Abstract
The metabolic syndrome (MetS), a cluster of dyslipidemia, hypertension, and diabetes, and an important contributor to cardiovascular morbidity and mortality, occurs in nearly 35% of adults and 50% of the aging population in the United States. However, the underlying mechanisms by which MetS orchestrates and amplifies cardiovascular events remain elusive. Furthermore, traditional therapeutic strategies addressing lifestyle modifications and individual components of MetS are often unsuccessful in decreasing morbidity due to MetS. The availability of an adequate experimental platform that mimics the complexity of MetS may allow development of novel management techniques. Swine models, including domestic pigs and minipigs, have made important contributions to our understanding of many aspects of MetS. Given their similarity to human anatomy and physiology, those models may have significant predictive power for elucidating the pathophysiology of MetS in a manner applicable to humans. Moreover, experimental maneuvers and drugs can be tested in these pre-clinical models before application in patients with MetS. This review highlights the utility of the pig as an animal model for metabolic disorders, which may play a crucial role in novel drug development to optimize management of MetS.
Keywords: Metabolic syndrome, insulin resistance, adiposity, swine, hypertension
Overview
The prevalence of obesity and metabolic syndrome (MetS) is increasing in the developed world (Eckel et al., 2005). MetS is defined by the constellation of obesity, dyslipidemia, hypertension, insulin resistance (IR), and increased levels of serum proinflammatory markers (Grundy et al., 2004). According to American Heart Association, individuals are diagnosed MetS when they show ≥3 of following components: 1) Central or abdominal obesity (measured by waist circumference); 2) Elevated triglyceride levels; 3) Low high-density lipoprotein (HDL); 4) Hypertension; 5) Elevated fasting glucose. The International Diabetes Federation criteria are similar, but more restrictive for central obesity (Parikh and Mohan, 2012). MetS is an important contributor to cardiovascular morbidity and mortality, which can be observed in nearly 35% of adults in the United States and 50% of the aging population (Aguilar et al., 2015). MetS patients are also at increased risk of developing microalbuminuria (Hoehner et al., 2002) and renal dysfunction (Chen et al., 2004).
Investigation of the pathogenesis of MetS and its adverse outcomes have been facilitated by development of animal models of MetS. Various species have been used as models of MetS, particularly rats and mice, whereas studies in swine models to date account for no more than 10% of the total number of publications in this area identified in a search of the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed; http://www.ncbi.nlm.nih.gov/pubmed?term=(%22Metabolic+Syndrome+X%22%5BMesh%5D)+AND+%22Swine%22%5BMesh%5D; year 1960–2015). However, although rodents are small and thus useful for multi-variable experiments, they show several important differences in metabolism and physiology from humans (Davis et al., 2013, Arner, 2005). For example, normal mouse lipoprotein profiles have primarily atheroprotective HDL, whereas normal human lipoprotein profiles contain primarily atherogenic low-density lipoprotein (LDL)(Kennedy et al., 2010). Adipsin, an adipokine that is increased in human Mets, is found lower in rodents. (Rosen et al., 1989, Napolitano et al., 1994) Unlike swine (Table 1), Rodents may not always develop hypertension (Mark et al., 1999). As they do not readily and simultaneously exhibit all the clinical signs of MetS, (Spurlock and Gabler, 2008) the translation of rodent data for humans have been hindered. In contrast, pigs possess many anatomical and physiological similarities to humans, as well as a high sequence and chromosome structure homology. (Vamathevan et al., 2013, Groenen et al., 2012) Comparisons of 317 known human drug target genes revealed less variation from minipigs (19 genes) than from beagle (41 genes). (Vamathevan et al., 2013) However, pigs do show some anatomical difference from humans. In the kidney, the avascular plane is transverse in swine, rather than longitudinal as in humans, and it has 2 large venous trunks (humans have 3) and no large veins on the dorsal surface of the pelvis. (Bagetti Filho et al., 2008) In the heart, innervation of the atrioventricular node and ventricular conduction tissues also differs from humans. (Crick et al., 1999) Nonetheless, many similarities to humans support the use of the pig model for investigating MetS (Spurlock and Gabler, 2008).
Table 1.
Nutrient compositions of different diets used to induce the metabolic syndrome in pigs
| Studies | Fat (%) |
Carbohydrate (%) |
Fructose (%) |
Sucrose (%) |
Cholesterol (/Kg) |
Sodium cholate (/Kg) |
|---|---|---|---|---|---|---|
| Lee et al., 2010 | 45 | 47 | 11g/Kg | |||
| Elmadhun et al., 2014a | 4 | 1.5 | ||||
| Borbouse et al., 2009 | 43 | 20 | 20 | 2 | 0.7 | |
| Li et al., 2012 | 43 | 40.8 | 17.8 | 1.05 | 2 | 0.7 |
| Pawar et al., 2015 | 43 | 40.8 | 17.8 | 1.05 | 2 | 0.7 |
| Zhang et al., 2013 | 43 | 40.8 | 17.8 | 1.05 | 2 | 0.7 |
| Robich et al., 2012 | 4 | 1.5 | ||||
| Bradley et al., 2015 | 43 | 19 | 2 | 0.7 | ||
| Christoffersen et al., 2013 | 30.7 | 30.9 | ||||
| Xi et al., 2004 | 12.5 | 69.6 | 37 | 10 | ||
| Sabe et al., 2014 | ||||||
| Ma et al., 2015 | 43 | 40.8 | 17.8 | 1.05 | 2 | 0.7 |
| te Pas et al., 2013 | 250/kg | 150/kg | 100/kg | 10/kg | ||
| Johansen et al., 2001 | 51.3 | |||||
| Lee et al., 2009 | 46 | 43 | 20 | 0.9 | ||
| Li et al., 2015 | 45.7 | 35.3 | 20 | |||
| McKenney et al., 2014 | 42.9 | 40.8 | 19 | 2.0 | 0.7 |
Inducing MetS in swine
MetS has been induced in different strains of pigs by high caloric diets (Table 1), mostly including 15–25% (by weight) fatty acids (mainly lard supplemented with hydrogenated soya bean and coconut oil), 1–2% cholesterol, 40% refined sugars (commonly 20% fructose and 20% sucrose), 17% protein, and 15% other carbohydrates like starches and fibers. In most MetS models, features of this disorder appear after a 3–6 months diet, but in some can emerge as early as after 2–5 weeks of high-fat/high-energy diet. (Christoffersen et al., 2013, Johansen et al., 2001) At 12 weeks after high-fat/high-sucrose diet there are usually apparent manifestations of MetS (Xi et al., 2004), which progressively aggravate over time (Pawar et al., 2015). At 6 months of high-fat/ high-fructose diet, some atherosclerotic plaque formation in the coronary artery may be evident (McKenney et al., 2014, Borbouse et al., 2009) (Table 2).
