Abstract
Aim
Assess the burden of congenital and perinatal cytomegalovirus (CMV) disease among infants hospitalized in neonatal intensive care units (NICUs).
Methods
CMV infection was defined as a report of positive CMV viral culture or PCR at any time since birth in an infant hospitalized in a NICU reporting to California Perinatal Quality Care Collaborative during 2005–2010.
Results
156 (1.7 per 1000) infants were reported with CMV infection, representing an estimated 5% of the expected number of live births with symptomatic CMV disease. Prevalence was higher among infants with younger gestational ages and lower birth weights. Infants with CMV infection had significantly longer hospital stays; 14 (9%) died.
Conclusions
Reported prevalence of CMV infection in NICUs represents a fraction of total expected disease burden from CMV in the newborn period, likely resulting from underdiagnosis and milder symptomatic cases that do not require NICU care. More complete ascertainment of infants with congenital CMV infection that would benefit from antiviral treatment may reduce the burden of CMV disease in this population.
Keywords: cytomegalovirus, congenital infection, acquired infection, prevalence, premature infant, very low birth weight infant
BACKGROUND
In the United States, an estimated 28,000 (0.7%) infants are born with congenital cytomegalovirus (CMV) infection annually, 3,600 (12.7%) of whom are estimated to be symptomatic at birth [6]. Symptomatic congenital CMV disease is characterized by petechiae, purpura, thrombocytopenia, hepatosplenomegaly, direct hyperbilirubinemia, microcephaly, intracranial calcifications, chorioretinitis, and hearing impairment [4, 10]. To confirm a diagnosis of congenital CMV infection, CMV must be detected in urine, saliva or blood within 2–3 weeks of birth [19, 27]. In a study of dried blood samples collected for newborn screening, the prevalence of congenital CMV infection was significantly higher among preterm and low birth weight infants [13]. As such, the prevalence of congenital CMV infection among infants admitted to neonatal intensive care units (NICUs) is likely higher than that of the overall population [22, 26, 28].
A subset of the NICU population, infants born <32 weeks gestational age or with a birth weight <1500g, is at risk for symptomatic CMV disease [7] from infection acquired peri- or postnatally through exposure to infected maternal genital secretions, breast milk, or blood transfusion [32]. The risk of CMV transmission by blood transfusion can be reduced by the use of CMV-seronegative or leukocyte-reduced blood components [1, 18, 35], which have become the standard of care for premature infants in the United States [1, 11, 31]; most CMV infections in neonates are likely transmitted by breast milk [7, 17, 34]. The clinical characteristics associated with postnatally-acquired CMV infection include hepatopathy, thrombocytopenia, neutropenia, petechiae, respiratory distress syndrome, and sepsis-like syndrome [7, 34].
Limited data are available on the burden of CMV disease in infancy and the extent to which it is severe enough to require hospitalization in NICUs. This study describes demographic and clinical characteristics of infants with reported CMV infection in California NICUs during 2005–2010 and estimate the proportion of expected live births with symptomatic congenital or acquired CMV disease identified in California NICUs during the study period.
METHODS
Study population
The California Perinatal Quality Care Collaborative (CPQCC) collects data for over 90% of infants cared for in California NICUs [8, 20]. Eligibility criteria include admission to a NICU at birth or within 28 days of life and one of the following: a) birth weight between 401g–1,500g, b) gestational age between 22 weeks and 29 weeks 6 days, or c) for infants with birth weight >1500g, either death, surgery, intubation for >4 hours, positive pressure support for >4 hours, hyperbilirubinemia, early bacterial sepsis or acute transfer.
A comprehensive questionnaire including demographics, maternal and delivery history, post-delivery diagnoses and interventions is used to collect data for each eligible infant. Data are abstracted by NICU personnel (i.e. physicians, nurses and other trained abstractors) and submitted electronically or on-line. Because the current definition of congenital CMV infection used by CPQCC is based on documentation of a positive viral culture or PCR for CMV at any time since birth, it is possible that infants with hospitalizations lasting beyond the first 3 weeks after birth had peri- or postanatally acquired CMV infection; 136 (87%) infants reported with CMV infection were discharged or died after 3 weeks of life. Specimen type and date of collection are not recorded, precluding our ability to verify the diagnosis and categorize infants as having congenital or acquired CMV infection.
