Abstract
Purpose
The 4π static noncoplanar radiation therapy delivery technique has demonstrated better normal tissue sparing and dose conformity than the clinically used volumetric modulated arc therapy (VMAT). It is unclear whether this is a fundamental limitation of VMAT delivery or the coplanar nature of its typical clinical plans. The dosimetry and the limits of normal tissue toxicity constrained dose escalation of coplanar VMAT, noncoplanar VMAT and 4π radiation therapy are quantified in this study.
Methods and materials
Clinical stereotactic body radiation therapy plans for 20 liver patients receiving 30 to 60 Gy using coplanar VMAT (cVMAT) were replanned using 3 to 4 partial noncoplanar arcs (nVMAT) and 4π with 20 intensity modulated noncoplanar fields. The conformity number, homogeneity index, 50% dose spillage volume, normal liver volume receiving >15 Gy, dose to organs at risk (OARs), and tumor control probability were compared for all 3 treatment plans. The maximum tolerable dose yielding a normal liver normal tissue control probability <1%, 5%, and 10% was calculated with the Lyman-Kutcher-Burman model for each plan as well as the resulting survival fractions at 1, 2, 3, and 4 years.
Results
Compared with cVMAT, the nVMAT and 4π plans reduced liver volume receiving >15 Gy by an average of 5 cm3 and 80 cm3, respectively. 4π reduced the 50% dose spillage volume by ∼23% compared with both VMAT plans, and either significantly decreased or maintained OAR doses. The 4π maximum tolerable doses and survival fractions were significantly higher than both cVMAT and nVMAT (P < .05) for all normal liver normal tissue control probability limits used in this study.
Conclusions
The 4π technique provides significantly better OAR sparing than both cVMAT and nVMAT and enables more clinically relevant dose escalation for tumor local control. Therefore, despite the current accessibility of nVMAT, it is not a viable alternative to 4π for liver SBRT.
Summary.
Liver stereotactic body radiation therapy volumetric modulated arc therapy (VMAT) plans with 3-4 partial noncoplanar arcs and beam orientation optimized noncoplanar 4π plans were compared with coplanar VMAT plans. 4π plans significantly reduced the normal liver volume receiving 15 Gy or higher and allowed clinically meaningful dose escalation with liver iso-toxicity. On the other hand, noncoplanar VMAT minimally improved the liver sparing and the prospect of dose escalation. In conclusion, noncoplanar VMAT cannot replace 4? radiation therapy despite its clinical availability.
Introduction
Liver cancer is responsible for over 600,000 deaths each year, according to the American Cancer Society, making it a leading cause of cancer deaths worldwide. Although surgical resection is considered the primary treatment option for hepatocellular carcinoma and oligometastases, 80% to 90% of patients present with unresectable tumors,1 which are treated with modalities including radiation therapy. Conventionally fractionated radiation therapy has shown ineffective to achieve local control,2 but stereotactic body radiation therapy (SBRT), with the delivery of fewer high-dose fractions, is a more effective treatment for patients with eligible conditions,3 which typically include inoperable liver tumors under the size of 6 cm and certain liver function criteria.4 The success of SBRT is largely owed to the higher biological effective doses (BED) as well as normal tissue sparing afforded by improved dose conformity from recent advances in radiation physics.1, 5, 6
Volumetric modulated arc therapy (VMAT) is a practical and effective delivery technique for liver SBRT, and has demonstrated normal tissue sparing and dose conformity superior to coplanar intensity modulated radiation therapy (IMRT).7 Although the incorporation of non-coplanar beams has improved normal tissue sparing in liver IMRT studies,7, 8, 9 typical clinical VMAT liver plans are still coplanar, consisting of a pair of (clockwise and counterclockwise) arcs using collimators with 90° rotational offset. To gain dose conformity and liver sparing, manually selected noncoplanar VMAT has been clinically used, but its benefit has not been quantified. Meanwhile, methods for the automatic selection of noncoplanar IMRT beams and optimized fluence maps have been developed for treatment sites including liver SBRT. Two separate groups have shown that by optimizing noncoplanar beam orientation selection, superior dosimetry to coplanar VMAT can be attained.9, 10 In this study, we refer to the beam orientation optimized noncoplanar IMRT plans as “4π static” because of the maximum of 4π steradian angles that can be used for noncoplanar beam orientation optimization. The usable angles are typically smaller than 4π because of collision concerns and couch pedestal occlusion.11 Because the delivery efficiency of noncoplanar IMRT plans is considered lower than that of VMAT without the use of automation, a function unavailable to most clinics, 1 important question is whether manually selected noncoplanar VMAT, which is the only clinically available method to incorporate these arcs, can offer dosimetry superior to coplanar VMAT while maintaining the advantage of clinical availability and efficiency. The purpose of this study is therefore to quantify and compare the dosimetry of these planning methods for liver SBRT.
Methods and Materials
Coplanar VMAT plans
Under institutional review board approval, treatment plans including computed tomography, dose, and contours were obtained for 20 liver SBRT patients (Table 1). These patients were treated with VMAT plans (RapidArc, Eclipse Treatment Planning System Version 10, Varian), typically consisting of 2 full coplanar arcs (cVMAT) with 90° collimator angle offset. The few original plans that included 1 partial noncoplanar arc were reoptimized in Eclipse with 1 full coplanar arcs to set a uniform baseline.