Table 2.
Pig characteristics and manifested metabolic syndrome (MetS) components induced by MetS diets.
|
Studies |
Breed |
Gender | Age | Diet duration |
Reported metabolic syndrome components |
|---|---|---|---|---|---|
| Lee et al., 2010 | Yucatan | male and female |
3 months | 6 months | Obesity, hypertension, increased cholesterol, increased TG, insulin resistance |
| Elmadhun et al., 2014a | Ossabaw | male | 6 weeks | 9 weeks | Obesity , hypertension, increased cholesterol, impaired glucose intolerance |
| Borbouse et al., 2009 | Ossabaw | male | 12 months |
3–6 months | Obesity, hyperglycemia, hyperinsulinemia, increased cholesterol, increased LDL/HDL ratio, increased TG |
| Li et al., 2012 | Ossabaw | female | 3 months | 14 weeks | Obesity, insulin resistance, increased cholesterol, increased LDL/HDL |
| Pawar et al., 2015 | Domestic pigs | female | 3 months | 16 weeks |
Insulin resistance, hypertension |
| Zhang et al., 2013 | Ossabaw | female | 3 months | 16 weeks |
Obesity, insulin resistance, inflammation |
| Robich et al., 2012 | Yorkshire | 11 weeks | Obesity, increased cholesterol impaired glucose tolerance |
||
| Bradley et al., 2015 | Ossabaw | male and female |
6–8 month |
6 months | Ossabaw, obesity, hypertension, hyperglycemia, increased cholesterol, increased TG, increased LD/cholesterol ration, |
| Christoffersen et al., 2013 | Göttingen | male and female |
9 weeks | 4 months | Obesity, insulin resistance, impaired glucose tolerance, increased cholesterol, increased TG, visceral adiposity. Changes appeared after 2 weeks. |
| Xi et al., 2004 | Chinese Guizhou |
male | 3–4 months |
6 months |
Obesity, hyperglycemia, hyperinsulinemia, increased cholesterol, increased TG, |
| Sabe et al., 2014 | Ossabaw | male | 6 months |
Obesity, impaired glucose tolerance, |
|
| Ma et al., 2015 | Domestic pigs | female | 3 months | 16 weeks |
Obesity, hypertension, increased cholesterol, insulin resistance, visceral adiposity |
| te Pas et al., 2013 | YorkshirexLand race |
11 weeks | 10 weeks | Hyperlipidemia, insulin resistance | |
| Johansen et al., 2001 | Göttingen | female | 9–10 months |
5 weeks | Obesity, increased TG, hyperinsulinemia, impaired glucose tolerance, |
| Lee et al., 2009 | Ossabaw | 5–10 months |
24 weeks | Obesity, insulin resistance, hyperglycemia, hypertension |
|
| Li et al., 2015 | Lee-Sung (LS) and Lanyu (LY) Lee-Sung |
male and female |
5 months | 6 months | Hypercholesterolemia, hypertriglyceridemia, obesity, hypertension, hyperglycemia |
| McKenney et al., 2014 | Ossabaw | male | 6 months | 6 months | Obesity, hypertension, increased LDL/HDL ratio, insulin resistance, increased TG, coronary artery atherosclerosis |
TG: triglyceride; LDL: low-density lipoprotein; HDL: high-density lipoprotein.
Minipig breeds frequently used to simulate MetS include Yucatan, (Lee et al., 2010) developed in the Yorkshire area of northern England and imported into the United States in 1890s; Sinclair, originally developed at the Hormel Institute of the University of Minnesota in the 1950s; Gӧttingen (Johansen et al., 2001), developed in the 1960s at the Institute of Animal Breeding and Genetics of the University of Gӧttingen, Germany, by crossbreeding the Vietnamese, Hormel, and German improved Landrace swine; the Chinese Guizhou (Xi et al., 2004) and Taiwan Lee-Sung (Li et al., 2015) strains, as well as Ossabaw pigs.
The Ossabaw strain encompasses descendants of minipigs brought from Spain that were isolated on the Ossabaw Islands off the coast of Georgia, (Brisbin et al., 1977), and develop MetS upon high-fat/high-sucrose feeding (Clark et al., 2011, Lee et al., 2009, Zhang et al., 2013) and readily exhibit obesity, insulin resistance, and hypertension, not often seen in other breeds (Litten-Brown et al., 2010). This unique strain has lived in relative genetic isolation for centuries, surviving on abundant food in the fall and famine conditions in the winter, a scenario that may have selected for a “thrifty genotype”. (Speakman, 2008) Hypothetically, in early evolutionary history genes promoting efficient fat deposition might have been advantageous by allowing survival at periods of famine. In modern society, such genes are disadvantageous because famine does not necessarily come, resulting in facilitated evolution of metabolic disorders. Ossabaw pigs have low insulin binding affinity for liver microenzymes and thereby are relatively insensitive to insulin (Meserole and Etherton, 1984) and susceptible to MetS. (Elmadhun et al., 2014a, Zhang et al., 2013) This strain, however, is costly, and does not grow to the full body size of domestic pigs, possibly due to lower plasma levels of growth hormone (Kasser et al., 1981, Wangsness et al., 1977), resulting in more limited sample collection capacity.
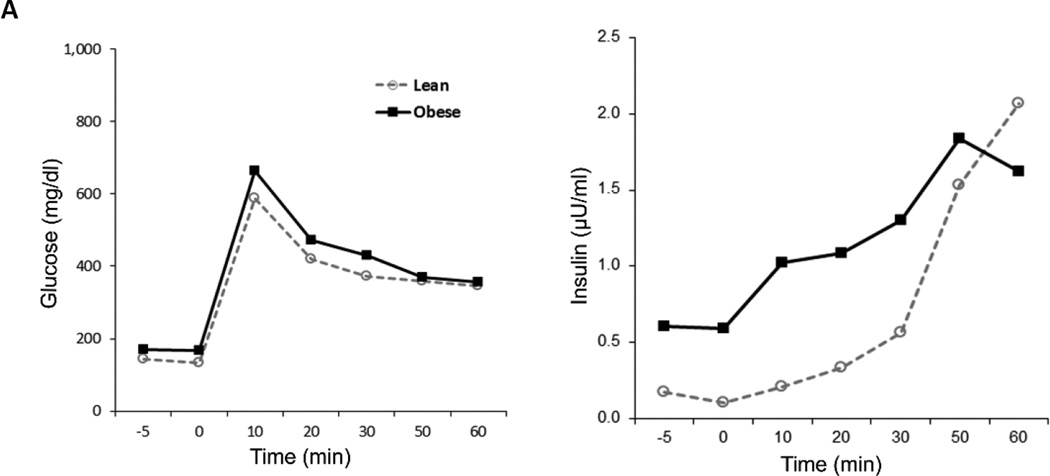
Recently, MetS was successfully induced in large domestic pigs such as American Yorkshire (Sus scrofa domesticus) using high saturated-fat/cholesterol/sugar (te Pas et al., 2013) or high-fat/high-fructose diet. (Pawar et al., 2015, Ma et al., 2015) In domestic pigs, a 2% high-cholesterol diet causes only endothelial dysfunction and early changes of atherosclerosis, but not obesity, IR, or hypertension (Eirin et al., 2014, Urbieta-Caceres et al., 2010). Contrarily, a 3–4 months ad-libitum high-fat/high-fructose diet (Table 1) induced significant manifestations of MetS resembling those observed in Ossabaw pigs. A 12–16 weeks of diet elicited a 20% increase in blood pressure, 2-fold increase in IR (Figure 1A), a 4-fold increase in serum cholesterol, and doubling of triglyceride levels. In addition, plasma tumor necrosis factor (TNF)-α levels were also elevated by 3-fold (Li et al., 2012, Pawar et al., 2015, Zhang et al., 2013). These findings suggest that the MetS model in domestic pigs is effective and may offer some advantages in cost and genetic heterogeneity.