Data analysis
This study utilized data from 2005–2010 to assess the prevalence of CMV infection per 1000 live births, using as a denominator the number of live births reported to the CPQCC. The following clinical characteristics were available for analysis: antenatal conditions (fetal distress, intrauterine growth retardation [IUGR], any anomaly diagnosed prior to birth); Apgar at 1st minute, respiratory distress syndrome, neurologic abnormalities (microcephaly, seizure, cystic periventricular leukomalacia, and periventricular-intraventricular hemorrhage [PIH]); death; and length of hospital stay.
Microcephaly was defined as reported head circumference measured on the day of birth or the following day ≤10th percentile according to U.S. growth charts of preterm infants [25]. Cystic periventricular leukomalacia and PIH were identified by cranial ultrasound, computerized tomographic scan or magnetic resonance imaging performed on or before 28 days of life. PIH was graded as follows: 0 for no subependymal or intraventricular hemorrhage; 1 for subependymal germinal matrix hemorrhage only; 2 for intraventricular blood, without ventricular dilation; 3 for intraventricular blood with ventricular dilation; and 4 for intraparenchymal hemorrhage.
In univariate analyses, clinical characteristics of infants with and without reported CMV infection were compared. Analyses of clinical characteristics were performed for very low birth weight (VLBW) infants (those with birth weight between 401g–1,500g) and infants weighing >1500g. Infants with other congenital or perinatal infections, such as herpes simplex virus and HIV, were excluded from the analyses. Statistical analyses, performed using SAS version 9.3 (SAS, Cary, NC), were based on Chi-square test or exact test for comparison of categorical variables, Chi-square for linear trend, and the Wilcoxon test for continuous variables. Statistical analyses were
The proportion of the expected number of live births with symptomatic CMV disease in California that was identified in California NICUs was estimated assuming all infants reported with CMV infection would have symptomatic disease, given that CMV-specific testing among infants in the United States appears to be conducted primarily for diagnostic purposes [21]. CPQCC average coverage of California NICUs of 87% (85% during 2005–2008 and 90% during 2009–2010) was used to extrapolate the number of live births reported with CMV infection in CPQCC-member hospitals to the entire state of California. The expected number of live births with symptomatic congenital CMV disease was derived assuming an annual birth prevalence of congenital CMV infection of 0.7%, with 12.7% of infected live births presenting symptomatic congenital CMV disease [6]. The expected number of live births with symptomatic acquired CMV disease was estimated assuming that symptomatic disease from peri- or postnatally acquired infection was limited to live births <32 weeks gestational age or with a birth weight <1500g, with an incidence rate of 2.4% [17].
The study protocol was submitted to the Institutional Review Boards at the Centers for Disease Control and Prevention and Stanford University and deemed exempt from further review because all data were de-identified.
RESULTS
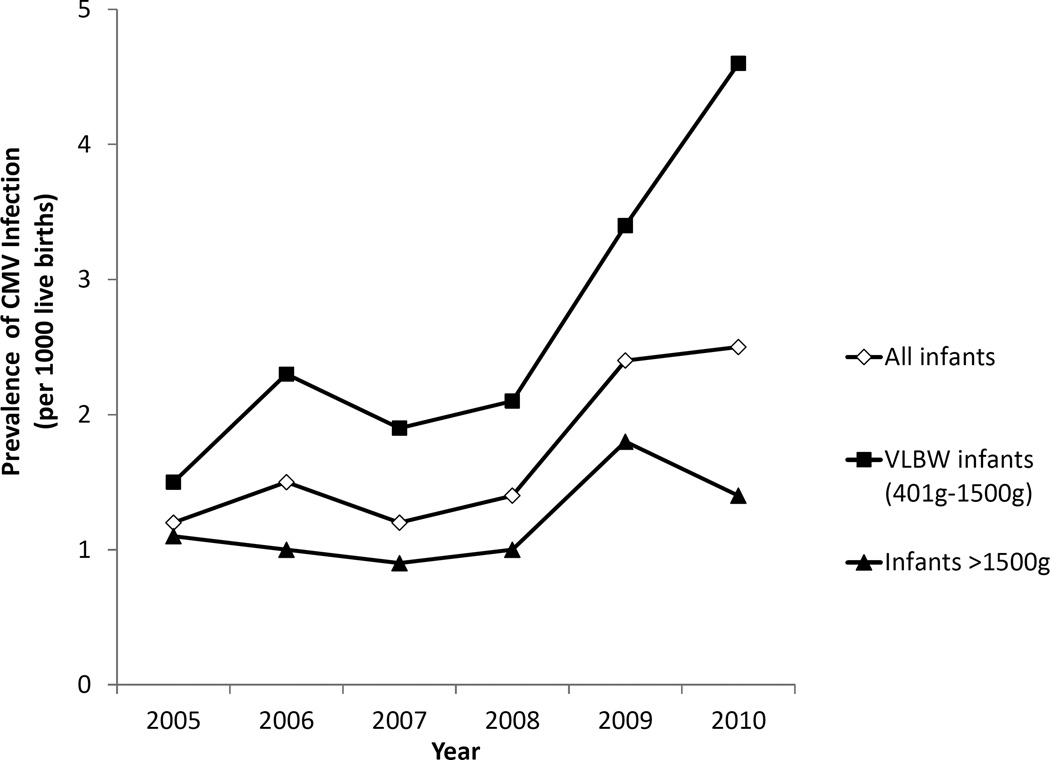
