Abstract
Aims
It is well-established that exercise diminishes cardiovascular risk, but whether humoral factors secreted by muscle confer these benefits has not been conclusively shown. We have shown that the secreted protein follistatin-like 1 (Fstl1) has beneficial actions on cardiac and endothelial function. However, the role of muscle-derived Fstl1 in proliferative vascular disease remains largely unknown. Here, we investigated whether muscle-derived Fstl1 modulates vascular remodelling in response to injury.
Methods and results
The targeted ablation of Fstl1 in muscle led to an increase in neointimal formation following wire-induced arterial injury compared with control mice. Conversely, muscle-specific Fstl1 transgenic (TG) mice displayed a decrease in the neointimal thickening following arterial injury. Muscle-specific Fstl1 ablation and overexpression increased and decreased, respectively, the frequency of BrdU-positive proliferating cells in injured vessels. In cultured human aortic smooth muscle cells (HASMCs), treatment with human FSTL1 protein decreased proliferation and migration induced by stimulation with PDGF-BB. Treatment with FSTL1 enhanced AMPK phosphorylation, and inhibition of AMPK abrogated the inhibitory actions of FSTL1 on HASMC responses to PDGF-BB. The injured arteries of Fstl1-TG mice exhibited an increase in AMPK phosphorylation, and administration of AMPK inhibitor reversed the anti-proliferative actions of Fstl1 on the vessel wall.
Conclusion
Our findings indicate that muscle-derived Fstl1 attenuates neointimal formation in response to arterial injury by suppressing SMC proliferation through an AMPK-dependent mechanism. Thus, the release of protein factors from muscle, such as Fstl1, may partly explain why the maintenance of muscle function can have a therapeutic effect on the cardiovascular system.
Keywords: Fstl1, Myokine, Vascular remodelling, Smooth muscle cell, AMPK
1. Introduction
Atherosclerotic vascular diseases including coronary artery disease are the major causes of morbidity and mortality worldwide.1 Pathological remodelling of vascular wall is a crucial characteristic during the development of various vascular diseases including atherosclerosis, in-stent restenosis, vein graft stenosis, and vasculopathy after transplantation.2–4 Vascular smooth muscle cell (SMC) proliferation and migration contribute to the neointimal formation and vessel stenosis during vascular remodelling.3,5,6 A better understanding of the factors that modulate SMC responses could be valuable for the prevention and/or treatment of vascular disorders.
It is well-established that exercise benefits the cardiovascular mortality and morbidity.7–9 Aerobic exercise training has benefits regarding the reduction of risk factors for cardiovascular disease.9–12 Aerobic exercise training also improves vascular tone and function, and reduces restenosis after percutaneous coronary intervention.13–17 A plausible explanation is that this type of exercise promotes shear stress leading to eNOS activation and nitric oxide production. However, it has also been shown that resistance exercise training, which is associated with glycolytic muscle hypertrophy and strengthening, can have vascular-protective effects as well.10,18–20 Whereas strength training is recommended as a complement to aerobic exercise for the reduction in cardiovascular risk,21 the regulatory mechanisms that contribute to the cardiovascular-protective actions of glycolytic muscle are unknown.
It has been suggested that skeletal muscle secretes various bioactive substances, also referred to as myokines, that may act on nearby and remote organs.22,23 Presumably, myokine release, in response to aerobic or resistance exercise, functions to promote systemic adaptations to the increase in muscle activity. Previously, we have shown that the secreted glycoprotein follistatin-like 1 (Fstl1), also referred to as TSC-36, exerts cardiovascular-protective activities in the models of ischaemia and pathological cardiac hypertrophy.24–27 Here, we document that skeletal muscle is a major source of secreted Fstl1 in plasma and show that skeletal muscle-derived Fstl1 is protective in a model of injury-induced vascular intima proliferation using genetic models that specifically ablate or overexpress this factor in muscle. We conclude that skeletal muscle secretes protein factors, such as Fstl1, that may contribute to the cardiovascular-protective actions.
2. Methods
Please refer an expanded Methods section in the Supplementary Material online.
2.1. Mouse model of vascular injury
To generate muscle-specific Fstl1 deficient mice (Fstl1-KO), mice with loxP sites flanking the exon 1 of the Fstl1 gene (Fstl-1flox/flox)26,28 were crossed with mice overexpressing Cre recombinase under the control of the MCK promoter (MCK-Cre) (Jackson Laboratory). Muscle-specific Fstl1 transgenic (Fstl1-TG) mice expressing full-length murine Fstl1 cDNA with MCK promoter in the background of C57BL/6J were used for this study.26
Mice at the ages of 8–12 weeks were subjected to wire injury operation as previously described.29 Briefly, after anaesthetization (pentobarbital 50 mg/kg i.p.), the adequacy of anaesthesia was confirmed by a lack of withdrawal response to toe pinch during the surgical procedure. Then, a straight spring wire was inserted into the left femoral artery to denude and dilate the artery. Buprenorphine at 0.25 mg/kg was administered before surgery and after surgery for analgesia every 8 h for 48 h. After 3 weeks, mice were sacrificed to analyses. Study protocols were approved by the Institutional Animal Care and Use Committee at Nagoya University. Our study conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication, 8th Edition, 2011).
2.2. Histological assessment
After mice were sacrificed using an overdose of pentobarbital, the arteries were excised and embedded in paraffin. Sections were stained with haematoxylin–eosin (H–E) or MOMA-2 antibody. The cross-sectional area of the blood vessel layers, including the intimal and medial areas, were measured as previously described.29 The number of infiltrated macrophages to injured arteries was evaluated by dividing the number of MOMA-2-positive cells by the number of total cells in neointima.
2.3. Detection of cell proliferation in vivo
In vivo bromodeoxyuridine (BrdU) labelling was performed to identify proliferating cells in wire-injured artery by detection of the DNA synthesis. BrdU (40 µg/g mouse) was injected intraperitoneally 24 h before preparation of the artery. BrdU-labelled and unlabelled cells in neointima were counted. The BrdU labelling index was calculated by dividing the number of BrdU-labelled cells by the number of total cells as described previously.29
2.4. Preparation of recombinant human FSTL1 protein
The pMIB/V5-His insect cell expression vector expressing full-length human FSTL1 cDNA lacking signal peptide tagged with FLAG at the C terminus was transfected into insect Sf9 cells, and a stable cell line was generated by blasticidin selection as previously described.27,30 The culture supernatants were collected and incubated with anti-FLAG M2 affinity gel (Sigma). FSTL1 protein was eluted by incubation with 3 × FLAG peptide (Sigma) and dialyzed with PBS.