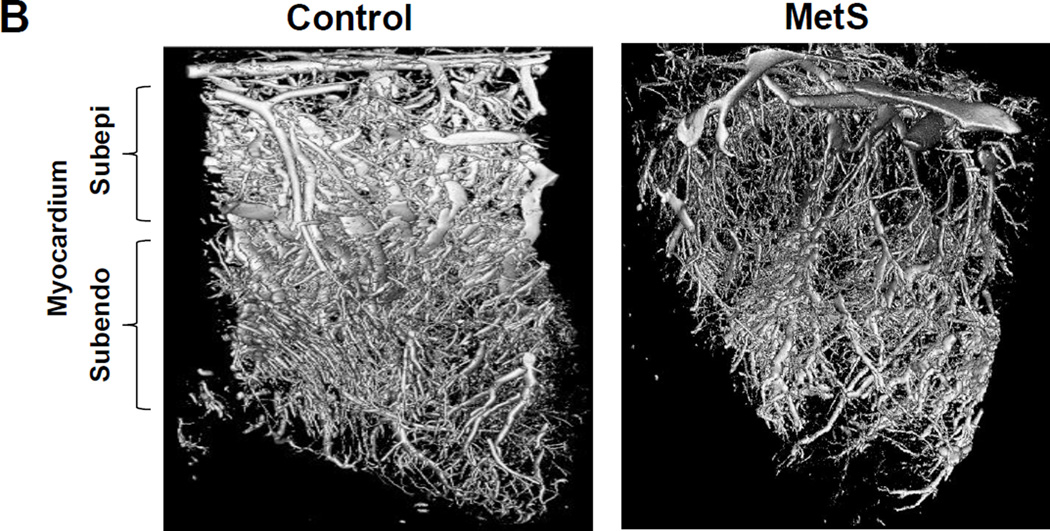
Figure 1.
A. Plasma insulin levels during an intravenous glucose tolerance test in Ossabaw pigs fed with MetS or control diets.
B. Metabolic syndrome in Ossabaw pigs decreased microvascular density in myocardium compared with control using three-dimensional micro-computed tomography.
Interestingly, female pigs seem to develop MetS models more readily than their counterpart male pigs. While male pigs also become obese and increase fasting blood glucose and insulin levels following a MetS diet (Larsen et al., 2001), female pigs develop a larger abdominal circumference and higher concentrations of plasma insulin, triglyceride, total cholesterol, and leptin. Compared with male pigs, females are more insulin-resistant and might therefore constitute better models for MetS (Christoffersen et al., 2013, Christoffersen et al., 2007).
In addition to diet-induced MetS, a genetically modified MetS model has been induced using a novel technique. Increased activity of proprotein convertase subtilisin/kexin type (PCSK)9 leads to lower liver LDL-receptor levels, a reduction in LDL uptake from the circulation, and thus in hypercholesterolemia and associated atherosclerosis. In human subjects, a PCSK9 gain-of function mutation (D374Y) can lead to LDL cholesterol levels increase (Abifadel et al., 2003). Recently, Yucatan minipigs induced with liver-specific expression of human D374Y-PCSK9 displayed reduced hepatic LDL receptor levels, severe hypercholesterolemia, and spontaneous development of progressive aortic atherosclerotic lesions with human-like histopathological features (Al-Mashhadi et al., 2013). This model may be useful for translational research in atherosclerosis, although it requires a relatively long induction period, often 12 months. Additional studies are also required to determine whether this model exhibits indices of MetS such as IR and hypertension.
MetS pig models have shown activation of distinct injurious features in many vital organs and tissues. Swine MetS has provided insight regarding obesity-induced alterations in the central nervous system, (Karmi et al., 2010) digestive system, (Liang et al., 2015) pancreas, (Fullenkamp et al., 2011) gut microbiome, (Pedersen et al., 2013) heart, (Neeb et al., 2010) kidney, (Li et al., 2011) reproductive, (Newell-Fugate et al., 2014) and musculoskeletal (Karmi et al., 2010) systems, as well as metabolic health in the offspring. (Arentson-Lantz et al., 2014) On the other hand, the higher cost of large compared to small animals may limit the experimental sample size, and assessment of central obesity in swine is limited by missing firm standards for waist circumference or body-mass index. Nevertheless, this model holds promise for exploring clinically-relevant pathways of metabolic disorders.
In recent years the impact of MetS on the heart and kidney has been characterized in swine models.
MetS and the swine heart
A recent study showed that normal weight patients with MetS had a 1.6-fold and obese patients with MetS a 2.5-fold increased risk for death from cardiovascular events as compared with normal weight patients without MetS (Arnlov et al., 2010). Pigs are useful models for the study of cardiovascular disease, owing to a 90% similarity of morphology and physical function of the cardiovascular system to human subjects (Smith and Swindle, 2006, Hughes, 1986).
We have shown in MetS Ossabaw pigs that a 16-week high-fat/high-fructose diet induced a 30% increase in the rate-pressure product, impaired myocardial perfusion, and blunted response to vasoactive challenge by adenosine (Li et al., 2012), and myocardial oxygenation was greatly reduced in both Ossabaw and domestic MetS models (Pawar et al., 2015, Li et al., 2012). MetS diet also accelerated formation of atherosclerotic plaque and aggravated in-stent stenosis in both Yucatan and Ossabaw pigs, but to a greater extent in the latter breed (Neeb et al., 2010). Ossabaw MetS pigs also showed decreased microvascular density in the sub-epicardial myocardium, as shown by micro-computed tomography (Li et al., 2014) (Figure 1B), indicating adverse effects of MetS in both the cardiac macro- and micro-circulation. Altered myocardial vascular function in Ossabaw pigs fed with atherogenic diet has been linked to decreased myocardial endothelial nitric oxide (NO) synthase functionality and NO bioavailability (Bradley et al., 2015), and in Yucatan pigs to impaired function of large-conductance Ca2+-activated K+ (BKCa) channels (Mokelke et al., 2005). MetS in Ossabaw pigs induced by high-cholesterol diet also impairs signaling of cardiac angiogenesis (Elmadhun et al., 2014a) thereby restricting the compensatory capacity of the myocardium for recruiting adequate blood and oxygen supplies.