During 2005–2010, 156 of 91,435 infants in CPQCC-member hospital NICUs were reported as having CMV infection; 87 of 33,588 VLBW infants and 69 of 57,847 infants >1500g. This corresponds to an overall prevalence of 1.7 infants reported with CMV infection per 1000 live births requiring NICU care; 2.6 per 1000 among VLBW infants and 1.2 per 1000 among infants >1500g. The prevalence of CMV infection increased from 1.1 to 2.5 per 1000 live births during 2005–2010 with marked increases among VLBW infants (1.5 to 4.6 per 1000 live births) (Figure 1). The prevalence of CMV infection was higher among infants with younger gestational age (<32 weeks: 2.4 per 1000, 32–36 weeks: 1.4 per 1000, and ≥37 weeks: 1.2 per 1000), and among infants with lower birth weight (<1500g: 2.6 per 1000, 1501g–2500g: 1.8 per 1000, and >2500g: 0.8 per 1000) (P<0.01).
Figure 1.
Prevalence of Reported CMV Infection by Year, CPQCC NICUs, California, 2005–2010
The majority of infants reported with CMV infection were born to mothers 20–29 years of age (44%) (Table 1). The prevalence of CMV infection was highest among infants born to mothers <20 years of age, 1.8-fold that of infants born to mothers 30–39 years of age. The highest prevalence of CMV infection was observed among infants born to Asian/Pacific Islander mothers (3.4 per 1000 live births), followed by those born to Hispanics (1.8 per 1000 live births) and non-Hispanic blacks (1.7 per 1000 live births). Among VLBW infants, the prevalence of CMV infection among infants born to Asian/Pacific Islander mothers was nearly three-fold that of infants born to non-Hispanic white mothers (PR=2.9; 95%CI-1.5–5.5). Among infants >1500g, the prevalence of CMV infection among infants born to Hispanic mothers was nearly two-fold that of infants born to non-Hispanic white mothers (PR=1.9; 95%CI=1.1–3.5).
Table 1.
NICU Care Level and Maternal Demographics of Infants Reported with CMV Infection in California, 2005–2010
| Characteristic | Infants reported with CMV infection n (%) |
Prevalence per 1000 |
Prevalence Ratio (95% CI) |
|
|---|---|---|---|---|
| Birth Hospital Level (NICU care level) | ||||
| Primary | 33 (21) | 1.2 | Reference | |
| Intermediate (II) | 10 (6) | 1.6 | 1.4 (0.7–2.8) | |
| Community (IIIA/B) | 86 (55) | 2.3 | 2.0 (1.3–2.9) | |
| Regional (IIIC/D) | 23 (15) | 1.5 | 1.3 (0.7–2.1) | |
| Non-CCS/Home Birth | 4 (3) | 0.8 | 0.7 (0.2–2.0) | |
| Total | 156 (100) | 1.7 | ||
| Maternal Age | ||||
| <20 | 24 (16) | 2.6 | 1.8 (1.1–3.0) | |
| 20–29 | 69 (44) | 1.7 | 1.2 (0.8–1.7) | |
| 30–39 | 50 (32) | 1.4 | Reference | |
| 40+ | 11 (7) | 2.2 | 1.5 (0.8–3.0) | |
| Total | 154 (100) | 1.7 | ||
| Maternal Race/Ethnicity | ||||
| Non-Hispanic White | 33 (21) | 1.2 | Reference | |
| Non-Hispanic Black | 14 (9) | 1.7 | 1.5 (0.8–2.8) | |
| Hispanic | 78 (50) | 1.8 | 1.6 (1.0–2.3) | |
| Asian/Pacific Islander | 27 (17) | 3.4 | 3.0 (1.8–4.9) | |
| Others | 3 (2) | 1.3 | 1.1 (0.3–3.5) | |
| Total | 155 (100) | 1.7 | ||
Overall, IUGR was diagnosed in 25 (17%) infants reported with CMV infection (Table 2), and was more common than in infants without CMV infection (Table 2). Among infants >1500g, 15 (23%) with CMV infection had anomalies detected prior to birth (brain anomalies, cardiac defects, or gastrointestinal malformations) two times as many as those without CMV infection (P<0.05). The proportion of infants with Apgar scores ≤3 did not differ by CMV status, even after stratification by birth weight. VLBW infants with CMV infection were more likely than those without CMV infection to have respiratory distress syndrome (OR=2.5; 95%CI=1.3–4.7) (P<0.01). Although only two infants reported with CMV infection had microcephaly listed as a congenital anomaly, 19 (14%) infants with CMV infection were determined to have head circumference ≤3rd percentile, and 42 (31%) had microcephaly (head circumference ≤ 10th percentile). Microcephaly was more common among infants with CMV infection (P<0.05).