2.5. Cell culture and treatment
Human aortic smooth muscle cells (HASMCs) were incubated in mitogen-free media containing 1% FBS for 12 h before experiments. HASMCs were treated with FSTL1 protein or vehicle for the indicated lengths of time. In some experiments, HASMCs were incubated in the presence or absence of FSTL1 protein followed by stimulation with PDGF-BB or vehicle for the indicated lengths of time. In some experiments, cells were infected with adenovirus vectors expressing c-myc-tagged dominant-negative mutant AMPK (Ad-dn-AMPK) or β-galactosidase (Ad-β-gal) at a multiplicity of infection (MOI) of 10 for 24 h.31–33
2.6. Proliferation assays
Cell number was assessed by MTS-based assay. DNA synthesis was measured with a BrdU proliferation assay kit according to the instruction of manufacturer.29 HASMCs were pre-treated with FSTL1 protein or vehicle for 10 h and stimulated with PDGF-BB or HB-EGF for the indicated lengths of time.
2.7. Cell migration assays
Directional cell migration of HASMCs was simulated using an in vitro scratch-wound assay as previously described.29,34 Chemotaxis of HASMCs was assessed by transwell assay with polycarbonate membranes coated with fibronectin.34 HASMCs were added to the upper chamber, and serum-deprived media supplemented with PDGF-BB or vehicle was added to the lower chamber. Cells were allowed to migrate through the pores of the membrane for 4 h.
2.8. Determination of mRNA levels
Gene expression levels were quantified by quantitative real-time PCR method. PCR procedure was performed with a Bio-Rad real-time PCR detection system using THUNDERBIRD SYBR qPCR Mix as a double-standard DNA-specific dye.
2.9. Western blot analysis
Tissue or cell samples were homogenized in lysis buffer containing 1 mm PMSF. The equal amounts of proteins or plasma were subjected to SDS–PAGE and transferred to PVDF membranes. The membranes were incubated with the indicated antibodies, followed by incubation with the secondary antibody conjugated with horseradish peroxidase.
2.10. Statistical analysis
Data are presented as mean ± SE. Differences between groups were evaluated by the Student's t-test or analysis of variance with Fisher's protected least significant difference test. A P-value <0.05 denoted the presence of a statistically significant difference.
3. Results
3.1. Disruption of Fstl1 in skeletal muscle leads to enhanced neointimal formation after arterial injury
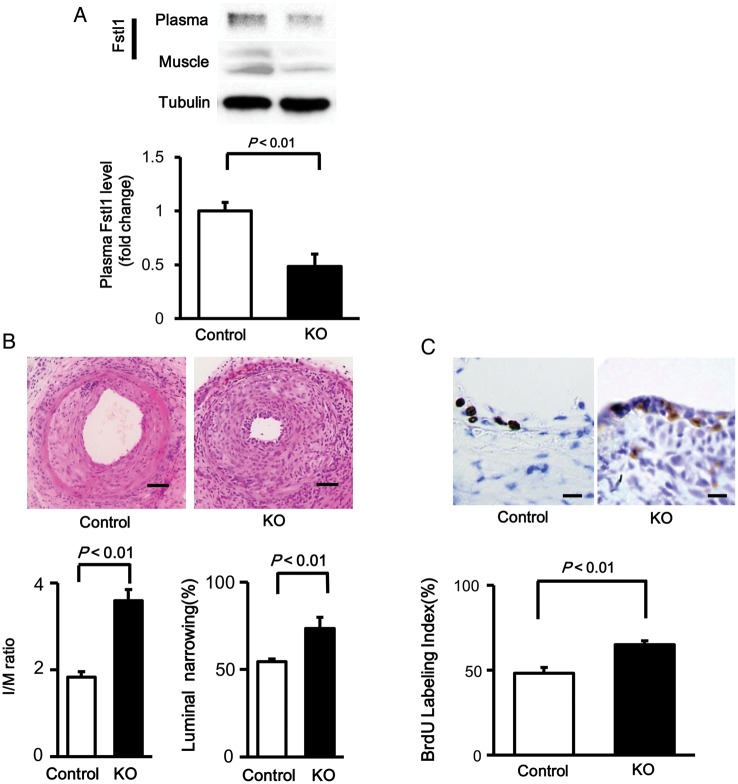
To investigate the role of muscle-derived Fstl1 in neointimal thickening after vascular injury in vivo, muscle-specific Fstl1-KO (MCK-Cre+/− × Fstl-1flox/flox) and control (MCK-Cre−/− × Fstl-1flox/flox) mice were subjected to wire-induced arterial injury. Plasma levels of Fstl1 in Fstl1-KO mice were decreased by 52% compared with those in control mice (Figure 1A). Fstl1 protein levels in skeletal muscle of Fstl1-KO mice were decreased by 90% compared with those in control mice. Thus, it is likely that skeletal muscle is a major source of circulating Fstl1. Figure 1B shows representative pictures for HE-stained sections of femoral arteries isolated from Fstl1-KO and control mice at 21 days after operation. Quantitative analysis of neointimal thickening revealed that the intimal/medial area (I/M) ratio was significantly increased by 85% in injured femoral artery of Fstl1-KO mice compared with that of control mice (Figure 1B). The percent luminal narrowing in injured vessels was also increased by 35% in Fstl1-KO mice compared with control mice.
Figure 1.
Fstl1-KO mice show enhanced neointimal formation following vascular injury. (A) Fstl1 protein levels in plasma and skeletal muscle tissue in muscle-specific Fstl1-KO and control littermate mice. Fstl1 levels in plasma (1.0 μl) and skeletal muscle were determined by western blot analysis. (B) Assessment of neointimal thickening in injured arteries in control and Fstl1-KO mice. Upper panels show the representative haematoxylin–eosin sections of femoral arteries from control and Fstl1-KO mice at 21 days after wire injury. Scale bar shows 50 μm. Lower panels show the quantitative analyses of I/M ratio (the ratio of intimal thickness/medial thickness) and luminal narrowing ratio (the ratio of intimal area/medial area). n = 8 in each group. (C) Assessment of vascular proliferation in the neointima after vascular injury. Upper panels show the representative BrdU staining of femoral arteries from control and Fstl1-KO mice at 7 days after surgery. Scale bar shows 10 μm. Quantitative data of BrdU labelling index are shown in the lower panel. The BrdU labelling index was calculated by dividing the number of BrdU-labelled cells by the number of total cells. n = 7 in each group. Data are presented as mean ± SE.
