV-associated cancers post-treatment include those treated with allogeneic or autologous transplant, pelvic radiation, or those diagnosed with Hodgkin's lymphoma [14-17]. One proposed mechanism for this increase in risk is treatment-related immunocompromise [14-15]. Despite their increased susceptibility for vaccine preventable diseases, only 61% of childhood cancer survivors have re-initiated booster vaccinations by three years post completion of treatment [18].
Given these risks, the Children's Oncology Group recommends HPV vaccination for all eligible females surviving childhood cancer [19]. Because HPV vaccination has only been approved by the FDA since 2006, little is known about vaccination uptake among childhood cancer survivors. The current study has been designed to estimate HPV vaccination initiation and completion across females surviving childhood cancer, and to identify sociodemographic and cancer-specific factors associated with HPV vaccination initiation and completion.
Methods
Participants
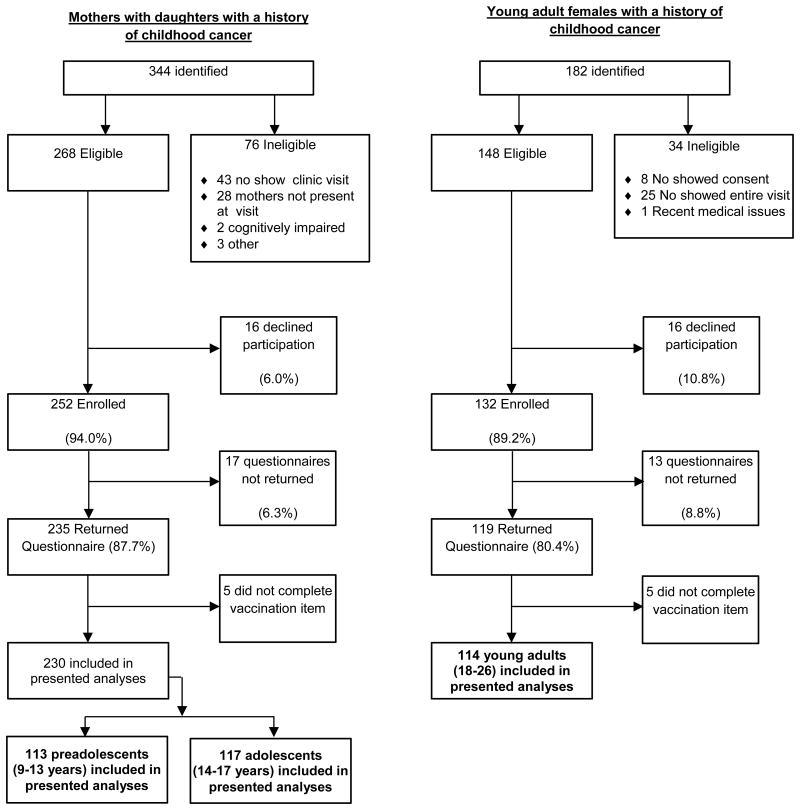
Young adults surviving childhood cancer and maternal caregivers for those <18 years of age were recruited from the After Completion of Therapy (ACT) Clinic at St. Jude Children's Research Hospital. Eligibility criteria for entry into the ACT Clinic includes being at least five years post cancer diagnosis, and two years disease free. Additional eligibility for study participation included: 1a) maternal caregiver with a daughter 9-17 years of age who survived childhood cancer; or, 1b) a female survivor of childhood cancer 18-26 years of age; 2) proficient in reading/writing English; and, 3) cognitive ability to complete study questionnaire. Over an 18-month interval, a total of 230 mothers and 114 young adult females with a childhood cancer history enrolled in the study and completed questionnaires (Figure 1). Cancer survivors differed significantly on vaccine outcomes by age (Table I) and were thus divided into three groups: (a) preadolescents aged 9-13 years (n = 113; mean age (Mage)= 10.9 years, standard deviation (SD) = 1.43), (b) adolescents aged 14-17 (n = 117; Mage = 15.6 years, SD = 1.13), and (c) young adults aged 18-26 (n = 114; Mage = 21.2 years, SD = 2.48) for subsequent analyses.
Figure 1. Flowchart Depicting Recruitment and Questionnaire Completion for Mothers of Childhood Cancer Survivors and Young Adult Survivors.