Table 2.
Clinical Characteristics of Infants by Reported CMV Infection Status, CPQCC NICUs, California, 2005–2010
| Characteristics | All infants reported with CMV infection (n=156) |
VLBW infants (401g–1500g) (N=33 511) |
Infants >1500g (N=57 683) |
||||
|---|---|---|---|---|---|---|---|
| CMV (n=87) |
No viral infection reported (n=33 424) |
OR (95% CI), P-value |
CMV (n=69) |
No viral infection reported (n=57 614) |
OR (95% CI), P-value |
||
| n(%) | n(%) | n(%) | n(%) | n(%) | |||
| Fetal distress | 37 (25) | 21 (24) | 7023 (22) | 16 (25) | 9 388 (17) | ||
| IUGR | 25 (17) | 19 (22) | 4 219 (13) | 1.9 (1.1–3.1) | 6 (9) | 1 881 (3) | 3.0 (1.3–6.9) |
| Anomaly | 15 (10) | 0 (0) | 925 (3) | 15 (23) | 6 833 (12) | 2.2 (1.2–3.9) | |
| Apgar ≤ 3,1st minute | 41 (27) | 28 (33) | 8 827 (27) | 13 (20) | 7 410 (13) | ||
| Respiratory distress syndrome | 99 (63) | 76 (87) | 22 906 (73) | 2.5 (1.3–4.7) | 23 (33) | 19 538 (34) | |
| Head circumference ≤3rdpercentile | 19 (14) | 11 (14) | 2206 (9) | 8 (14) | 1478 (3) | 4.8 (2.2–10.1) | |
| Microcephaly | 42 (31) | 24 (31) | 5 051 (20) | 1.8 (1.1–3.0) | 18 (32) | 4 531 (10) | 3.9 (2.2–6.9) |
| Seizure | 6 (4) | 3 (3) | 1206 (4) | 3 (4) | 2922 (5) | ||
| Neural imaging | 147 (94) | 87 (100) | 29039 (93) | 60 (87) | 25588 (45) | 8.2 (4.1–16.5) | |
| Cystic periventricular leukomalacia | 6 (4) | 3 (3) | 762 (3) | 3 (5) | 340 (1) | 3.8 (1.2–12.1) | |
| PIH Grades 1–4 | 42 (28) | 27 (31) | 7764 (27) | 15 (25) | 2214 (9) | 3.5 (1.9–6.2) | |
| Death | 14 (9) | 8 (9) | 2929 (9) | 6 (9) | 2169 (4) | 2.4 (1.1–5.6) | |
| Gestational age (weeks), median (range) | 31 (22–41) | 27 (22–35) | 28 (17–41) | P<0.05 | 37 (30–41) | 37 (22–44) | P>0.05 |
| Birth weight (grams), median (range) | 840 (430–1495) |
1060 (120–1500) |
P<0.01 | 2410 (1505–4224) |
2810 (1501–6940) |
P<0.01 | |
Notes: odds ratio, 95% confidence interval are shown only when significant association was observed (P<0.05). IUGR: intra-uterine growth retardation; Microcephaly defined as head circumference ≤10th percentile; PIH: periventricular-intraventricular hemorrhage graded as follows: 0 for no subependymal or intraventricular hemorrhage which constituted the reference group; 1 for subependymal germinal matrix hemorrhage only; 2 for intraventricular blood, without ventricular dilation; 3 for intraventricular blood with ventricular dilation; and 4 for intraparenchymal hemorrhage. Wilcoxon test was used to compare continuous variables.