To evaluate the proliferative status of neointimal cells, BrdU staining was performed in sections of wire-injured femoral artery from Fstl1-KO and control mice. The number of BrdU-positive proliferating cells in the neointima was significantly higher by 35% in Fstl1-KO mice than in control mice (Figure 1C). These findings indicate that reduced levels of plasma Fstl1 leads to enhancement of cell proliferation and exacerbation of neointimal thickening in response to vascular injury.
To assess the contribution of inflammatory status to increased neointima formation after injury in Fstl1-KO mice, macrophage frequency was determined by staining with an antibody for MOMA-2, a marker of monocytes and macrophages. Fstl1-KO mice showed 3.0-fold increase in the frequency of MOMA-2-positive cells in the neointima compared with control mice (Supplementary material online, Figure S1A and B). Consistently, mRNA level of another macrophage marker CD68 was significantly higher in injured arteries of Fstl1-KO mice than in those from control mice, which was accompanied with increased expression levels of TNF-α, IL1β, and MCP-1 (Supplementary material online, Figure S1C). These results indicate that reduced plasma levels of Fstl1 leads to increased proinflammatory responses in injured arteries.
3.2. Overexpression of Fstl1 in skeletal muscle leads to attenuated neointimal hyperplasia in response to injury
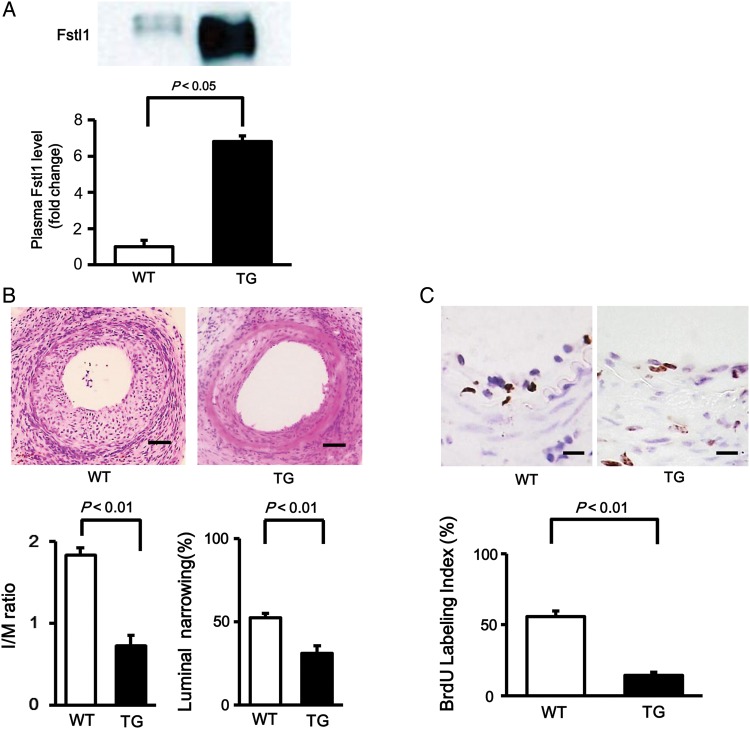
To investigate the effects of muscle-derived Fstl1 overexpression on neointimal thickening after vascular injury in vivo, muscle-specific Fstl1-TG and control WT mice were subjected to wire-induced arterial injury. Plasma levels of Fstl1 were 6.9-fold higher in Fstl1-TG mice than in WT mice (Figure 2A). Figure 2B shows representative pictures for HE-stained sections of femoral arteries isolated from Fstl1-TG and WT mice at 21 days after operation. Quantitative analysis of neointimal hyperplasia demonstrated that the I/M ratio was significantly reduced by 64% in injured femoral artery of Fstl1-TG mice compared with that of WT mice (Figure 2B). The percent luminal narrowing in response vascular injury was also suppressed by 40% in Fstl1-TG mice compared with control mice. Furthermore, the number of BrdU-positive proliferating cells in the neointima was significantly lower by 74% in Fstl1-TG mice than in WT mice (Figure 2C). These observations indicate that overproduction of plasma Fstl1 inhibits cell proliferation in damaged vessels, thereby leading to attenuation of neointimal hyperplasia following vascular injury.
Figure 2.
Fstl1-TG mice exhibit attenuated neointimal hyperplasia after arterial injury. (A) Plasma Fstl1 level in muscle-specific Fstl1-TG and littermate wild-type (WT) mice. Fstl1 level in plasma (1.0 μl) was determined by western blot analysis. n = 4 in each group. (B) Evaluation of neointimal thickening in wire-injured vessels in WT and Fstl1-TG mice. Upper panels show the representative haematoxylin–eosin sections of femoral arteries from WT and Fstl1-TG mice at 21 days after surgery. Scale bar shows 50 μm. Lower panels show the quantitative analyses of I/M ratio (the ratio of intimal thickness/medial thickness) and luminal narrowing ratio (the ratio of intimal area/medial area). n = 8 in each group. (C) Measurement of neointimal vascular proliferation in response to injury. Upper panels show the representative BrdU staining of femoral arteries from WT and Fstl1-TG mice at 7 days after injury. Scale bar shows 10 μm. The lower panel shows quantitative data of BrdU labelling index as calculated by dividing the number of BrdU-labelled cells by the number of total cells. n = 7 in each group. Data are presented as mean ± SE.
3.3. FSTL1 attenuates proliferation and migration of SMCs
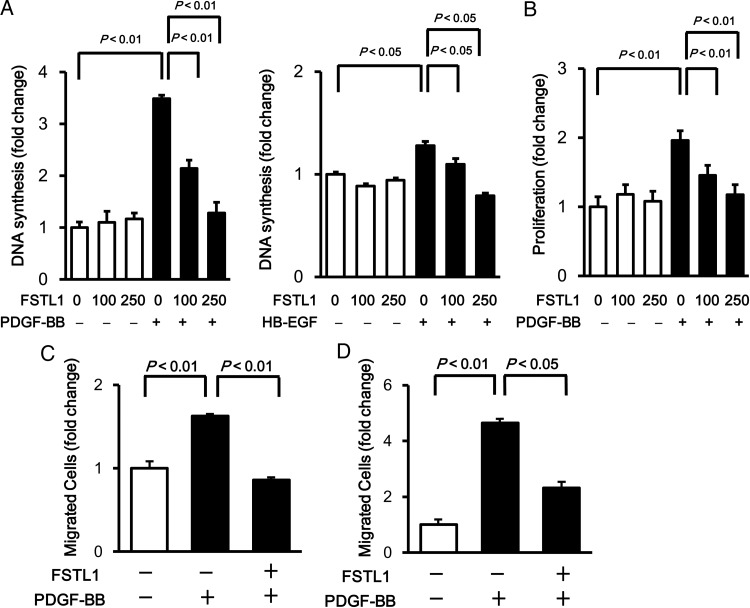
To investigate the effects of Fstl1 on SMC growth at a mechanistic level, HASMCs were treated with recombinant human FSTL1 protein, produced from insect cells, or vehicle followed by stimulation with PDGF-BB. The dose of FSTL1 protein used for these experiments (100 and 250 ng/ml) is similar to levels of circulating FSTL1 in healthy subjects and patients with acute coronary syndrome or systemic juvenile idiopathic arthritis.35,36 Levels of DNA synthesis in HASMCs were measured by BrdU incorporation assay. Pre-treatment of HASMCs with FSTL1 protein reduced PDGF-BB-stimulated DNA synthesis in a dose-dependent manner (Figure 3A). FSTL1 treatment also decreased HB-EGF-induced DNA synthesis in HASMCs (Figure 3A). Similarly, pre-treatment with FSTL1 protein significantly attenuated the PDGF-BB-stimulated increase in HASMC number as assessed by MTS-based assay in a dose-dependent manner (Figure 3B).