In Ossabaw MetS models, myocardial oxidative stress was found to be increased and antioxidant enzymes reduced (Bradley et al., 2015). Moreover, MetS attenuated myocardial autophagy in response to overwhelming nutrient supplies which were observed at both early (3 months) (Li et al., 2012) and late (9 months)(Sabe et al., 2014) stages of MetS. Therefore, attenuation of autophagy, a housekeeping process to maintain cellular energy homeostasis, may have profound influence on myocardial viability and cardiac adaptability in MetS. Additionally, in Ossabaw pigs with superimposed renovascular hypertension, we found that MetS magnified downregulation of mitochondrial proteins and activity, (Zhang et al., 2015) and synergistically exacerbated the diastolic dysfunction and myocardial fibrosis induced by hypertension (Zhang et al., 2015). Importantly, in Yucatan pigs chronic (7 months) MetS may lead to cardiac IR, along with impaired insulin signaling such as PI3-kinase activation, Akt phosphorylation and abnormal phosphorylation of insulin receptor substrate-1 (Lee et al., 2010). These effects may further inhibit proper cardiac energy utilization and function. These findings suggest multiple potential pathways that mediate the myocardial injury in MetS, yet their roles in directing pharmacological exploration need to be carefully examined.
MetS and the swine kidney
Patients with 1–2 and those with ≥3 traits of MetS are, respectively, 80 and 130% more likely to have microalbuminuria than those without the syndrome (Hoehner et al., 2002). The incidence of chronic kidney disease in patients with MetS increases progressively with the individual number of the MetS components (Chen et al., 2004). These studies suggest a close link between MetS and renal dysfunction, but their mechanistic relationship is incompletely understood, partly because of the need for appropriate experimental platforms. One of the advantages of pigs as experimental models of kidney research, compared with rats and dogs, is that their kidneys are more similar in anatomy and physiology to the human kidney (Yokota et al., 1985, Schook and Tumbleson, 1996). The porcine kidney is multi-pyramidal with an undivided cortex, and has several different medullary structures. Each medullary pyramid forms a separate papilla and fusion results in the formation of some compound papillae. Pig also possesses maximal urine concentration, glomerular filtration rate (GFR), and renal blood flow (RBF) similar to humans (Sachs, 1994). These important properties render the swine a suitable model to study renal disease, including the effects of MetS on the kidney.
Both Mets Ossabaw and domestic pig models almost double their RBF and GFR upon MetS diet feeding (Li et al., 2011, Ma et al., 2015). In Ossabaw pigs, the augmented renal hemodynamic indices are associated with increased renal cortical volume and microvascular density (Li et al., 2012), linking augmented hemodynamics and microcirculatory remodeling in the development of kidney hypertrophy (Li et al., 2011). Interestingly, neither circulating nor renal inflammatory and oxidative stress markers in the MetS Ossabaw pigs were different from the control group at 10 weeks (Li et al., 2011), but increased renal deposit of triglycerides was positively associated with increased GFR (Li et al., 2011), suggesting a possible role for excessive lipid deposit in kidney injury in early MetS.
Nevertheless, the enhanced renal hemodynamic state does not seem to be reno-protective. Instead, the proximal and Henle's tubular flow in the MetS kidneys was found to be slower than in healthy pig kidneys, and associated with marked proximal tubular vacuolization, suggesting tubular degenerative changes and atrophy (Li et al., 2011). Furthermore, tubulo-interstitial fibrosis becomes evident in Ossabaw pigs after 16 weeks of diet, although RBF and GFR remain elevated (Zhang et al., 2013). By 16 weeks of diets, MetS Ossabaw pigs exhibited increased renal inflammatory macrophages and plasma ox-LDL levels (Zhang et al., 2013), indicating that inflammation may play a role at later stages of renal injury. Both MetS Ossabaw and domestic pig kidneys are also encased in greater amounts of perirenal fat compared with control kidneys, enriched in inflammatory macrophages and cytokines, including TNF-α(Zhang et al., 2013, Ma et al., 2015). At least in the domestic pig MetS model, the peri-renal fat may not only serve as an inflammatory depot but may also impair renal artery endothelial function (Ma et al., 2015). These findings may have implications in the setting of therapeutic management of MetS-induced kidney disease.
MetS and the adipose tissue
Visceral obesity is a major component of MetS. In high-energy diet induced-MetS impairments in glucose and insulin metabolism are associated with an increase in accumulation of body fat (Christoffersen et al., 2013). We have recently shown that in domestic pigs both abdominal fat tissue volume and adipocyte sizes progressively increased over 16 weeks of MetS diet, accompanied by a parallel increase in intra-adipose capillary count and fibrosis (Pawar et al., 2015). The adipokines adiponectin and leptin are reciprocally regulated by obesity (Spurlock et al., 1998, Jacobi et al., 2004, Pawar et al., 2015). Indeed, MetS upregulates in porcine adipose tissue the release of abundant inflammatory adipokines from the adipose tissue, such as TNF-α, interleukin-6, and monocyte chemotactic protein-1 (Pawar et al., 2015), and stimulates macrophage infiltration (Ma et al., 2015, Zhang et al., 2013). Furthermore, MetS upregulates the expression of toll-like receptor in porcine adipose tissue (Gabler et al., 2008), which is closely associated with development of IR (Shi et al., 2006). Importantly, in the domestic MetS model, the inflamed adipose tissue may affect the organ it encapsulates, at least partly by disrupting the vascular endothelial function, which can be restored by TNF-α inhibition (Ma et al., 2015). Similarly, in Ossabaw MetS, perivascular fat impairs coronary endothelial function, (Payne et al., 2010) and is linked to atherosclerosis formation in the coronary artery wall (McKenney et al., 2014). Whether these changes are similar to human events is unclear and further investigations would be useful to provide more insight in this area.
Experimental Treatment in MetS swine model
Lifestyle changes are the essential and fundamental management strategies in MetS. In a swine model, contemporary pigs fed a Paleolithic diet consistent with the hunter-gather lifestyle of our ancestors are leaner, more sensitive to insulin, and have lower circulating concentrations of C-reactive protein than their counterparts fed a cereal diet reflective of modern-day habits (Jonsson et al., 2006), signifying the importance of dietary modification in alleviating the progression of MetS.