Table I. Univariate Differences in HPV Vaccine Initiation among Females Surviving Childhood Cancer by Age Group.
| 9-13 years (n=113) | 14-17 years (n=117) | 18-26 years (n=114) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Not Initiated | Initiated | Not Initiated | Initiated | Not Initiated | Initiated | |
| n=91 | n=22 | n=64 | n=53 | n=70 | n=44 | |
|
| ||||||
| Variable | Freq (%) | Freq (%) | Freq (%) | Freq (%) | Freq (%) | Freq (%) |
| Race/Ethnicity | ||||||
| White | 77 (84.6)* | 15 (68.2) | 48 (75) | 35 (66) | 51 (72.9) | 34 (77.3) |
| Non-White | 14 (15.4) | 7 (31.8) | 16 (25) | 18 (34) | 19 (27.1) | 10 (22.7) |
|
| ||||||
| Household Income | ||||||
| Less than $20,000 | 14 (16.1)** | 8 (40) | 5 (7.9) | 10 (20.4) | 17 (27.4) | 12 (29.3) |
| $20,000 to $59,999 | 28 (32.2) | 3 (15) | 24 (38.1) | 19 (38.8) | 25 (40.3) | 15 (36.6) |
| $60,000 and above | 45 (51.7) | 9 (45) | 34 (54) | 20 (40.8) | 20 (32.3) | 14 (34.1) |
|
| ||||||
| Cancer Diagnosis | ||||||
| Leukemia/Lymphoma | 35 (38.5) | 10 (45.5) | 28 (43.8) | 15 (28.3) | 33 (47.1) | 20 (45.5) |
| Brain/CNS | 14 (15.4) | 4 (18.2) | 14 (21.9) | 12 (22.6) | 17 (24.3) | 8 (18.2) |
| Solid Tumors | 42 (46.2) | 8 (36.4) | 22 (34.4) | 26 (49.1) | 20 (28.6) | 16 (36.4) |
|
| ||||||
| Cancer Tx Intensity | ||||||
| Least/Mod Intense (1/2) | 52 (57.8) | 13 (59.1) | 39 (60.9)* | 21 (42.9) | 40 (57.1) | 28 (63.6) |
| Very/Most Intense (3/4) | 38 (42.2) | 9 (40.9) | 25 (39.1) | 28 (57.1) | 30 (42.9) | 16 (36.4) |
|
| ||||||
| Number Treatments | ||||||
| 1 Tx modality | 35 (39.3) | 13 (59.1) | 29 (45.3)* | 15 (28.3) | 26 (37.1) | 18 (40.9) |
| 2 Tx modalities | 28 (31.5) | 5 (22.7) | 21 (32.8) | 28 (52.8) | 34 (48.6) | 18 (40.9) |
| 3 Tx modalities | 26 (29.2) | 4 (18.2) | 14 (21.9) | 10 (18.9) | 10 (14.3) | 8 (18.2) |
|
| ||||||
| Median Age at Dxa | ||||||
| Low | 38 (43.2) | 5 (23.8) | 33 (54.1) | 26 (51) | 25 (37.3)** | 24 (61.5) |
| High | 50 (56.8) | 16 (76.2) | 28 (45.9) | 25 (49) | 42 (62.7) | 15 (38.5) |
|
| ||||||
| Visited OB/GYN | ||||||
| No | 82 (92.1) | 20 (90.9) | 49 (79)*** | 28 (54.9) | 20 (29) | 7 (16.7) |
| Yes | 7 (7.9) | 2 (9.1) | 13 (21) | 23 (45.1) | 49 (71) | 35 (83.3) |
|
| ||||||
| Get yearly Pap test | ||||||
| No | 84 (94.4) | 20 (90.9) | 56 (91.8)** | 37 (74) | 30 (44.1) | 17 (40.5) |
| Yes | 5 (5.6) | 2 (9.1) | 5 (8.2) | 13 (26) | 38 (55.9) | 25 (59.5) |
|
| ||||||
| Physician recommended vaccine | ||||||
| No | 64 (73.6)*** | 5 (25) | 32 (56.1)*** | 8 (15.7) | 48 (71.6)*** | 7 (17.1) |
| Yes | 23 (26.4) | 15 (75) | 25 (43.9) | 43 (84.3) | 19 (28.4) | 34 (82.9) |
Median cut-points for age at diagnosis differed for each age group: 9-13 years group, 0-1.9 vs. 2-7.9; 14-17 years group, 0-3.9 vs. 4-11.9; 18-26 years group, 0-11.9 vs. 12-19.9;
p<.05,
p<.01,
p<.001
Participants were recruited during regularly scheduled ACT Clinic visits. Trained research team members approached eligible participants, explained the purpose of the study, and obtained informed consent as approved by the institutional review board. After obtaining consent, participants completed questionnaires. Surveys were essentially identical across age groups, aside from referent (e.g., you vs. your daughter). After questionnaire completion, all participants were provided with HPV vaccination and HPV information sheets.
Outcome Variables
HPV vaccine initiation/non-initiation was defined as a binary variable such that participants who reported receiving one or more vaccine doses were categorized as “initiated,” whereas those reporting zero doses were categorized as “non-initiated.” HPV vaccine completion/non-completion was defined as a binary variable such that participants who reported receiving all three vaccine doses were categorized as “completers,” whereas those who reported receiving at least one but less than three vaccine dose were categorized as “non-completers.” Non-initiated participants were excluded in models of vaccine completion.