Figure 3.
FSTL1 suppresses PDGF-BB-induced proliferation and migration of HASMCs. (A) Effect of FSTL1 on DNA synthesis of HASMCs. HASMCs were treated with or without recombinant human FSTL1 protein (100 or 250 ng/ml) for 10 h followed by stimulation with PDGF-BB (10 ng/ml), HB-EGF (10 ng/ml), or vehicle for 24 h. Cells were labelled with BrdU during the last 22 h of the culture period. (B) Effect of FSTL1 on the number of HASMCs. HASMCs were treated with or without recombinant FSTL1 protein (100 or 250 ng/ml) for 10 h followed by stimulation with PDGF-BB (10 ng/ml) or vehicle for 20 h. The numbers of HASMCs were assessed by MTS-based assay. (C) Effect of FSTL1 on the migratory activity of HASMCs. HASMCs were cultured on fibronectin-coated glass dishes, pre-treated with FSTL1 protein (250 ng/ml) or vehicle for 10 h, and scratched with a pipette tip. Wounded cells were incubated with PDGF-BB (10 ng/ml) or vehicle for 10 h. n = 3 in each group. (D) Effect of FSTL1 on the chemotactic activity of HASMCs. HASMCs were pre-incubated in the transwell in the absence or presence of FSTL1 protein (250 ng/ml) for 10 h followed by treatment with PDGF-BB (10 ng/ml) or vehicle for 4 h. n = 6 in each group. Data are presented as mean ± SE.
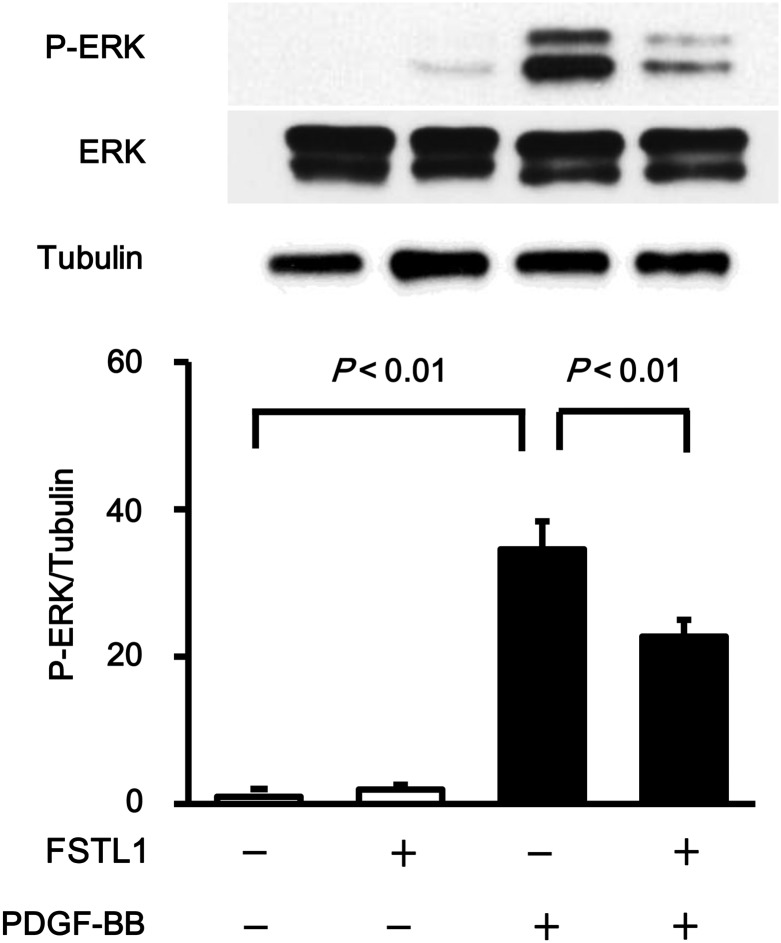
To examine the effect of FSTL1 on HASMC migration, in vitro scratch-wound and transwell chamber assays were performed. Treatment with FSTL1 protein significantly reduced the number of migrated HASMCs following stimulation with PDGF-BB in the scratch-wound assay (Figure 3C). Likewise, transwell assay revealed that FSTL1 decreased HASMC migration in response to PDGF-BB (Figure 3D). Because ERK activation is essential for proliferative properties of SMCs,37 the phosphorylation levels of ERK at Thr202/Tyr204 were evaluated by western blot analysis. Treatment of HASMCs with FSTL1 protein significantly suppressed ERK phosphorylation levels in response to PDGF-BB stimulation (Figure 4). Collectively, these data show that treatment with human FSTL1 protein suppresses proliferative and migratory activities of HASMCs.
Figure 4.
FSTL1 inhibits PDGF-BB-induced ERK activation HASMCs were pre-incubated in the presence or absence of FSTL1 (250 ng/ml) for 12 h and treated with PDGF-BB (10 ng/ml) or vehicle for 15 min. Phosphorylation levels of ERK (P-ERK) were determined by western blot analysis. Representative blots are shown in upper panels. ERK phosphorylation levels were quantified by using Image J, and quantitative data are shown in the lower panel. n = 3 in each group. Data are presented as mean ± SE.