Additionally, medications have been shown to modulate cellular turnover and improve tissue viability. The antidiabetic drug metformin confers a survival advantage in patients with cardiovascular disease, and was recently found to selectively downregulate the apoptosis pathway in MetS pigs and upregulate cardioprotective proteins including mitogen-activated kinase proteins p38 and extracellular signal-regulated protein kinases 1 and 2. (Elmadhun et al., 2014b) Atorvastatin elicits a net decrease in apoptosis as well, (Sabe et al., 2015) and also prevents myocardial autophagic dysfunction produced by MetS, which may in part account for its cardioprotective effect. (Sabe et al., 2014) On the other hand, cholesterol levels in domestic pigs are less responsive to statins than humans (Hasler-Rapacz et al., 1996), possibly due to some differences from humans in lipid metabolism. Like rats and dogs, pigs have low plasma activity of cholesteryl-ester transport protein, and manifest a high HDL and low LDL distribution. (Yin et al., 2012) Many traditional models, including rabbit, Zucker diabetic fatty rat, and mice, do not show overall similarity to dyslipidemic humans. Non-human primates exhibit the most similar lipid profile compared to humans with respect to basal plasma total cholesterol, LDL/HDL ratio, and lipoprotein traces, but ethical issues restrict their widespread use as experimental models. Furthermore, inter-species differences in cholesterol handling might also be related to the primary site for fatty acid synthesis, which is the liver in humans, the adipose tissue in pigs, and both the liver and adipose tissue in rodents and rabbits (Nafikov RA, Beitz DC: Carbohydrate and lipid metabolism in domestic animals. (Nafikov and Beitz, 2007)
Treatment with sustained-release-nitrite reduces myocardial oxidative stress while increasing myocardial antioxidant capacity and NO bioavailability, and is associated with marked improvement in vasoreactivity of coronary arteries. (Bradley et al., 2015) In addition, resveratrol polyphenol, which is often found in high concentrations in red wine and considered cardioprotective, improves regional ejection fraction and myocardial perfusion in the high-cholesterol diet induced-MetS heart with or without chronic ischemia (Robich et al., 2012, Sabe et al., 2013), as well as body mass index (Sabe et al., 2014). Such beneficial effects are thought to be mediated through binding to its primary target protein sirtuin-1, a key regulator of energy metabolism (Li, 2013), and activating NO synthase (Robich et al., 2012). In a Chinese Guizhou pig MetS model, a lipoprotein lipase activator (ibrolipim) decreased ectopic lipid deposition, improved IR, and alleviated the beta cell damage (Yin et al., 2004).
Clearly, these studies have markedly advanced the understanding and identification new pathways contributing to MetS and their interaction with targeted pharmacological agents. Nevertheless, drug metabolism in MetS pigs requires cautious assessment. More studies are therefore needed to gauge optimal dosages of anti-MetS drugs and to decrease the potential of MetS subjects of experiencing life-threatening toxicity or lack of effects.
Conclusion
It is becoming increasingly apparent that both minipigs and larger pig strains are valuable models to mimic human MetS and test new treatment strategies. Additional advantages of the pig are the ability to use standard diagnostic and treatment technologies like in humans, and collect body fluid and tissues in adequate quantity especially in larger swine, thereby accelerating clinical translation. Future studies need to continue exploring the molecular basis of MetS and its comorbidities, as well as pathogenesis of MetS in other vital organs such as liver (steatohepatitis). Studies that focus on validation of the MetS models by controlling the severity of symptoms of MetS and organ-specific complications through developing and optimizing novel therapeutics would be of great significance.
Acknowledgments
Funding
Partly supported by NIH Grants DK104273, DK102325, DK73608 and HL123160.
Footnotes
Disclosure
None.
REFERENCES
- Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derre A, Villeger L, Farnier M, Beucler I, Bruckert E, Chambaz J, Chanu B, Lecerf JM, Luc G, Moulin P, Weissenbach J, Prat A, Krempf M, Junien C, Seidah NG, Boileau C. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313:1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- Al-Mashhadi RH, Sorensen CB, Kragh PM, Christoffersen C, Mortensen MB, Tolbod LP, Thim T, Du Y, Li J, Liu Y, Moldt B, Schmidt M, Vajta G, Larsen T, Purup S, Bolund L, Nielsen LB, Callesen H, Falk E, Mikkelsen JG, Bentzon JF. Familial hypercholesterolemia and atherosclerosis in cloned minipigs created by DNA transposition of a human PCSK9 gain-of-function mutant. Sci Transl Med. 2013;5:166ra161. doi: 10.1126/scitranslmed.3004853. [DOI] [PubMed] [Google Scholar]
- Arentson-Lantz EJ, Buhman KK, Ajuwon K, Donkin SS. Excess pregnancy weight gain leads to early indications of metabolic syndrome in a swine model of fetal programming. Nutr Res. 2014;34:241–249. doi: 10.1016/j.nutres.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Arner P. Resistin: yet another adipokine tells us that men are not mice. Diabetologia. 2005;48:2203–2205. doi: 10.1007/s00125-005-1956-3. [DOI] [PubMed] [Google Scholar]
- Arnlov J, Ingelsson E, Sundstrom J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010;121:230–236. doi: 10.1161/CIRCULATIONAHA.109.887521. [DOI] [PubMed] [Google Scholar]
- Bagetti Filho HJ, Pereira-Sampaio MA, Favorito LA, Sampaio FJ. Pig kidney: anatomical relationships between the renal venous arrangement and the kidney collecting system. J Urol. 2008;179:1627–1630. doi: 10.1016/j.juro.2007.11.040. [DOI] [PubMed] [Google Scholar]
