Abstract
Cardiovascular disease (CVD) continues to be the leading cause of death among women in the United States, accounting for approximately one of every three female deaths. Sex-specific data focused on CVD has been increasing steadily, yet is not routinely collected nor translated into practice. This comprehensive review focuses on novel and unique aspects of cardiovascular health in women and sex-differences as they relate to clinical practice in the prevention, diagnosis, and treatment of CVD. This review also provides current approaches to the evaluation and treatment of acute coronary syndromes that are more prevalent in women, including: myocardial infarction associated with non-obstructive coronary arteries, spontaneous coronary artery dissection, and stress-induced cardiomyopathy (Takotsubo Syndrome). Other CVD entities with higher prevalence or unique considerations in women, such as heart failure with preserved ejection fraction, peripheral arterial disease and abdominal aortic aneurysms, are also briefly reviewed. Lastly, recommendations for cardiac rehabilitation are addressed.
Keywords: Primary Prevention, Women, Risk Factors, Cardiovascular Disease, Acute Coronary Syndromes
Subject Terms: Cardiovascular Disease, Women, Risk Factors, Prevention
I. Introduction
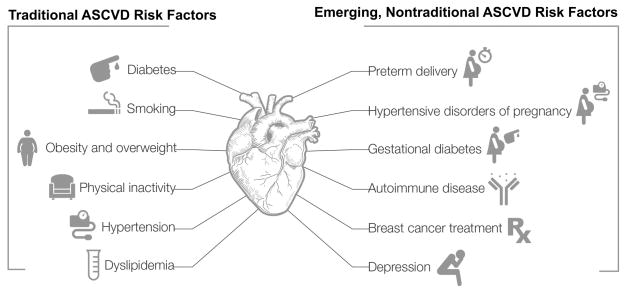
Cardiovascular disease (CVD) remains the leading cause of death in women, and according to the most recently released United States statistics accounted for 398,086 female deaths in 2013.1 For the past three decades, dramatic declines in heart disease mortality for both men and women have been observed, especially in the >65 age group. However, recent data suggest stagnation in the improvements in incidence and mortality of coronary heart disease, specifically among younger women (<55 years).2 It is imperative that we understand the mechanisms that contribute to worsening risk factor profiles in young women, in order to reduce future atherosclerotic cardiovascular disease (ASCVD) morbidity and mortality. Increased recognition of the prevalence of traditional ASCVD risk factors, and their differential impact in women, as well as emerging, nontraditional risk factors unique to, or more common in women, contribute to new understanding of mechanisms leading to these worsening outcomes for women (Figure 1). Lastly, diagnosis of acute coronary syndromes (ACS) is often challenging in women, especially young women, and it is important to recognize differences in the signs and symptoms at presentation, in order to improve patient management and outcomes.
Figure 1. Traditional and Non-traditional ASCVD risk factors in women.
Increasing among women and more impactful traditional ASCVD risk factors include: diabetes, hypertension, dyslipidemia, smoking, obesity and physical inactivity. Emerging, nontraditional ASCVD risk factors include: preterm delivery, hypertensive pregnancy disorders, gestational diabetes, breast cancer treatments, autoimmune diseases and depression.
Awareness of CVD as the primary cause of mortality in women has been slowly increasing. In 1997, only 30% of American women surveyed were aware that CVD was the leading cause of death in women; this increased to 54% in 2009, and has subsequently plateaued when last surveyed in 2012.3 Women are less likely to receive preventive treatment or guidance, such as lipid-lowering therapy, aspirin, and therapeutic lifestyle changes, than are men at similar ASCVD risk.4, 5 When medications are prescribed, treatment is less likely to be aggressive or to achieve optimal effects, for example, women with hypertension are less likely to have their blood pressure at goal; and hyperlipidemic women, especially those with coexisting diabetes, are less likely to be treated with statins to lower low-density lipoprotein (LDL) cholesterol.6–8 Also, cardiac rehabilitation (CR) is underused,9–11 with women being 55% less likely to participate in CR than men9, the reasons for which are multifactorial, but partly due to lack of referral by their treating physician.12
Coronary artery disease (CAD) can be defined as vascular disease limited to the epicardial coronary arteries and should not be confused with ischemic heart disease (IHD), which includes ischemic disease originating in the coronary arteries, the microcirculation, or from an imbalance in myocardial oxygen supply and demand. Particularly in women, use of the terminology “IHD” has advantages over “CAD” due to the lower prevalence of anatomically obstructive coronary artery disease, yet greater rates of myocardial ischemia and associated mortality in females, compared with similarly aged males.13–17 The Women’s Ischemia Syndrome Evaluation (WISE) and other related studies have implicated abnormal coronary reactivity18, microvascular dysfunction19, and plaque erosion/distal microembolization20, 21 as causative to female-specific IHD pathophysiology. Women with IHD have a persistent suboptimal treatment pattern, higher mortality and poorer CVD outcomes compared to men.22–25 In an environment where cardiologists have traditionally been trained to equate IHD with angiographically-defined obstructive CAD, failure to recognize those unique aspects of IHD in women has contributed to less aggressive lifestyle and medical preventive interventions in women relative to men, and may contribute to the observed sex-based mortality gap. Thus, a paradigm shift beyond solely an anatomical description of obstructive CAD is needed to translate into earlier IHD risk detection and treatment for women.
Biological variances among women and men are called sex differences and are frequently reproducible in animal models. Sex differences in the CV system are due to differences in gene expression from the sex chromosomes which may be further modified by sex differences in hormones resulting sex-unique gene expression and function. These differences result in variations in prevalence and presentation of CV conditions, including those associated with autonomic regulation, hypertension, diabetes, and vascular and cardiac remodeling. In contrast, gender differences are unique to the human and arise from sociocultural practices (behaviors, environment, lifestyle, nutrition). In order to facilitate quality improvement in sex- and gender- specific care, this review will examine the latest clinical perspectives on CVD in women, focusing on novel and unique aspects of cardiovascular health in women and sex- and gender- differences as they relate to clinical practice in the prevention, diagnosis, and treatment of CVD. This review will also provide current approaches to the evaluation and treatment of ACS and other CVD entities that have greater prevalence or unique considerations in women.
II. Traditional ASCVD Risk Factors in Women (Table 1.)
Table 1.
Traditional ASCVD Risk factors – Sex based differences and Recommendations
| RISK FACTOR | SEX-BASED DIFFERENCES | RECOMMENDATION |
|---|---|---|
Diabetes
|
DM- women with DM have a 3-fold excess risk of fatal CAD compared to non-diabetic women. MI –earlier occurrence and higher mortality in diabetic women compared to diabetic men. Lower revascularization rates in diabetic women compared to diabetic men. HF- diabetic women have a higher risk of developing HF compared with diabetic men. Stroke- DM is a stronger risk factor for stroke in women compared with men. PAD- DM is a stronger risk factor for the development of claudication in women compared with men. Decreased long-term survival in women undergoing revascularization and increased postsurgical mortality are seen in diabetic women with PAD compared to diabetic men with PAD. |
Both women and men with DM should have aggressive management of their CVD risk factors. Observational studies suggest that women may require greater frequency/intensity of physical activity than men to reduce CVD events. |
Hypertension
|
Higher prevalence of HTN in women over age 60 than in men. Less well controlled in women than men. |
Encourage optimal BP through diet, exercise and avoidance of excess alcohol and sodium. Pharmacotherapy is indicated when blood pressure is >140/90. |
|
Dyslipidemia |
Among women, dyslipidemia has the highest PAR at 47.1%, compared with all other known risk factors for CVD. Atheroma regression and LDL lowering may be even greater among women on statins than in men. |
Statins are equally effective for secondary CVD prevention in both men and women, however statins may contribute to a greater likelihood of developing DM and myalgias in women. Statins are recommended for primary prevention in women, however randomized trial evidence in women is limited. |
|
Obesity |
The impact of obesity on the development of CAD appears to be greater in women than in men. In the Framingham Heart Study, obesity increased the risk of CAD by 64% in women, compared to 46% in men. | Women should maintain or lose weight through an appropriate balance of physical activity and diet. Women who need to lose weight should be advised to accumulate a minimum of 60 to 90 min of at least moderate- intensity physical activity preferably all days of the week. |
Physical inactivity
|
The prevalence of inactivity and sedentary behaviors is higher among women than men. | Overwhelming evidence indicates that regular physical activity is one of the most powerful health-promoting practices that clinicians can recommend for patients. Women should be advised to accumulate at least 150 min/wk of moderate exercise, 75 min/wk of vigorous exercise, or an equivalent combination. |
|
Smoking |
In a recent meta-analysis by Huxley et al it was reported that in all age groups with the exception of the youngest (30–44 years), women had a significant 25% increased risk for CAD conferred by cigarette smoking compared with men. | Smoking is associated with a decade of lost life, and cessation reduces that loss by about 90%. Women should be advised not to smoke and to avoid environmental tobacco smoke. Provide counseling at each encounter, nicotine replacement, and other pharmacotherapy/behavioral therapy as indicated. |
Ischemic heart disease, IHD; Heart failure, HF; Peripheral arterial disease, PAD; Population attributable risk, PAR; Diabetes mellitus, DM; Coronary artery disease, CAD; Cardiovascular disease, CVD; Hypertension, HTN.
Diabetes
More than 13.4 million US women have a diagnosis of DM and 90% to 95% of these women have type 2 DM (T2DM).26 The rate of T2DM in Hispanic women is more than double when compared with non-Hispanic white women (12.7% versus 6.45%, respectively).27 The increasing prevalence of T2DM is concerning because it is a potent risk factor for ASCVD and has long been recognized to confer greater risk for ASCVD death in women compared with men.28
There is a 3-fold excess fatal CAD risk in women with T2DM compared with nondiabetic women (95% confidence interval [CI], 1.9–4.8).29 Women with T2DM have a higher adjusted hazard ratio (HR) of fatal CAD (HR=14.74; 95% CI, 6.16–35.27) compared with T2DM men (HR=3.77; 95% CI, 2.52–5.65)30. In a meta-analysis of over 850,000 individuals the relative risk for CVD was 44% greater in women with DM than in similarly affected men.31
The presence of DM thus represents an imperative for aggressive CVD prevention strategies in women. Growing evidence suggests that diabetic women have more adverse ASCVD risk factor status than diabetic men, consisting of impaired endothelium-dependent vasodilation, a hypercoagulable state, worse atherogenic dyslipidemia, and more metabolic syndrome.32–34 As the detrimental effects of glucose already occur at glycemic levels below the threshold for the diagnosis of diabetes, the transition from normoglycemia to impaired glucose tolerance and overt diabetes may be more detrimental in women than in men. Accumulating evidence suggests that these adverse changes in metabolic and vascular risk factor profile in pre-diabetic individuals are greater in women than they are in men.35, 36
Smoking
Although there are fewer adult (≥18 years) women smokers (15% vs. 19% of men)37, a recent meta-analysis reported that in all age groups, with the exception of the youngest (30–44 years), women had a 25% increased risk for CAD conferred by cigarette smoking compared with men.38 The combination of smoking with oral contraceptive use has a synergistic effect of on risk of acute MI, stroke and venous thromboembolism.39, 40
Obesity and overweight
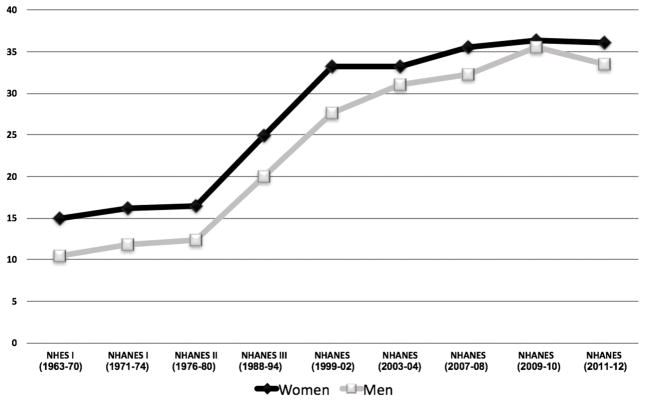
More than 2 in 3 adults in the US are considered to be overweight or obese, and the prevalence of obesity is higher among women than men (Figure 2).41 The impact of obesity on the development of CAD appears to be greater in women than in men. In the Framingham Heart Study, obesity increased the relative risk of CAD by 64% in women, as opposed to 46% in men.42 Weight gain during adult years is highly related to developing a greater ASCVD risk factor burden, and this has been observed with relatively modest weight gain in prospective studies such as the Framingham Offspring Study.43
Figure 2.
Percentage of US Adults Classified as Obese (BMI >30 kg/m2) in Health Surveys from 1963–2012.216–219
Physical Inactivity
The Physical Activity Guidelines for Americans recommend that adults get at least 150 minutes/week of moderate-intensity aerobic activity such as walking, or 75 minutes/week of vigorous-intensity aerobic activity, such as jogging, or a combination of both. Muscle strength training activities are also recommended on two or more days per week.44 According to data from a 2011 National Health Interview Survey (NHIS) in adults, inactivity was higher among women than men (33.2% versus 29.9%, age-adjusted) and increased with age from 26.1% to 33.4%, 40.0%, and 52.4% among adults 18–44, 45–64, 65–74, and ≥75 years of age, respectively.45 Observational data demonstrate an association between higher levels of physical activity and lower rates of many chronic diseases, including CVD, as well as enhanced longevity. Furthermore, an inverse dose-response relation exists, with higher levels of activity associated with commensurately lower rates of ASCVD in a curvilinear fashion.46, 47
Hypertension
Endogenous estrogens maintain vasodilation and contribute to blood pressure control in premenopausal women. Women develop hypertension about a decade after men, becoming more prevalent in elderly women than elderly men.48 No sex differences in the clinical manifestation of hypertension, outside of pregnancy-related hypertension have been described.49 Hypertension is often poorly controlled in older women; only 23% of women vs. 38% of men >80 years have a blood pressure (BP) <140/90 mm Hg.50 There is currently no evidence that antihypertensive treatments differentially affect BP response but many trials of antihypertensive agents do not report sex-specific analysis for efficacy or adverse effect profiles.
In 2013, the Eighth Joint National Committee (JNC8) released new guidelines on the management of adult hypertension, and recommended treating all hypertensive persons 60 years or older to a BP goal of <150/90 mm Hg and hypertensive persons aged 30–59 year, or with presence of DM or chronic kidney disease (CKD) at any age to a goal of 140/90 mm Hg.51
More recently, the most appropriate targets for systolic blood pressure (SBP) to reduce CVD morbidity and mortality among persons without diabetes were analyzed in a randomized controlled multicenter clinical trial, Systolic Blood Pressure Intervention Trial (SPRINT). Subjects with a SBP of 130 mm Hg or higher and increased CVD risk, but without diabetes, were randomly assigned to an “intensive treatment group” (BP target of less than 120 mm Hg achieved with an average of 3 medications) or to a “standard treatment group” (BP target of less than 140 mm Hg achieved with an average of 2 medications). The intensive treatment group resulted in 25% lower relative risk of fatal and nonfatal major CVD events and death from any cause HR=0.75 (95% CI: 0.64–0.89, p<0.001), although with notably higher rates of adverse events.52 These results may lead to a reassessment of the current JNC8 guidelines.