Among infants reported with CMV infection, 99% of VLBW infants and 81% of infants >1500g were hospitalized in a NICU beginning on in the 1st day of life. Fourteen (9%) infants reported with CMV infection died, including 8 (9%) of the VLBW infants and 6 (9%) of the infants >1500g (Table 2), at a median of 38 (range: 3–99) and 9 (range: 3–107) days of life, respectively. Among infants >1500g, the frequency of death was significantly higher among those with CMV infection. Among surviving infants with CMV infection, the median length of hospital stay was 99 (interquartile range: 72–125) days for VLBW infants and 48 (18–76) days for infants >1500g, which was significantly longer than for those without CMV infection, 60 (42–86) days for VLBW infants and 14 (8–27) days for infants >1500g (P<0.01).
Of a total of 3 272 565 live births in California during 2005–2010, an estimated 3834 would have symptomatic CMV disease - 2909 live births with symptomatic congenital CMV disease, and 925 VLBW live births with symptomatic acquired CMV disease (Table 3). The total number of live births with symptomatic CMV disease identified in California NICUs extrapolated from CPQCC data (n=179) represented 5% of the expected number of live births with symptomatic CMV disease in the newborn period during 2005–2010; this proportion increased from 3% in 2005 to 7% in 2010.
Table 3.
Estimated Proportion of Expected Live Births with Symptomatic Congenital or Acquired CMV Disease Identified in California NICUs, 2005–2010
| n | |
|---|---|
| Total number of live births in California during 2005–2010 | 3 272 565 |
| Number of VLBW live births | 38 539 |
| Expected number of live births in California with | |
| Symptomatic congenital CMV disease (LB*0.7%*12.7%)§ | 2909(a) |
| Symptomatic acquired CMV disease among VLBW live births (VLBW*2.4%)¥ | 925(b) |
| Total number of live births with either symptomatic congenital or acquired CMV disease | 3834(a+b) |
| Number of live births reported with CMV infection in CPQCC-member NICUs | 156 |
| Number of live births identified with CMV infection in California NICUs extrapolated from CPQCC£ | 179(c) |
| Proportion of expected number of live births with symptomatic congenital or acquired CMV disease identified in California NICUs | 5%(c/(a+b)) |
Notes:
Estimates of birth prevalence of congenital CMV infection and proportion of symptomatic infants at birth according to a published systematic review of the literature [6]
Estimates of postnatal CMV disease acquired via consumption of frozen breast milk [17]
Based on average CPQCC coverage of 87% during 2005–2010
DISCUSSION
Limited data are available on the burden of disease caused by congenital or acquired CMV infection among infants in the NICU setting [22, 26, 28]. While congenital CMV infection can result in severe disease [4, 10, 15], the proportion of infants with disease severe enough to require NICU care is unknown. The overall reported prevalence of CMV infection was 1.7 per 1000 live births requiring NICU care in California, higher than the estimated prevalence of symptomatic congenital CMV disease in the general population (0.9 per 1000 live births) [6]. However, the proportion of infants identified with CMV infection in NICUs represents only a fraction of the total expected burden from symptomatic CMV disease, likely resulting from milder symptomatic cases that do not require NICU care. Nonetheless, CMV infection was associated with prolonged NICU stays, higher mortality rates, and neurological abnormalities.
Data on the prevalence of congenital or acquired CMV infection among NICU infants based on screening studies are limited. A study in Canada [33] found a prevalence of congenital CMV infection among VLBW infants of 1.5%. Another study in Spain [2] diagnosed 2.3% of VLBW infants with congenital CMV infection and 10.2% with peri- or postnatally acquired CMV infection. In our study, the true prevalence of CMV disease in NICUs was likely underestimated because even clinically apparent CMV infections may not be suspected or tested for in a timely manner, laboratory techniques have variable performance depending on the specimen type, and not all infections diagnosed may have been reported. Typical practice for considering and diagnosing CMV infection among NICU infants is currently unknown; the increasing prevalence of CMV infection in recent years of CPQCC data could reflect changing practices in CMV testing, diagnosis, and/or reporting, and merits further investigation. Some of the infants reported in our study may have had asymptomatic CMV infection. Our major limitation was the inability to classify reported infants as having congenital or acquired CMV infection. Because acquired CMV infection might occur in up to approximately 10% of VLBW infants [2, 17], screening VLBW infants for CMV infection at birth would allow for distinguishing between congenital and acquired CMV infection, which have different prognoses for long-term sequelae and might simplify follow-up of these infants.