Table 1.
Patient data
| Diagnosis | Prescription dose (Gy) | Fractions | PTV (cm3) | Normal liver volume (cm3) | Liver volume (cm3) | |
|---|---|---|---|---|---|---|
| 1 | Metastatic esophageal cancer | 60 | 5 | 53.3 | 1491.0 | 1544.5 |
| 2 | Metastatic squamous cell carcinoma | 60 | 5 | 64.9 | 2169.9 | 2234.7 |
| 3 | Metastatic adenocarcinoma of the lung | 60 | 5 | 46.7 | 1194.2 | 1239.0 |
| 4 | Metastatic colorectal cancer | 60 | 5 | 35.6 | 1168.6 | 1187.5 |
| 5 | Metastatic colon cancer | 60 | 5 | 59.2 | 1404.7 | 1454.3 |
| 6 | Metastatic colorectal cancer | 50 | 5 | 123.0 | 1324.1 | 1447.1 |
| 7 | Metastatic uterus carcinosarcoma | 50 | 5 | 35.1 | 1768.8 | 1803.8 |
| 8 | Metastatic transitional cell from the kidney | 60 | 5 | 30.2 | 1241.2 | 1270.1 |
| 9 | Metastatic colon cancer | 50 | 5 | 179.0 | 1752.7 | 1909.4 |
| 10 | Hepatocellular carcinoma | 36 | 3 | 54.4 | 1133.4 | 1187.9 |
| 11 | Metastatic colon cancer | 60 | 5 | 88.4 | 1302.7 | 1391.1 |
| 12 | Metastatic prostate adenocarcinoma | 60 | 5 | 10.5 | 1442.7 | 1453.3 |
| 13 | Metastatic cholangiocarcinoma | 40 | 5 | 48.8 | 1837.0 | 1837.0 |
| 14 | Metastatic high-grade cholangiocarcinoma | 48 | 3 | 19.4 | 1027.0 | 1047.8 |
| 15 | Cholangiocarcinoma | 40 | 5 | 88.7 | 1787.0 | 1852.0 |
| 16 | Cholangiocarcinoma | 40 | 5 | 67.0 | 2517.6 | 2577.3 |
| 17 | Cholangiocarcinoma | 40 | 5 | 141.8 | 1809.6 | 1926.6 |
| 18 | Metastatic primitive germ cell tumor | 30 | 5 | 192.9 | 3342.8 | 3346.1 |
| 19 | Metastatic gall bladder small-cell carcinoma | 60 | 5 | 109.4 | 1533.5 | 1616.2 |
| 20 | Metastatic bile duct adenocarcinoma | 50 | 5 | 57.3 | 1107.8 | 1148.6 |
PTV, planning target volume.
Noncoplanar VMAT plans
These clinical plans were replanned in Eclipse with 3 to 4 manually selected partial noncoplanar arcs (nVMAT), as shown in Figure 1 (middle), with collimator angle offsets between 45° and 135°. The average total arc length for the 20 nVMAT plans was 553°. These arcs were empirically chosen to maximize noncoplanar angles while avoiding collision. Each plan typically consisted of 1 arc with a 0° couch rotation, 2 arcs with an average couch rotation of ±21° (−40° to +35° range), and for a few patients with tumors located near the mid-sagittal plane, 1 arc with a nearly perpendicular couch kick (86° on average). The optimization objectives were set up to minimize dose to liver, kidneys, spinal cord, and stomach, with the highest priority placed on the normal liver dose. All plans were normalized to deliver 100% of the prescribed dose to 95% of the planning target volume (PTV), and PTV hot spots were limited to those of the respective coplanar plans. A source-to-axis distance of 100 cm was maintained for all plans.
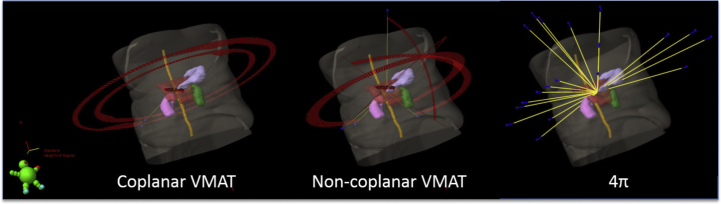
Figure 1.
Beam geometry comparison for a typical liver stereotactic body radiation therapy case. Beam orientation is shown for the coplanar volumetric modulated arc therapy (VMAT) plan (left), 3 to 4 noncoplanar partial arcs (center), and 20 noncoplanar, optimized beams (right).