Dyslipidemia
Dyslipidemia has the highest population-adjusted risk among women, at 47.1%, compared with all other known risk factors for ASCVD.53 However, this greater ASCVD risk is typically not observed prior to menopause, even if cholesterol levels are quite elevated. Lifestyle modifications, including diet and exercise, are of critical importance in the primary and secondary prevention of ASCVD. Pharmacologic therapy of hyperlipidemia for secondary prevention has clearly been shown to be equally effective in women and men for reduction of recurrent cardiac events and ASCVD mortality.54, 55 In primary prevention, data in women are more limited. Primary prevention guidelines for statin initiation have recently been tailored to be sex-specific, with inclusion of sex in the American Heart Association (AHA)/American College of Cardiology (ACC) pooled cohort formula for ASCVD risk determination. Statins should be used in subjects with moderate or high ASCVD risk according to the new AHA/ACC guidelines.56 Of note, many more women will now qualify for treatment with statins according to these guidelines. In a Dutch study of 4854 people (mean age 65 years), of which 54% were women, the ACC/AHA guidelines recommended statin therapy in 66% of women, in contrast to the older ATP-III guidelines which would have recommended treatment in 36% of women.57 However, the ACC/AHA pooled cohort risk score guidelines were developed specifically for the American population and we can therefore expect this tool to perform differently in other populations.
Recent data from the Center for Disease Control and Prevention (CDC) indicated that between 2005 and 2012, only 45% of 78.1 million adults eligible for cholesterol-lowering medications actually took them.58 Of even more concern though, is that recent reports have identified sex-specific differences in both treatment and adherence to lipid-lowering medications; women are less likely to be prescribed statin therapy59, 60, and compliance is variable.61 Reasons for this disparity are unclear at the present time, but underscore the need for additional physician and patient awareness of the benefits of lipid-lowering therapy in women. In a recent review, there was a suggestion that women had a greater likelihood of developing DM on statins62, which may contribute to some uncertainty, and needs further exploration. Evolving insights into the impact of sex and ethnicity on indication for, and interpretation of advanced lipid testing (such as Lp-PLA2 activity determined by PLAC testing63) in the prediction of ASCVD events, may play a role in refinement of risk stratification of certain individuals considered for statin therapy.64 Indeed, for the first time ever, the Food and Drug Administration advised that labeling for the PLAC test contain separate performance data for black women, black men, white women, and white men. The sex-specific aspects of other biomarkers and imaging studies, such as coronary artery calcium measurements (CAC), and roles in ASCVD risk stratification continue to be debated.
III. Nontraditional ASCVD Risk Factors in Women
Pregnancy Related Disorders and CVD Risk Association
Preterm delivery
Preterm delivery (PTD) defined as birth at <37 weeks gestation complicates 5–12.7% of deliveries worldwide.65 The underlying causes and mechanisms of PTD delivery are not yet completely understood. The main mechanisms that have been suggested are inflammation, infection and vascular diseases. A recent study concluded that PTD is an independent risk factor for subsequent long-term CV morbidity and CV-related hospitalizations. The risk for ASCVD was further increased with a history of early PTD (<34 weeks’ gestation). 66
Hypertensive pregnancy disorders
Hypertensive pregnancy disorders include gestational hypertension, chronic hypertension and pre-eclampsia. Gestational hypertension is defined as new onset hypertension (>140/90 mmHg) after 20 weeks gestation in a woman who was originally normotensive. Women who develop hypertension prior to 20 weeks of gestation are diagnosed with chronic hypertension. Women who suffer severe hypertension (>160/110 mmHg) are at greater risk of progressing to pre-eclampsia. Pre-eclampsia is defined as new onset hypertension (>140/90 mmHg) after 20 weeks gestation, and proteinuria (0.3 g/24 h) and/or end organ dysfunction. There is growing consensus that the associated CVD risk persists into later life, far beyond the affected pregnancy period. In a meta-analysis with 198,252 pre-eclamptic women, it was concluded that in comparison to women with normotensive pregnancies, women with pre-eclampsia had a 3.7-fold (95% CI: 2.70–5.05) relative risk for developing hypertension 14 years after pregnancy, a 2.16 (95% CI: 1.86–2.52) relative risk for IHD after 12 years, a 1.81 (95% CI: 1.45–2.27) relative risk of stroke after 10 years and a 1.79 (95% CI: 1.37–2.33) relative risk for venous thromboembolism after 5 years.67 Earlier occurrence of pre-eclampsia in pregnancy is associated with poorer outcomes; in addition, the severity of pre-eclampsia is correlated with the severity of CVD later in life.
Gestational diabetes
For many years, gestational diabetes mellitus (GDM) was defined as any degree of glucose intolerance with onset or first recognition during pregnancy. 68 However, the ongoing epidemic of obesity and DM has led to more T2DM in women of childbearing age, resulting in an increase in the number women with undiagnosed T2DM at pregnancy, and thus women found to have DM in the first trimester are classified as having T2DM.69. GDM is defined as newly diagnosed DM beyond the first trimester of pregnancy.70 GDM increases the risk of developing T2DM by 7-fold, which is a major risk factor for subsequent ASCVD, but also raises CVD risk (2-fold for stroke, 4-fold for MI) independently of the overt development of T2DM.71, 72
Persistence of weight gain after pregnancy
Pregnancy is the only normal physiologic setting in which body weight increases by 20% or more during a 9-month period. After delivery, maternal capacity for restoring normal weight regulation is enhanced by breastfeeding, but may be disrupted by lifestyle factors, including lack of time for exercise; dietary changes and limited sleep duration. Weight at one year postpartum is a stronger predictor of the likelihood of being overweight 15 years later than the weight gained during the pregnancy itself.73 A recent study observed that weight trend in the first year post-partum reported that an adverse cardiometabolic profile emerges as early as one year postpartum in women who do not lose weight between 3 and 12 months after delivery.74
Autoimmune Diseases: Rheumatoid Arthritis and Systemic Lupus Erythematosus
Numerous population studies have demonstrated an association between inflammatory diseases and increased mortality, in both men and women, mainly as a consequence of ASCVD.75 In autoimmune diseases the immune response to self-antigens results in damage or dysfunction of tissues, which can occur systemically or affect specific organs or body systems. For most systemic autoimmune disorders there is a clear sex difference in prevalence, making this a more common ASCVD risk factor in women. The microvasculature in women may play an important role in the predisposition of women with autoimmune diseases to develop accelerated CVD.76 The female to male ratio for rheumatoid arthritis (RA) is 2.5:1, and for systemic lupus erythematosus (SLE) is 9:1. Patients with RA have a 2- to 3-fold higher risk of MI and a 50% higher risk of stroke.77 For SLE, recent case- control series have indicated that the risk of MI is increased between 9- to 50-fold over that in the general population.78, 79 It has been recognized that well known CV risk scoring systems underestimate the burden of CV risk in patients with RA and SLE, and an empiric European League Against Rheumatism (EULAR) multiplier of 1.5 has been suggested.80
Radiation and Chemotherapy for Breast Cancer
Radiotherapy for breast cancer often involves incidental exposure of the heart to ionizing radiation, increasing the subsequent rate of IHD. The increase is proportional to the mean dose to the heart, beginning within a few years after exposure, and continuing for at least 20 years.81 Women with preexisting cardiac risk factors have greater absolute increases in risk from radiotherapy. In a recent population based case-control study, women irradiated for cancer of the left breast had higher rates of CAD events than women receiving radiation to the right breast. Moreover, the rate of CAD events increased by 7.4% per gray of the mean radiation dose delivered.81 Radiation-induced heart disease can also manifest as valvular and cardiomyopathic processes.
There has been a tremendous improvement in the survival rates of breast cancer. Unfortunately, this improvement in outcome has been associated with chemotherapy dose-dependent acute, subacute and late cardiotoxicity. Breast cancer patients treated with chemotherapy may be at risk for either or both Type I (anthracycline-like agents) and Type II (Trastuzumab-like agents) cardiotoxicity, for which prevention and monitoring is a contemporary issue of recent significant controversy and attention.82 Patients with breast cancer who have undergone anthracycline-based therapy and patients who have had mediastinal radiation therapy are candidates for long-term cardiac surveillance programs. An expert consensus statement from the European Association of Cardiovascular Imaging and the American Society of Echocardiography recommends evaluation based on signs and symptoms and echocardiographic surveillance continuing 5 years after treatment in high-risk patients and 10 years in all other patients. It has also been recommended that high-risk patients should receive a functional noninvasive stress test within 5 to 10 years of completion of chest radiation therapy.83
Depression
Depression is a prevalent and increasingly recognized risk factor for development of CAD; it’s presence also portending unfavorable outcomes after a CAD event.84 Limited evidence suggests that depression and other psychosocial risk factors might be more powerful risk factors in younger individuals,85 and especially in young women.86–88 Although few women develop CVD at a young age,89 the lifetime risk in women at age 50 is about 40%, and therefore identification of risk factors in young populations may provide long-term benefit by facilitating early prevention.90 Furthermore, young women have been underrepresented in studies of CVD,91 have higher rates of depression,92, 93 and have higher mortality rates after acute MI compared with men.94 While CVD mortality rates have declined in the United States, this decline is less pronounced among young women in recent years,2 a time period when rates of depression have been increasing.
IV. Menopause and CVD
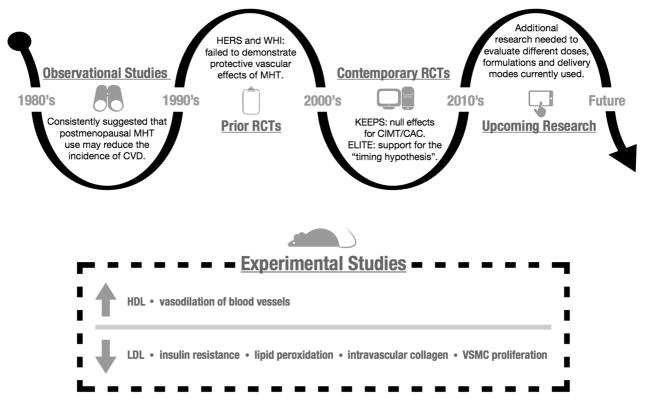
Premenopausal women are relatively protected against CVD, compared with age-matched men. However, this sex-gap narrows after menopause. This long-standing observation led to a hypothesis that ovarian steroid hormones and, in particular, estrogens, were cardioprotective, initially supported by retrospective observational studies95–100. However, such conclusions were refuted by randomized clinical trials (RCTs) of both primary and secondary prevention of ASCVD.101, 102 The discordance was surprising in light of the beneficial physiologic effects of estrogen on the vascular endothelium at the cellular and molecular levels, on blood vessels in animal CVD models, and on lipids and insulin resistance biomarkers; as such menopausal hormone therapy (MHT) became one of the most controversial areas in women’s health.103, 104 The results of the major RCTs, the Women’s Health Initiative (WHI) and the Heart Estrogen/Progestin Replacement Study (HERS), led to dramatic changes in clinical practice in the mid-2000’s, with marked declines in the use of MHT worldwide
Since then, clinicians and scientists have reviewed the RCT’s with a critical eye, attempting to explain the discordance with the observational studies. The average WHI enrollment age was 63 years, greater than 12 years older than the age at which MHT is commonly initiated in clinical practice, for the indication of postmenopausal vasomotor symptom management. When the WHI investigators analyzed the results by age groups (50–59, 60–69, 70–79 years), CAD outcomes with MHT were found to be more favorable in younger than older women, especially in the E-alone trial.102, 105 Consistent with these trends, a meta-analysis of more than 39,000 women enrolled in 23 clinical trials concluded that MHT reduces CAD risk in women younger than 60 years, but not in older women.106 Debate about the “timing hypothesis” continues, with recent RCT’s focused on surrogate endpoints such as carotid intimal medial thickness (CIMT) and CAC. These trials have also yielded inconsistent findings, including null results for CIMT and CAC in the Kronos Early Estrogen Prevention Study (KEEPS)107 and evidence supportive of the timing hypothesis in the Early Versus Late Intervention Trial with Estradiol (ELITE) (Figure 3).108 Overall, a consensus has emerged that MHT, at the lowest effective dose, remains an appropriate treatment for menopausal symptoms in early (i.e. within 5 years) menopause, in the absence of contraindications, but should never be prescribed for the express purpose of preventing CVD. 109, 110
Figure 3. Menopausal Hormone Therapy Timeline.
Experimental studies have consistently demonstrated beneficial physiologic effects of estrogen on the vascular endothelium at the cellular and molecular level. This long-standing observation led to a hypothesis that estrogens were cardioprotective, which was initially supported by retrospective and prospective observational studies, followed by disappointment from HERS, WHI and other RCTs that failed to demonstrate reduced risks of clinical CVD events with MHT. More recent RCTs include KEEPS (null results) and ELITE (which has supported the “timing hypothesis”). MHT is contraindicated for the primary and secondary prevention of CVD.
BRCA Carriers, Prophylactic Salpingo-Oophorectomy and Menopause: Clinical Management Considerations and Recommendations
Women who inherit a mutation in either the BRCA1 or BRCA2 gene have greatly elevated lifetime risks of ovarian cancer, fallopian tube cancer and breast cancer. Risk-reducing surgery with mastectomies and bilateral salpingo-oophorectomy (BSO) is recommended, often prior to natural menopause, to prevent cancer.111
There are no published guidelines specifically for the management of BRCA-mutation carriers after prophylactic BSO. In the general population, studies of surgical menopause in young women have demonstrated increased risk for development of premature CVD, low bone density and, an increase in cognitive impairment.112–115 A positive association between BSO and increased risk of CVD has been observed in a number of observational studies, including the Nurse’s Health Study and the Mayo Clinic Cohort of Oophorectomy and Aging.113, 116, 117
The appropriate management of BRCA-positive women who elect to undergo prophylactic BSO is an important clinical issue. The National Comprehensive Cancer Network guidelines state that the increased risk of osteoporosis and CVD associated with premature menopause should be addressed, as well as possible effects of cognitive changes and vasomotor symptoms on quality of life; counseling also includes a discussion of possible short-term MHT up to the average age of natural menopause. Specific guidelines for the appropriate care of BRCA-positive women after prophylactic BSO are needed. Further studies are required to determine the optimal management of young BRCA-positive women who elect to undergo prophylactic BSO.
V. Primary Prevention Guidelines
Over the last decade, substantial progress has been made in improvement of the awareness of CVD as the major cause of morbidity and mortality in women. Concurrently, an emerging understanding of the sex-unique approaches required to recognize, diagnose, treat and, ideally prevent, CVD has evolved. The focus is on recognizing lifetime risk for CVD in women and prevention of disease development. For the first time in 2007, the AHA published “evidence-based” guidelines focused on the primary prevention of CVD in women, which were subsequently updated in 2011 as “effectiveness-based” guidelines.118 Early screening and a complete CVD risk assessment were advised to reduce the pervasiveness of CVD in women, who were previously largely excluded, or minimally represented in CV research. The transformation from evidence-based to effectiveness-based guidelines denoted a shift from pure clinical research as the basis of recommendations to an approach that encompasses benefits and risks observed in clinical practice.