3.4. FSTL1 suppresses SMC proliferation through activation of AMPK
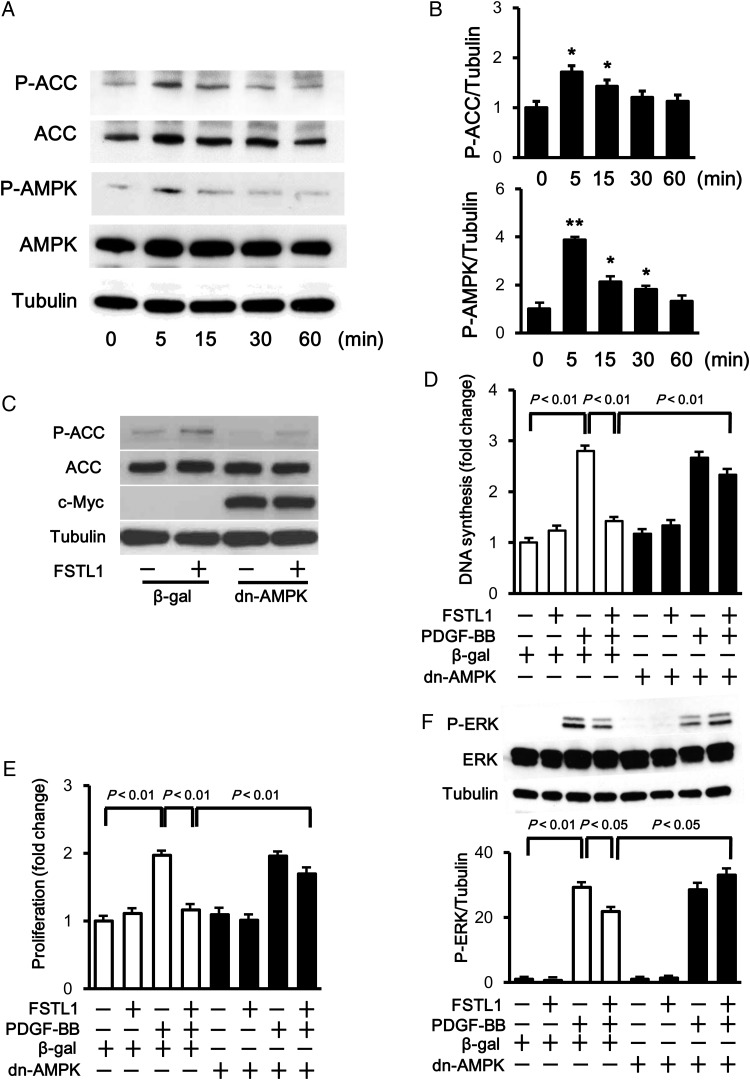
Because AMPK activation is reported to modulate SMC function,38–41 the phosphorylation levels of AMPK at Thr-172 were assessed by western blot analysis. Treatment of HASMCs with FSTL1 protein increased AMPK phosphorylation, which was accompanied by enhanced phosphorylation of an AMPK downstream molecule, acetyl-coenzyme A carboxylase (ACC), at Ser-79 (Figure 5A). Quantitative analysis showed that FSTL1 treatment significantly increased the phosphorylation of AMPK and ACC in a time-dependent fashion with maximal levels occurring at 5 min after stimulation with Fstl1 (Figure 5B).
Figure 5.
AMPK is essential for the suppressive action of FSTL1 on HASMC growth. (A) Time-dependent changes in the phosphorylation of AMPK and ACC in HASMCs following stimulation with FSTL1 (250 ng/ml). The phosphorylation levels of AMPK (P-AMPK) and ACC (P-ACC) were determined by western blot analysis. (B) Quantitative analysis of phosphorylation levels of ACC and AMPK. Phosphorylation levels were quantified by using Image J, and quantitative data are shown. n = 3 in each group. *P < 0.05 vs. basal. **P < 0.01 vs. basal. (C) Inhibition of AMPK activation reverses FSTL1-stimulated ACC phosphorylation. HASMCs were transduced with c-Myc tagged-Ad-dn-AMPK or Ad-β-gal at an MOI of 10 for 24 h and treated with FSTL1 (250 ng/ml) or vehicle for 5 min. The phosphorylation levels of ACC (P-ACC) were assessed by western blot analysis. (D and E) Effect of AMPK inactivation on FSTL1-induced inhibition of HASMC growth. HASMCs were transduced with Ad-dn-AMPK or Ad-β-gal for 24 h, and treated with or without FSTL1 (250 ng/ml) for 10 h followed by stimulation with PDGF-BB (10 ng/ml) or vehicle for 20 h. DNA synthesis and number of HASMCs were quantified by using BrdU cell proliferation (D) and MTS-based (E) assays, respectively. n = 8 in each group. (F) Involvement of AMPK in the inhibitory effect of FSTL1 on PDGF-BB-induced ERK phosphorylation. HASMCs were transduced with Ad-dn-AMPK or Ad-β-gal for 24 h and treated with or without FSTL1 (250 ng/ml) for 10 h followed by stimulation with PDGF-BB (10 ng/ml) or vehicle for 15 min. Data are presented as mean ± SE.
To investigate the involvement of AMPK signalling in anti-proliferative function of FSTL1, HASMCs were transduced with adenoviral vectors producing a dominant-negative mutant protein of AMPK tagged with c-myc (Ad-dn-AMPK) or β-galactosidase (Ad-β-gal), followed by treatment with FSTL1 protein or vehicle. Transduction with Ad-dn-AMPK abolished FSTL1-induced phosphorylation of ACC in HASMCs (Figure 5C). Transduction of HASMCs with Ad-dn-AMPK also abrogated the inhibitory effects of FSTL1 on PDGF-BB-stimulated DNA synthesis (Figure 5D). Similarly, transduction with Ad-dn-AMPK reversed FSTL1-mediated inhibition of HASMC proliferation assessed by MTS assay (Figure 5E). Finally, transduction with Ad-dn-AMPK also restored the suppressive effects of FSTL1 on ERK phosphorylation levels in response to PDGF-BB (Figure 5F). Collectively, these results show that FSTL1 inhibits SMC proliferation through an AMPK-dependent mechanism.