Independent Variables
All participants completed questionnaires regarding their/their daughters' HPV vaccination history and sociodemographic and medical history information. Cancer specific data were abstracted from patients' medical records.
Medical and Sociodemographic Variables
Mothers and young adult participants provided familial demographic information, including age, race/ethnicity, marital status, education level, and household income. Medical and treatment history obtained included history of gynecological care, cervical cancer screening, cancer type, variety and type of treatment modalities, time since diagnosis, and age at diagnosis. Items were adapted from instruments previously used in the HPV vaccine literature [19,20].
Cancer Treatment Intensity
The Intensity of Treatment Rating Scale 2.0 (ITR-2) measured disease severity and cancer treatment intensity [21]. The ITR-2 utilizes diagnosis, stage/risk level, and treatment modality to produce ratings from least (1) to most intensive (4) treatment.
Statistical Analysis
Preliminary univariate analyses were conducted to examine differences between age groups (preadolescents, adolescents, and young adults) for HPV vaccine initiation and completion. Univariate differences were assessed as a function of sociodemographic and medical factors (cancer type, treatment modality, treatment intensity, age at diagnosis, and physician recommendation). Univariate comparisons with P<0.10 were included in each of the multivariable models (vaccine initiation and completion) for each age group. Differences for continuous variables were assessed using univariate one-way analysis of variance and categorical variables were assessed using Chi-square tests and Fisher's Exact test. Multivariable logistic regression models with potential predictors from the univariate analyses entered in one block were used to calculate odds ratios (OR) and 95% confidence intervals (CI) for vaccine outcomes. All statistical tests were considered statistically significant at P<0.05.
Results
Prevalence
Overall, 34.6% (119/344) of survivors reported having initiated the HPV vaccine series. Among those cancer survivors who had initiated the vaccine, 60.5% (72/119) completed the series. However, the rate of initiation differed significantly by age group (χ2=18.16, P<.001). Subsequent Chi-square tests revealed that the proportion of preadolescents who initiated the vaccine (19.5% or 22/113) was significantly lower than those in the adolescent (45.3% or 53/117, P<.001) and young adult group (38.6% or 44/114, P=.002). No significant differences emerged in the rates of vaccine completion by age group among those who had initiated the vaccine series.
Univariate Age Group Comparisons
Univariate differences emerged between age groups on household income (P=0.01), number of treatment modalities received during active therapy (P=0.06), age at cancer diagnosis (P<0.01), ever having visited an OB/GYN (P<0.01), yearly Papanicolaou (Pap) test (P<0.01), and having ever received a physician recommendation to get the HPV vaccine (P<0.01). Specifically, young adult participants were more likely than those in the other groups to be in the lowest income group (less than $20,000), to have visited an OB/GYN, and to have received yearly Pap tests. Adolescents were more likely than preadolescents to have visited an OB/GYN and to have received yearly Pap tests. Adolescents were also more likely than other groups to report receiving a physician recommendation for HPV vaccination. Preadolescents were more likely than the older females to have received a single cancer treatment modality. Mean age at diagnosis increased significantly across all three age groups. Based on the many univariate differences on the outcomes by age, analyses examining the association between these variables with vaccination outcomes were completed separately for each age group.
HPV Vaccine Initiation
Univariate analyses revealed significant differences between those who have/have not initiated HPV vaccination (Table I) within each of the three age groups. However, the specific predictors varied for each group. The final models are presented in Table II and described below.
Table II. Multivariate Logistic Regression for Factors Associating with HPV Vaccination Initiation among Females Surviving Childhood Cancera.
| Variable | OR | 95% CIb | p |
|---|---|---|---|
| 9-13 year olds | |||
| Physician recommended HPV vaccine | |||
| No | 1.00 | ||
| Yes | 9.72 | [2.80, 33.80] | <.001 |
| 14-17 year olds | |||
| Physician recommended HPV vaccine | |||
| No | 1.00 | ||
| Yes | 6.81 | [2.38, 19.47] | <.001 |
| 18-26 year olds | |||
| Age at Diagnosis | |||
| 0.0-11.9 years | 1.00 | ||
| 12.0-19.9 years | 0.28 | [0.10, 0.76] | .012 |
| Physician recommended HPV vaccine | |||
| No | 1.00 | ||
| Yes | 11.96 | [4.22, 33.93] | <.001 |
Only statistically significant variables are reported;
CI=confidence interval for odds ratio (OR).