4π static plans
The first step in the 4π static optimization process, the details of which have been previously reported,10 is to eliminate any beams out of the 1162 beam 4π static solid angle space that would cause collisions between the gantry and couch or patient. From the remaining beams, an integrated beam orientation and fluence map optimization is performed using a greedy column generation method.12 Briefly, the remaining candidate beams were subdivided into 5 × 5 mm2 beamlets and the dose distribution matrices of each beamlet were calculated using collapsed-cone convolution/superposition codes and 6-MV x-ray polyenergetic kernels with heterogeneity corrections. The dose calculation model was tuned to match 6-MV machine commissioning data. The dose calculation resolution was 2.5 × 2.5 × 2.5 mm3. The first-order derivative of the quadratic cost function with each additional beam from the candidate pool was evaluated and the beam that contributed most was kept. The process was iteratively performed until the desired number of beams was selected. Full fluence map optimization was performed after each beam selection. Although the cost function value did not converge, the dosimetric gains with more than 20 beams were modest. Considering a balance between plan deliverability and plan quality, In this study, 20 beams were selected with the 4π static algorithm for each patient, such as those in Figure 1 (right), the gantry and couch angles for each beam were imported into Eclipse to develop a clinically deliverable treatment plan using identical dose calculation engine as the VMAT plans. The organs at risk (OAR) constraints, plan normalization, source-to-axis distance, and PTV hotspots matched those described earlier for the nVMAT plans.
Additional notes on the optimization implementation
For each plan optimization, dose constraints were placed on the liver, kidney, spinal cord, and stomach. When necessary, additional optimization constraints were placed on other OARs such as the bowel or heart, depending on the location of the tumor. Although the constraints and penalties were initially the same for each, these parameters were adjusted throughout the optimization process to further reduce OAR doses for all techniques. Because plan optimizations with Eclipse depend on both the sequence of the planning parameters used along with the process and the timing of when they are used, maintaining a single set of constraints and penalties generally would result in poorer plan quality. Instead, the objectives for each plan were pushed as hard as possible to reduce OAR doses, particularly to the normal liver, while maintaining acceptable PTV doses. The normal tissue objective was also used, with a 0.1-cm distance from target border, 99% start dose, 50% end dose, and 0.2 falloff. Each plan was reoptimized until no further OAR dose reduction could be achieved without compromising PTV coverage (typically between 2 and 4 runs).
Plan comparison
The cVMAT, nVMAT, and 4π static plans were evaluated by comparing various metrics of normal tissue sparing. The minimum dose to 98% of the PTV (D98%) was compared among the 3 plans to evaluate any underdosage to the target. The conformity of each plan was evaluated by the conformity number (CN), defined by van’t Riet et al as
| (1) |
where TVRI is the target volume covered by the prescription isodose, TV is the target volume, and VRI is the volume of the prescription isodose.13 R50, defined as the ratio of the 50% isodose volume to the PTV, was used to evaluate the dose gradient outside the PTV. The homogeneity index was also calculated as 1 + (D2% – D98%)/(prescription dose). The normal liver volume receiving >15 Gy (VL>15) was quantified to assess normal tissue dose, as were the mean and maximum doses to OARs.
A radiobiological modeling study was performed to compare the probabilities of tumor control, normal tissue complication, and patient survival. The tumor control probability (TCP) was first calculated for each plan. Because TCP calculation parameters vary widely between studies and for different tumor types,14, 15 and because a variety of tumor origins were included in this study, high tumor radioresistance was assumed for the parameter selection (α = 0.2 Gy−1, α/β = 10 Gy) for a conservative TCP estimate. The Kutcher-Burman dose-volume histogram (DVH) reduction scheme16 was used to calculate the normal liver effective volume, the percentage of the normal liver volume that, if irradiated uniformly to the prescription dose, would give the same normal tissue complication probability (NTCP) as the nonuniform dose distribution. The effective volume was then used to calculate the normal liver NTCP using the Lyman model.17 The Lyman-Kutcher-Burman (LKB) NTCP model parameters from Dawson et al were used (TD50 = 45.8 Gy [metastases], TD50 = 39.8 Gy [primary tumors], n = 0.97, m = 0.12).18 Because these parameters were obtained from fractionated treatment plans, the SBRT plans in this study were normalized to 1.5 Gy per fraction using α/β = 2.5 Gy.19
Dose escalation
The prescription dose for each plan was escalated (or reduced) to the maximum tolerable dose (MTD) yielding a normal liver NTCP below the desired limit. The number of fractions for the clinical plans was maintained, with an escalated dose per fraction. In this study, MTDs for NTCP limits of 1%, 5%, and 10% were calculated. The BED for each MTD was determined by
| (2) |
with number of fractions N, dose per fraction d, total treatment time T, α = 0.01, α/β = 15, and tumor doubling time Td = 120 days from Tai et al.20 The survival model developed by Tai et al was used to predict the survival rates (SR) for each plan based on the BED:
| (3) |
where D is the prescription dose, τ is the elapsed time since treatment, δ describes the tumor growth rate, K0 is the initial number of tumor clonogens, K50 is the critical number of tumor clonogens corresponding to death in 50% of patients, σk is the Gaussian width for the critical clonogen number distribution, and γ = ln2/Td. The fitting parameters from Tai et al are K50/K0 = 2.03, σk/K0 = 0.65, and δ = 0.2.20 Survival fractions at 1, 2, 3, and 4 years were calculated for each plan before and after dose escalation.