Findings from the longitudinal, observational Nurses’ Health Study highlighted the critical importance of lifestyle modifications in CAD prevention, demonstrating that women can reduce their risk of coronary events by more than 80% by not smoking, maintaining healthy body weight (body mass index [BMI <25 kg/m2]), consuming a healthy diet, participating in moderate to vigorous exercise for 30 minutes a day, and consuming no more than a moderate amount of alcohol.119, 120 The INTERHEART study was a large case-control study that screened all patients admitted to the coronary care unit or equivalent cardiology ward for a first MI at 262 participating centers in 52 countries. INTERHEART identified 9 easily measured risk factors (smoking, lipids, hypertension, diabetes, obesity, diet, physical activity, alcohol consumption, and psychosocial factors) that account for over 90% of the risk for acute MI.53 Importantly, the magnitude of the ASCVD risks for men and women were similar, but the impact of modifying the risks was greater in women. Thus, large studies have demonstrated that lifestyle intervention for primary prevention can decrease the incidence of ASCVD as well as the associated mortality rates in both women and men.
Aspirin
Aspirin (ASA) has proven to be effective for both men and women in the secondary prevention of CVD and in the treatment of acute MI. However, for primary prevention of CVD in women, data have been more limited. In the large-scale Women’s Health Study (WHS), almost 40,000 healthy women over the age of 45 were randomly assigned to low dose ASA (100 mg every other day) or to placebo for ten years, and major CVD events were evaluated.
Overall, the trial showed a statistically non-significant 9% reduction in the primary composite outcome of major CVD events with low-dose aspirin.121 ASA significantly lowered the risk of total stroke by 17% (CI, 0.01–0.31) and the risk of ischemic stroke by 24% (CI, 0.07–0.37) in women, but did not lower the risk of MI or CV death.121 This contrasts to the significant reduction in MI and neutral effect on stroke for primary prevention in men, observed in the Physicians’ Health Study.122 Moreover, as with men, ASA increased gastrointestinal bleeding risks and the risk of hemorrhagic stroke. However, in subgroup analyses, the CVD risk/benefit ratio appeared to be directly linked to a woman’s age; in WHS participants over age 65, ASA was clearly associated with evidence of benefit for both ischemic stroke and MI. The AHA “effectiveness-based” guideline recommendations for the prevention of CVD in women were thus derived to state that for primary prevention, ASA therapy (81mg daily or 100 mg every other day) can be useful in women ≥ 65 years of age if blood pressure is controlled and benefit for stroke and MI prevention is likely to outweigh risk of gastrointestinal bleeding and hemorrhagic stroke (Class IIa, Level of Evidence B), and may be reasonable for women < 65 years of age for ischemic stroke prevention (Class IIb, Level of Evidence B). The US Preventive Services Task Force (USPSTF) is reviewing their prior 2007 and 2009 recommendations (for aspirin use in the prevention of colorectal cancer and CVD, respectively), and have proposed a draft of primary prevention guidelines. In the present format, a pragmatic approach is suggested, without sex-specific differentiation, using 81 mg of ASA in both men and women aged 50 to 59 (Grade B=offer to all) and 60–69 (grade C=selective offering) who have a ≥10% 10yr-ASCVD risk, are not at increased risk for bleeding, have a life expectancy of at least 10 years, and are willing to take low dose ASA for at least 10 years. It is the judgment of the USPSTF that there is some certainty that the net benefit of aspirin use is at least moderate for adults aged 50 to 59 years who are at average risk for bleeding; adults who have little potential of benefit or high risk for GI bleeding should be discouraged from aspirin use.
Aspirin in Women with Diabetes Mellitus
The use of ASA to prevent ASCVD events in women with DM is controversial and the evidence for benefit is far from conclusive. There have been several meta-analyses of aspirin use in DM; most did not show a benefit for aspirin treatment in DM for primary CVD prevention.123–126 Moreover, three trials that have examined ASA use among patients with DM, demonstrated no overall benefit in the treatment group.127–129 However, in the subgroup of DM in the Women’s Health Study, women who received ASA had a lower risk of stroke, compared with those without DM.121 A 2010 consensus by the AHA, the ACC Foundation, and the American Diabetes Association made the following recommendations130 for adults with DM and without pre-existing CVD:
Low-dose aspirin (75–162 mg/d) should be considered for individuals with a 10-year risk of CVD of at least 10% who do not have an increased risk of bleeding; this group consists of men at least 50 years of age and women at least 60 years of age with at least 1 additional CVD risk factor.
Aspirin should not be recommended for adults with DM at low risk (men <50 years of age and women <60 years of age with no additional CVD risk factors).
It is important for physicians to be aware that, despite the increased risk for ASCVD in female patients with diabetes, having diabetes alone does not qualify them for ASA therapy. Physicians must still perform a proper ASCVD and bleeding risk assessment before making recommendations.
Statins
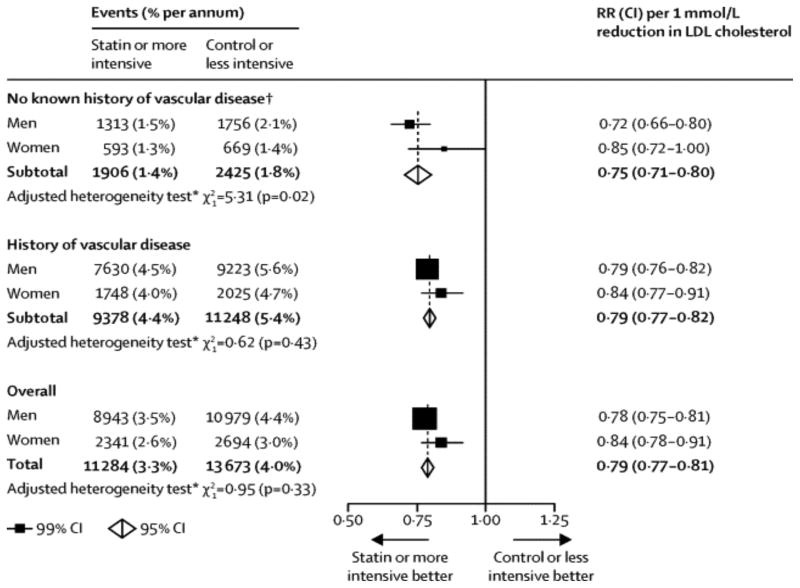
It is well established that statin therapy is as effective in women as in men for secondary prevention of ASCVD.131 What has been more controversial is the effectiveness of statins in primary prevention in women.132 A recent meta-analysis of 27 trials of statin therapy concluded that the proportional reduction in major vascular events per 1·0 mmol/L reduction in LDL cholesterol was similar for men and women [risk ratio [RR] for women 0.84, (99% CI 0.78–0.91); RR for men 0.78, (99% CI 0.75–0.81)], irrespective of the baseline level of ASCVD risk or subtype of ASCVD outcome assessed.133 Although the results were slightly more favorable for men than women (p for heterogeneity by sex <0.05), the guidelines for statin use are the same for both sexes (Figure 4)
Figure 4. Effects on major vascular events per 1·0 mmol/L reduction in LDL cholesterol, subdivided by history of vascular disease and sex.
Proportional reduction in major vascular events per 1·0 mmol/L reduction in LDL cholesterol was similar for men and women irrespective of the baseline level of ASCVD risk or subtype of ASCVD outcome assessed. The results were slightly more favorable for men than women (p, heterogeneity by sex <0.05). Reused with permission from the Cholesterol Treatment Trialists (CTT) Collaboration133
In 2013, ACC/AHA jointly released new guidelines on the treatment of cholesterol to reduce ASCVD in adults; recommending statin use in asymptomatic adults ages 40 to 75 years without a history of CVD who have: 1) LDL cholesterol level > 189, 2) LDL cholesterol level of 70 to 189 mg/dL, if they also have DM (moderate- to high-dose statin use is recommended, depending on 10-year ASCVD event risk) or 3) an estimated 10-year ASCVD event risk of 7.5% or greater, as calculated on the pooled cohort equation risk calculator. Moderate to high-dose statin use occurs only after clinician-patient risk/benefit discussion that addresses other risk factors and optimal lifestyle, the potential for benefit vs. potential for adverse effects, and drug-drug interactions. Instead of treating to a specific LDL cholesterol target, the ACC/AHA recommends fixed-dose statin therapy.56 In response, the Mayo Clinic established a task force, and concluded similar recommendations, although emphasizing lifestyle modifications over immediate initiation of statin therapy in those adults age 40 years and older with an LDL cholesterol level of 70 to 189 mg/dL, without DM, yet with and ASCVD event risk > 7.5%, in cases where the patient is sufficiently motivated to reduce their ASCVD event risk to less than 7.5%, especially if the LDL cholesterol level is less than 100 mg/dL.56, 134 Critics of the new guidelines have suggested that the risk score overestimates risk. Nonetheless, the ASCVD risk calculator was based on more than one population and was validated in Caucasian and African American men and women. Therefore, when applied in Hispanic-American, Asian-American and South Asian-American populations, misclassification of risk category may be more likely.
The USPSTF is reviewing their prior 2008 guideline recommendations on statin use for primary prevention of ASCVD. The draft USPSTF recommendation (Grade B-offer to all) includes that all adults without a history of ASCVD (i.e., symptomatic coronary artery disease or thrombotic stroke) use a low- to moderate-dose statin for the prevention of ASCVD events when all of the following criteria are met: ages 40 to 75 years, one or more ASCVD risk factors (i.e., dyslipidemia, diabetes, hypertension, or smoking), and a calculated 10-year ASCVD risk of 10% or greater. At a lower level of recommendation (Grade C-selective offering), a calculated 10-year ASCVD risk of 7.5–10% is suggested.
A recent report from the CDC found that there were significant differences in the percentage of men (40.8%) and women (32.9%) on or eligible for statin treatment. Among persons on or eligible for treatment there were major differences in the proportion of men (52.9%) and women (58.6) taking cholesterol-lowering medication.58 There is no compelling evidence to support that statins are less safe in women than in men. The guidelines recommend baseline ALT level assessment, but unless there is suspected hepatic dysfunction, monitoring is not needed. In the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER), which enrolled more women than any other statin trial to date, no differences in the rates of myopathies between men and women were found. The JUPITER trial, however, demonstrated that women taking rosuvastatin had a greater increase in their HbA1c compared with placebo (HbA1c 5.9 vs. 5.8, P = 0.001), in addition to a greater risk of developing new diabetes (1.53 vs. 1.03 per 100 person-years, respectively; HR = 1.49; 95% CI: 1.11–2.01; P = 0.008) compared with men (1.36 vs. 1.20 per 100 person-years, respectively; HR = 1.14; 95% CI: 0.91–1.43; P = 0.24).135 Of note, 80% of incident DM occurred in those with impaired fasting glucose at study entry. In the Women’s Health Initiative, reported statin use was associated with an increased risk of self-reported new-onset diabetes in postmenopausal women (HR = 1.48, 95% CI: 1.38–1.59).136 A recent meta-analysis, including 13 statin trials with 91,140 participants, found that statin therapy was associated with a 9% increased risk of developing incident DM, OR 1.09 (95% CI 1.02–1.17); however no sex-specific analysis was performed.137 Overall, the benefit of statins from reduction in coronary events appears to exceed the risk related to DM in both men and women.
VI. Ischemic Heart Disease in women
In medicine, the proper distinction between sex and gender effects is usually unachievable which is why these are often compiled for clinical purposes.49 Sex- and gender-specific CVD research has led to a new understanding of the pathophysiology of coronary disease in women, which includes, but is not limited to, our conventional understanding of atherosclerosis. IHD in women includes not only atherosclerotic obstructive CAD, but also an expanded spectrum of coronary disease, including coronary microvascular dysfunction (CMD), endothelial dysfunction, vasomotor abnormalities, spontaneous coronary artery dissection (SCAD) and stress-induced cardiomyopathy. 138
Certainly, there are marked differences in the prevalence, incidence and burden of IHD in women when compared to men139, such that an awareness of uniquely “female-pattern of IHD” is emerging, although some have suggested that the “Yentl syndrome is alive and well” 15 years after these initial observations.140 This literature described that when women look like men (with ‘male-pattern’ obstructive CAD), they are more likely to be diagnosed and treated like men. Dr. Bernadine Healy used the term ‘Yentl syndrome’ in 2001, as depicted in the Barbra Streisand movie of the same name, to call attention to the paradox of adverse outcomes of women with IHD, as well as the underdiagnosis and undertreatment of women.
The three most important characteristics of IHD in women are that they have: 1) a higher prevalence of angina 2) a lower burden of obstructive CAD on angiography and 3) a poorer prognosis in comparison to men.141 Additionally, current risk scores, based on ACS thresholds determined in predominantly male-based populations, do not accurately predict risk in women, showing the need for sex-specific biomarker ranges and risk stratification tools in order to improve the diagnosis, treatment, and follow-up in female populations142 In a recent prospective cohort study, the high sensitivity troponin I assay noticeably increased the diagnosis of MI in women (from 11% to 22%, P<0.001) but had a minimal effect on men (19% to 21%, P=0.002).143 Other biomarkers, such as proneurotensin, are also found to be sex-specific and related to incident CVD only in women, affirming the need for more research in this area. 144
Clinical Presentation
Optimal recognition and timely management of acute MI, especially for reducing patient delay in seeking acute medical care, is critical. In a comprehensive review of the presenting symptoms of ACS in women, women were more likely than men to present without chest pain and had higher mortality than men, especially among younger age groups; sex differences in clinical presentation without chest pain and in mortality were attenuated with increasing age.145
Although it has been recognized that a wide range of atypical symptoms occur more frequently in women including weakness, fatigue, nausea, dyspnea, as well as unconventional descriptors, triggers and locations of chest-related symptoms, such as in the neck, jaw, and back the most common presenting symptom of ACS is chest pain in both men and women.146, 147
Obstructive versus Nonobstructive CAD
Recognition of IHD, both acute and chronic, is often delayed or deferred in women. Consequently, many women at risk for related adverse outcomes are not provided specific diagnostic, preventive, and/or treatment strategies. In part, this lack of recognition is related to sex-specific CVD pathophysiology in women that differs from the traditional male-pattern model (flow-limiting atherosclerotic CAD). This nonobstructive CAD pattern and the tendency among women to have plaque erosion with subsequent thrombus formation, along with CMD, are not well recognized. Importantly, data are emerging to show that more extensive nonobstructive CAD involvement is associated with a rate of major adverse cardiovascular events that may approximate that of obstructive CAD.148 However, there are many limitations to our understanding of nonobstructive CAD and gaps in current knowledge.