3.5. Fstl1 attenuates neointimal hyperplasia through AMPK-dependent mechanism
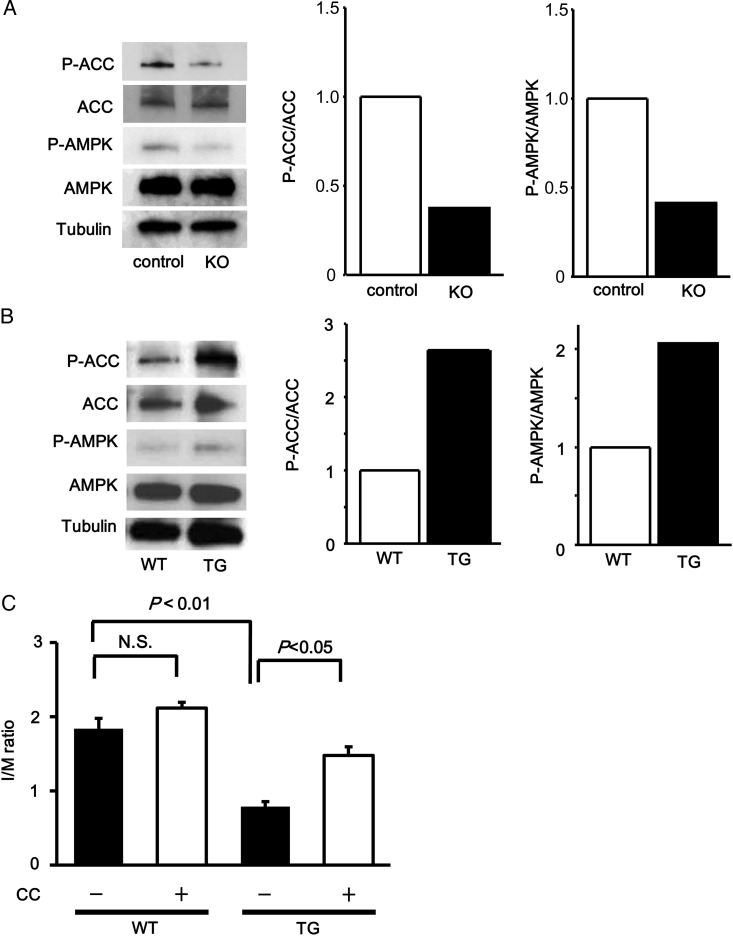
To investigate the involvement of AMPK signalling in Fstl1-mediated suppression of neointimal hyperplasia in vivo, phosphorylation levels of AMPK and ACC in wire-injured femoral artery were assessed by western blot analysis. Fstl1-KO mice exhibited a reduced phosphorylation of AMPK and ACC in injured arteries compared with control mice (Figure 6A). In contrast, Fstl1-TG mice showed an enhanced phosphorylation of AMPK and ACC in injured arteries compared with WT mice (Figure 6B). We also injected compound C, an AMPK inhibitor, or vehicle into the peritoneal cavity of WT and Fstl1-TG mice. Although administration of compound C led to a trend towards an increase in I/M ratio in injured artery in WT mice, it was not statistically significant. On the other hand, treatment with compound C significantly reversed the reduced I/M ratio of Fstl1-TG mice in response to injury (Figure 6C). These findings suggest that Fstl1 attenuates the process of neointimal hyperplasia through AMPK-dependent mechanism in vivo.
Figure 6.
Fstl1 ameliorates neointimal thickenings via AMPK-dependent manner. (A and B) Phosphorylation of AMPK and ACC in injured arteries. Phosphorylation levels of ACC (P-ACC) and AMPK (P-AMPK) in injured arteries (pooled samples, n = 3) from control and Fstl1-KO mice (A), and from WT and Fstl1-TG mice (B), at 3 days after surgery were evaluated by western blot analysis. Representative blots are shown. Right panels show quantitative analyses of phosphorylation levels of ACC (P-ACC/ACC) and AMPK (P-AMPK/AMPK). (C) Role of AMPK signalling in Fstl1-mediated attenuation of neointimal thickenings. AMPK inhibitor compound C or vehicle was intraperitoneally injected into WT or Fstl1-TG mice. Quantitative analysis of I/M ratio (the ratio of intimal area/medial area) is shown. n = 6 in each group. N.S., not significant. Data are presented as mean ± SE.
4. Discussion
The current study provides the first evidence that muscle-derived Fstl1 negatively regulates the development of pathological vascular remodelling in a mouse model of arterial injury. Muscle-specific deficiency of Fstl1 led to the reduction of circulating Fstl1 levels and enhancement of neointimal hyperplasia and vascular cell proliferation in wire-injured arteries. Conversely, muscle-specific overexpression of Fstl1 led to the elevation of plasma Fstl1 levels and reduction of neointimal formation and vascular cell proliferation in response to injury. Furthermore, recombinant FSTL1 protein significantly suppressed SMC proliferative and migratory activities in response to growth factor stimulation. These data suggest that Fstl1 acts as a myokine that can protect against the progression of vascular diseases by directly affecting SMC behaviour.
We have previously shown that systemic administration of Fstl1 to mice attenuates pathological myocardial remodelling following acute ischaemia or pressure overload.25–27 We have also demonstrated that Fstl1 inhibits the apoptotic activities of endothelial cells and cardiomyocytes.24,27 The present study makes two novel observations. First, these data show that Fstl1 can function to negatively regulate the formation of neointimal vascular lesions following injury, at least in part, by its ability to inhibit SMC proliferation. Secondly, these data show that skeletal muscle is a major source of Fstl1 production that is capable of secreting physiologically relevant levels of this factor. Thus, Fstl1 appears to function as a myokine, exerting cardiovascular-protective actions in an endocrine manner.
It has been shown that several myokines play roles in inter-tissue communication and that changes in circulating myokine production contribute to the whole-body homeostasis. Physical exercise increases the production of IL-6 in skeletal muscle, which can regulate glucose metabolism by acting on the remote tissues including liver, intestine and pancreatic islet.22,42 Muscle-derived overproduction of IL-15 contributes to reduction of adiposity.43 The insulin-sensitizing plasma protein FGF-21 is abundantly expressed in murine skeletal muscle and is also secreted from human skeletal muscle.44,45 Furthermore, aerobic exercise-inducible elevation of PGC-1α expression in skeletal muscle leads to increased production of irisin, which has been reported to drive brown-fat-like development of white fat and improves glucose metabolism,46 however, recent studies have cast doubt about the induction of irisin by exercise.47 Thus, muscle tissue can communicate with other metabolic organs by releasing these myokines, and some of these metabolic myokines appear to mediate the salutary effects of exercise on metabolic diseases.