Results
Plan comparison
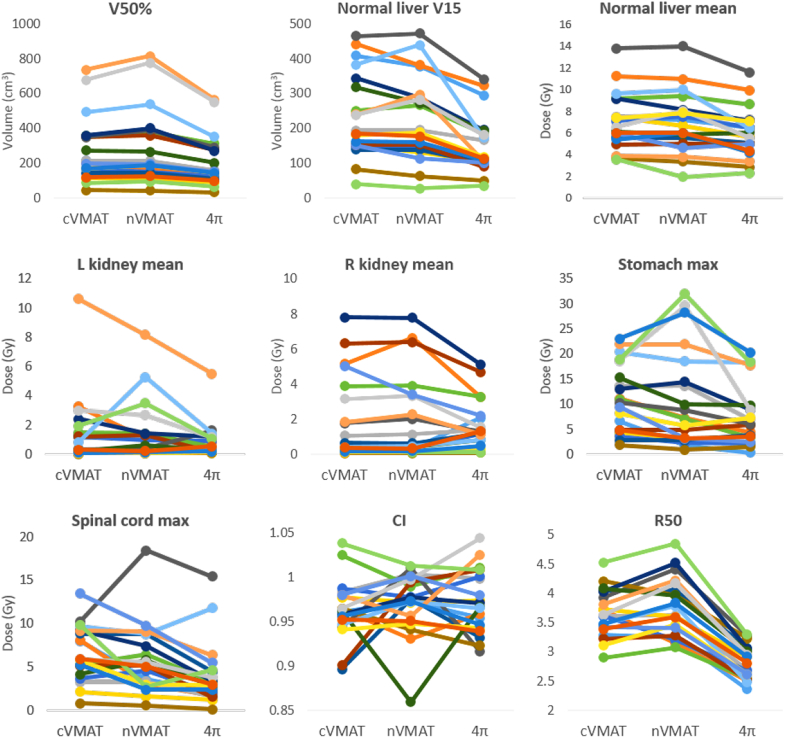
The average dose statistics for each plan type, using paired, 2-tailed t tests, are given in Table 2. On average, the nVMAT plans delivered higher kidney doses than the cVMAT plans and lower doses to the other OARs, but the only statistically significant difference was an increased V50%. However, there were large improvements with 4π static compared with both VMAT techniques. The mean dose to the normal liver was significantly lower with the 4π static plan, and the liver volume receiving >15 Gy was reduced from the cVMAT and nVMAT plans by 79.8 cm3 and 74.8 cm3, respectively (>32%). Although nVMAT enabled reductions in maximum stomach and spinal cord doses (compared with cVMAT) of approximately 2% and 14%, respectively, 4π static reduced these doses by 32% and 40%. The homogeneity index and CN values were similar for all plans. The individual dosimetric parameter comparison is shown in Figure 2. Consistent reduction in the R50 from cVMAT to 4π static was observed for all patients and the reduction is correlated to that of liver V15 and mean liver dose. In comparison, R50 of nVMAT slightly increased from cVMAT, indicating no improvement in the dose compactness.
Table 2.
Average dose statistics for coplanar VMAT, noncoplanar VMAT, and 4π plans for all 20 patients
| Plan type | OAR doses (Gy) |
|||||
|---|---|---|---|---|---|---|
| Left kidney (mean) | Right kidney (mean) | Normal liver (mean) | Stomach (maximum) | Cord (maximum) | Body (mean) | |
| cVMAT | 1.42a (2.4) | 2.04 (2.4) | 7.07a (2.6) | 11.15 (6.7) | 6.69a (3.2) | 1.51a (0.6) |
| nVMAT | 1.46a (2.1) | 2.09 (2.5) | 6.97a (2.8) | 10.95a (10.0) | 5.74a (4.0) | 1.51a (0.6) |
| 4π | 0.82 (1.2) | 1.70 (1.4) | 6.01 (2.3) | 7.58 (6.3) | 4.00 (3.7) | 1.46 (0.6) |
| PTV D98% (Gy) | VL>15 (cm3) | V50% (cm3) | R50 | HI | CN | |
|---|---|---|---|---|---|---|
| cVMAT | 49.62a (9.6) | 233.7a (120.8) | 271.5b (185.6) | 3.66b (0.4) | 0.11 (0.03) | 0.94 (0.03) |
| nVMAT | 49.59 (9.7) | 228.7a (122.3) | 287.5b (213.4) | 3.77a (0.5) | 0.10 (0.02) | 0.93 (0.04) |
| 4π | 49.42 (9.7) | 153.9 (84.5) | 210.8 (146.4) | 2.82 (0.3) | 0.11 (0.03) | 0.93 (0.03) |
cVMAT, coplanar volumetric modulated arc therapy; nVMAT, noncoplanar volumetric modulated arc therapy; OAR, organ at risk; PTV, planning target volume; VL>15, volume receiving >15 Gy.
Significantly different from 4π with P < .05 (paired, 2-tailed t test).
Significantly different from 4π and other VMAT plan type with P < .05 (paired, 2-tailed t test).
Figure 2.
Individual patient dosimetric results comparison. CI, confidence interval; cVMAT, coplanar volumetric modulated arc therapy; L, left; max, maximum; nVMAT, noncoplanar volumetric modulated arc therapy; R, right.