With the widespread use of coronary angiography in the early clinical management of MI, multicenter MI registries have evolved and reported that as many as 10% of MI patients have no evidence of obstructive CAD.149 These patients with MI and nonobstructive coronary arteries (MINOCA)150 represent an enigma because the underlying cause of the MI is not immediately apparent. In a recent systematic review it was determined that MINOCA is characterized by: (1) a 6% prevalence of all MI presentations, [95% CI, 5%–7%] with a median patient age of 55 years and 40% women. (2) No diagnostic distinguishing clinical presentation features compared with MI-with obstructive CAD, (3) a better 12-month all-cause mortality compared with MI-with obstructive CAD, although its prognosis should be considered as guarded, and (4) structural dysfunction, coronary spasm, and thrombotic disorders as potential underlying causes. Given that MINOCA has similar features to MI- with obstructive CAD, it should be considered a working diagnosis that requires further evaluation of potential underlying causes.151
Acute coronary syndromes in women
ACS refers to a spectrum of clinical presentations including ST-segment elevation myocardial infarction (STEMI), non–ST-segment elevation MI (NSTEMI) and unstable angina. Symptoms of ACS in women may differ from those in men, which may lead to delays and misdiagnosis. Young women with acute MI represent a relatively large yet understudied population. Nearly 16,000 U.S. women 55 years or younger die from IHD each year. These women account for 40,000 hospitalizations for acute MI annually and have greater risks for morbidity and mortality compared with both young men and older women with acute MI.23, 152 The Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study, is an observational study of acute MI patients aged ≤55 years in the United States and Spain. In this study, young women with STEMI were less likely to receive reperfusion therapy and more likely to have reperfusion delays than similarly aged men. Sex disparities were more pronounced among patients transferred to percutaneous coronary intervention (PCI) institutions or who received fibrinolytic therapy.153
Coronary microvascular dysfunction
CMD, is defined as limited coronary flow reserve and/or coronary endothelial dysfunction and is associated with worse outcomes, with increased rate of cardiac death, stroke or heart failure.154, 155 An annual major adverse cardiovascular event rate of 2.5% is present in women with CMD and risk factors for CMD have not been fully elucidated. 156 CMD is characterized by a decrease in the size of epicardial vessels and microvasculature, diffuse atherosclerotic disease, increased arterial stiffness and fibrosis, altered remodeling, and the presence of endothelial or smooth muscle dysfunction.157 The microcirculation cannot be investigated by angiogram, thus, several techniques for functional assessment, of coronary flow reserve (non-invasive and invasive) have evolved, however the gold standard is an invasive coronary reactivity test. The WISE study highlighted the importance of CMD in women 141 and supported the use of invasive coronary vasomotor testing as a safe method for definitive diagnosis and assessment of prognosis in high-risk women. 156 Early detection of endothelial dysfunction, measured by brachial artery flow-mediated vasodilation, has also been associated with a substantial increase in IHD in women.158 Additional simpler noninvasive techniques have emerged, with specially designed fingertip probes to measure the peripheral reactive hyperemia index, a measure thought to reflect endothelial function.159 Positron emission tomography (PET) and cardiac magnetic resonance (CMR) imaging are growing noninvasive modalities to detect sub-endocardial ischemia. It is now well established that the prognosis is worse in women with CMD and should not be underestimated by clinicians.160
Treatment of microvascular angina in women starts with risk factor modification and lifestyle changes to achieve optimal coronary risk factor control. Exercise training and CR is often recommended. Statins, by their anti-inflammatory properties, are especially beneficial in improving endothelial function. The first step in medical treatment includes traditional anti-ischemic drugs such as: nitrates, beta-blockers, ACEI and calcium channels blockers. Non-traditional anti-ischemic medications such as ranolazine or aminophylline (xanthine derivative) have been evaluated, but do not show consistent benefit. Xanthines and tricyclic antidepressants may be helpful for altered cardiac pain perception.161
Spontaneous Coronary Artery Dissection
Spontaneous Coronary Artery Dissection (SCAD) is defined as a sudden separation between the layers of a coronary artery wall, creating an intimal flap and intramural hematoma thus obstructing intraluminal blood flow distally, and resulting in acute myocardial ischemia.162 80% of SCAD patients are female with average age of 42 years, with 20% to 25% of cases occurring in the peripartum period.163 An association with occult fibromuscular dysplasia (FMD) has been observed in approximately 50% of patients leading to routine screening with CT-angiography from “base of skull to pelvis”, as well as MR or CT screening for detection of occult cerebral aneurysms.162 The classic presentation is of a young healthy woman, without traditional ASCVD risk factors, and sudden onset of ACS. Ongoing substantial progress of SCAD research is taking place due to recent increases in patient engagement through social media and creation of disease-specific online communities. The establishment of a large registry database, 164 provided preliminary evidence that there may be a genetic predisposition to SCAD.165
The diagnosis of SCAD most importantly requires a high degree of suspicion with careful angiographic study. Accurate differentiation of ACS due to SCAD from ACS due to atherosclerosis is crucial, because the approaches to both acute and long-term management are different. The most important reasons for accurately diagnosing SCAD are that acute SCAD patients undergoing PCI have markedly reduced technical success rates compared with PCI success rates for atherosclerotic ACS (62% vs. 92%)162 Moreover, the substantial rate of spontaneous vascular healing162, 166 suggests a role for conservative management in stable SCAD patients with preserved distal coronary flow. Conservative management has generally been associated with favorable outcomes,166 however careful inpatient monitoring (4–5 days) is needed due to a small early threat of dissection progression and the consequent need for acute intervention.
Ten-year recurrence rates of up to 20%, predominantly in women,162 underscore the need for close and long-term follow-up, as well as the imperative for more research. In a retrospective case series, statins were associated with recurrent SCAD; therefore statins are discouraged, and recommended only when hyperlipidemia is documented.162 Although evidence of benefit is lacking, the administration of low dose aspirin is routinely recommended. CR should be recommended to all SCAD patients.167
Stress Cardiomyopathy (Takotsubo/Broken Heart Syndrome)
Stress-induced cardiomyopathy was first described in Japan in 1990 and was named after the octopus trapping pot with a round bottom and narrow neck, which resembles the left ventriculogram during systole in these patients. It is characterized by transient systolic and diastolic left ventricular dysfunction with a variety of wall-motion abnormalities, but classically noted is mid to apical akinesis, and basal hyperdynamic function.168 It mainly affects post-menopausal women and is often preceded by extreme physical or emotional triggers.169 The clinical presentation, electrocardiographic findings, and biomarker profiles are often similar to those of ACS, but the coronary artery anatomy is found to be without significant obstructive disease at angiography. 169
The cause of Takotsubo cardiomyopathy remains unknown, but is thought to be related to a disproportionate distribution and activation of myocardial sympathetic receptors. The ventricular dysfunction, which usually involves the left, but may also involve the right ventricle, generally resolves within several weeks with supportive therapy, including beta-blockade; however, especially in the presence of significant comorbidities, the outcome may not be benign. Patients remain at risk for recurrence, even years after the first event.170–172 Beta-blockers have been proposed as a therapeutic strategy.173 In a recently published large international registry, patients with stress-induced cardiomyopathy were found to more likely present with neurologic and psychiatric comorbidities.169
Medical Anti-Ischemic Therapy
Despite their beneficial effect, medical therapy such as aspirin, angiotensin converting enzyme inhibitors (ACE-I), angiotensin receptor blockers (ARBs), β-blockers, aldosterone inhibitors and statins are frequently delayed in women. The EuroHeart Survey demonstrated that in the treatment of stable angina women were significantly less likely to receive aspirin.22 Upon hospital discharge for non-ST-elevation MI, women were about 3 % less likely to receive aspirin and beta-blockers and about 13% less likely to receive statin therapy compared to men.25 Recent evidence suggests that many drugs that we commonly use to treat CVD in women, including especially antithrombotic and antiarrhythmic agents, are metabolized differently in women, and put them at risk for increased adverse effects, and potential need for dose adjustment, a neglected area of understanding which requires further research.
Invasive Testing for IHD
In women and men with a high probability of CAD or with evidence of ACS, coronary angiography is indicated for diagnosis and, when appropriate, catheter-based therapy. Large-scale observations from the CRUSADE initiative showed that despite these recommendations, women with ACS are treated less aggressively, with fewer cardiac catheterizations, catheter-based interventions, fibrinolytic and bypass surgical procedures, resulting in less favorable clinical outcomes with higher mortality and lower health related quality of life compared to men25. A recent meta-analysis comparing early invasive versus conservative treatment strategies in men and women with NSTEMI and unstable angina ACS showed a comparable benefit of an early invasive strategy in men and high-risk women for reducing the composite end point of death, MI, or rehospitalization with ACS; however, lower risk women, without biomarker elevation, did not show a benefit174. Regarding potential risks associated with invasive procedures, women have been shown to have more bleeding complications. However, dose-adjusting of antithrombotic/antiplatelet therapies and newer technical approaches (radial access) may result in reduced bleeding and vascular complications in women.175, 176
Noninvasive Testing for IHD
The 2014 AHA Consensus Statement on the Role of Noninvasive Testing in the Clinical Evaluation of Women with Suspected Ischemic Heart Disease provides evidence-based guidelines on diagnosis of IHD in women by noninvasive testing177. The options for non-invasive tests are similar for both men and women and pretest probability must be taken into account when “Choosing Wisely” according to testing appropriateness (Table 2).177 In women unable to perform activities of daily living or to perform adequately on exercise treadmill testing (ETT), a pharmacological stress test is the preferred method of risk assessment. Stress imaging tests provide information about wall motion abnormalities or perfusion, and provide assessment of ventricular function.
Table 2. Pretest probability for Coronary Artery Disease by Age, Sex and Symptoms.
Reused with permission from Gibbons et al. 215
| Pretest Probability of Coronary Artery Disease by Age, Gender and Symptoms1 | |||||
|---|---|---|---|---|---|
| Age (y) | Gender | Typical/Definite Angina Pectoris | Atypical/Probable Angina Pectoris | Nonanginal Chest Pain | Asymptomatic |
| 30–39 | Men | Intermediate | Intermediate | Low | Very low |
| Women | Intermediate | Very low | Very low | Very low | |
| 40–49 | Men | High | Intermediate | Intermediate | Low |
| Women | Intermediate | Low | Very low | Very low | |
| 50–59 | Men | High | Intermediate | Intermediate | Low |
| Women | Intermediate | Intermediate | Low | Very low | |
| 60–69 | Men | High | Intermediate | Intermediate | Low |
| Women | High | Intermediate | Intermediate | Low | |
High indicates >90%; Intermediate 10–90%, Low <10%, Very low <5%
No data exists for patients <30 or >69 y but it can be assumed that prevalence of coronary artery disease increases with age. In a few cases, patients with ages at the extremes of the decades listed may have probabilities slightly outside the high or low range.
Functional Testing
Functional tests include ETT with electrocardiogram (ECG), exercise/pharmacologic stress echocardiography, exercise/pharmacologic cardiac nuclear imaging with single-photon emission computed tomography (SPECT) or PET, pharmacologic stress CMR, CT perfusion and CT or Doppler ultrasound-derived flow reserve measurements.139 ETT is the most common method of diagnosing CAD in women despite a higher false-positive rate compared to men. ETT is recommended as the diagnostic test of choice in symptomatic, intermediate risk women who are able to exercise and have a normal resting ECG. Exercise stress testing provides valuable information about exercise capacity, and hemodynamic response to exercise and recovery.
Anatomic Testing
Evidence regarding the usefulness of cardiac CT has grown. Coronary computed tomographic angiography (CCTA) and CAC score provide additional tools for the clinical assessment of CAD. Recently published studies include the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial comparing functional vs. anatomic assessment tests, demonstrating no significant differences in outcomes by test used.178 The Rule Out Myocardial Infarction using Computer Assisted Tomography (ROMICAT) trial demonstrated that CCTA predicts major CV events.179 ROMICAT II trial found that found that women who undergo CCTA compared to standard cardiac evaluation had less hospital admissions, shorter length of hospital stay and lower total radiation dose compared with men.180
VII. Heart Failure in women
Heart Failure with preserved ejection fraction
Heart failure is major health threat in the US. In most studies Heart failure in women occurs in older age and with less ischemic causes. Women are approximately two times more likely than men to develop heart failure with preserved ejection fraction (HFpEF). This syndrome was historically considered to be caused exclusively by left ventricular diastolic dysfunction, as demonstrated on echocardiography, but research has identified several other contributory factors, including limitations in left ventricular systolic reserve, systemic and pulmonary vascular function, coronary microvascular endothelial inflammation and reduction of nitric oxide bioavailability, chronotropic reserve, right heart function, autonomic tone, left atrial function, and peripheral impairments.181, 182 These impairments in cardiac, vascular, and peripheral reserve can be caused by common risk factors for HFpEF, such as aging, adiposity, hypertension, and metabolic stress. HFpEF is a clinical diagnosis, and is subject to under-detection due to the lack of specific diagnostic biomarkers.
In contrast to heart failure with reduced ejection fraction (HFrEF), unfortunately no treatment has been proven effective for HFpEF in clinical trials.183,184 Blood pressure control concordant with existing hypertension guidelines remains the most important recommendation in treating patients with HFpEF (Recommendation Class I-B); in addition, use of diuretics to relieve volume overload symptoms (Recommendation Class I-C), coronary revascularization for CAD with angina/ischemia despite optimal medical therapy (Recommendation Class IIa-C), management of atrial fibrillation (AF) (Recommendation Class IIa-C), and ARB’s may also be considered to reduce hospitalizations, (Recommendation Class IIb-B).185 Women exhibit a worse quality of life after diagnosis of HF and more frequently exhibit depression.49 As this poorly understood entity disproportionately affects women, and particularly elderly women, it is in dire need of research efforts to elucidate pathophysiology and treatment strategies.
Peripartum cardiomyopathy (PPCM)
PPCM, also known as pregnancy-associated cardiomyopathy186, is an uncommon condition in which an idiopathic form of left ventricular systolic dysfunction develops during pregnancy or the postpartum period in women without previous heart disease 187. The incidence of this condition in the United States is approximately 1 in 3,000 deliveries, with a significantly higher incidence in African Americans, women older than 30 years of age, those with a history of pregnancy-associated hypertension, and in those with multifetal pregnancies.188 The etiology of PPCM remains unknown and is a diagnosis of exclusion, therefore all patients should be thoroughly investigated.
The majority of women demonstrate a partial or complete recovery within 2 to 6 months after the diagnosis of PPCM. A recurring concern is the potential risk during or following subsequent pregnancies, even if LV function returns to normal. Despite the critical importance of this issue, it is only briefly discussed in the most recent guidelines for the management of pregnancy-related heart disease.189 In advanced HF with hemodynamic instability, urgent delivery, irrespective of gestation may need to be considered.190
Upon urgent delivery, the principles of managing acute HF due to PPCM do not differ than those applying to acute HF from other causes, including: diuretics (thiazide diuretics appear to be safe191), β-blockers, ACE I/ARB’s, and hydralazine/nitrates.187 Inotropes may be considered in patients with severely reduced cardiac output states; anticoagulation may be indicated if ejection fraction falls <35%. Further research is needed before subsequent pregnancy recommendations and firm breastfeeding recommendations can be made for PPCM patients.