Preadolescent Initiation Model
Based on the univariate findings, the multivariable model for HPV vaccine initiation/non-initiation among 9-13 year-olds included race, household income, and physician recommendation for vaccine. The final multivariable logistic regression model predicting binary vaccine initiation outcome indicated that physician recommendation significantly associated with increased vaccine initiation (OR, 9.72; 95% CI, 2.80–33.80, P<0.001).
Adolescent Initiation Model
Based on the univariate findings, the multivariate model for HPV vaccine initiation/non-initiation among 14-17 year-olds included treatment intensity, number of treatment modalities, OB/GYN care history, annual Pap test history, and physician recommendation for vaccine. The final multivariable logistic regression model predicting binary vaccine initiation outcome indicated that physician recommendation was also associated with increased vaccine initiation (OR, 6.81; 95% CI, 2.38–19.47, P<0.001).
Young Adult Initiation Model
Based on the univariate findings, the multivariable model for HPV vaccine initiation/non-initiation among 18-26 year-olds included age at diagnosis and physician recommendation for vaccine. The final multivariable logistic regression model predicting binary vaccine initiation outcome indicated older age at diagnosis (≥12 years of age [OR, 0.28; 95% CI, 0.10–0.76, P=0.01]) was associated with a decreased likelihood of vaccine initiation, whereas physician recommendation (OR, 11.96; 95% CI, 4.22–33.93, P<0.001) was associated with increased vaccine initiation.
HPV Vaccine Completion
Univariate analyses for participants who had initiated the vaccine revealed significant differences between those who have/have not completed the vaccine series across age groups (Table III). Due to a very low rate of HPV vaccine initiation (22/113) for the preadolescent group, there were not enough participants to examine the predictors of vaccine completion versus non-completion. Final models for the adolescent and young adult groups are described below and presented in Table IV.
Table III. Univariate Differences in Vaccine Completion among Female Survivors of Childhood Cancer by Age Group.
| 9-13 years (n=22) | 14-17 years (n=53) | 18-26 years (n=44) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Initiated, not completed | Completed | Initiated, not completed | Completed | Initiated, not completed | Completed | |
| n=11 | n=11 | n=22 | n=31 | n=14 | n=30 | |
|
| ||||||
| Variable | Freq (%) | Freq (%) | Freq (%) | Freq (%) | Freq (%) | Freq (%) |
| Race/Ethnicity | ||||||
| White | 7 (63.6) | 8 (72.7) | 10 (45.5)*** | 25 (80.6) | 10 (71.4) | 24 (80) |
| Non-White | 4 (36.4) | 3 (27.3) | 12 (54.5) | 6 (19.4) | 4 (28.6) | 6 (20) |
|
| ||||||
| Household Income | ||||||
| Less than $20,000 | 4 (40) | 4 (40) | 2 (10) | 8 (27.6) | 5 (38.5) | 7 (25) |
| $20,000 to $59,999 | 2 (20) | 1 (10) | 11 (55) | 8 (27.6) | 5 (38.5) | 10 (35.7) |
| $60,000 and above | 4 (40) | 5 (50) | 7 (35) | 13 (44.8) | 3 (23.1) | 11 (39.3) |
|
| ||||||
| Cancer Diagnosis | ||||||
| Leukemia/Lymphoma | 7 (63.6) | 3 (27.3) | 6 (27.3) | 9 (29) | 7 (50) | 13 (43.3) |
| Brain/CNS | 1 (9.1) | 3 (27.3) | 6 (27.3) | 6 (19.4) | 3 (21.4) | 5 (16.7) |
| Solid Tumors | 3 (27.3) | 5 (45.5) | 10 (45.5) | 16 (51.6) | 4 (28.6) | 12 (40) |
|
| ||||||
| Cancer Tx Intensity | ||||||
| Least/Mod Intense (1/2) | 8 (72.7) | 5 (45.5) | 8 (40) | 13 (44.8) | 12 (85.7)** | 16 (53.3) |
| Very/Most Intense (3/4) | 3 (27.3) | 6 (54.5) | 12 (60) | 16 (55.2) | 2 (14.3) | 14 (46.7) |
|
| ||||||
| Number Treatments | ||||||
| 1 Tx modality | 8 (72.7)* | 5 (45.5) | 7 (31.8) | 8 (25.8) | 8 (57.1) | 10 (33.3) |
| 2 Tx modalities | 3 (27.3) | 2 (18.2) | 9 (40.9) | 19 (61.3) | 4 (28.6) | 14 (46.7) |
| 3 Tx modalities | 0 (0) | 4 (36.4) | 6 (27.3) | 4 (12.9) | 2 (14.3) | 6 (20) |
|
| ||||||
| Median Age at Dxa | ||||||
| Low (younger) | 3 (27.3) | 2 (20) | 9 (42.9) | 17 (56.7) | 9 (75) | 15 (55.6) |
| High (older) | 8 (72.7) | 8 (80) | 12 (57.1) | 13 (43.3) | 3 (25) | 12 (44.4) |
|
| ||||||
| Visited OB/GYN | ||||||
| No | 10 (90.9) | 10 (90.9) | 10 (50) | 18 (58.1) | 2 (15.4) | 5 (17.2) |
| Yes | 1 (9.1) | 1 (9.1) | 10 (50) | 13 (41.9) | 11 (84.6) | 24 (82.8) |
|
| ||||||
| Get yearly Pap test | ||||||
| No | 10 (90.9) | 10 (90.9) | 14 (73.7) | 23 (74.2) | 5 (35.7) | 12 (42.9) |
| Yes | 1 (9.1) | 1 (9.1) | 5 (26.3) | 8 (25.8) | 9 (64.3) | 16 (57.1) |
|
| ||||||
| Physician recommended vaccine | ||||||