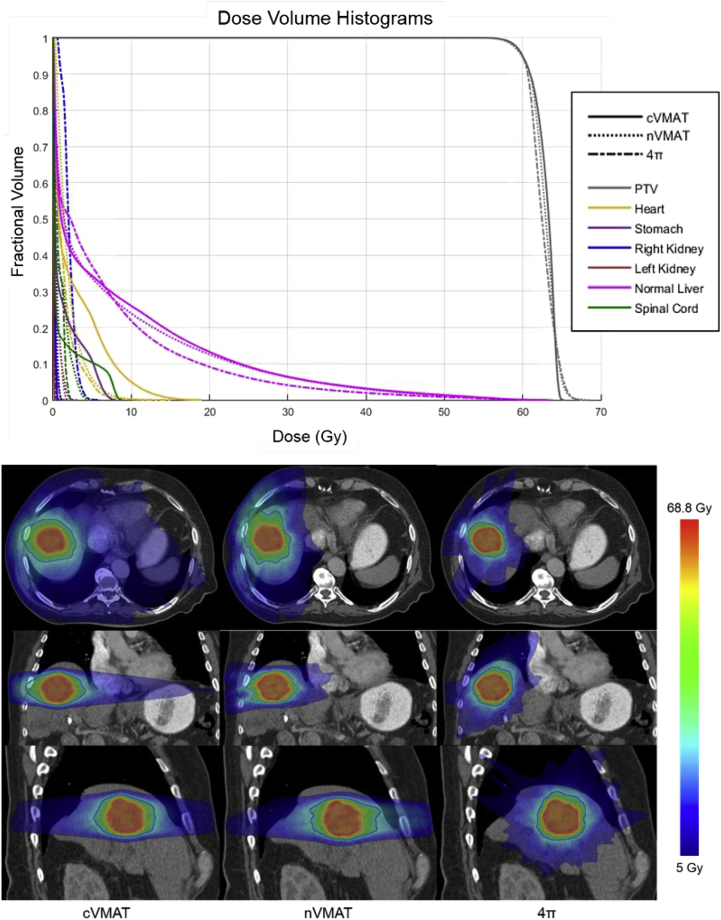
These reductions in OAR dose are evident in the DVHs in Figure 3 (top). Additionally, the 4π static technique reduced the 50% dose spillage volume by >22% compared with both VMAT plans. The major reduction in 50% isodose volume with the 4π static technique is evident in the dose washes in Figure 3 (bottom). Although the dose washes show less low dose spillage for the nVMAT plan than the cVMAT plan, the isodose volume above 15 Gy is very similar. There was a statistically significant difference between the mean cVMAT and 4π static PTV D98% doses, but only by 0.2 Gy.
Figure 3.
(Top) Dose-volume histograms for a typical liver SBRT case. (Bottom) Dose color washes for each plan type. The orange contours represent the PTV volume, and the blue represent the 50% isodose lines. cVMAT, coplanar volumetric modulated arc therapy; nVMAT, noncoplanar volumetric modulated arc therapy; PTV, planning target volume; SBRT, stereotactic body radiation therapy.
The average TCP values (calculated with the LKB model), given in Table 3, were very similar for nVMAT and cVMAT plans and slightly (∼1.1%) lower for 4π static. Although the optimization constraints on the maximum PTV dose were relaxed, 4π static plans still tend to result in more homogenous PTV doses, as seen in the DVH in Figure 2 (top), and slightly lower TCP.
Table 3.
Biological modeling parameter results for cVMAT, nVMAT, and 4π plans for all 20 patients (standard deviation in parentheses)
| TCP | Veff | NTCPa | |
|---|---|---|---|
| cVMAT | 7.37% (11.5%) | 13.79%b (5.2%) | 5.29% (18.8%) |
| nVMAT | 7.40%b (11.1%) | 14.00%b (5.9%) | 6.22% (22.2%) |
| 4π | 6.25% (9.7%) | 11.63% (4.35%) | 1.65% (6.3%) |
cVMAT, coplanar volumetric modulated arc therapy; NTCP, normal tissue complication probability; nVMAT, noncoplanar volumetric modulated arc therapy; Veff, effective volume.
The mean NTCP is drastically increased by 2 outlying patients, without which the mean NTCP values are 0.06%, 0.53%, and 0.02% for cVMAT, nVMAT, and 4π, respectively.
Significantly different from 4π plans with P < .05 (paired, 2-tailed t test).
The average normal liver effective volume was very similar for the VMAT plans, but was significantly reduced with 4π static by >15%. The resultant NTCP was therefore also much lower, with reductions of 69% from cVMAT and 73% from nVMAT.
Dose escalation
The results of the dose escalation are given in Table 4. For every NTCP limit, 4π static enabled significantly higher average MTDs than both cVMAT and nVMAT. The escalated doses for 4π static were approximately 20% and 14% higher than those for cVMAT and nVMAT, respectively. The corresponding BEDs for 4π static were higher than cVMAT and nVMAT for every NTCP limit by about 39% and 19%, respectively, with a statistically significant difference between 4π static and cVMAT at the 5% level (paired, 2-tailed t test). The average MTD and BED for nVMAT were higher than cVMAT for every NTCP limit, but not statistically significant at the 5% level.
Table 4.