VIII. Cardiac Rehabilitation in women
CR is a multidisciplinary outpatient program that reduces overall and CV-related mortality by 13% and 26% respectively, when compared to usual care.192 CR is indicated following ACS, post-intervention (PCI and CABG) and heart failure diagnoses. Despite women-specific clinical practice guideline recommendations for CR referral as a Class 1, Level A indication,192, 193 a recent meta-analysis showed that men were a third more likely to be enrolled in CR compared with women (P < 0.00001).194
The reasons for women being under-represented in CR programs are multifactorial. Physician referral patterns, program structure, and patient preferences influence the degree of CR participation among women.118, 195 Recommendations to attend CR programs need to be consistently offered to all women and reinforced by all health staff, including physicians.
IX. Other Vascular Diseases in Women
Stroke
In the United States, 53.5% of the estimated new or recurrent strokes occur among women annually, resulting in ≈55 000 more stroke events in women than in men.44 Women have an increased lifetime incidence of stroke compared to men, largely due to a sharp increase in stroke risk in older postmenopausal women. Women also have an increased lifetime prevalence of stroke risk factors, including hypertension, as well as abdominal obesity and metabolic syndrome, especially in middle-aged women. Incidence of AF is lower in women compared to men196; however, women suffering from AF show a higher incidence of stroke and a higher mortality rate with respect to men. A recently published meta-analysis evaluated 30 studies with 4,371,714 participants addressed whether AF is a stronger risk factor for stroke, CVD death, all-cause mortality, and other outcomes in women compared with men. This analysis found that the pooled relative risks for stroke was associated with twice the relative risk of stroke in women than in men (relative risk ratio 1.99, 95% confidence interval 1.46 to 2.71). AF was associated with a higher relative risk of all cause mortality, stroke, CV mortality, cardiac events, and heart failure in women compared with men.197 Active screening for AF, especially in women >75 years of age, in primary care settings using vital sign assessment followed by confirmatory ECG when heart rate irregularity is detected is recommended (Class I; level of evidence B).198 Although female sex is incorporated as a risk factor for stroke in the widely used CHA2DS2-VASc score, AF seems to affect women and men differently.197 The AHA recently recommended the development of a specific risk score for stroke in women as some risk factors for stroke are unique to, more prevalent or differently impact women.198 Lastly, when stroke risk stratification indicates the need for anticoagulation, women should receive treatment. Pregnancy and the postpartum period represent a time of increased risk of stroke, presenting challenges for stroke management. Recognition of these issues is critical to improving acute care and functional recovery after stroke in women.
Peripheral Arterial Disease in Women
Atherosclerotic lower extremity peripheral arterial disease (PAD) is now known to be associated with equal morbidity and mortality to CAD and stroke, and is associated with significantly reduced quality-of-life.199–201 Recent studies have shown a high prevalence of PAD in women,202 particularly women at the extremes of ages (> 80 years and < 40 years), who represent a greater estimated population burden of PAD.202 Intermittent claudication has been considered the hallmark feature of PAD, women may often be asymptomatic203, or present with atypical symptoms204. Non-invasive ankle-brachial index (ABI) can diagnose lower extremity PAD205 and AHA/ACC guidelines recommend screening for PAD in all adults > 65 years, or if there is a history of any tobacco use or DM, screening should commence earlier (at > 50 years). 206 An ABI < 0.90 is abnormal and indicates the presence of PAD. An ABI of 0.90–1.0 is borderline for PAD205, but represents an increased risk for CVD.207
Abdominal Aortic Aneurysms
Abdominal aortic aneurysms (AAAs) are four to six times more common in men than in women.208, 209 In addition, AAAs develop in women approximately 10 years later than in men.210 As with coronary heart disease, there is evidence that women with AAA also have a worse prognosis. Even in the absence of adjustment for AAA diameter, a meta-analysis showed that the annual risk of rupture of large AAA (≥5 cm in diameter) was 18% (95% CI, 8% to 26%) in women versus 12% (95% CI, 5% to 20%) in men.211
In a population-based study, it was reported that in the event of rupture, men were more likely to be treated with surgery than women (odds ratio, 1.4; 95% CI, 1.14 to 1.9). 212 Women with ruptured AAAs, irrespective of age, were less likely to be admitted to the hospital.213 Female sex was also an independent predictor (hazard ratio, 1.69; 95% CI, 1.28 to 2.22) of in-hospital death after surgery for ruptured AAA.214 As is the case for CAD, AAAs are underdiagnosed and undertreated in women. All clinicians need to be aware that although women are inherently less likely than men to develop an AAA, those who develop an AAA fare worse than men.
X. Conclusion
CVD continues to be the leading cause of death for women in the United States. The average lifetime risk of developing CVD in women at 50 years of age is about 40%, and this percentage rises as the number of risk factors increases. A focus on primary prevention of CVD is necessary to reduce CVD mortality and the overall CVD burden. Identifying and treating risk factors, including hypertension, dyslipidemia, diabetes, smoking, obesity and physical inactivity, has become a major focus of the AHA in order to accomplish this goal. Unfortunately, many of these risk factors are increasing in prevalence and severity, especially in young women. Further research into the mechanisms responsible for the observed sex differences in traditional risk factor effects would not only improve our understanding of the etiology of CVD, but could also inform health policy makers and clinical guideline committees in tailoring sex-specific interventions for the treatment and management of these risk factors. Moreover there are additional, female-specific risk factors (preterm delivery, hypertensive pregnancy disorders, gestational diabetes, menopausal transition) that can be identified during reproductive life that may improve current risk assessment strategies for primary prevention of CVD. However, considerable challenges remain in incorporating this information into current risk assessment tools.
Frequently unrecognized, and often undiagnosed CVD presentations that are either more prevalent in, or unique to women, include coronary microvascular dysfunction, spontaneous coronary artery dissection, stress-induced cardiomyopathy, and heart failure with preserved ejection fraction. There is yet much more to learn, and this requires sex- and gender-specific approaches to research, with appropriate representation of women in clinical cardiovascular trials. For many decades, CVD research has focused primarily on men, thus leading to an under-appreciation of sex differences from an etiological, diagnostic, and therapeutic perspective. As long as women are under-represented in clinical trials, we will continue to lack data to make accurate clinical decisions on 51% of the world’s population. Recent initiatives have raised awareness that CVD and its optimal management may differ between men and women. We encourage a new era in research, where cardiovascular studies are designed with adequate power for sex-specific analysis to understand mechanisms and develop optimal treatments for cardiovascular diseases in both sexes.
Supplementary Material
Acknowledgments
We graciously acknowledge the graphical assistance of Raul Reyeszumeta and Eduardo Garcia in preparation of the Figures.
Sources of Funding
This effort was supported by National Institute of Health grant HL34594 (JEM).
Non-standard Abbreviations and Acronyms
- ACS
Acute coronary syndromes
- ACC
American College of Cardiology
- AHA
American Heart Association
- ACE-I
Angiotensin converting enzyme inhibitors
- ARBs
Angiotensin receptor blockers
- ABI
Ankle-brachial index
- ASCVD
Atherosclerotic cardiovascular disease
- AF
Atrial fibrillation
- BRFSS
Behavioral Risk Factor Surveillance System
- BSO
Bilateral salpingo-oophorectomy
- CMR
Cardiac magnetic resonance
- CR
Cardiac rehabilitation
- CV
Cardiovascular
- CVD
Cardiovascular disease
- CIMT
Carotid intimal medial thickness
- CDC
Center for Disease Control and Prevention
- CKD
Chronic kidney disease
- CI
Confidence interval
- CAC
Coronary artery calcium
- CAD
Coronary artery disease
- CCTA
Coronary computed tomographic angiography
- CMD
Coronary microvascular dysfunction
- DM
Diabetes Mellitus
- ELITE
Early Versus Late Intervention Trial with Estradiol
- JNC 8
Eighth Joint National Committee
- ECG
Electrocardiogram
- EULAR
European League Against Rheumatism
- ETT
Exercise Treadmill Test
- GDM
Gestational diabetes mellitus
- HR
Hazard ratio
- HERS
Heart and Estrogen/Progestin Replacement Study
- IHD
Ischemic heart disease
- KEEPS
Kronos Early Estrogen Prevention Study
- LDL
Low-density lipoprotein
- MHT
Menopausal hormone therapy
- MI
Myocardial infarction
- NHIS
National Health Interview Survey
- PCI
Percutaneous coronary intervention
- PPCM
Peripartum cardiomyopathy
- PAD
Peripheral arterial disease
- PET
Positron emission tomography
- PTD
Preterm delivery
- PROMISE
Prospective Multicenter Imaging Study for Evaluation of Chest Pain
- RR
Relative risk
- RA
Rheumatoid arthritis
- ROMICAT
Rule Out Myocardial Infarction using Computer Assisted Tomography
- SPECT
Single-photon emission computed tomography
- STEMI
ST-segment-elevation myocardial infarction
- SLE
Systemic lupus erythematosus
- SBP
Systolic blood pressure
- T2DM
Type 2 DM
- USPSTF
United States Preventive Services Task Force
- VIRGO
Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients
- WHI
Women’s Health Initiative
- WISE
Women’s Ischemia Syndrome Evaluation
Footnotes
Disclosures
None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the united states from 1979 through 2011: Evidence for stagnation in young adults, especially women. Circulation. 2015;132:997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosca L, Mochari-Greenberger H, Dolor RJ, Newby LK, Robb KJ. Twelve-year follow-up of american women’s awareness of cardiovascular disease risk and barriers to heart health. Circ Cardiovasc Qual Outcomes. 2010;3:120–127. doi: 10.1161/CIRCOUTCOMES.109.915538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abuful A, Gidron Y, Henkin Y. Physicians’ attitudes toward preventive therapy for coronary artery disease: Is there a gender bias? Clin Cardiol. 2005;28:389–393. doi: 10.1002/clc.4960280809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosca L, Linfante AH, Benjamin EJ, Berra K, Hayes SN, Walsh BW, Fabunmi RP, Kwan J, Mills T, Simpson SL. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation. 2005;111:499–510. doi: 10.1161/01.CIR.0000154568.43333.82. [DOI] [PubMed] [Google Scholar]
- 6.Gu Q, Burt VL, Paulose-Ram R, Dillon CF. Gender differences in hypertension treatment, drug utilization patterns, and blood pressure control among us adults with hypertension: Data from the national health and nutrition examination survey 1999–2004. Am J Hypertens. 2008;21:789–798. doi: 10.1038/ajh.2008.185. [DOI] [PubMed] [Google Scholar]
- 7.Bird CE, Fremont AM, Bierman AS, Wickstrom S, Shah M, Rector T, Horstman T, Escarce JJ. Does quality of care for cardiovascular disease and diabetes differ by gender for enrollees in managed care plans? Womens Health Issues. 2007;17:131–138. doi: 10.1016/j.whi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Chou AF, Scholle SH, Weisman CS, Bierman AS, Correa-de-Araujo R, Mosca L. Gender disparities in the quality of cardiovascular disease care in private managed care plans. Womens Health Issues. 2007;17:120–130. doi: 10.1016/j.whi.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Witt BJ, Jacobsen SJ, Weston SA, Killian JM, Meverden RA, Allison TG, Reeder GS, Roger VL. Cardiac rehabilitation after myocardial infarction in the community. J Am Coll Cardiol. 2004;44:988–996. doi: 10.1016/j.jacc.2004.05.062. [DOI] [PubMed] [Google Scholar]
- 10.Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116:1653–1662. doi: 10.1161/CIRCULATIONAHA.107.701466. [DOI] [PubMed] [Google Scholar]
- 11.Thomas RJ, Miller NH, Lamendola C, Berra K, Hedback B, Durstine JL, Haskell W. National survey on gender differences in cardiac rehabilitation programs. Patient characteristics and enrollment patterns. J Cardiopulm Rehabil. 1996;16:402–412. doi: 10.1097/00008483-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Ghisi GL, Polyzotis P, Oh P, Pakosh M, Grace SL. Physician factors affecting cardiac rehabilitation referral and patient enrollment: A systematic review. Clin Cardiol. 2013;36:323–335. doi: 10.1002/clc.22126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smilowitz NR, Sampson BA, Abrecht CR, Siegfried JS, Hochman JS, Reynolds HR. Women have less severe and extensive coronary atherosclerosis in fatal cases of ischemic heart disease: An autopsy study. Am Heart J. 2011;161:681–688. doi: 10.1016/j.ahj.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Moriel M, Rozanski A, Klein J, Berman DS, Merz CN. The limited efficacy of exercise radionuclide ventriculography in assessing prognosis of women with coronary artery disease. Am J Cardiol. 1995;76:1030–1035. doi: 10.1016/s0002-9149(99)80290-2. [DOI] [PubMed] [Google Scholar]
- 15.Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G. Insights from the nhlbi-sponsored women’s ischemia syndrome evaluation (wise) study: Part i: Gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47:S4–S20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 16.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G. Insights from the nhlbi-sponsored women’s ischemia syndrome evaluation (wise) study: Part ii: Gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–29. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 17.Shaw LJ, Shaw RE, Merz CN, Brindis RG, Klein LW, Nallamothu B, Douglas PS, Krone RJ, McKay CR, Block PC, Hewitt K, Weintraub WS, Peterson ED. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the american college of cardiology-national cardiovascular data registry. Circulation. 2008;117:1787–1801. doi: 10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 18.von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: Results from the national heart, lung, and blood institute-sponsored women’s ischemia syndrome evaluation (wise) Circulation. 2004;109:722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 19.Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The atherosclerosis risk in communities study. JAMA. 2002;287:1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 20.Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97:2110–2116. doi: 10.1161/01.cir.97.21.2110. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GB, Feit F, Pena-Sing I, Axel L, Attubato MJ, Yatskar L, Kalhorn RT, Wood DA, Lobach IV, Hochman JS. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–1425. doi: 10.1161/CIRCULATIONAHA.111.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daly C, Clemens F, Lopez Sendon JL, Tavazzi L, Boersma E, Danchin N, Delahaye F, Gitt A, Julian D, Mulcahy D, Ruzyllo W, Thygesen K, Verheugt F, Fox KM. Gender differences in the management and clinical outcome of stable angina. Circulation. 2006;113:490–498. doi: 10.1161/CIRCULATIONAHA.105.561647. [DOI] [PubMed] [Google Scholar]
- 23.Vaccarino V, Parsons L, Peterson ED, Rogers WJ, Kiefe CI, Canto J. Sex differences in mortality after acute myocardial infarction: Changes from 1994 to 2006. Arch Intern Med. 2009;169:1767–1774. doi: 10.1001/archinternmed.2009.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jneid H, Fonarow GC, Cannon CP, Hernandez AF, Palacios IF, Maree AO, Wells Q, Bozkurt B, Labresh KA, Liang L, Hong Y, Newby LK, Fletcher G, Peterson E, Wexler L. Sex differences in medical care and early death after acute myocardial infarction. Circulation. 2008;118:2803–2810. doi: 10.1161/CIRCULATIONAHA.108.789800. [DOI] [PubMed] [Google Scholar]