| No | 3 (30) | 2 (20) | 6 (30) | 2 (6.5)** | 2 (16.7) | 5 (17.2) |
| Yes | 7 (70) | 8 (80) | 14 (70) | 29 (93.5) | 10 (83.3) | 24 (82.8) |
Median cut-points for age at diagnosis differed for each age group: 9-13 years group, 0-1.9 vs. 2-7.9; 14-17 years group, 0-3.9 vs. 4-11.9; 18-26 years group, 0-11.9 vs. 12-19.9;
p<.05,
p<.01,
p<.001
Table IV. Multivariate Logistic Regression for Factors Associating with HPV Vaccination Completion among Female Survivors of Childhood Cancera.
| Variable | OR | 95% CIb | p |
|---|---|---|---|
| 14-17 year olds | |||
| Race/Ethnicity | |||
| White | 1.00 | ||
| Non-White | 0.17 | [0.05, 0.66] | .010 |
| Physician recommended HPV vaccine | |||
| No | 1.00 | ||
| Yes | 7.54 | [1.19, 47.69] | .032 |
| 18-26 year olds | |||
| Cancer Treatment Intensity | |||
| Least/Moderate Intense (1/2) | 1.00 | ||
| Very/Most Intense (3/4) | 5.25 | [1.00, 27.61] | .050 |
Only statistically significant variables are reported;
CI=confidence interval for odds ratio (OR).
Adolescent Completion Model
The final multivariable logistic regression model for HPV vaccine completion among 14-17 year-olds included race and physician recommendation for HPV vaccination. Among vaccine-initiated participants, those who were non-White were less likely to have completed the HPV vaccine series post initiation (OR, 0.17; 95% CI, 0.05–0.66, P=0.01) relative to White participants. Additionally, those who had received a physician recommendation for the HPV vaccine were more likely to have completed the HPV vaccine series post initiation (OR, 7.54; 95% CI, 1.19–47.69, P=0.03).
Young Adult Completion Model
The final multivariable logistic regression model for HPV vaccine completion among 18-26 year-olds included only treatment intensity rating. Among vaccine-initiated participants, those who received very or most intensive treatment were more likely to have completed the HPV vaccine series as compared to those with least intensive or moderately intensive treatments (OR, 5.25; 95% CI, 1.00–27.61, P=0.05).
Discussion
Over the past 50 years, survival rates for childhood cancer have dramatically improved, but survivorship comes at a cost. By age 45, 95.5% of survivors will experience some type of chronic health condition with 80.5% experiencing severe, life threatening, or disabling conditions including pulmonary dysfunction, hearing loss, endocrine and reproductive problems, cardiac dysfunction, and neurocognitive impairment, with physiologic frailty adding to the risk of chronic health conditions and death [22,23]. Additionally, 10-13% of childhood cancer survivors will experience a subsequent malignant neoplasm by young adulthood, placing this group at high risk for late health complications [22]. Few mechanisms exist for reducing the risk of second cancers in this population, but HPV vaccination is one such option.
Although our findings indicated that 34.6% of survivors between the ages of 9-26 years initiate HPV vaccination (with 20.9% completing the vaccine series), age group disparities made it necessary to analyze initiation and completion rates within age groups. Data from the Centers for Disease Control and Prevention indicate that 53.8% of US females between the ages of 13-17 years have initiated the vaccine, and 33.4% have completed the three dose vaccine series [24]. The results of our study suggest that cancer survivors in this adolescent age group are less likely to be vaccinated, with 45.3% initiating and 26.5% completing the series. This equates to a completion rate of 58.5% among survivors who initiate the vaccine, compared to 62.1% in the US population. Also in 2012, 34.5% of young adult females between the ages of 19-26 reported initiating the HPV vaccine [25]. Furthermore, these study findings suggest that rates of vaccination among young adults surviving childhood cancer (38.6% initiation rate and 26.3% completion rate) are marginally higher relative to population norms. Although rates of vaccine initiation are lower than those outlined in the Health People 2020 objectives, it is promising that 68.1% of young adult cancer survivors who initiated the vaccine completed the full series, which may be due (in part) to advocacy by medical professionals regarding the benefits of immunization [26]. As providers are now required to deliver HPV vaccination without cost-sharing as part of the Affordable Care Act, future research should examine the effect of this approach on vaccine rates in survivors.