Dose escalation based on the normal liver effective volume and desired normal liver LKB NTCP for all 20 patients (standard deviation in parentheses)
| 1% NTCP |
5% NTCP |
10% NTCP |
||||
|---|---|---|---|---|---|---|
| MTD (Gy) | BED (Gy) | MTD (Gy) | BED (Gy) | MTD (Gy) | BED (Gy) | |
| cVMAT | 76.80a (27.6) | 170.40a (97.8) | 85.51a (30.7) | 202.70a (117.6) | 90.14a (32.4) | 220.94a (129.0) |
| nVMAT | 80.63a (37.7) | 198.32 (177.7) | 89.76a (41.6) | 236.78 (215.5) | 94.65a (43.8) | 258.67 (237.4) |
| 4π | 91.98 (36.2) | 235.80 (164.8) | 102.43 (40.3) | 281.91 (199.8) | 107.99 (42.5) | 308.08 (219.8) |
cVMAT, coplanar volumetric modulated arc therapy; NTCP, normal tissue complication probability; LKB, Lyman-Kutcher-Burman; MTD, maximum tolerated dose; nVMAT, noncoplanar volumetric modulated arc therapy.
Significantly different from 4π plans with P < .05 (paired, 2-tailed t test).
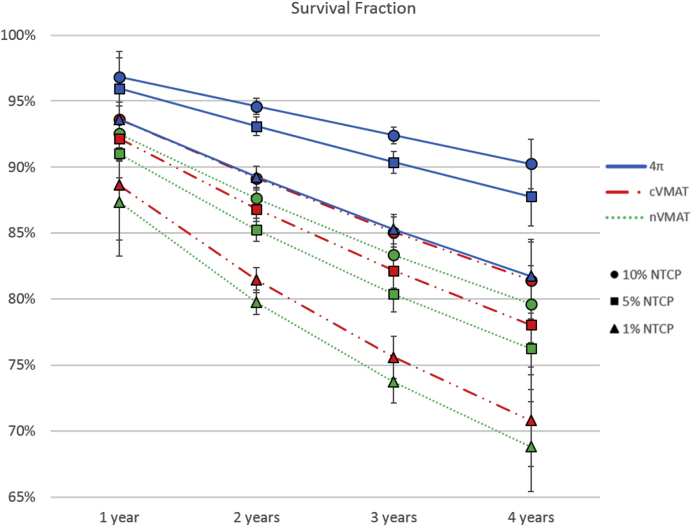
The average survival fractions at 1, 2, 3, and 4 years for each NTCP limit (1%, 5%, and 10%) showed a statistically significant increase for the 4π static plans compared with both VMAT plans, as shown in Figure 4. These improvements were most significant for the 4π static 4-year survival fraction, which was more than 7% higher than both VMAT plans at the 10% and 5% NTCP limits and more than 9% higher at the 1% NTCP limit. Even for conservative treatments allowing for only a 1% probability of liver complications, 4π static technique enabled an average 4-year survival fraction of 86%. This increased to 93% when a 10% NTCP was tolerated.
Figure 4.
Average survival predicted with the model from Tai et al20 for the maximum tolerable doses yielding 1%, 5%, and 10% normal liver NTCPs. All fractions for nVMAT and cVMAT were significantly different than 4π static at the 5% significance level (paired, 2-tailed t test). cVMAT, coplanar volumetric modulated arc therapy; NTCP, normal tissue control probability; nVMAT, noncoplanar volumetric modulated arc therapy; PTV, planning target volume.
Delivery time
The delivery time of 2 arc cVMAT for SBRT treatment was estimated as 5 to 7 minutes. The time increased by an additional 1 to 3 minutes for nVMAT, depending on whether remote couch rotation was used. Delivery of the 4π treatment with 20 beams can take 45 minutes with manual couch rotation and translation. A significant portion of the time was used by the therapist to enter and exit the shielded vault. Consequently, the time can be reduced to 12 minutes with automated delivery,11 in line with the nVMAT delivery time.
Discussion
The success of SBRT is largely attributed to the improved physical dose conformity enabled by recent advances in radiation therapy techniques, including intensity modulated radiation therapy, image-guided radiation therapy, and volumetric modulated arc therapy. A previous study showed that tumor local control can benefit from further dose escalation,5 but is limited by normal tissue toxicity.
Experience from intracranial radiosurgery suggests that an effective way to achieve a steeper dose gradient outside the tumor is by adding non-coplanar beams and arcs.21 The usefulness of noncoplanar beams in SBRT has been far less clear. R50 has been commonly used to evaluate the dose compactness in SBRT treatment,22 the minimization of which leads to more effective SBRT.23 Lung Radiation Therapy Oncology Group protocols24 recommend using either noncoplanar static or coplanar arc beams to achieve the R50 dosimetric goal. Lim et al25 showed that compared against coplanar plans, lower R50 could be achieved in 81% of patients when noncoplanar beam arrangement was employed. However, in a different lung study, no dosimetric improvements were found comparing coplanar and noncoplanar IMRT plans.26 In the case of liver radiation therapy, it was found that the advantage of using noncoplanar beams can be matched by coplanar plans with a large number of beams.27 Noncoplanar VMAT has been used on an ad hoc basis in liver SBRT7 with the expectation that these arcs should improve normal organ sparing without quantification of the dosimetric improvement.
