Abstract
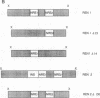
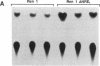
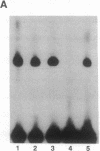
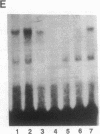
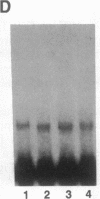
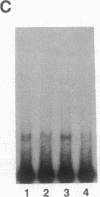
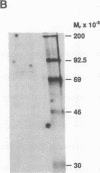
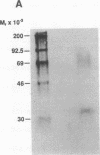
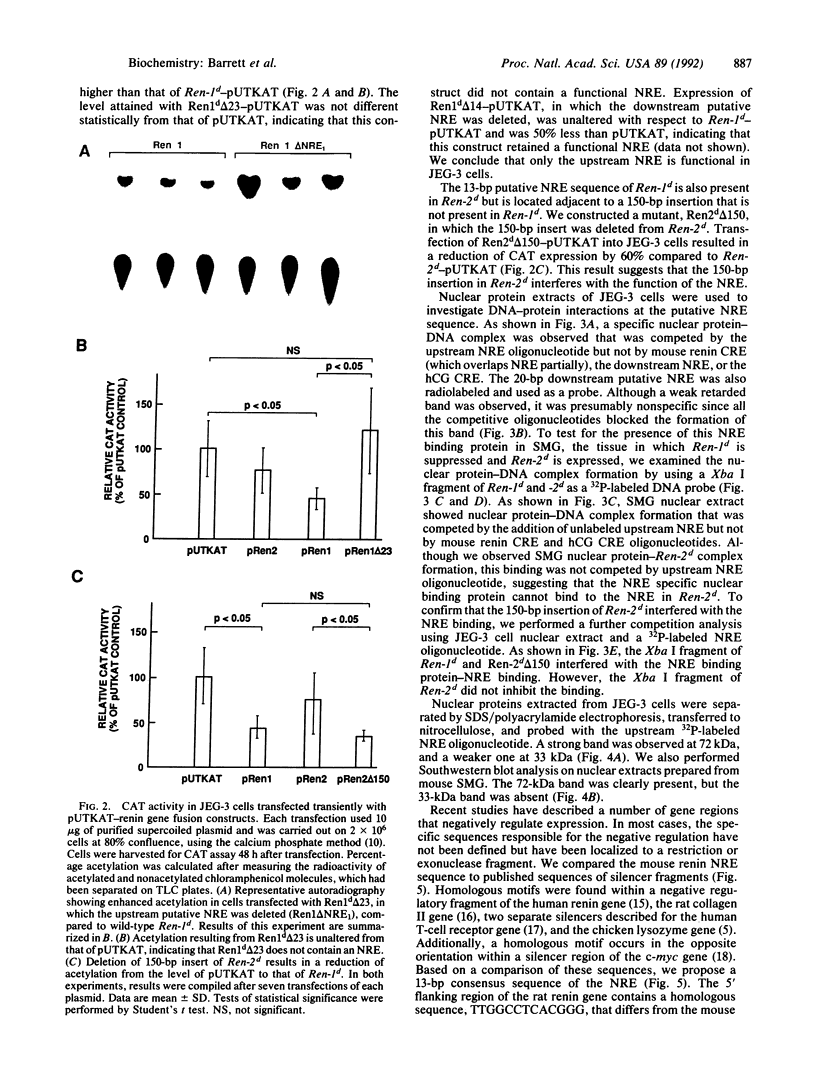
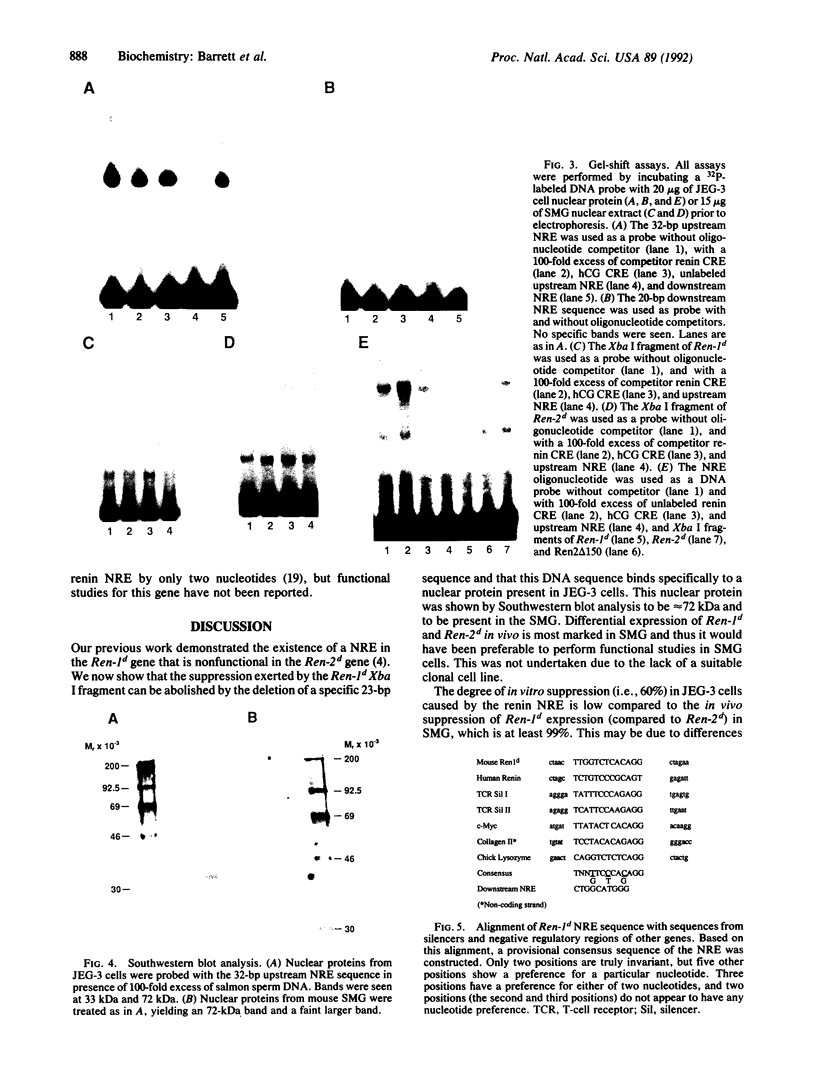
The 5' flanking region of the mouse renin genes (Ren-1d and Ren-2d) contains two motifs that are homologous to known negative regulatory elements (NREs). Ren-2d has a 150-base-pair (bp) insertion 5' to the upstream putative NRE (NRE-1), which is lacking in Ren-1d. We tested the functionality of these sequences by using site-directed mutagenesis to delete individually each putative NRE from Ren-1d and to delete the 150-bp insertion from Ren-2d. We examined the effect of these mutations on the expression of the reporter gene chloramphenicol acetyltransferase, which was expressed from a truncated thymidine kinase promoter fused to the renin regulatory region. This plasmid was transfected into human choriocarcinoma JEG-3 cells. Only the upstream NRE (positions -619 to -597) was found to be functional in Ren-1d. The deletion of a 150-bp insertion from Ren-2d resulted in the suppression of chloramphenicol acetyltransferase activity to the level of Ren-1d expression. These data suggest that the upstream NRE that is functional in Ren-1d, but not in Ren-2d, may be partly responsible for differential expression of the renin genes in various tissues. The molecular mechanism of the NRE was examined by studying its interaction with nuclear proteins in submandibular gland and JEG-3 cells by gel-mobility-shift assays. Specific nuclear protein binding was observed only to the upstream NRE and the molecular mass of this protein was approximately 72 kDa as determined by Southwestern blot analysis. Thus our results suggest that both Ren-1d and Ren-2d conserve a cis-acting NRE in the 5' flanking region. In Ren-1d, this NRE could bind a specific nuclear protein resulting in the inhibition of Ren-1d expression in these tissues. On the other hand, the NRE in Ren-2d is nonfunctional due to interference by an adjacent 150-bp insertion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baniahmad A., Muller M., Steiner C., Renkawitz R. Activity of two different silencer elements of the chicken lysozyme gene can be compensated by enhancer elements. EMBO J. 1987 Aug;6(8):2297–2303. doi: 10.1002/j.1460-2075.1987.tb02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniahmad A., Steiner C., Köhne A. C., Renkawitz R. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell. 1990 May 4;61(3):505–514. doi: 10.1016/0092-8674(90)90532-j. [DOI] [PubMed] [Google Scholar]