The results of our study show that Fstl1 inhibits the proliferation of SMC through the activation of AMPK signalling. Other studies have shown that AMPK signalling plays a crucial role in the regulation of SMC function.38–41 AMPK activator 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) reduces SMC proliferation induced by PDGF-BB and fetal calf serum.38 AICAR also inhibits angiotensin II-induced proliferation and ERK activation of SMCs.39 Moreover, SMCs isolated from aorta of AMPKα2-deficient mice exhibit an increase in proliferative property.40 Furthermore, it has been shown that systemic injection of AICAR suppresses neointimal thickening after wire injury of rat femoral artery.39 Conversely, AMPKα2-deficiency contributes to the augmented neointimal hyperplasia after wire injury of carotid artery, which is accompanied with reduced levels of P27Kip1, a negative regulator of cell cycle.40 Our data demonstrate that Fstl1 enhances the activating phosphorylation of AMPK in SMCs and that blockade of AMPK activation reverses the effects of Fstl1 on growth activity and ERK phosphorylation in SMCs. Our pilot data also showed that FSTL1 increased the protein levels of P27Kip1 (data not shown). Thus, Fstl1 may suppress the proliferative responses of SMCs in vitro, at least in part, via activation of AMPK. Our in vivo data showed that overproduction of circulating Fstl1 leads to activated AMPK signalling and reduced neointimal formation in injured arteries, whereas the pharmacological inhibition of AMPK blocked the anti-proliferative actions of Fstl1. Taken together, these data suggest that Fstl1 exerts a negative impact on the development of neointimal hyperplasia following vascular injury in vivo through its ability to activate AMPK in SMCs. Consistent with our observations, Fstl1 has been shown to influence cellular phenotype and function via modulation of AMPK signalling in other contexts. Fstl1 has been shown to inhibit hypertrophy and promote the survival of cardiomyocytes via the activation of AMPK.26,27 Furthermore, Fstl1 attenuates agonist-stimulated production of inflammatory cytokines in myocytes and macrophages through its ability to activate AMPK.27 Taken together with our present findings, the Fstl1-AMPK regulatory axis may play a role in the control of various disease processes including cardiovascular remodelling and inflammatory responses.
Notably, the effect of Fstl1-deficiency on I/M ratio is greater than that on BrdU labelling index (Figure 1). Because Fstl1 displays an anti-migratory function in SMCs, these effects may have a greater contribution to Fstl1's effect on neointima formation than its anti-proliferative activity. Furthermore, inflammatory responses play a crucial role in the vascular remodelling after mechanical arterial injury, and systemic depletion of macrophages are reported to reduce neointimal hyperplasia and restenosis.48 In the present study, Fstl1-KO mice exhibited an increased accumulation of macrophages in injured artery with accompanying increases in proinflammatory cytokine expression. Thus, it is plausible that exacerbation of inflammatory response contributes to the progression of neointimal thickening caused by Fstl1-deficiency.
We note that there are a number of limitations in the interpretation of our study. A relatively aggressive model for vascular disease (i.e. wire-induced injury model) was used in this study, and it is unknown whether exercise can affect vascular stenosis in this model. Furthermore, there is no evidence that resistance exercise training in human can increase circulating Fstl1 concentrations to the levels that are observed in mice overexpressing Fstl1. Our data demonstrated that Fstl1-KO mice show reduced phosphorylation of AMPK in the injured vessel wall, and the systemic delivery of an AMPK inhibitor attenuated the suppressive effects of Fstl1 on neointimal formation in response to injury. However, it is conceivable that in addition to SMCs, Fstl1 may affect AMPK signalling in other types of cells in injured vasculature and its nearby or remote organs, including macrophages. Thus, further investigation is required to understand the mechanisms by which muscle-derived Fstl1 mediates the effects of exercise on cardiovascular disorders.
In conclusion, we demonstrated that Fstl1 reduces neointimal hyperplasia in injured arteries in vivo and that Fstl1 inhibits proliferation and migration of SMCs in vitro. Fstl1 has been shown to display salutary actions on endothelial cell function, acute cardiac injury, and myocardial hypertrophic response.24,26,27 Taken together, these data indicate that Fstl1 could have utility as a therapeutic target for manipulation of pathological conditions of cardiovascular systems.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by Grant-in-Aid for Scientific Research, Grant-in-Aid for Challenging Exploratory Research and grants from Takeda Science Foundation, the Uehara Memorial Foundation, Daiichi-Sankyo Foundation of Life Science, AstraZeneca Research & Development Grant, and SENSHIN Medical Research Foundation to N.O. R.S. was supported with the Grant-in-Aid for Young Scientists B and the Uehara Memorial Foundation. K.O. was supported with Grant-in-Aid for Scientific Research and The Cardiovascular Research Fund, Tokyo, Japan.
Supplementary Material
Acknowledgements
We gratefully thank for the technical assistance of Yoko Inoue and Miho Sakai.
Conflict of interest: none declared.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8:1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 3.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 4.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 6.Bauters C, Isner JM. The biology of restenosis. Prog Cardiovasc Dis. 1997;40:107–116. doi: 10.1016/s0033-0620(97)80003-5. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, Wilson P, Blair SN. Epidemiological assessment of the role of physical activity and fitness in development of cardiovascular disease. Am Heart J. 1985;109:876–885. doi: 10.1016/0002-8703(85)90653-2. [DOI] [PubMed] [Google Scholar]
- 8.Bijnen FC, Caspersen CJ, Feskens EJ, Saris WH, Mosterd WL, Kromhout D. Physical activity and 10-year mortality from cardiovascular diseases and all causes: The Zutphen Elderly Study. Arch Intern Med. 1998;158:1499–1505. doi: 10.1001/archinte.158.14.1499. [DOI] [PubMed] [Google Scholar]
- 9.Thompson PD. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:1319–1321. doi: 10.1161/01.ATV.0000087143.33998.F2. [DOI] [PubMed] [Google Scholar]
- 10.Umpierre D, Ribeiro PA, Kramer CK, Leitao CB, Zucatti AT, Azevedo MJ, Gross JL, Ribeiro JP, Schaan BD. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576. [DOI] [PubMed] [Google Scholar]
- 11.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 12.Couillard C, Despres JP, Lamarche B, Bergeron J, Gagnon J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Effects of endurance exercise training on plasma HDL cholesterol levels depend on levels of triglycerides: evidence from men of the Health, Risk Factors, Exercise Training and Genetics (HERITAGE) Family Study. Arterioscler Thromb Vasc Biol. 2001;21:1226–1232. doi: 10.1161/hq0701.092137. [DOI] [PubMed] [Google Scholar]