In this study, we compared 3 clinically deliverable planning methods for liver SBRT. Our study shows that the incorporation of this type of arrangement of noncoplanar VMAT arcs offered modest dosimetric gains over coplanar VMAT. However, by fully using the freedom of the noncoplanar beam geometry solution space and automated beam orientation selection, the 4π static technique achieved major reductions in the dose to OARs, particularly the normal liver, stomach, and spinal cord, as well as a much more compact dose distribution.
There are several reasons for the minimal plan quality improvement from nVMAT over cVMAT. First, in practice, the number of VMAT arcs is limited. Because of the continuity of the arcs, large noncoplanar angles are prohibited to avoid collision. This is different from intracranial stereotactic radiation surgery, where a large noncoplanar beam solution space can be nearly uniformly sampled and included. It has been shown that the inclusion of small noncoplanar arc angles in a rotational IMRT system does not significantly improve the plan quality.28 Second, selection of noncoplanar arcs is unintuitive to the planner. It is difficult, if not impossible, for a human operator to optimally select segments of noncoplanar arcs, considering the freedoms of arc lengths and locations. One way to overcome this limitation is by optimizing the noncoplanar arc selection. However, a method to globally search the noncoplanar arc trajectories, or “4π arc,” is unavailable. Papp et al29 reported a noncoplanar arc optimization method based on static beam orientation optimization and then use these beams to anchor noncoplanar segments. However, this method does not promise arcs between the anchoring beams are optimal. Furthermore, the method would result in arcs that require simultaneous couch and gantry rotation and are undeliverable in the clinical mode. Both limitations are effectively overcome using 4π static planning, where the collision-free beam geometry solution space is individually mapped and the beam orientations are optimized. Therefore, even though nVMAT is currently available and more efficient to deliver, it is not a reasonable substitution for 4π static.
A major difference between the current study and the previously published report of using 4π static for liver SBRT10 is that the liver SBRT plans are generated in Eclipse using identical dose calculation engine to ensure an unbiased comparison. Compared with the previous study, the liver VL>15 was further reduced by an additional ∼30 cm3 to 80 cm3. This increased normal tissue sparing is crucial for liver SBRT because it enables escalation of the prescription dose to the PTV without further complications from high doses to normal tissue. Our dose escalation study showed that for the same normal liver complication probability, prescription doses delivered with the 4π static technique could be escalated 20% higher than cVMAT plans and 14% higher than nVMAT plans, leading to significant increases in predicted survival fractions.
Improved liver tumor motion management can be combined with 4π static radiation therapy for additional dosimetric gains, but the gain may be less significant. For example, Velec et al30 showed that by reducing the PTV volume using the mean respiratory position, the isotoxicity PTV dose can be escalated by 4 Gy, a moderate number compared to the dose escalation using 4π static.
Conclusions
4π static radiation therapy using optimized noncoplanar beams significantly reduces normal liver doses that can facilitate safe tumor dose escalation. Despite its clinical availability, VMAT using noncoplanar arcs is not a viable replacement of 4π static radiation therapy for compact dose liver SBRT.
Footnotes
Conflicts of interest: Ke Sheng receives research grants from Varian Medical Systems.
References
- 1.Chang D.T., Swaminath A., Kozak M. Stereotactic body radiotherapy for colorectal liver metastases: A pooled analysis. Cancer. 2011;117:4060–4069. doi: 10.1002/cncr.25997. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence T.S., Robertson J.M., Anscher M.S. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237–1248. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 3.Wahl DR, Stenmark MH, Tao Y, et al Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma [e-pub ahead of print]. J Clin Oncol. Accessed January 10, 2016. [DOI] [PMC free article] [PubMed]
- 4.Price T.R., Perkins S.M., Sandrasegaran K. Evaluation of response after stereotactic body radiotherapy for hepatocellular carcinoma. Cancer. 2012;118:3191–3198. doi: 10.1002/cncr.26404. [DOI] [PubMed] [Google Scholar]
- 5.Rusthoven K.E., Kavanagh B.D., Cardenes H. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 6.Mornex F., Girard N., Merle P. [Tolerance and efficacy of conformal radiotherapy for hepatocellular carcinoma in cirrhotic patients. Results of the French RTF1 phase II trial] Cancer Radiother. 2005;9:470–476. doi: 10.1016/j.canrad.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Wang P.M., Hsu W.C., Chung N.N. Feasibility of stereotactic body radiation therapy with volumetric modulated arc therapy and high intensity photon beams for hepatocellular carcinoma patients. Radiat Oncol. 2014;9:18. doi: 10.1186/1748-717X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas E., Chapet O., Kessler M.L. Benefit of using biologic parameters (EUD and NTCP) in IMRT optimization for treatment of intrahepatic tumors. Int J Radiat Oncol Biol Phys. 2005;62:571–578. doi: 10.1016/j.ijrobp.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 9.de Pooter J.A., Mendez Romero A., Wunderink W. Automated non-coplanar beam direction optimization improves IMRT in SBRT of liver metastasis. Radiother Oncol. 2008;88:376–381. doi: 10.1016/j.radonc.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Dong P., Lee P., Ruan D. 4pi non-coplanar liver SBRT: A novel delivery technique. Int J Radiat Oncol Biol Phys. 2012;85:1360–1366. doi: 10.1016/j.ijrobp.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Yu V.Y., Tran A., Nguyen D. The development and verification of a highly accurate collision prediction model for automated noncoplanar plan delivery. Med Phys. 2015;42:6457–6467. doi: 10.1118/1.4932631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romeijn HE, Ahuja RK, Dempsey JF, Kumar A. A column generation approach to radiation therapy treatment planning using aperture modulation. Available at: http://dx.doi.org/10.1137/040606612. Accessed January 10, 2016.