- Borbouse L, Dick GM, Asano S, Bender SB, Dincer UD, Payne GA, Neeb ZP, Bratz IN, Sturek M, Tune JD. Impaired function of coronary BK(Ca) channels in metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;297:H1629–H1637. doi: 10.1152/ajpheart.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley JM, Islam KN, Polhemus DJ, Donnarumma E, Brewster LP, Tao YX, Goodchild TT, Lefer DJ. Sustained release nitrite therapy results in myocardial protection in a porcine model of metabolic syndrome with peripheral vascular disease. Am J Physiol Heart Circ Physiol. 2015;309:H305–H317. doi: 10.1152/ajpheart.00163.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisbin I, Geiger RA, Graves H, Pinder JE, III, Sweeney JM, Sweeney JR. Morphological characterizations of two population of feral swine. Acta theriol. 1977;22:75–85. [Google Scholar]
- Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- Christoffersen B, Golozoubova V, Pacini G, Svendsen O, Raun K. The young Gottingen minipig as a model of childhood and adolescent obesity: influence of diet and gender. Obesity (Silver Spring) 2013;21:149–158. doi: 10.1002/oby.20249. [DOI] [PubMed] [Google Scholar]
- Christoffersen BO, Grand N, Golozoubova V, Svendsen O, Raun K. Gender-associated differences in metabolic syndrome-related parameters in Gottingen minipigs. Comp Med. 2007;57:493–504. [PubMed] [Google Scholar]
- Clark BA, Alloosh M, Wenzel JW, Sturek M, Kostrominova TY. Effect of diet-induced obesity and metabolic syndrome on skeletal muscles of Ossabaw miniature swine. Am J Physiol Endocrinol Metab. 2011;300:E848–E857. doi: 10.1152/ajpendo.00534.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick SJ, Sheppard MN, Ho SY, Anderson RH. Localisation and quantitation of autonomic innervation in the porcine heart I: conduction system. J Anat. 1999;195(Pt 3):341–357. doi: 10.1046/j.1469-7580.1999.19530341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JE, Cain J, Banz WJ, Peterson RG. Age-Related Differences in Response to High-Fat Feeding on Adipose Tissue and Metabolic Profile in ZDSD Rats. ISRN Obes. 2013;2013:584547. doi: 10.1155/2013/584547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Eirin A, Ebrahimi B, Zhang X, Zhu XY, Woollard JR, He Q, Textor SC, Lerman A, Lerman LO. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res. 2014;103:461–472. doi: 10.1093/cvr/cvu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmadhun NY, Sabe AA, Lassaletta AD, Chu LM, Kondra K, Sturek M, Sellke FW. Metabolic syndrome impairs notch signaling and promotes apoptosis in chronically ischemic myocardium. J Thorac Cardiovasc Surg. 2014a;148:1048–1055. doi: 10.1016/j.jtcvs.2014.05.056. discussion 1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmadhun NY, Sabe AA, Lassaletta AD, Chu LM, Sellke FW. Metformin mitigates apoptosis in ischemic myocardium. J Surg Res. 2014b;192:50–58. doi: 10.1016/j.jss.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullenkamp AM, Bell LN, Robbins RD, Lee L, Saxena R, Alloosh M, Klaunig JE, Mirmira RG, Sturek M, Chalasani N. Effect of different obesogenic diets on pancreatic histology in Ossabaw miniature swine. Pancreas. 2011;40:438–443. doi: 10.1097/MPA.0b013e3182061583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabler NK, Spencer JD, Webel DM, Spurlock ME. n-3 PUFA attenuate lipopolysaccharide-induced down-regulation of toll-like receptor 4 expression in porcine adipose tissue but does not alter the expression of other immune modulators. J Nutr Biochem. 2008;19:8–15. doi: 10.1016/j.jnutbio.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Groenen MA, Archibald AL, Uenishi H, Tuggle CK, Takeuchi Y, Rothschild MF, Rogel-Gaillard C, Park C, Milan D, Megens HJ, Li S, Larkin DM, Kim H, Frantz LA, Caccamo M, Ahn H, Aken BL, Anselmo A, Anthon C, Auvil L, Badaoui B, Beattie CW, Bendixen C, Berman D, Blecha F, Blomberg J, Bolund L, Bosse M, Botti S, Bujie Z, Bystrom M, Capitanu B, Carvalho-Silva D, Chardon P, Chen C, Cheng R, Choi SH, Chow W, Clark RC, Clee C, Crooijmans RP, Dawson HD, Dehais P, De Sapio F, Dibbits B, Drou N, Du ZQ, Eversole K, Fadista J, Fairley S, Faraut T, Faulkner GJ, Fowler KE, Fredholm M, Fritz E, Gilbert JG, Giuffra E, Gorodkin J, Griffin DK, Harrow JL, Hayward A, Howe K, Hu ZL, Humphray SJ, Hunt T, Hornshoj H, Jeon JT, Jern P, Jones M, Jurka J, Kanamori H, Kapetanovic R, Kim J, Kim JH, Kim KW, Kim TH, Larson G, Lee K, Lee KT, Leggett R, Lewin HA, Li Y, Liu W, Loveland JE, Lu Y, Lunney JK, Ma J, Madsen O, Mann K, Matthews L, McLaren S, Morozumi T, Murtaugh MP, Narayan J, Nguyen DT, Ni P, Oh SJ, Onteru S, Panitz F, Park EW, et al. Analyses of pig genomes provide insight into porcine demography and evolution. Nature. 2012;491:393–398. doi: 10.1038/nature11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Hasler-Rapacz J, Kempen HJ, Princen HM, Kudchodkar BJ, Lacko A, Rapacz J. Effects of simvastatin on plasma lipids and apolipoproteins in familial hypercholesterolemic swine. Arterioscler Thromb Vasc Biol. 1996;16:137–143. doi: 10.1161/01.atv.16.1.137. [DOI] [PubMed] [Google Scholar]
- Hoehner CM, Greenlund KJ, Rith-Najarian S, Casper ML, McClellan WM. Association of the insulin resistance syndrome and microalbuminuria among nondiabetic native Americans. The Inter-Tribal Heart Project. J Am Soc Nephrol. 2002;13:1626–1634. doi: 10.1097/01.asn.0000015762.92814.85. [DOI] [PubMed] [Google Scholar]
- Hughes HC. Swine in cardiovascular research. Lab Anim Sci. 1986;36:348–350. [PubMed] [Google Scholar]
- Jacobi SK, Ajuwon KM, Weber TE, Kuske JL, Dyer CJ, Spurlock ME. Cloning and expression of porcine adiponectin, and its relationship to adiposity, lipogenesis and the acute phase response. J Endocrinol. 2004;182:133–144. doi: 10.1677/joe.0.1820133. [DOI] [PubMed] [Google Scholar]
- Johansen T, Hansen HS, Richelsen B, Malmlof R. The obese Gottingen minipig as a model of the metabolic syndrome: dietary effects on obesity, insulin sensitivity, and growth hormone profile. Comp Med. 2001;51:150–155. [PubMed] [Google Scholar]
- Jonsson T, Ahren B, Pacini G, Sundler F, Wierup N, Steen S, Sjoberg T, Ugander M, Frostegard J, Goransson L, Lindeberg S. A Paleolithic diet confers higher insulin sensitivity, lower C-reactive protein and lower blood pressure than a cereal-based diet in domestic pigs. Nutr Metab (Lond) 2006;3:39. doi: 10.1186/1743-7075-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmi A, Iozzo P, Viljanen A, Hirvonen J, Fielding BA, Virtanen K, Oikonen V, Kemppainen J, Viljanen T, Guiducci L, Haaparanta-Solin M, Nagren K, Solin O, Nuutila P. Increased brain fatty acid uptake in metabolic syndrome. Diabetes. 2010;59:2171–2177. doi: 10.2337/db09-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasser TR, Martin RJ, Gahagan JH, Wangsness PJ. Fasting plasma hormones and metabolites in feral and domestic newborn pigs. J Anim Sci. 1981;53:420–426. doi: 10.2527/jas1981.532420x. [DOI] [PubMed] [Google Scholar]