In our study, the prevalence of CMV infection increased with younger gestational age and lower birth weight. In addition, CMV infection was associated with IUGR both in VLBW infants and infants >1500g. The mechanisms by which CMV infection may be associated with prematurity and IUGR are not completely understood. Altered placental cytokine expression is associated with IUGR, prematurity, and early membrane rupture in uninfected pregnancies [3]. Recent studies suggest that CMV infection could result in proinflammatory changes with important consequences for placental development and function, virus transmission, and fetal viability [9, 30]. Understanding immune mechanisms at the level of the placenta associated with CMV transmission is an active area of research with the potential to aid the development of vaccines and immunotherapies [29].
Microcephaly is a condition frequently reported among infants with congenital CMV disease [4, 10], but it was reported as a congenital anomaly for only 2 infants with CMV infection in our study. When the criteria of head circumference ≤10th percentile using appropriate population-based growth charts was applied, 31% of infants with CMV infection were classified as having microcephaly. Microcephaly was more common among infants with reported CMV infection compared to those without CMV infection both among VLBW infants and infants >1500g. While nearly all VLBW infants were routinely screened for brain abnormalities, among infants >1500g, it was more likely among those reported with CMV infection than those without, suggesting that this group more likely has true congenital CMV infection for which evaluation for brain abnormalities might be a routine practice. The role of neuroimaging is likely to evolve as more experience is gained with use of antiviral treatments for infants with congenital CMV-related neurologic abnormalities.
In populations with high maternal CMV seroprevalence, the birth prevalence of congenital CMV infection tends to be higher as does the acquisition of CMV through breastfeeding [5, 12]. A study using dried blood spots from a representative sample of newborns in California found the highest maternal IgG CMV seroprevalence in Hispanics (89%), followed by Asians (87%), blacks (77%) and whites (57%) [13]. In our study, the prevalence of CMV infection was highest among infants born to Asian/Pacific Islander mothers (3.4 per 1000), followed by Hispanics (1.8 per 1000), non-Hispanic blacks (1.7 per 1000), and non-Hispanic whites (1.2 per 1000). Information on country of origin and other maternal sociodemographic variables were not available which could have helped to better understand the higher prevalence of CMV infection observed among infants born to Asian/Pacific Islander mothers.
Population-based studies are needed to better understand the spectrum of symptomatic congenital and acquired CMV disease as well as the sub-groups of infants in whom disease is most prevalent. Understanding the proportion of cases of congenital and acquired CMV infection that are severe and require NICU care is important for assessing the burden and costs of CMV disease in infancy. In the absence of universal newborn screening most cases of congenital CMV infection, both asymptomatic and symptomatic, are likely to be missed. Newborn screening for CMV has the potential to identify infants at risk of hearing loss and cognitive delays although more data are needed on the interventions these infants would need and the benefit that prompt identification through early intervention could provide. More complete ascertainment of infants with symptomatic congenital CMV disease may facilitate appropriate selection of infants with central nervous system disease who would benefit from treatment with antivirals [14, 16, 23, 24]. In addition, data on burden of acquired CMV disease in VLBW and premature infants are important for guiding use of breast milk in this population [17].
Acknowledgments
Funding Source: No external funding was used for this study.