- 25.Blomkalns AL, Chen AY, Hochman JS, Peterson ED, Trynosky K, Diercks DB, Brogan GX, Jr, Boden WE, Roe MT, Ohman EM, Gibler WB, Newby LK. Gender disparities in the diagnosis and treatment of non-st-segment elevation acute coronary syndromes: Large-scale observations from the crusade (can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the american college of cardiology/american heart association guidelines) national quality improvement initiative. J Am Coll Cardiol. 2005;45:832–837. doi: 10.1016/j.jacc.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 26.Center for disease control and prevention. National diabetes statistics report. Estimates of diabetes and its burden in the united states, 2014. Atlanta, GA: U.S Department of Health and Human Services; 2014. [Google Scholar]
- 27.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: A report from the american heart association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The rancho bernardo study. Jama. 1991;265:627–631. [PubMed] [Google Scholar]
- 29.Manson JE, Colditz GA, Stampfer MJ, et al. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Archives of internal medicine. 1991;151:1141–1147. [PubMed] [Google Scholar]
- 30.Juutilainen A, Kortelainen S, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care. 2004;27:2898–2904. doi: 10.2337/diacare.27.12.2898. [DOI] [PubMed] [Google Scholar]
- 31.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: Meta-analysis of 37 prospective cohort studies. Bmj. 2006;332:73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinberg HO, Paradisi G, Cronin J, Crowde K, Hempfling A, Hook G, Baron AD. Type ii diabetes abrogates sex differences in endothelial function in premenopausal women. Circulation. 2000;101:2040–2046. doi: 10.1161/01.cir.101.17.2040. [DOI] [PubMed] [Google Scholar]
- 33.Carr ME. Diabetes mellitus: A hypercoagulable state. Journal of diabetes and its complications. 2001;15:44–54. doi: 10.1016/s1056-8727(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 34.Pradhan AD. Sex differences in the metabolic syndrome: Implications for cardiovascular health in women. Clinical chemistry. 2014;60:44–52. doi: 10.1373/clinchem.2013.202549. [DOI] [PubMed] [Google Scholar]
- 35.Haffner SM, Miettinen H, Stern MP. Relatively more atherogenic coronary heart disease risk factors in prediabetic women than in prediabetic men. Diabetologia. 1997;40:711–717. doi: 10.1007/s001250050738. [DOI] [PubMed] [Google Scholar]
- 36.Donahue RP, Rejman K, Rafalson LB, Dmochowski J, Stranges S, Trevisan M. Sex differences in endothelial function markers before conversion to pre-diabetes: Does the clock start ticking earlier among women? The western new york study. Diabetes Care. 2007;30:354–359. doi: 10.2337/dc06-1772. [DOI] [PubMed] [Google Scholar]
- 37.Current cigarette smoking among u.S. Adults aged 18 years and older. 2012 [Google Scholar]
- 38.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: A systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378:1297–1305. doi: 10.1016/S0140-6736(11)60781-2. [DOI] [PubMed] [Google Scholar]
- 39.Lidegaard O. Smoking and use of oral contraceptives: Impact on thrombotic diseases. Am J Obstet Gynecol. 1999;180:S357–363. doi: 10.1016/s0002-9378(99)70696-4. [DOI] [PubMed] [Google Scholar]
- 40.Pomp ER, Rosendaal FR, Doggen CJ. Smoking increases the risk of venous thrombosis and acts synergistically with oral contraceptive use. American journal of hematology. 2008;83:97–102. doi: 10.1002/ajh.21059. [DOI] [PubMed] [Google Scholar]
- 41.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among us adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 42.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: The framingham experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 43.Wilson PW, Kannel WB, Silbershatz H, D’Agostino RB. Clustering of metabolic factors and coronary heart disease. Arch Intern Med. 1999;159:1104–1109. doi: 10.1001/archinte.159.10.1104. [DOI] [PubMed] [Google Scholar]
- 44.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiller JSLJ, Ward BW, Peregoy JA. Summary health statistics for u.S. Adults: National health interview survey 2010. 2012 [PubMed] [Google Scholar]
- 46.Sattelmair J, Pertman J, Ding EL, Kohl HW, 3rd, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: A meta-analysis. Circulation. 2011;124:789–795. doi: 10.1161/CIRCULATIONAHA.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shiroma EJ, Lee IM. Physical activity and cardiovascular health: Lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation. 2010;122:743–752. doi: 10.1161/CIRCULATIONAHA.109.914721. [DOI] [PubMed] [Google Scholar]
- 48.Giralt D, Domingues-Montanari S, Mendioroz M, Ortega L, Maisterra O, Perea-Gainza M, Delgado P, Rosell A, Montaner J. The gender gap in stroke: A meta-analysis. Acta Neurol Scand. 2012;125:83–90. doi: 10.1111/j.1600-0404.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- 49.Regitz-Zagrosek V, Oertelt-Prigione S, Prescott E, Franconi F, Gerdts E, Foryst-Ludwig A, Maas AH, Kautzky-Willer A, Knappe-Wegner D, Kintscher U, Ladwig KH, Schenck-Gustafsson K, Stangl V. Gender in cardiovascular diseases: Impact on clinical manifestations, management, and outcomes. Eur Heart J. 2016;37:24–34. doi: 10.1093/eurheartj/ehv598. [DOI] [PubMed] [Google Scholar]
- 50.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: Current outcomes and control in the community. JAMA. 2005;294:466–472. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- 51.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 52.Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the interheart study): Case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 54.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM., Jr Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of afcaps/texcaps. Air force/texas coronary atherosclerosis prevention study. Jama. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 55.Pedersen TR, Kjekshus J, Berg K, Haghfelt T, Faergeman O, Faergeman G, Pyorala K, Miettinen T, Wilhelmsen L, Olsson AG, Wedel H. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The scandinavian simvastatin survival study (4s). 1994. Atherosclerosis Supplements. 2004;5:81–87. doi: 10.1016/j.atherosclerosissup.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 56.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Tomaselli GF. 2013 acc/aha guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 57.Kavousi M, Leening MJ, Nanchen D, Greenland P, Graham IM, Steyerberg EW, Ikram MA, Stricker BH, Hofman A, Franco OH. Comparison of application of the acc/aha guidelines, adult treatment panel iii guidelines, and european society of cardiology guidelines for cardiovascular disease prevention in a european cohort. Jama. 2014;311:1416–1423. doi: 10.1001/jama.2014.2632. [DOI] [PubMed] [Google Scholar]
- 58.Mercado C, DeSimone AK, Odom E, Gillespie C, Ayala C, Loustalot F. Prevalence of cholesterol treatment eligibility and medication use among adults - united states, 2005–2012. MMWR. Morbidity and mortality weekly report. 2015;64:1305–1311. doi: 10.15585/mmwr.mm6447a1. [DOI] [PubMed] [Google Scholar]
- 59.Virani SS, Woodard LD, Ramsey DJ, Urech TH, Akeroyd JM, Shah T, Deswal A, Bozkurt B, Ballantyne CM, Petersen LA. Gender disparities in evidence-based statin therapy in patients with cardiovascular disease. Am J Cardiol. 2015;115:21–26. doi: 10.1016/j.amjcard.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 60.Safford MM, Gamboa CM, Durant RW, Brown TM, Glasser SP, Shikany JM, Zweifler RM, Howard G, Muntner P. Race-sex differences in the management of hyperlipidemia: The reasons for geographic and racial differences in stroke study. Am J Prev Med. 2015;48:520–527. doi: 10.1016/j.amepre.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parris ES, Lawrence DB, Mohn LA, Long LB. Adherence to statin therapy and ldl cholesterol goal attainment by patients with diabetes and dyslipidemia. Diabetes Care. 2005;28:595–599. doi: 10.2337/diacare.28.3.595. [DOI] [PubMed] [Google Scholar]
- 62.Aiman U, Najmi A, Khan RA. Statin induced diabetes and its clinical implications. Journal of pharmacology & pharmacotherapeutics. 2014;5:181–185. doi: 10.4103/0976-500X.136097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Persson M, Hedblad B, Nelson JJ, Berglund G. Elevated lp-pla2 levels add prognostic information to the metabolic syndrome on incidence of cardiovascular events among middle-aged nondiabetic subjects. Arterioscler Thromb Vasc Biol. 2007;27:1411–1416. doi: 10.1161/ATVBAHA.107.142679. [DOI] [PubMed] [Google Scholar]
- 64.Judd SE, Fang K, Safford M, Cushman M. Abstract 19268: Robust performance of lppla2 activity in predicting cardiovascular disease risk in a national, diverse, sample. Circulation. 2015;132:A19268. [Google Scholar]
- 65.Gotsch F, Gotsch F, Romero R, Erez O, Vaisbuch E, Kusanovic JP, Mazaki-Tovi S, Kim SK, Hassan S, Yeo L. The preterm parturition syndrome and its implications for understanding the biology, risk assessment, diagnosis, treatment and prevention of preterm birth. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2009;22(Suppl 2):5–23. doi: 10.1080/14767050902860690. [DOI] [PubMed] [Google Scholar]
- 66.Kessous R, Shoham-Vardi I, Pariente G, Holcberg G, Sheiner E. An association between preterm delivery and long-term maternal cardiovascular morbidity. Am J Obstet Gynecol. 2013;209:368, e361–368. doi: 10.1016/j.ajog.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 67.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. Bmj. 2007;335:1. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 69.Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008;31:899–904. doi: 10.2337/dc07-2345. [DOI] [PubMed] [Google Scholar]
- 70.Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl):S8–s16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 71.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 72.Vrachnis N, Augoulea A, Iliodromiti Z, Lambrinoudaki I, Sifakis S, Creatsas G. Previous gestational diabetes mellitus and markers of cardiovascular risk. Int J Endocrinol. 2012;2012:458610. doi: 10.1155/2012/458610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Linne Y, Dye L, Barkeling B, Rossner S. Long-term weight development in women: A 15-year follow-up of the effects of pregnancy. Obesity research. 2004;12:1166–1178. doi: 10.1038/oby.2004.146. [DOI] [PubMed] [Google Scholar]
- 74.Kew S, Ye C, Hanley AJ, Connelly PW, Sermer M, Zinman B, Retnakaran R. Cardiometabolic implications of postpartum weight changes in the first year after delivery. Diabetes Care. 2014;37:1998–2006. doi: 10.2337/dc14-0087. [DOI] [PubMed] [Google Scholar]
- 75.del Rincon I, Polak JF, O’Leary DH, Battafarano DF, Erikson JM, Restrepo JF, Molina E, Escalante A. Systemic inflammation and cardiovascular risk factors predict rapid progression of atherosclerosis in rheumatoid arthritis. Ann Rheum Dis. 2015;74:1118–1123. doi: 10.1136/annrheumdis-2013-205058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gianturco L, Bodini BD, Atzeni F, Colombo C, Stella D, Sarzi-Puttini P, Drago L, Galaverna S, Turiel M. Cardiovascular and autoimmune diseases in females: The role of microvasculature and dysfunctional endothelium. Atherosclerosis. 2015;241:259–263. doi: 10.1016/j.atherosclerosis.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 77.Zhang J, Chen L, Delzell E, Muntner P, Hillegass WB, Safford MM, Millan IY, Crowson CS, Curtis JR. The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis. 2014;73:1301–1308. doi: 10.1136/annrheumdis-2013-204715. [DOI] [PubMed] [Google Scholar]
- 78.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, Cote R, Grover SA, Fortin PR, Clarke AE, Senecal JL. Traditional framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 79.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr, Jansen-McWilliams L, D’Agostino RB, Kuller LH. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: Comparison with the framingham study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 80.Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, McInnes IB, Haentzschel H, Gonzalez-Gay MA, Provan S, Semb A, Sidiropoulos P, Kitas G, Smulders YM, Soubrier M, Szekanecz Z, Sattar N, Nurmohamed MT. Eular evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–331. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 81.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen M-B, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. New England Journal of Medicine. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 82.Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: Cardio-oncology. Mayo Clin Proc. 2014;89:1287–1306. doi: 10.1016/j.mayocp.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lancellotti P, Nkomo VT, Badano LP, Bergler-Klein J, Bogaert J, Davin L, Cosyns B, Coucke P, Dulgheru R, Edvardsen T, Gaemperli O, Galderisi M, Griffin B, Heidenreich PA, Nieman K, Plana JC, Port SC, Scherrer-Crosbie M, Schwartz RG, Sebag IA, Voigt JU, Wann S, Yang PC. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: A report from the european association of cardiovascular imaging and the american society of echocardiography. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2013;26:1013–1032. doi: 10.1016/j.echo.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 84.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: Epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 85.Shah AJ, Veledar E, Hong Y, Bremner JD, Vaccarino V. Depression and history of attempted suicide as risk factors for heart disease mortality in young individuals. Arch Gen Psychiatry. 2011;68:1135–1142. doi: 10.1001/archgenpsychiatry.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nabi H, Hall M, Koskenvuo M, Singh-Manoux A, Oksanen T, Suominen S, Kivimaki M, Vahtera J. Psychological and somatic symptoms of anxiety and risk of coronary heart disease: The health and social support prospective cohort study. Biol Psychiatry. 2010;67:378–385. doi: 10.1016/j.biopsych.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Korkeila J, Vahtera J, Korkeila K, Kivimaki M, Sumanen M, Koskenvuo K, Koskenvuo M. Childhood adversities as predictors of incident coronary heart disease and cerebrovascular disease. Heart. 2010;96:298–303. doi: 10.1136/hrt.2009.188250. [DOI] [PubMed] [Google Scholar]
- 88.Wyman L, Crum RM, Celentano D. Depressed mood and cause-specific mortality: A 40-year general community assessment. Ann Epidemiol. 2012;22:638–643. doi: 10.1016/j.annepidem.2012.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the u.S. From 1980 through 2002: Concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50:2128–2132. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 90.Lloyd-Jones DM, Leip EP, Larson MG, D’Agostino RB, Beiser A, Wilson PW, Wolf PA, Levy D. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–798. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 91.Kim ES, Carrigan TP, Menon V. Enrollment of women in national heart, lung, and blood institute-funded cardiovascular randomized controlled trials fails to meet current federal mandates for inclusion. J Am Coll Cardiol. 2008;52:672–673. doi: 10.1016/j.jacc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 92.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of dsm-iv disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 93.Mallik S, Spertus JA, Reid KJ, Krumholz HM, Rumsfeld JS, Weintraub WS, Agarwal P, Santra M, Bidyasar S, Lichtman JH, Wenger NK, Vaccarino V. Depressive symptoms after acute myocardial infarction: Evidence for highest rates in younger women. Arch Intern Med. 2006;166:876–883. doi: 10.1001/archinte.166.8.876. [DOI] [PubMed] [Google Scholar]