- Kennedy AJ, Ellacott KL, King VL, Hasty AH. Mouse models of the metabolic syndrome. Dis Model Mech. 2010;3:156–166. doi: 10.1242/dmm.003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen MO, Rolin B, Wilken M, Carr RD, Svendsen O, Bollen P. Parameters of glucose and lipid metabolism in the male Gottingen minipig: influence of age body weight, and breeding family. Comp Med. 2001;51:436–442. [PubMed] [Google Scholar]
- Lee J, Xu Y, Lu L, Bergman B, Leitner JW, Greyson C, Draznin B, Schwartz GG. Multiple abnormalities of myocardial insulin signaling in a porcine model of diet-induced obesity. Am J Physiol Heart Circ Physiol. 2010;298:H310–H319. doi: 10.1152/ajpheart.00359.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Alloosh M, Saxena R, Van Alstine W, Watkins BA, Klaunig JE, Sturek M, Chalasani N. Nutritional model of steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Hepatology. 2009;50:56–67. doi: 10.1002/hep.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Liu CH, Chang CW, Chu HP, Chen KJ, Mersmann HJ, Ding ST, Chu CH, Chen CY. Development of a dietary-induced metabolic syndrome model using miniature pigs involvement of AMPK and SIRT1. Eur J Clin Invest. 2015;45:70–80. doi: 10.1111/eci.12370. [DOI] [PubMed] [Google Scholar]
- Li X. SIRT1 and energy metabolism. Acta Biochim Biophys Sin (Shanghai) 2013;45:51–60. doi: 10.1093/abbs/gms108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Woollard JR, Wang S, Korsmo MJ, Ebrahimi B, Grande JP, Textor SC, Lerman A, Lerman LO. Increased glomerular filtration rate in early metabolic syndrome is associated with renal adiposity and microvascular proliferation. Am J Physiol Renal Physiol. 2011;301:F1078–F1087. doi: 10.1152/ajprenal.00333.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZL, Ebrahimi B, Zhang X, Eirin A, Woollard JR, Tang H, Lerman A, Wang SM, Lerman LO. Obesity-metabolic derangement exacerbates cardiomyocyte loss distal to moderate coronary artery stenosis in pigs without affecting global cardiac function. Am J Physiol Heart Circ Physiol. 2014;306:H1087–H1101. doi: 10.1152/ajpheart.00052.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZL, Woollard JR, Ebrahimi B, Crane JA, Jordan KL, Lerman A, Wang SM, Lerman LO. Transition from obesity to metabolic syndrome is associated with altered myocardial autophagy and apoptosis. Arterioscler Thromb Vasc Biol. 2012;32:1132–1141. doi: 10.1161/ATVBAHA.111.244061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T, Alloosh M, Bell LN, Fullenkamp A, Saxena R, Van Alstine W, Bybee P, Werling K, Sturek M, Chalasani N, Masuoka HC. Liver injury and fibrosis induced by dietary challenge in the Ossabaw miniature Swine. PLoS One. 2015;10:e0124173. doi: 10.1371/journal.pone.0124173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten-Brown JC, Corson AM, Clarke L. Porcine models for the metabolic syndrome, digestive and bone disorders: a general overview. Animal. 2010;4:899–920. doi: 10.1017/S1751731110000200. [DOI] [PubMed] [Google Scholar]
- Ma S, Zhu XY, Eirin A, Woollard JR, Jordan KL, Tang H, Lerman A, Lerman LO. Perirenal fat promotes renal arterial endothelial dysfunction in obese swine through tumor necrosis factor-alpha. J Urol. 2015 doi: 10.1016/j.juro.2015.08.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark AL, Shaffer RA, Correia ML, Morgan DA, Sigmund CD, Haynes WG. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens. 1999;17:1949–1953. doi: 10.1097/00004872-199917121-00026. [DOI] [PubMed] [Google Scholar]
- McKenney ML, Schultz KA, Boyd JH, Byrd JP, Alloosh M, Teague SD, Arce-Esquivel AA, Fain JN, Laughlin MH, Sacks HS, Sturek M. Epicardial adipose excision slows the progression of porcine coronary atherosclerosis. J Cardiothorac Surg. 2014;9:2. doi: 10.1186/1749-8090-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meserole VK, Etherton TD. Insulin binding to liver microsomes from lean and obese swine during growth to market weight. J Anim Sci. 1984;59:650–657. doi: 10.2527/jas1984.593650x. [DOI] [PubMed] [Google Scholar]
- Mokelke EA, Dietz NJ, Eckman DM, Nelson MT, Sturek M. Diabetic dyslipidemia and exercise affect coronary tone and differential regulation of conduit and microvessel K+ current. Am J Physiol Heart Circ Physiol. 2005;288:H1233–H1241. doi: 10.1152/ajpheart.00732.2004. [DOI] [PubMed] [Google Scholar]
- Nafikov RA, Beitz DC. Carbohydrate and lipid metabolism in farm animals. J Nutr. 2007;137:702–705. doi: 10.1093/jn/137.3.702. [DOI] [PubMed] [Google Scholar]
- Napolitano A, Lowell BB, Damm D, Leibel RL, Ravussin E, Jimerson DC, Lesem MD, Van Dyke DC, Daly PA, Chatis P, et al. Concentrations of adipsin in blood and rates of adipsin secretion by adipose tissue in humans with normal, elevated and diminished adipose tissue mass. Int J Obes Relat Metab Disord. 1994;18:213–218. [PubMed] [Google Scholar]
- Neeb ZP, Edwards JM, Alloosh M, Long X, Mokelke EA, Sturek M. Metabolic syndrome and coronary artery disease in Ossabaw compared with Yucatan swine. Comp Med. 2010;60:300–315. [PMC free article] [PubMed] [Google Scholar]
- Newell-Fugate AE, Taibl JN, Clark SG, Alloosh M, Sturek M, Krisher RL. Effects of diet-induced obesity on metabolic parameters and reproductive function in female Ossabaw minipigs. Comp Med. 2014;64:44–49. [PMC free article] [PubMed] [Google Scholar]