- Barrett G. L., Morgan T. O., Smith M., Alcorn D., Aldred P. Effect of converting enzyme inhibition on renin synthesis and secretion in mice. Hypertension. 1989 Oct;14(4):385–395. doi: 10.1161/01.hyp.14.4.385. [DOI] [PubMed] [Google Scholar]
- Boam D. S., Clark A. R., Docherty K. Positive and negative regulation of the human insulin gene by multiple trans-acting factors. J Biol Chem. 1990 May 15;265(14):8285–8296. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burt D. W., Mullins L. J., George H., Smith G., Brooks J., Pioli D., Brammar W. J. The nucleotide sequence of a mouse renin-encoding gene, Ren-1d, and its upstream region. Gene. 1989 Dec 7;84(1):91–104. doi: 10.1016/0378-1119(89)90143-1. [DOI] [PubMed] [Google Scholar]
- Burt D. W., Nakamura N., Kelley P., Dzau V. J. Identification of negative and positive regulatory elements in the human renin gene. J Biol Chem. 1989 May 5;264(13):7357–7362. [PubMed] [Google Scholar]
- Cockerill P. N., Yuen M. H., Garrard W. T. The enhancer of the immunoglobulin heavy chain locus is flanked by presumptive chromosomal loop anchorage elements. J Biol Chem. 1987 Apr 15;262(11):5394–5397. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzau V. J., Burt D. W., Pratt R. E. Molecular biology of the renin-angiotensin system. Am J Physiol. 1988 Oct;255(4 Pt 2):F563–F573. doi: 10.1152/ajprenal.1988.255.4.F563. [DOI] [PubMed] [Google Scholar]
- Fabian J. R., Field L. J., McGowan R. A., Mullins J. J., Sigmund C. D., Gross K. W. Allele-specific expression of the murine Ren-1 genes. J Biol Chem. 1989 Oct 15;264(29):17589–17594. [PubMed] [Google Scholar]
- Field L. J., Gross K. W. Ren-1 and Ren-2 loci are expressed in mouse kidney. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6196–6200. doi: 10.1073/pnas.82.18.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N., Takimoto M., Bishop J. M. A FOS protein is present in a complex that binds a negative regulator of MYC. Genes Dev. 1989 Mar;3(3):293–303. doi: 10.1101/gad.3.3.293. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Rollier A., Tronche F., Ott M. O., Yaniv M., Weiss M. C. The rat albumin promoter is composed of six distinct positive elements within 130 nucleotides. Mol Cell Biol. 1989 Nov;9(11):4750–4758. doi: 10.1128/mcb.9.11.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hofmann J. F., Laroche T., Brand A. H., Gasser S. M. RAP-1 factor is necessary for DNA loop formation in vitro at the silent mating type locus HML. Cell. 1989 Jun 2;57(5):725–737. doi: 10.1016/0092-8674(89)90788-5. [DOI] [PubMed] [Google Scholar]
- Horiuchi M., Nakamura N., Tang S. S., Barrett G., Dzau V. J. Molecular mechanism of tissue-specific regulation of mouse renin gene expression by cAMP. Identification of an inhibitory protein that binds nuclear transcriptional factor. J Biol Chem. 1991 Aug 25;266(24):16247–16254. [PubMed] [Google Scholar]
- Lee C. Q., Miller H. A., Schlichter D., Dong J. N., Wicks W. D. Evidence for a cAMP-dependent nuclear factor capable of interacting with a specific region of a eukaryotic gene. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4223–4227. doi: 10.1073/pnas.85.12.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N., Burt D. W., Paul M., Dzau V. J. Negative control elements and cAMP responsive sequences in the tissue-specific expression of mouse renin genes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):56–59. doi: 10.1073/pnas.86.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prost E., Moore D. D. CAT vectors for analysis of eukaryotic promoters and enhancers. Gene. 1986;45(1):107–111. doi: 10.1016/0378-1119(86)90138-1. [DOI] [PubMed] [Google Scholar]
- Rice D. A., Aitken L. D., Vandenbark G. R., Mouw A. R., Franklin A., Schimmer B. P., Parker K. L. A cAMP-responsive element regulates expression of the mouse steroid 11 beta-hydroxylase gene. J Biol Chem. 1989 Aug 25;264(24):14011–14015. [PubMed] [Google Scholar]
- Savagner P., Miyashita T., Yamada Y. Two silencers regulate the tissue-specific expression of the collagen II gene. J Biol Chem. 1990 Apr 25;265(12):6669–6674. [PubMed] [Google Scholar]
- Schütte J., Viallet J., Nau M., Segal S., Fedorko J., Minna J. jun-B inhibits and c-fos stimulates the transforming and trans-activating activities of c-jun. Cell. 1989 Dec 22;59(6):987–997. doi: 10.1016/0092-8674(89)90755-1. [DOI] [PubMed] [Google Scholar]
- Winoto A., Baltimore D. Alpha beta lineage-specific expression of the alpha T cell receptor gene by nearby silencers. Cell. 1989 Nov 17;59(4):649–655. doi: 10.1016/0092-8674(89)90010-x. [DOI] [PubMed] [Google Scholar]