Abbreviations
- CMV
cytomegalovirus
- VLBW
very low birth weight
- NICU
neonatal intensive care units
- CPQCC
California Perinatal Quality Care Collaborative
- IUGR
intrauterine growth retardation
- PIH
periventricular-intraventricular hemorrhage
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.American Association of Blood Banks. Association Bulletin #97-2. Leukocyte reduction for the prevention of trasfusion-transmitted cytomegalovirus (TT-CMV) [Google Scholar]
- 2.Alvarez Dominguez E, Figueras Aloy J, Botet Mussons F, Marcos Maeso MA, Perez Fernandez JM. Cribado de la infeccion por citomegalovirus en recien nacidos de muy bajo peso [Screening for cytomegalovirus infection in very low birth weight infants] An Pediatr (Barc) 2013;79(1):3–9. doi: 10.1016/j.anpedi.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Ayatollahi M, Geramizadeh B, Yazdani M, Azarpira N. Effect of the immunoregulatory cytokines on successful pregnancy depends upon the control of graft rejection mechanisms. Transplant Proc. 2007;39(1):244–245. doi: 10.1016/j.transproceed.2006.10.223. [DOI] [PubMed] [Google Scholar]
- 4.Boppana SB, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11(2):93–99. doi: 10.1097/00006454-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Britt W. Infectious Diseases of the Fetus and Newborn. 7th. Philadelphia: W.B. Saunders; 2011. Cytomegalovirus; pp. 706–755. [Google Scholar]
- 6.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17(5):355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 7.Dworsky M, Yow M, Stagno S, Pass RF, Alford C. Cytomegalovirus infection of breast milk and transmission in infancy. Pediatrics. 1983;72(3):295–299. [PubMed] [Google Scholar]
- 8.Gould JB. The role of regional collaboratives: the California Perinatal Quality Care Collaborative model. Clin Perinatol. 2010;37(1):71–86. doi: 10.1016/j.clp.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton ST, Scott G, Naing Z, Iwasenko J, Hall B, Graf N, et al. Human cytomegalovirus-induces cytokine changes in the placenta with implications for adverse pregnancy outcomes. PLoS One. 2012;7(12):e52899. doi: 10.1371/journal.pone.0052899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Istas AS, Demmler GJ, Dobbins JG, Stewart JA. Surveillance for congenital cytomegalovirus disease: a report from the National Congenital Cytomegalovirus Disease Registry. Clin Infect Dis. 1995;20(3):665–670. doi: 10.1093/clinids/20.3.665. [DOI] [PubMed] [Google Scholar]
- 11.Josephson CD, Castillejo MI, Caliendo AM, Waller EK, Zimring J, Easley KA, et al. Prevention of transfusion-transmitted cytomegalovirus in low-birth weight infants (</=1500 g) using cytomegalovirus-seronegative and leukoreduced transfusions. Transfus Med Rev. 2011;25(2):125–132. doi: 10.1016/j.tmrv.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 13.Kharrazi M, Hyde T, Young S, Amin MM, Cannon MJ, Dollard SC. Use of screening dried blood spots for estimation of prevalence, risk factors, and birth outcomes of congenital cytomegalovirus infection. J Pediatr. 2010;157(2):191–197. doi: 10.1016/j.jpeds.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Kimberlin DW, Lin CY, Sanchez PJ, Demmler GJ, Dankner W, Shelton M, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143(1):16–25. doi: 10.1016/s0022-3476(03)00192-6. [DOI] [PubMed] [Google Scholar]
- 15.Kylat RI, Kelly EN, Ford-Jones EL. Clinical findings and adverse outcome in neonates with symptomatic congenital cytomegalovirus (SCCMV) infection. Eur J Pediatr. 2006;165(11):773–778. doi: 10.1007/s00431-006-0172-6. [DOI] [PubMed] [Google Scholar]
- 16.Lackner A, Acham A, Alborno T, Moser M, Engele H, Raggam RB, et al. Effect on hearing of ganciclovir therapy for asymptomatic congenital cytomegalovirus infection: four to 10 year follow up. J Laryngol Otol. 2009;123(4):391–396. doi: 10.1017/S0022215108003162. [DOI] [PubMed] [Google Scholar]
- 17.Lanzieri TM, Dollard SC, Josephson CD, Schmid DS, Bialek SR. Breast Milk-Acquired Cytomegalovirus Infection and Disease in VLBW and Premature Infants. Pediatrics. 2013;131(6):e1937–e1945. doi: 10.1542/peds.2013-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laupacis A, Brown J, Costello B, Delage G, Freedman J, Hume H, et al. Prevention of posttransfusion CMV in the era of universal WBC reduction: a consensus statement. Transfusion. 2001;41(4):560–569. doi: 10.1046/j.1537-2995.2001.41040560.x. [DOI] [PubMed] [Google Scholar]