- 94.Champney KP, Frederick PD, Bueno H, Parashar S, Foody J, Merz CN, Canto JG, Lichtman JH, Vaccarino V. The joint contribution of sex, age and type of myocardial infarction on hospital mortality following acute myocardial infarction. Heart. 2009;95:895–899. doi: 10.1136/hrt.2008.155804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen therapy and cardiovascular disease. New England Journal of Medicine. 1991;325:756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 96.Grodstein F, Stampfer M. The epidemiology of coronary heart disease and estrogen replacement in postmenopausal women. Prog Cardiovasc Dis. 1995;38:199–210. doi: 10.1016/s0033-0620(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 97.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 98.Wolf PH, Madans JH, Finucane FF, Higgins M, Kleinman JC. Reduction of cardiovascular disease-related mortality among postmenopausal women who use hormones: Evidence from a national cohort. Am J Obstet Gynecol. 1991;164:489–494. doi: 10.1016/s0002-9378(11)80006-2. [DOI] [PubMed] [Google Scholar]
- 99.Henderson BE, Paganini-Hill A, Ross RK. Decreased mortality in users of estrogen replacement therapy. Arch Intern Med. 1991;151:75–78. [PubMed] [Google Scholar]
- 100.Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. Jama. 1991;265:1861–1867. [PubMed] [Google Scholar]
- 101.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and estrogen/progestin replacement study (hers) research group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 102.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women’s health initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 103.Chandrasekar B, Nattel S, Tanguay JF. Coronary artery endothelial protection after local delivery of 17beta-estradiol during balloon angioplasty in a porcine model: A potential new pharmacologic approach to improve endothelial function. J Am Coll Cardiol. 2001;38:1570–1576. doi: 10.1016/s0735-1097(01)01552-2. [DOI] [PubMed] [Google Scholar]
- 104.Mori T, Durand J, Chen Y, Thompson JA, Bakir S, Oparil S. Effects of short-term estrogen treatment on the neointimal response to balloon injury of rat carotid artery. Am J Cardiol. 2000;85:1276–1279. doi: 10.1016/s0002-9149(00)00748-7. [DOI] [PubMed] [Google Scholar]
- 105.Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, Pettinger M, Heckbert SR, Greep N, Crawford S, Eaton CB, Kostis JB, Caralis P, Prentice R. Conjugated equine estrogens and coronary heart disease: The women’s health initiative. Arch Intern Med. 2006;166:357–365. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 106.Salpeter SR, Walsh JM, Greyber E, Salpeter EE. Brief report: Coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. J Gen Intern Med. 2006;21:363–366. doi: 10.1111/j.1525-1497.2006.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, Hopkins PN, Lobo RA, Manson JE, Merriam GR, Miller VM, Neal-Perry G, Santoro N, Taylor HS, Vittinghoff E, Yan M, Hodis HN. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal womena randomized trialcardiovascular disease and menopausal hormone therapy. Annals of Internal Medicine. 2014;161:249–260. doi: 10.7326/M14-0353. [DOI] [PubMed] [Google Scholar]
- 108.Hodis HN, Mack WJ, Shoupe D, Azen SP, Stanczyk FZ, Hwang-Levine J, Budoff MJ, Henderson VW. Methods and baseline cardiovascular data from the early versus late intervention trial with estradiol testing the menopausal hormone timing hypothesis. Menopause. 2015;22:391–401. doi: 10.1097/GME.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, Santen RJ. Treatment of symptoms of the menopause: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975–4011. doi: 10.1210/jc.2015-2236. [DOI] [PubMed] [Google Scholar]
- 110.Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126:859–876. doi: 10.1097/AOG.0000000000001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chai X, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, Garber JE, Neuhausen SL, Matloff E, Eeles R, Tung N, Weitzel JN, Couch FJ, Hulick PJ, Ganz PA, Daly MB, Olopade OI, Tomlinson G, Blum JL, Domchek SM, Chen J, Rebbeck TR. Use of risk-reducing surgeries in a prospective cohort of 1,499 brca1 and brca2 mutation carriers. Breast cancer research and treatment. 2014;148:397–406. doi: 10.1007/s10549-014-3134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tuppurainen M, Kroger H, Honkanen R, Puntila E, Huopio J, Saarikoski S, Alhava E. Risks of perimenopausal fractures--a prospective population-based study. Acta Obstet Gynecol Scand. 1995;74:624–628. doi: 10.3109/00016349509013475. [DOI] [PubMed] [Google Scholar]
- 113.Rivera CM, Grossardt BR, Rhodes DJ, Brown RD, Jr, Roger VL, Melton LJ, 3rd, Rocca WA. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15–23. doi: 10.1097/gme.0b013e31818888f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, Shoupe D, Berek JS, Hankinson S, Manson JE. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol. 2009;113:1027–1037. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ., 3rd Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 116.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 117.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: A meta-analysis. Menopause. 2006;13:265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 118.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC, Jr, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: A guideline from the american heart association. Circulation. 2011;123:1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu FB, Stampfer MJ, Manson JE, Grodstein F, Colditz GA, Speizer FE, Willett WC. Trends in the incidence of coronary heart disease and changes in diet and lifestyle in women. N Engl J Med. 2000;343:530–537. doi: 10.1056/NEJM200008243430802. [DOI] [PubMed] [Google Scholar]
- 120.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. New England Journal of Medicine. 2000;343:16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 121.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 122.Final report on the aspirin component of the ongoing physicians’ health study. Steering committee of the physicians’ health study research group. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 123.Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: A meta-analysis. J Gen Intern Med. 2011;26:1336–1344. doi: 10.1007/s11606-011-1757-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang C, Sun A, Zhang P, Wu C, Zhang S, Fu M, Wang K, Zou Y, Ge J. Aspirin for primary prevention of cardiovascular events in patients with diabetes: A meta-analysis. Diabetes research and clinical practice. 2010;87:211–218. doi: 10.1016/j.diabres.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 125.Younis N, Williams S, Ammori B, Soran H. Role of aspirin in the primary prevention of cardiovascular disease in diabetes mellitus: A meta-analysis. Expert opinion on pharmacotherapy. 2010;11:1459–1466. doi: 10.1517/14656561003792538. [DOI] [PubMed] [Google Scholar]
- 126.De Berardis G, Sacco M, Strippoli GF, Pellegrini F, Graziano G, Tognoni G, Nicolucci A. Aspirin for primary prevention of cardiovascular events in people with diabetes: Meta-analysis of randomised controlled trials. Bmj. 2009;339:b4531. doi: 10.1136/bmj.b4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott R, Lee R, Bancroft J, MacEwan S, Shepherd J, Macfarlane P, Morris A, Jung R, Kelly C, Connacher A, Peden N, Jamieson A, Matthews D, Leese G, McKnight J, O’Brien I, Semple C, Petrie J, Gordon D, Pringle S, MacWalter R. The prevention of progression of arterial disease and diabetes (popadad) trial: Factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. Bmj. 2008;337:a1840. doi: 10.1136/bmj.a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, Jinnouchi H, Sugiyama S, Saito Y. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: A randomized controlled trial. Jama. 2008;300:2134–2141. doi: 10.1001/jama.2008.623. [DOI] [PubMed] [Google Scholar]
- 129.Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early treatment diabetic retinopathy study report 14. Etdrs investigators. Jama. 1992;268:1292–1300. doi: 10.1001/jama.1992.03490100090033. [DOI] [PubMed] [Google Scholar]
- 130.Pignone M, Alberts MJ, Colwell JA, Cushman M, Inzucchi SE, Mukherjee D, Rosenson RS, Williams CD, Wilson PW, Kirkman MS. Aspirin for primary prevention of cardiovascular events in people with diabetes: A position statement of the american diabetes association, a scientific statement of the american heart association, and an expert consensus document of the american college of cardiology foundation. Diabetes Care. 2010;33:1395–1402. doi: 10.2337/dc10-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Walsh JM, Pignone M. Drug treatment of hyperlipidemia in women. JAMA. 2004;291:2243–2252. doi: 10.1001/jama.291.18.2243. [DOI] [PubMed] [Google Scholar]
- 132.Abramson J, Wright JM. Are lipid-lowering guidelines evidence-based? Lancet. 2007;369:168–169. doi: 10.1016/S0140-6736(07)60084-1. [DOI] [PubMed] [Google Scholar]
- 133.Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A. Efficacy and safety of ldl-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 134.Lopez-Jimenez F, Simha V, Thomas RJ, Allison TG, Basu A, Fernandes R, Hurst RT, Kopecky SL, Kullo IJ, Mulvagh SL, Thompson WG, Trejo-Gutierrez JF, Wright RS. A summary and critical assessment of the 2013 acc/aha guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: Filling the gaps. Mayo Clin Proc. 2014;89:1257–1278. doi: 10.1016/j.mayocp.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 135.Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity c-reactive protein or dyslipidemia: Results from the justification for the use of statins in prevention: An intervention trial evaluating rosuvastatin (jupiter) and meta-analysis of women from primary prevention trials. Circulation. 2010;121:1069–1077. doi: 10.1161/CIRCULATIONAHA.109.906479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Culver AL, Ockene IS, Balasubramanian R, Olendzki BC, Sepavich DM, Wactawski-Wende J, Manson JE, Qiao Y, Liu S, Merriam PA, Rahilly-Tierny C, Thomas F, Berger JS, Ockene JK, Curb JD, Ma Y. Statin use and risk of diabetes mellitus in postmenopausal women in the women’s health initiative. Arch Intern Med. 2012;172:144–152. doi: 10.1001/archinternmed.2011.625. [DOI] [PubMed] [Google Scholar]
- 137.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 138.Shaw LJ, Bugiardini R, Merz CNB. Women and ischemic heart diseaseevolving knowledge. Journal of the American College of Cardiology. 2009;54:1561–1575. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brewer LC, Svatikova A, Mulvagh SL. The challenges of prevention, diagnosis and treatment of ischemic heart disease in women. Cardiovasc Drugs Ther. 2015;29:355–368. doi: 10.1007/s10557-015-6607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Merz CN. The yentl syndrome is alive and well. Eur Heart J. 2011;32:1313–1315. doi: 10.1093/eurheartj/ehr083. [DOI] [PubMed] [Google Scholar]
- 141.Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: Results from the nhlbi wise study. Am Heart J. 2001;141:735–741. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 142.Agrawal S, Van Eyk J, Sobhani K, Wei J, Bairey Merz CN. Sex, myocardial infarction, and the failure of risk scores in women. J Womens Health (Larchmt) 2015 doi: 10.1089/jwh.2015.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shah AS, Griffiths M, Lee KK, McAllister DA, Hunter AL, Ferry AV, Cruikshank A, Reid A, Stoddart M, Strachan F, Walker S, Collinson PO, Apple FS, Gray AJ, Fox KA, Newby DE, Mills NL. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: Prospective cohort study. Bmj. 2015;350:g7873. doi: 10.1136/bmj.g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Melander O, Maisel AS, Almgren P, et al. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA. 2012;308:1469–1475. doi: 10.1001/jama.2012.12998. [DOI] [PubMed] [Google Scholar]
- 145.Canto JG, Rogers WJ, Goldberg RJ, Peterson ED, Wenger NK, Vaccarino V, Kiefe CI, Frederick PD, Sopko G, Zheng ZJ. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA. 2012;307:813–822. doi: 10.1001/jama.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mieres JH, Heller GV, Hendel RC, Gulati M, Boden WE, Katten D, Shaw LJ. Signs and symptoms of suspected myocardial ischemia in women: Results from the what is the optimal method for ischemia evaluation in women? Trial. J Womens Health (Larchmt) 2011;20:1261–1268. doi: 10.1089/jwh.2010.2595. [DOI] [PubMed] [Google Scholar]
- 147.Eastwood JA, Johnson BD, Rutledge T, Bittner V, Whittaker KS, Krantz DS, Cornell CE, Eteiba W, Handberg E, Vido D, Bairey Merz CN. Anginal symptoms, coronary artery disease, and adverse outcomes in black and white women: The nhlbi-sponsored women’s ischemia syndrome evaluation (wise) study. J Womens Health (Larchmt) 2013;22:724–732. doi: 10.1089/jwh.2012.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bittencourt MS, Hulten E, Ghoshhajra B, O’Leary D, Christman MP, Montana P, Truong QA, Steigner M, Murthy VL, Rybicki FJ, Nasir K, Gowdak LH, Hainer J, Brady TJ, Di Carli MF, Hoffmann U, Abbara S, Blankstein R. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7:282–291. doi: 10.1161/CIRCIMAGING.113.001047. [DOI] [PubMed] [Google Scholar]
- 149.Gehrie ER, Reynolds HR, Chen AY, Neelon BH, Roe MT, Gibler WB, Ohman EM, Newby LK, Peterson ED, Hochman JS. Characterization and outcomes of women and men with non-st-segment elevation myocardial infarction and nonobstructive coronary artery disease: Results from the can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the acc/aha guidelines (crusade) quality improvement initiative. Am Heart J. 2009;158:688–694. doi: 10.1016/j.ahj.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 150.Beltrame JF. Assessing patients with myocardial infarction and nonobstructed coronary arteries (minoca) Journal of internal medicine. 2013;273:182–185. doi: 10.1111/j.1365-2796.2012.02591.x. [DOI] [PubMed] [Google Scholar]
- 151.Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861–870. doi: 10.1161/CIRCULATIONAHA.114.011201. [DOI] [PubMed] [Google Scholar]
- 152.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National registry of myocardial infarction 2 participants. N Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 153.D’Onofrio G, Safdar B, Lichtman JH, Strait KM, Dreyer RP, Geda M, Spertus JA, Krumholz HM. Sex differences in reperfusion in young patients with st-segment-elevation myocardial infarction: Results from the virgo study. Circulation. 2015;131:1324–1332. doi: 10.1161/CIRCULATIONAHA.114.012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ, Merz CN. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: A report from the women’s ischemia syndrome evaluation study and the st james women take heart project. Arch Intern Med. 2009;169:843–850. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the national heart, lung and blood institute wise (women’s ischemia syndrome evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, Azarbal B, Petersen J, Sharaf B, Handberg E, Shufelt C, Kothawade K, Sopko G, Lerman A, Shaw L, Kelsey SF, Pepine CJ, Merz CN. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: Results from the nhlbi-sponsored wise (women’s ischemia syndrome evaluation) study. JACC Cardiovasc Interv. 2012;5:646–653. doi: 10.1016/j.jcin.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Herrmann J, Kaski JC, Lerman A. Coronary microvascular dysfunction in the clinical setting: From mystery to reality. Eur Heart J. 2012;33:2771–2782b. doi: 10.1093/eurheartj/ehs246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol. 2008;51:997–1002. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 159.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 160.Sedlak TL, Lee M, Izadnegahdar M, Merz CN, Gao M, Humphries KH. Sex differences in clinical outcomes in patients with stable angina and no obstructive coronary artery disease. Am Heart J. 2013;166:38–44. doi: 10.1016/j.ahj.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 161.Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: An update. Eur Heart J. 2014;35:1101–1111. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, Gersh BJ, Khambatta S, Best PJ, Rihal CS, Gulati R. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579–588. doi: 10.1161/CIRCULATIONAHA.112.105718. [DOI] [PubMed] [Google Scholar]
- 163.Hayes SN. Spontaneous coronary artery dissection (scad): New insights into this not-so-rare condition. Tex Heart Inst J. 2014;41:295–298. doi: 10.14503/THIJ-14-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tweet MS, Gulati R, Aase LA, Hayes SN. Spontaneous coronary artery dissection: A disease-specific, social networking community-initiated study. Mayo Clin Proc. 2011;86:845–850. doi: 10.4065/mcp.2011.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Goel K, Tweet M, Olson TM, Maleszewski JJ, Gulati R, Hayes SN. Familial spontaneous coronary artery dissection: Evidence for genetic susceptibility. JAMA Intern Med. 2015;175:821–826. doi: 10.1001/jamainternmed.2014.8307. [DOI] [PubMed] [Google Scholar]