Similar to previous research, these study findings indicated that younger girls were significantly less likely to have initiated the vaccine as compared to adolescent and young adult survivor groups [27]. Given that the preadolescent group was comprised of children ages 9-13 years and that the recommended age range for obtaining the vaccine is 11-12 years of age, it is not surprising that the preadolescents had lower vaccine initiation rates. We suggest, however, that efforts to increase vaccine initiation among younger survivors are warranted. In our sample, only 14.2% (n=49) of participants were ages 9-10 years. Thus the remaining 85.8% (n=295) should have initiated the 6-month vaccine series. By age 13, all of our female survivors would ideally have completed the vaccine series. The World Health Organization (WHO) emphasized administration of the HPV vaccination prior the initiation of sexual activity if it is to be effective in preventing cervical cancer [27]. The WHO also indicates that national HPV immunization efforts for females ages 9-13 should not only be a priority but are also feasible, financially sustainable, and cost-effective [28].
Our study aligns with previous research demonstrating that physician recommendation for HPV immunization is a significant predictor of vaccine initiation [29]. For preadolescents and adolescents, only physician recommendation significantly predicted vaccine initiation in the multivariable model. Pediatric health care providers (HCPs) have multiple opportunities to recommend HPV immunization prior to sexual debut (and initial HPV exposure) during early adolescent preventative health visits [30]. Furthermore, physician recommendation has been described as an influential “cue to action” within the Health Belief Model [29,31]. Specific to HPV vaccination, a physician recommendation may be perceived as a situational factor prompting action on the part of the adolescent or parent to initiate vaccination [29]. Pediatric HCPs who foster and maintain an alliance with their patients and families may more likely to influence the vaccination decisions of parents. Thus, HCPs who are able to initiate tailored discussions of the benefits of HPV vaccination (cancer prevention and maximized vaccine effectiveness) within a developmentally appropriate social/cultural context may be more successful in influencing parental decisions to have their children vaccinated [32].
Despite the robust association between physician recommendation and vaccine outcome reported in this study, less than 52% of families/survivors reported having had the vaccine recommended by a doctor. This highlights the confusion that is often reported among primary and specialty care providers and begs the question, who is responsible for HPV vaccine management in survivors of childhood cancer? Whereas survivorship specialists frequently believe that primary care providers (PCP) are responsible for this aspect of care, PCPs often report that oncologists should be responsible, citing the vaccine's indication. Although guidelines for vaccinations in children surviving cancer have been developed, future iterations should specifically recommend management responsibilities by specialty, while promoting vaccine communication across providers [19,33].
Interestingly, being White also predicted vaccine completion beyond the impact of other variables within the adolescent age group. Despite comparable or higher rates of vaccine initiation, African-American and Hispanic adolescents are less likely to complete the HPV vaccine series than their White counterparts [24,34]. Three administrations are necessary for vaccination completion. Thus, the likelihood of vaccine completion should increase with a combination of vaccine education, rapport with HCPs, and financial resources. These additional features may also create additional barriers to vaccine completion. The extent to which these barriers differ across socio-economic and ethnicity/race strata within the childhood cancer survivorship population is currently unknown and should be examined in future research.
Although this study provides useful information on demographic and psychosocial factors related to HPV vaccine initiation and completion, a number of study limitations must be considered. Limitations include: a) single US site cross-sectional design; b) inclusion of only females who were five or more years post-diagnosis; and c) self-report or maternal-report only. Single site, cross-sectional designs that allow for self-selection in participation carry inherent biases, and a larger sample size would have been beneficial when developing the completion models. Furthermore, potential participants who did not attend their clinic appointment (and therefore could not participate in the study) may be less adherent to other appointments and medical recommendations, making it less likely that they would have received the HPV vaccine. Finally, the generalizability of these findings may be limited to US survivors secondary to variability in international health care systems, and differences in vaccine timing, dosing, scheduling, and licensing. Future studies should consider the utilization of a multi-site international study design, the inclusion of survivors less than five years post treatment completion, and HPV vaccination among male survivors of childhood cancer. Although the HPV vaccine was approved for males in 2009, rates of vaccine uptake remain quite low [35].