- 13.van't Riet A., Mak A.C., Moerland M.A., Elders L.H., van der Zee W. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: Application to the prostate. Int J Radiat Oncol Biol Phys. 1997;37:731–736. doi: 10.1016/s0360-3016(96)00601-3. [DOI] [PubMed] [Google Scholar]
- 14.Deacon J., Peckham M.J., Steel G.G. The radioresponse of human tumors and the initial slope of the cell survival curve. Radiother Oncology. 1984;2:317–323. doi: 10.1016/s0167-8140(84)80074-2. [DOI] [PubMed] [Google Scholar]
- 15.Fertil B., Malaise E.P. Intrinsic radiosensitivity of human cell lines is correlated with radioresponsiveness of human tumors: Analysis of 101 published survival curves. Int J Radiat Oncol Biol Phys. 1985;11:1699–1707. doi: 10.1016/0360-3016(85)90223-8. [DOI] [PubMed] [Google Scholar]
- 16.Kutcher G.J., Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: The effective volume method gerald. Int J Radiat Oncol Biol Phys. 1989;16:1623–1630. doi: 10.1016/0360-3016(89)90972-3. [DOI] [PubMed] [Google Scholar]
- 17.Lyman J.T. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–S19. [PubMed] [Google Scholar]
- 18.Dawson L.A., Normolle D., Balter J.M., McGinn C.J., Lawrence T.S., Ten Haken R.K. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 19.Dawson L.A., Eccles C., Craig T. Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncol. 2006;45:856–864. doi: 10.1080/02841860600936369. [DOI] [PubMed] [Google Scholar]
- 20.Tai A., Erickson B., Khater K.A., Li X.A. Estimate of radiobiologic parameters from clinical data for biologically based treatment planning for liver irradiation. Int J Radiat Oncol Biol Phys. 2008;70:900–907. doi: 10.1016/j.ijrobp.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 21.Podgorsak E.B., Pike G.B., Olivier A., Pla M., Souhami L. Radiosurgery with high energy photon beams: A comparison among techniques. Int J Radiat Oncol Biol Phys. 1989;16:857–865. doi: 10.1016/0360-3016(89)90506-3. [DOI] [PubMed] [Google Scholar]
- 22.Timmerman R.D., Park C., Kavanagh B.D. The North American experience with stereotactic body radiation therapy in non-small cell lung cancer. Jo Thoracic Oncol. 2007;2:S101–S112. doi: 10.1097/JTO.0b013e318074e4fa. [DOI] [PubMed] [Google Scholar]
- 23.Yang J., Fowler J.F., Lamond J.P., Lanciano R., Feng J., Brady L.W. Red shell: Defining a high-risk zone of normal tissue damage in stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2010;77:903–909. doi: 10.1016/j.ijrobp.2009.12.069. [DOI] [PubMed] [Google Scholar]
- 24.Timmerman R., Kavanagh B. Lippincott Williams & Wilkins; Philadelphia, PA: 2004. Stereotactic Body Radiation Therapy. [Google Scholar]
- 25.Derycke S., Van Duyse B., De Gersem W., De Wagter C., De Neve W. Non-coplanar beam intensity modulation allows large dose escalation in stage III lung cancer. Radiother Oncol. 1997;45:253–261. doi: 10.1016/s0167-8140(97)00132-1. [DOI] [PubMed] [Google Scholar]
- 26.Christian J.A., Bedford J.L., Webb S., Brada M. Comparison of inverse-planned three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;67:735–741. doi: 10.1016/j.ijrobp.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh C.H., Liu C.Y., Shueng P.W. Comparison of coplanar and noncoplanar intensity-modulated radiation therapy and helical tomotherapy for hepatocellular carcinoma. Radiat Oncol. 2010;5:40. doi: 10.1186/1748-717X-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang W., Jones R., Lu W. Feasibility of non-coplanar tomotherapy for lung cancer stereotactic body radiation therapy. Technol Cancer Res Treat. 2011;10:307–315. doi: 10.7785/tcrt.2012.500207. [DOI] [PubMed] [Google Scholar]
- 29.Papp D., Bortfeld T., Unkelbach J. A modular approach to intensity-modulated arc therapy optimization with noncoplanar trajectories. Phys Med Biol. 2015;60:5179–5198. doi: 10.1088/0031-9155/60/13/5179. [DOI] [PubMed] [Google Scholar]
- 30.Velec M., Moseley J.L., Dawson L.A., Brock K.K. Dose escalated liver stereotactic body radiation therapy at the mean respiratory position. Int J Radiat Oncol Biol Phys. 2014;89:1121–1128. doi: 10.1016/j.ijrobp.2014.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]