- 166.Alfonso F, Paulo M, Lennie V, Dutary J, Bernardo E, Jimenez-Quevedo P, Gonzalo N, Escaned J, Banuelos C, Perez-Vizcayno MJ, Hernandez R, Macaya C. Spontaneous coronary artery dissection: Long-term follow-up of a large series of patients prospectively managed with a “conservative” therapeutic strategy. JACC Cardiovasc Interv. 2012;5:1062–1070. doi: 10.1016/j.jcin.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 167.Silber TC, Tweet MS, Bowman MJ, Hayes SN, Squires RW. Cardiac rehabilitation after spontaneous coronary artery dissection. J Cardiopulm Rehabil Prev. 2015 doi: 10.1097/HCR.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 168.Medeiros K, O’Connor MJ, Baicu CF, Fitzgibbons TP, Shaw P, Tighe DA, Zile MR, Aurigemma GP. Systolic and diastolic mechanics in stress cardiomyopathy. Circulation. 2014;129:1659–1667. doi: 10.1161/CIRCULATIONAHA.113.002781. [DOI] [PubMed] [Google Scholar]
- 169.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss H-P, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck K-H, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KEJ, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. New England Journal of Medicine. 2015;373:929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 170.Citro R, Rigo F, D’Andrea A, Ciampi Q, Parodi G, Provenza G, Piccolo R, Mirra M, Zito C, Giudice R, Patella MM, Antonini-Canterin F, Bossone E, Piscione F, Salerno-Uriarte J. Echocardiographic correlates of acute heart failure, cardiogenic shock, and in-hospital mortality in tako-tsubo cardiomyopathy. JACC Cardiovasc Imaging. 2014;7:119–129. doi: 10.1016/j.jcmg.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 171.Previtali M, Repetto A, Camporotondo R, Citro R, Faggiano P, Bovelli D, Baldini E, Pasquetto G, Ascione L, Vignali L, Rosso R, Baralis G, Rossi ML, Ferlini M, Bossone E, Panciroli C, Rovere FD, Visconti LO, Klersy C. Clinical characteristics and outcome of left ventricular ballooning syndrome in a european population. Am J Cardiol. 2011;107:120–125. doi: 10.1016/j.amjcard.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 172.Sharkey SW, Windenburg DC, Lesser JR, Maron MS, Hauser RG, Lesser JN, Haas TS, Hodges JS, Maron BJ. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol. 2010;55:333–341. doi: 10.1016/j.jacc.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 173.Kyuma M, Tsuchihashi K, Shinshi Y, Hase M, Nakata T, Ooiwa H, Abiru M, Hikita N, Adachi T, Shoji T, Fujise Y, Shimamoto K. Effect of intravenous propranolol on left ventricular apical ballooning without coronary artery stenosis (ampulla cardiomyopathy): Three cases. Circ J. 2002;66:1181–1184. doi: 10.1253/circj.66.1181. [DOI] [PubMed] [Google Scholar]
- 174.O’Donoghue M, Boden WE, Braunwald E, Cannon CP, Clayton TC, de Winter RJ, Fox KA, Lagerqvist B, McCullough PA, Murphy SA, Spacek R, Swahn E, Wallentin L, Windhausen F, Sabatine MS. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-st-segment elevation myocardial infarction: A meta-analysis. JAMA. 2008;300:71–80. doi: 10.1001/jama.300.1.71. [DOI] [PubMed] [Google Scholar]
- 175.Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, Van de Werf F, Aylward P, Topol EJ, Califf RM. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global use of strategies to open occluded coronary arteries in acute coronary syndromes iib investigators. N Engl J Med. 1999;341:226–232. doi: 10.1056/NEJM199907223410402. [DOI] [PubMed] [Google Scholar]
- 176.Rao SV, Hess CN, Barham B, Aberle LH, Anstrom KJ, Patel TB, Jorgensen JP, Mazzaferri EL, Jr, Jolly SS, Jacobs A, Newby LK, Gibson CM, Kong DF, Mehran R, Waksman R, Gilchrist IC, McCourt BJ, Messenger JC, Peterson ED, Harrington RA, Krucoff MW. A registry-based randomized trial comparing radial and femoral approaches in women undergoing percutaneous coronary intervention: The safe-pci for women (study of access site for enhancement of pci for women) trial. JACC Cardiovasc Interv. 2014;7:857–867. doi: 10.1016/j.jcin.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 177.Mieres JH, Gulati M, Bairey Merz N, Berman DS, Gerber TC, Hayes SN, Kramer CM, Min JK, Newby LK, Nixon JV, Srichai MB, Pellikka PA, Redberg RF, Wenger NK, Shaw LJ. Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: A consensus statement from the american heart association. Circulation. 2014;130:350–379. doi: 10.1161/CIR.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 178.Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–1300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, Truong QA, Cury RC, Abbara S, Shapiro MD, Moloo J, Butler J, Ferencik M, Lee H, Jang IK, Parry BA, Brown DF, Udelson JE, Achenbach S, Brady TJ, Nagurney JT. Coronary computed tomography angiography for early triage of patients with acute chest pain: The romicat (rule out myocardial infarction using computer assisted tomography) trial. J Am Coll Cardiol. 2009;53:1642–1650. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Truong QA, Hayden D, Woodard PK, Kirby R, Chou ET, Nagurney JT, Wiviott SD, Fleg JL, Schoenfeld DA, Udelson JE, Hoffmann U. Sex differences in the effectiveness of early coronary computed tomographic angiography compared with standard emergency department evaluation for acute chest pain: The rule-out myocardial infarction with computer-assisted tomography (romicat)-ii trial. Circulation. 2013;127:2494–2502. doi: 10.1161/CIRCULATIONAHA.113.001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: Pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 183.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 184.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 accf/aha guideline for the management of heart failurea report of the american college of cardiology foundation/american heart association task force on practice guidelines. Journal of the American College of Cardiology. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 186.Elkayam U, Akhter MW, Singh H, Khan S, Bitar F, Hameed A, Shotan A. Pregnancy-associated cardiomyopathy: Clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111:2050–2055. doi: 10.1161/01.CIR.0000162478.36652.7E. [DOI] [PubMed] [Google Scholar]
- 187.Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, Regitz-Zagrosek V, Schaufelberger M, Tavazzi L, van Veldhuisen DJ, Watkins H, Shah AJ, Seferovic PM, Elkayam U, Pankuweit S, Papp Z, Mouquet F, McMurray JJ. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: A position statement from the heart failure association of the european society of cardiology working group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12:767–778. doi: 10.1093/eurjhf/hfq120. [DOI] [PubMed] [Google Scholar]
- 188.Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the united statesdiagnosis, prognosis, and management. Journal of the American College of Cardiology. 2011;58:659–670. doi: 10.1016/j.jacc.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 189.Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke-Baerwolf C, Gorenek B, Iung B, Kirby M, Maas AH, Morais J, Nihoyannopoulos P, Pieper PG, Presbitero P, Roos-Hesselink JW, Schaufelberger M, Seeland U, Torracca L. Esc guidelines on the management of cardiovascular diseases during pregnancy: The task force on the management of cardiovascular diseases during pregnancy of the european society of cardiology (esc) Eur Heart J. 2011;32:3147–3197. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 190.McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, Modi K, Alexis JD, Ramani GV, Semigran MJ, Haythe J, Markham DW, Marek J, Gorcsan IIIJ, Wu W-C, Lin Y, Halder I, Pisarcik J, Cooper LT, Fett JD. Clinical outcomes for peripartum cardiomyopathy in north americaresults of the ipac study (investigations of pregnancy-associated cardiomyopathy) Journal of the American College of Cardiology. 2015;66:905–914. doi: 10.1016/j.jacc.2015.06.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tomasulo P. Lactmed-new nlm database on drugs and lactation. Med Ref Serv Q. 2007;26:51–58. doi: 10.1300/J115v26S01_04. [DOI] [PubMed] [Google Scholar]
- 192.Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, Thompson DR, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011:CD001800. doi: 10.1002/14651858.CD001800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren WM, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte Op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F. european guidelines on cardiovascular disease prevention in clinical practice (version 2012). The fifth joint task force of the european society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) G Ital Cardiol (Rome) 2013;14:328–392. doi: 10.1714/1264.13964. [DOI] [PubMed] [Google Scholar]
- 194.Samayoa L, Grace SL, Gravely S, Scott LB, Marzolini S, Colella TJ. Sex differences in cardiac rehabilitation enrollment: A meta-analysis. Can J Cardiol. 2014;30:793–800. doi: 10.1016/j.cjca.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 195.McCarthy MM, Vaughan Dickson V, Chyun D. Barriers to cardiac rehabilitation in women with cardiovascular disease: An integrative review. J Cardiovasc Nurs. 2011;26:E1–E10. doi: 10.1097/JCN.0b013e3181f877e9. [DOI] [PubMed] [Google Scholar]
- 196.Poli D, Antonucci E. Epidemiology, diagnosis, and management of atrial fibrillation in women. Int J Womens Health. 2015;7:605–614. doi: 10.2147/IJWH.S45925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M, Odutayo AA. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: Systematic review and meta-analysis of cohort studies. Bmj. 2016;532:h7013. doi: 10.1136/bmj.h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, Howard VJ, Lichtman JH, Lisabeth LD, Pina IL, Reeves MJ, Rexrode KM, Saposnik G, Singh V, Towfighi A, Vaccarino V, Walters MR. Guidelines for the prevention of stroke in women: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2014;45:1545–1588. doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.McDermott MM, Ferrucci L, Guralnik JM, Dyer AR, Liu K, Pearce WH, Clark E, Liao Y, Criqui MH. The ankle-brachial index is associated with the magnitude of impaired walking endurance among men and women with peripheral arterial disease. Vasc Med. 2010;15:251–257. doi: 10.1177/1358863X10365181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.McDermott MM, Greenland P, Liu K, Criqui MH, Guralnik JM, Celic L, Chan C. Sex differences in peripheral arterial disease: Leg symptoms and physical functioning. J Am Geriatr Soc. 2003;51:222–228. doi: 10.1046/j.1532-5415.2003.51061.x. [DOI] [PubMed] [Google Scholar]
- 201.McDermott MM, Feinglass J, Slavensky R, Pearce WH. The ankle-brachial index as a predictor of survival in patients with peripheral vascular disease. J Gen Intern Med. 1994;9:445–449. doi: 10.1007/BF02599061. [DOI] [PubMed] [Google Scholar]
- 202.Hirsch AT, Allison MA, Gomes AS, Corriere MA, Duval S, Ershow AG, Hiatt WR, Karas RH, Lovell MB, McDermott MM, Mendes DM, Nussmeier NA, Treat-Jacobson D. A call to action: Women and peripheral artery disease: A scientific statement from the american heart association. Circulation. 2012;125:1449–1472. doi: 10.1161/CIR.0b013e31824c39ba. [DOI] [PubMed] [Google Scholar]
- 203.McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: The women’s health and aging study. Circulation. 2000;101:1007–1012. doi: 10.1161/01.cir.101.9.1007. [DOI] [PubMed] [Google Scholar]
- 204.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D, Martin GJ. Leg symptoms in peripheral arterial disease: Associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 205.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jonsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D American Heart Association Council on Peripheral Vascular D Council on E and Prevention, Council on Clinical C, Council on Cardiovascular N, Council on Cardiovascular R Intervention Council on Cardiovascular S Anesthesia. Measurement and interpretation of the ankle-brachial index: A scientific statement from the american heart association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 206.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. Acc/aha 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the american association for vascular surgery/society for vascular surgery, society for cardiovascular angiography and interventions, society for vascular medicine and biology, society of interventional radiology, and the acc/aha task force on practice guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease): Endorsed by the american association of cardiovascular and pulmonary rehabilitation; national heart, lung, and blood institute; society for vascular nursing; transatlantic inter-society consensus; and vascular disease foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 207.Hirsch AT, Allison MA, Gomes AS, Corriere MA, Duval S, Ershow AG, Hiatt WR, Karas RH, Lovell MB, McDermott MM, Mendes DM, Nussmeier NA, Treat-Jacobson D American Heart Association Council on Peripheral Vascular D, Council on Cardiovascular N, Council on Cardiovascular R, Intervention Council on Cardiovascular S, Anesthesia Council on Clinical C, Council on E and Prevention. A call to action: Women and peripheral artery disease: A scientific statement from the american heart association. Circulation. 2012;125:1449–1472. doi: 10.1161/CIR.0b013e31824c39ba. [DOI] [PubMed] [Google Scholar]
- 208.Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg. 1995;82:1066–1070. doi: 10.1002/bjs.1800820821. [DOI] [PubMed] [Google Scholar]
- 209.Lederle FA, Johnson GR, Wilson SE. Abdominal aortic aneurysm in women. J Vasc Surg. 2001;34:122–126. doi: 10.1067/mva.2001.115275. [DOI] [PubMed] [Google Scholar]
- 210.McFarlane MJ. The epidemiologic necropsy for abdominal aortic aneurysm. JAMA. 1991;265:2085–2088. [PubMed] [Google Scholar]
- 211.Screening for abdominal aortic aneurysm: Recommendation statement. Ann Intern Med. 2005;142:198–202. doi: 10.7326/0003-4819-142-3-200502010-00011. [DOI] [PubMed] [Google Scholar]
- 212.Katz DJ, Stanley JC, Zelenock GB. Gender differences in abdominal aortic aneurysm prevalence, treatment, and outcome. J Vasc Surg. 1997;25:561–568. doi: 10.1016/s0741-5214(97)70268-4. [DOI] [PubMed] [Google Scholar]
- 213.Semmens JB, Norman PE, Lawrence-Brown MM, Holman CD. Influence of gender on outcome from ruptured abdominal aortic aneurysm. Br J Surg. 2000;87:191–194. doi: 10.1046/j.1365-2168.2000.01346.x. [DOI] [PubMed] [Google Scholar]
- 214.Norman PE, Powell JT. Abdominal aortic aneurysm: The prognosis in women is worse than in men. Circulation. 2007;115:2865–2869. doi: 10.1161/CIRCULATIONAHA.106.671859. [DOI] [PubMed] [Google Scholar]
- 215.Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, Mark DB, Marwick TH, McCallister BD, Thompson PD, Winters WL, Jr, Yanowitz FG, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A, Jr, Lewis RP, O’Rourke RA, Ryan TJ. Acc/aha guidelines for exercise testing: Executive summary. A report of the american college of cardiology/american heart association task force on practice guidelines (committee on exercise testing) Circulation. 1997;96:345–354. doi: 10.1161/01.cir.96.1.345. [DOI] [PubMed] [Google Scholar]
- 216.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among us adults, 1999–2000. Jama. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 217.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among us adults, 1999–2008. Jama. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 218.Fitzgerald KR. Review of article: Prevalence of obesity and trends in the distribution of body mass index among us adults, 1999–2010 by katherine m. Flegal, phd; margaret d. Carroll, msph; brian k. Kit, md; cynthia l. Ogden, phd (jama 2012;307:491–7) Journal of vascular nursing: official publication of the Society for Peripheral Vascular Nursing. 2013;31:131–132. doi: 10.1016/j.jvn.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 219.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the united states, 2011–2012. Jama. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.