- 13.Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 14.Hambrecht R, Walther C, Mobius-Winkler S, Gielen S, Linke A, Conradi K, Erbs S, Kluge R, Kendziorra K, Sabri O, Sick P, Schuler G. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation. 2004;109:1371–1378. doi: 10.1161/01.CIR.0000121360.31954.1F. [DOI] [PubMed] [Google Scholar]
- 15.Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 16.Lee HY, Kim JH, Kim BO, Byun YS, Cho S, Goh CW, Ahn H, Rhee KJ, Kim C. Regular exercise training reduces coronary restenosis after percutaneous coronary intervention in patients with acute myocardial infarction. Int J Cardiol. 2013;167:2617–2622. doi: 10.1016/j.ijcard.2012.06.122. [DOI] [PubMed] [Google Scholar]
- 17.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 18.Kelley GA, Kelley KS. Progressive resistance exercise and resting blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2000;35:838–843. doi: 10.1161/01.hyp.35.3.838. [DOI] [PubMed] [Google Scholar]
- 19.Heffernan KS, Fahs CA, Iwamoto GA, Jae SY, Wilund KR, Woods JA, Fernhall B. Resistance exercise training reduces central blood pressure and improves microvascular function in African American and white men. Atherosclerosis. 2009;207:220–226. doi: 10.1016/j.atherosclerosis.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 20.Anton MM, Cortez-Cooper MY, DeVan AE, Neidre DB, Cook JN, Tanaka H. Resistance training increases basal limb blood flow and vascular conductance in aging humans. J Appl Physiol. 2006;101:1351–1355. doi: 10.1152/japplphysiol.00497.2006. [DOI] [PubMed] [Google Scholar]
- 21.Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, Gulanick M, Laing ST, Stewart KJ. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572–584. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 23.Walsh K. Adipokines, myokines and cardiovascular disease. Circ J. 2009;73:13–18. doi: 10.1253/circj.cj-08-0961. [DOI] [PubMed] [Google Scholar]
- 24.Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. 2008;283:32802–32811. doi: 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–3108. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimano M, Ouchi N, Nakamura K, van Wijk B, Ohashi K, Asaumi Y, Higuchi A, Pimentel DR, Sam F, Murohara T, van den Hoff MJ, Walsh K. Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure overload. Proc Natl Acad Sci USA. 2011;108:E899–E906. doi: 10.1073/pnas.1108559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogura Y, Ouchi N, Ohashi K, Shibata R, Kataoka Y, Kambara T, Kito T, Maruyama S, Yuasa D, Matsuo K, Enomoto T, Uemura Y, Miyabe M, Ishii M, Yamamoto T, Shimizu Y, Walsh K, Murohara T. Therapeutic impact of follistatin-like 1 on myocardial ischemic injury in preclinical animal models. Circulation. 2012;126:1728–1738. doi: 10.1161/CIRCULATIONAHA.112.115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sylva M, Li VS, Buffing AA, van Es JH, van den Born M, van der Velden S, Gunst Q, Koolstra JH, Moorman AF, Clevers H, van den Hoff MJ. The BMP antagonist follistatin-like 1 is required for skeletal and lung organogenesis. PLoS One. 2011;6:e22616. doi: 10.1371/journal.pone.0022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uemura Y, Shibata R, Ohashi K, Enomoto T, Kambara T, Yamamoto T, Ogura Y, Yuasa D, Joki Y, Matsuo K, Miyabe M, Kataoka Y, Murohara T, Ouchi N. Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. FASEB J. 2013;27:25–33. doi: 10.1096/fj.12-213744. [DOI] [PubMed] [Google Scholar]
- 30.Ouchi N, Asaumi Y, Ohashi K, Higuchi A, Sono-Romanelli S, Oshima Y, Walsh K. DIP2A functions as a FSTL1 receptor. J Biol Chem. 2010;285:7127–7134. doi: 10.1074/jbc.M109.069468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278:31000–31006. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- 32.Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyake H, Maeda K, Asai N, Shibata R, Ichimiya H, Isotani-Sakakibara M, Yamamura Y, Kato K, Enomoto A, Takahashi M, Murohara T. The actin-binding protein Girdin and its Akt-mediated phosphorylation regulate neointima formation after vascular injury. Circ Res. 2011;108:1170–1179. doi: 10.1161/CIRCRESAHA.110.236174. [DOI] [PubMed] [Google Scholar]
- 35.Widera C, Horn-Wichmann R, Kempf T, Bethmann K, Fiedler B, Sharma S, Lichtinghagen R, Leitolf H, Ivandic B, Katus HA, Giannitsis E, Wollert KC. Circulating concentrations of follistatin-like 1 in healthy individuals and patients with acute coronary syndrome as assessed by an immunoluminometric sandwich assay. Clin Chem. 2009;55:1794–1800. doi: 10.1373/clinchem.2009.129411. [DOI] [PubMed] [Google Scholar]
- 36.Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci WS, Sam F, Ouchi N, Walsh K. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007;42:1065–1074. doi: 10.1016/j.yjmcc.2007.03.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gennaro G, Menard C, Michaud SE, Deblois D, Rivard A. Inhibition of vascular smooth muscle cell proliferation and neointimal formation in injured arteries by a novel, oral mitogen-activated protein kinase/extracellular signal-regulated kinase inhibitor. Circulation. 2004;110:3367–3371. doi: 10.1161/01.CIR.0000147773.86866.CD. [DOI] [PubMed] [Google Scholar]
- 38.Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, Matsumoto K, Toyonaga T, Asano T, Nishikawa T, Araki E. Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res. 2005;97:837–844. doi: 10.1161/01.RES.0000185823.73556.06. [DOI] [PubMed] [Google Scholar]
- 39.Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T, Hirata Y. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation. 2004;110:444–451. doi: 10.1161/01.CIR.0000136025.96811.76. [DOI] [PubMed] [Google Scholar]
- 40.Song P, Wang S, He C, Liang B, Viollet B, Zou MH. AMPKalpha2 deletion exacerbates neointima formation by upregulating Skp2 in vascular smooth muscle cells. Circ Res. 2011;109:1230–1239. doi: 10.1161/CIRCRESAHA.111.250423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Ferri N. AMP-activated protein kinase and the control of smooth muscle cell hyperproliferation in vascular disease. Vascul Pharmacol. 2012;56:9–13. doi: 10.1016/j.vph.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes. 2004;53:1643–1648. doi: 10.2337/diabetes.53.7.1643. [DOI] [PubMed] [Google Scholar]
- 43.Quinn LS, Anderson BG, Strait-Bodey L, Stroud AM, Argiles JM. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am J Physiol Endocrinol Metab. 2009;296:E191–E202. doi: 10.1152/ajpendo.90506.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K. FGF21 is an Akt-regulated myokine. FEBS Lett. 2008;582:3805–3810. doi: 10.1016/j.febslet.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hojman P, Pedersen M, Nielsen AR, Krogh-Madsen R, Yfanti C, Akerstrom T, Nielsen S, Pedersen BK. Fibroblast growth factor-21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes. 2009;58:2797–2801. doi: 10.2337/db09-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, Ashare AB, Lathi K, Isner JM. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–2804. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 48.Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-o M. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA. 2007;104:7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.