- 19.Lazzarotto T, Guerra B, Lanari M, Gabrielli L, Landini MP. New advances in the diagnosis of congenital cytomegalovirus infection. J Clin Virol. 2008;41(3):192–197. doi: 10.1016/j.jcv.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Lee HC, Green C, Hintz SR, Tyson JE, Parikh NA, Langer J, et al. Prediction of death for extremely premature infants in a population-based cohort. Pediatrics. 2010;126(3):e644–e650. doi: 10.1542/peds.2010-0097. [DOI] [PubMed] [Google Scholar]
- 21.Leung J, Cannon MJ, Grosse SD, Bialek SR. Laboratory testing for cytomegalovirus among pregnant women in the United States: a retrospective study using administrative claims data. BMC Infect Dis. 2012;12:334. doi: 10.1186/1471-2334-12-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald H, Tobin JH. Congenital cytomegalovirus infection: a collaborative study on epidemiological, clinical and laboratory findings. Dev Med Child Neurol. 1978;20(4):471–482. doi: 10.1111/j.1469-8749.1978.tb15248.x. [DOI] [PubMed] [Google Scholar]
- 23.Michaels MG, Greenberg DP, Sabo DL, Wald ER. Treatment of children with congenital cytomegalovirus infection with ganciclovir. Pediatr Infect Dis J. 2003;22(6):504–509. doi: 10.1097/01.inf.0000069767.43169.2d. [DOI] [PubMed] [Google Scholar]
- 24.Nassetta L, Kimberlin D, Whitley R. Treatment of congenital cytomegalovirus infection: implications for future therapeutic strategies. J Antimicrob Chemother. 2009;63(5):862–867. doi: 10.1093/jac/dkp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2):e214–e224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 26.Panhani S, Heinonen KM. Screening for congenital cytomegalovirus infection among preterm infants born before the 34th gestational week in Finland. Scand J Infect Dis. 1994;26(4):375–378. doi: 10.3109/00365549409008607. [DOI] [PubMed] [Google Scholar]
- 27.Revello MG, Gerna G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin Microbiol Rev. 2002;15(4):680–715. doi: 10.1128/CMR.15.4.680-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos DV, Souza MM, Goncalves SH, Cotta AC, Melo LA, Andrade GM, et al. Congenital cytomegalovirus infection in a neonatal intensive care unit in brazil evaluated by PCR and association with perinatal aspects. Rev Inst Med Trop Sao Paulo. 2000;42(3):129–132. doi: 10.1590/s0036-46652000000300003. [DOI] [PubMed] [Google Scholar]
- 29.Schleiss MR. Cytomegalovirus in the Neonate: Immune Correlates of Infection and Protection. Clin Dev Immunol. 2013;2013:501801. doi: 10.1155/2013/501801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott GM, Chow SS, Craig ME, Pang CN, Hall B, Wilkins MR, et al. Cytomegalovirus infection during pregnancy with maternofetal transmission induces a proinflammatory cytokine bias in placenta and amniotic fluid. J Infect Dis. 2012;205(8):1305–1310. doi: 10.1093/infdis/jis186. [DOI] [PubMed] [Google Scholar]
- 31.Spinella PC, Dressler A, Tucci M, Carroll CL, Rosen RS, Hume H, et al. Survey of transfusion policies at US and Canadian children's hospitals in 2008 and 2009. Transfusion. 2010;50(11):2328–2335. doi: 10.1111/j.1537-2995.2010.02708.x. [DOI] [PubMed] [Google Scholar]
- 32.Stagno S, Reynolds DW, Pass RF, Alford CA. Breast milk and the risk of cytomegalovirus infection. N Engl J Med. 1980;302(19):1073–1076. doi: 10.1056/NEJM198005083021908. [DOI] [PubMed] [Google Scholar]
- 33.Vaudry W, Rosychuk RJ, Lee BE, Cheung PY, Pang X, Preiksaitis JK. Congenital cytomegalovirus infection in high-risk Canadian infants: Report of a pilot screening study. Can J Infect Dis Med Microbiol. 2010;21(1):e12–e19. doi: 10.1155/2010/942874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vochem M, Hamprecht K, Jahn G, Speer CP. Transmission of cytomegalovirus to preterm infants through breast milk. Pediatr Infect Dis J. 1998;17(1):53–58. doi: 10.1097/00006454-199801000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Yeager AS, Grumet FC, Hafleigh EB, Arvin AM, Bradley JS, Prober CG. Prevention of transfusion-acquired cytomegalovirus infections in newborn infants. J Pediatr. 1981;98(2):281–287. doi: 10.1016/s0022-3476(81)80662-2. [DOI] [PubMed] [Google Scholar]