In conclusion, female survivors of childhood cancer are at increased risk for later HPV-related complications including second cancers; therefore, HPV vaccination is recommended as primary prevention of these and other HPV-related health issues. Our results suggest that the majority of childhood cancer survivors do not initiate HPV vaccination. Age group disparities were identified, with preadolescents being less likely than their older counterparts to have initiated vaccination and completed the vaccine series. The results of the current study also identified physician recommendation as the most significant factor for initiation of vaccination across all three age groups, with older age at diagnosis being associated with non-initiation among the young adult group only. Factors predicting completion of the vaccine series differed in the adolescent and young adult groups, with physician recommendation and White race being associated with vaccine completion among the adolescents, whereas more intensive cancer treatment associated with completion among young adults. Findings drawn from the present study may inform future efforts to increase vaccination initiation and completion rates among females surviving childhood cancer.
Acknowledgments
This work was supported in part by the Cancer Center Support (CORE) (grant number CA21765); and The American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflict of Interest Statement: There are no conflict of interest disclaimers by any of the authors.
References
- 1.Weinstock H, Berman S, Cates W. Sexually transmitted diseases among American youth: Incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36:6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 2.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 3.Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, Markowitz LE. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 4.Myers ER, McCrory DC, Nanda K, Bastian L, Matchar DB. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am J Epidemiol. 2000;151:1158–1171. doi: 10.1093/oxfordjournals.aje.a010166. [DOI] [PubMed] [Google Scholar]
- 5.Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, Bocchini JA, Unger ER. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 6.Klein NP, Hansen J, Chao C, Velicer C, Emery M, Slezak J, Lewis N, Deosaransingh K, Sy L, Ackerson B, Cheetham TC, Liaw KL, Takhar H, Jacobsen SJ. Safety of quadrivalent human papillomavirus vaccine administered routinely to females. Arch Pediatr Adolesc Med. 2012;166:1140–1148. doi: 10.1001/archpediatrics.2012.1451. [DOI] [PubMed] [Google Scholar]
- 7.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G HPV Vaccine Study Group. Sustained efficacy up to 4-5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration. [Accessed June 21, 2014];FDA licenses new vaccine for prevention of cervical cancer and other diseases in females caused by human papillomavirus. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108666.htm Published June 8, 2008, Updated April 8, 2013.
- 9.Villa LL, Costa RLR, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koustsky LA, Malm C, Lehtinen M, Skjeldestad FE, Olsson SE, Steinwall M, Brown DR, Kurman RJ, Ronnett BM, Stoler MH, Ferenczy A, Harper DM, Tamms GM, Yu J, Lupinacci L, Railkar R, Taddeo FJ, Jansen KU, Esser MT, Sings HL, Saah AJ, Barr E. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: A randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years - United States, 2012. MMWR Morb Mortal Wkly Rep. 2012;61:1–4. [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration. [June 20, 2014];Product approval-prescribing information [package insert] Gardasil® [human papillomavirus quadrivalent (types 6, 11, 16, and 18) vaccine, recombinant], Merck and Co, Inc. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM094042 Published 2006, Updated March 2014.
- 12.Saslow D, Castle PE, Cox JT, Davey DD, Einstein MH, Ferris DG, Goldie SJ, Harper DM, Kinney W, Moscicki AB, Noller KL, Wheeler CM, Ades T, Andrews KS, Doroshenk MK, Kahn KG, Schmidt C, Shafey O, Smith RA, Partridge EE, Gynecology Cancer Advisory Group. Garcia F. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57:7–28. doi: 10.3322/canjclin.57.1.7. [DOI] [PubMed] [Google Scholar]
- 13.Ojha RP, Tota JE, Offutt-Powell TN, Klosky JL, Minniear TD, Jackson BE, Gurney JG. Human papillomavirus-associated subsequent malignancies among long-term survivors of pediatric and young adult cancers. PLoS One. 2013 doi: 10.1371/journal.pone.0070349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatia S, Louie AD, Bhatia R, O'Donnell MR, Fung H, Kashyap A, Krishnan A, Molina A, Nademanee A, Niland JC, Parker PA, Snyder DS, Spielberger R, Stein A, Forman SJ. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19:464–471. doi: 10.1200/JCO.2001.19.2.464. [DOI] [PubMed] [Google Scholar]
- 15.Sasadeusz J, Kelly H, Szer J, Schwarer AP, Mitchell H, Grigg A. Abnormal cervical cytology in bone marrow transplant receipts. Bone Marrow Transplant. 2001;28:393–397. doi: 10.1038/sj.bmt.1703141. [DOI] [PubMed] [Google Scholar]
- 16.Fujimura M, Ostrow RS, Okagaki T. Implication of human papillomavirus in postirradiation dysplasia. Cancer. 1991;68:2181–2185. doi: 10.1002/1097-0142(19911115)68:10<2181::aid-cncr2820681016>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Katz RL, Veanattukalathil S, Weiss KM. Human papillomavirus infection and neoplasia of the cervix and anogenital region in women with Hodgkin's disease. Acta Cytol. 1987;31:845–854. [PubMed] [Google Scholar]
- 18.Crawford NW, Heath JA, Ashley D, Downie P, Buttery JP. Survivors of childhood cancer: an Australian audit of vaccination status after treatment. Pediatr Blood Cancer. 2010:128–33. doi: 10.1002/pbc.22256. [DOI] [PubMed] [Google Scholar]
- 19.Children's Oncology Group. [June 20, 2014];Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http://www.survivorshipguidelines.org/pdf/LTFUGuidelines.pdf Published October 2008.