- Parikh RM, Mohan V. Changing definitions of metabolic syndrome. Indian J Endocrinol Metab. 2012;16:7–12. doi: 10.4103/2230-8210.91175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar AS, Zhu XY, Eirin A, Tang H, Jordan KL, Woollard JR, Lerman A, Lerman LO. Adipose tissue remodeling in a novel domestic porcine model of diet-induced obesity. Obesity (Silver Spring) 2015;23:399–407. doi: 10.1002/oby.20971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, Tune JD. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler Thromb Vasc Biol. 2010;30:1711–1717. doi: 10.1161/ATVBAHA.110.210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen R, Ingerslev HC, Sturek M, Alloosh M, Cirera S, Christoffersen BO, Moesgaard SG, Larsen N, Boye M. Characterisation of gut microbiota in Ossabaw and Gottingen minipigs as models of obesity and metabolic syndrome. PLoS One. 2013;8:e56612. doi: 10.1371/journal.pone.0056612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robich MP, Chu LM, Burgess TA, Feng J, Han Y, Nezafat R, Leber MP, Laham RJ, Manning WJ, Sellke FW. Resveratrol preserves myocardial function and perfusion in remote nonischemic myocardium in a swine model of metabolic syndrome. J Am Coll Surg. 2012;215:681–689. doi: 10.1016/j.jamcollsurg.2012.06.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen BS, Cook KS, Yaglom J, Groves DL, Volanakis JE, Damm D, White T, Spiegelman BM. Adipsin and complement factor D activity: an immune-related defect in obesity. Science. 1989;244:1483–1487. doi: 10.1126/science.2734615. [DOI] [PubMed] [Google Scholar]
- Sabe AA, Elmadhun NY, Robich MP, Dalal RS, Sellke FW. Does resveratrol improve insulin signaling in chronically ischemic myocardium? J Surg Res. 2013;183:531–536. doi: 10.1016/j.jss.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabe AA, Elmadhun NY, Sadek AA, Chu LM, Bianchi C, Sellke FW. Differential effects of atorvastatin on autophagy in ischemic and nonischemic myocardium in Ossabaw swine with metabolic syndrome. J Thorac Cardiovasc Surg. 2014;148:3172–3178. doi: 10.1016/j.jtcvs.2014.07.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabe AA, Elmadhun NY, Sadek AA, Dalal RS, Chu LM, Bianchi C, Sellke FW. Atorvastatin regulates apoptosis in chronically ischemic myocardium. J Card Surg. 2015;30:218–223. doi: 10.1111/jocs.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs DH. The pig as a potential xenograft donor. Vet Immunol Immunopathol. 1994;43:185–191. doi: 10.1016/0165-2427(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Schook LB, Tumbleson ME. Advances in swine in biomedical research. Plenum Press; 1996. [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Swindle MM. Preparation of swine for the laboratory. ILAR J. 2006;47:358–363. doi: 10.1093/ilar.47.4.358. [DOI] [PubMed] [Google Scholar]
- Speakman JR. Thrifty genes for obesity, an attractive but flawed idea, and an alternative perspective: the 'drifty gene' hypothesis. Int J Obes (Lond) 2008;32:1611–1617. doi: 10.1038/ijo.2008.161. [DOI] [PubMed] [Google Scholar]
- Spurlock ME, Frank GR, Cornelius SG, Ji S, Willis GM, Bidwell CA. Obese gene expression in porcine adipose tissue is reduced by food deprivation but not by maintenance or submaintenance intake. J Nutr. 1998;128:677–682. doi: 10.1093/jn/128.4.677. [DOI] [PubMed] [Google Scholar]
- Spurlock ME, Gabler NK. The development of porcine models of obesity and the metabolic syndrome. J Nutr. 2008;138:397–402. doi: 10.1093/jn/138.2.397. [DOI] [PubMed] [Google Scholar]
- te Pas MF, Koopmans SJ, Kruijt L, Calus MP, Smits MA. Plasma proteome profiles associated with diet-induced metabolic syndrome and the early onset of metabolic syndrome in a pig model. PLoS One. 2013;8:e73087. doi: 10.1371/journal.pone.0073087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbieta-Caceres VH, Lavi R, Zhu XY, Crane JA, Textor SC, Lerman A, Lerman LO. Early atherosclerosis aggravates the effect of renal artery stenosis on the swine kidney. Am J Physiol Renal Physiol. 2010;299:F135–F140. doi: 10.1152/ajprenal.00159.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamathevan JJ, Hall MD, Hasan S, Woollard PM, Xu M, Yang Y, Li X, Wang X, Kenny S, Brown JR, Huxley-Jones J, Lyon J, Haselden J, Min J, Sanseau P. Minipig and beagle animal model genomes aid species selection in pharmaceutical discovery and development. Toxicol Appl Pharmacol. 2013;270:149–157. doi: 10.1016/j.taap.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Wangsness PJ, Martin RJ, Gahagan JH. Insulin and growth hormone in lean and obese pigs. Am J Physiol. 1977;233:E104–E108. doi: 10.1152/ajpendo.1977.233.2.E104. [DOI] [PubMed] [Google Scholar]
- Xi S, Yin W, Wang Z, Kusunoki M, Lian X, Koike T, Fan J, Zhang Q. A minipig model of high-fat/high-sucrose diet-induced diabetes and atherosclerosis. Int J Exp Pathol. 2004;85:223–231. doi: 10.1111/j.0959-9673.2004.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Carballo-Jane E, McLaren DG, Mendoza VH, Gagen K, Geoghagen NS, McNamara LA, Gorski JN, Eiermann GJ, Petrov A, Wolff M, Tong X, Wilsie LC, Akiyama TE, Chen J, Thankappan A, Xue J, Ping X, Andrews G, Wickham LA, Gai CL, Trinh T, Kulick AA, Donnelly MJ, Voronin GO, Rosa R, Cumiskey AM, Bekkari K, Mitnaul LJ, Puig O, Chen F, Raubertas R, Wong PH, Hansen BC, Koblan KS, Roddy TP, Hubbard BK, Strack AM. Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. J Lipid Res. 2012;53:51–65. doi: 10.1194/jlr.M019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Liao D, Kusunoki M, Xi S, Tsutsumi K, Wang Z, Lian X, Koike T, Fan J, Yang Y, Tang C. NO-1886 decreases ectopic lipid deposition and protects pancreatic beta cells in diet-induced diabetic swine. J Endocrinol. 2004;180:399–408. doi: 10.1677/joe.0.1800399. [DOI] [PubMed] [Google Scholar]
- Yokota SD, Benyajati S, Dantzler WH. Comparative aspects of glomerular filtration in vertebrates. Ren Physiol. 1985;8:193–221. doi: 10.1159/000173055. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li ZL, Eirin A, Ebrahimi B, Pawar AS, Zhu XY, Lerman A, Lerman LO. Cardiac metabolic alterations in hypertensive obese pigs. Hypertension. 2015;66:430–436. doi: 10.1161/HYPERTENSIONAHA.115.05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li ZL, Woollard JR, Eirin A, Ebrahimi B, Crane JA, Zhu XY, Pawar AS, Krier JD, Jordan KL, Tang H, Textor SC, Lerman A, Lerman LO. Obesity-metabolic derangement preserves hemodynamics but promotes intrarenal adiposity and macrophage infiltration in swine renovascular disease. Am J Physiol Renal Physiol. 2013;305:F265–F276. doi: 10.1152/ajprenal.00043.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]