- 20.Brabin L, Roberts SA, Farzaneh F, Kitchener HC. Future acceptance of adolescent human papillomavirus vaccination: A survey of parental attitudes. Vaccine. 2006;24:3087–3094. doi: 10.1016/j.vaccine.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 21.Werba BE, Hobbie W, Kazak AE, Ittenbach RF, Reilly AF, Meadows AT. Classifying the intensity of pediatric cancer treatment protocols: The intensity of treatment rating scale 2.0 (ITR-2) Pediatr Blood Cancer. 2007;48:673–677. doi: 10.1002/pbc.21184. [DOI] [PubMed] [Google Scholar]
- 22.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, Green DM, Armstrong GT, Nottage KA, Jones KE, Sklar CA, Srivastava DK, Robison LL. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ness KK, Krull KR, Jones KE, Mulrooney DA, Armstrong GT, Green DM, Chemaitilly W, Smith WA, Wilson CL, Sklar CA, Shelton K, Srivastava DK, Ali S, Robison LL, Hudson MM. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort Study. J Clin Oncol. 2013;31:4496–4503. doi: 10.1200/JCO.2013.52.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13-17 years – United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:685–693. [PMC free article] [PubMed] [Google Scholar]
- 25.Williams WW, Lu PJ, O'Halloran A, Bridges CB, Pilishvili T, Hales CM, Markowitz LE. Noninfluenza vaccination coverage among adults – United States, 2012. MMWR Morb Mortal Wkly Rep. 2014;63:95–102. [PMC free article] [PubMed] [Google Scholar]
- 26.Department of Health and Human Services. [June 22, 2014];Healthy people 2020: Improving the health of Americans. http://www.healthypeople.gov/2020/default.aspx.
- 27.Kessels SJ, Marshall HS, Watson M, Braunack-Mayer AJ, Reuzel R, Tooher RL. Factors associated with HPV vaccine uptake in teenage girls: A systematic review. Vaccine. 2012;30:3546–3556. doi: 10.1016/j.vaccine.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 28.GAVI Alliance. [June 22, 2014];Human papillomavirus vaccine support. http://www.gavialliance.org/support/nvs/human-papillomavirus-vaccine-support/
- 29.Reiter PL, Brewer NT, Gottlieb SL, McRee AL, Smith JS. Parents' health beliefs and HPV vaccination of their adolescent daughters. Soc Sci Med. 2009;69:475–480. doi: 10.1016/j.socscimed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Vadaparampil ST, Kahn JA, Salmon D, Lee JH, Quinn GP, Roetzheim R, Bruder K, Malo TL, Proveaux T, Zhao X, Halsey N, Giuliano AR. Missed clinical opportunities: Provider recommendations for HPV vaccination for 11-12 year old girls are limited. Vaccine. 2011;29:8634–8641. doi: 10.1016/j.vaccine.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker MH. The Health Belief Model and personal health behavior. Health Educ Monogr. 1974;2:324–473. [Google Scholar]
- 32.Griffioen AM, Glynn S, Mullins TK, Zimet GD, Rosenthal SL, Fortenberry JD, Kahn JA. Perspectives on decision making about human papillomavirus vaccination among 11- to 12-year old girls and their mothers. Clin Pediatr (Phila) 2012;51:560–568. doi: 10.1177/0009922812443732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esposito S, Cecinati V, Brescia L, Principi N. Vaccinations in children with cancer. Vaccine. 2010;28:3278–84. doi: 10.1016/j.vaccine.2010.02.096. [DOI] [PubMed] [Google Scholar]
- 34.Niccolai LM, Mehta NR, Hadler JL. Racial/ethnic and poverty disparities in human papillomavirus vaccination completion. Am J Prev Med. 2011;41:428–433. doi: 10.1016/j.amepre.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 35.Stokley S, Jeyarajah J, Yankey D, Cano M, Gee J, Roark J, Curtis RC, Markowitz L Immunization Services Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescents, 2007-2013, and post-licensure vaccine safety monitoring, 2006-2014 – United States. MMWR. 2014;63:620–624. [PMC free article] [PubMed] [Google Scholar]