Abstract
Aims: Cholesterol plays a pivotal role in many aspects of brain development; reduced cholesterol levels during brain development, as a consequence of genetic defects in cholesterol biosynthesis, leads to severe brain damage, including microcephaly and mental retardation, both of which are also hallmarks of the fetal alcohol syndrome. We had previously shown that ethanol up-regulates the levels of two cholesterol transporters, ABCA1 (ATP binding cassette-A1) and ABCG1, leading to increased cholesterol efflux and decreased cholesterol content in astrocytes in vitro. In the present study we investigated whether similar effects could be seen in vivo. Methods: Pregnant Sprague-Dawley rats were fed liquid diets containing 36% of the calories from ethanol from gestational day (GD) 6 to GD 21. A pair-fed control groups and an ad libitum control group were included in the study. ABCA1 and ABCG1 protein expression and cholesterol and phospholipid levels were measured in the neocortex of female and male fetuses at GD 21. Results: Body weights were decreased in female fetuses as a consequence of ethanol treatments. ABCA1 and ABCG1 protein levels were increased, and cholesterol levels were decreased, in the neocortex of ethanol-exposed female, but not male, fetuses. Levels of phospholipids were unchanged. Control female fetuses fed ad libitum displayed an up-regulation of ABCA1 and a decrease in cholesterol content compared with pair-fed controls, suggesting that a compensatory up-regulation of cholesterol levels may occur during food restriction. Conclusion: Maternal ethanol consumption may affect fetal brain development by increasing cholesterol transporters’ expression and reducing brain cholesterol levels.
INTRODUCTION
Maternal alcohol consumption during pregnancy may lead to fetal alcohol syndrome (FAS), a dysmorphogenic neuropathological syndrome characterized by growth deficiency, craniofacial malformations and central nervous system (CNS) dysfunction (Clarren et al., 1978; Riley et al., 2011). Damages to the CNS by in utero exposure to alcohol can result in a variety of neurobehavioral disturbances, ranging from hyperactivity/attention deficit disorder and learning disabilities in childhood (Streissguth and O'Malley, 2000), to major depressive and psychotic disorders in adulthood (Famy et al., 1998).
Morphological and behavioral abnormalities seen in FAS are also seen in Smith–Lemli–Opitz syndrome (SLOS) (Guizzetti et al., 2007). SLOS is a disorder originating from a genetic mutation in a gene encoding for the enzyme 7-dehydrocholesterol-Δ7-reductase which catalyzes the last step of the cholesterol synthesis, leading to the biosynthesis of an inactive enzyme and to deficiency of cholesterol in the whole body (Nowaczyk et al., 1999). Both FAS and SLOS exhibit similar patterns of growth retardation, abnormal facial features, limb malformations and behavioral dysfunctions (Streissguth et al., 1980; Nowaczyk et al., 1999; Roux et al., 2000; Cohen and Shiota, 2002). Prominent similarities in facial abnormalities were found in rodent models of FAS and SLOS, such as hypoplasia of the frontal lobes and corpus callosum, anteverted nares, small upturned nose, long upper lip and abnormal cell rounding (Dehart et al., 1997; Lanoue et al., 1997). Given the similarities between FAS and SLOS, we had hypothesized that in utero ethanol exposure may disrupt cholesterol homeostasis in the brain (Guizzetti et al., 2011).
The brain has the highest cholesterol content compared with other organs (25% of total body cholesterol), while representing only about 5% of total body weight (Dietschy and Turley, 2001). Cholesterol in the brain is synthesized endogenously, as its uptake from the circulation is prevented by the blood–brain barrier (Turley et al., 1996). In the developing CNS, cholesterol plays a role in the Sonic Hedgehog signaling (Porter et al., 1996; Incardona and Roelink, 2000; Marti and Bovolenta, 2002; Cooper et al., 2003), and is essential for neuronal survival (Michikawa and Yanagisawa, 1999), massive synaptogenesis in CNS neurons (Mauch et al., 2001) and proliferation of glial cells (Dobbing and Sands, 1973).
Cholesterol homeostasis in the brain is maintained through two processes: cholesterol synthesis and cholesterol clearance. A mechanism of brain cholesterol clearance involves cholesterol efflux from brain cells to astrocyte-released lipoproteins, which exit the brain after passing from the brain parenchyma into the cerebrospinal fluid and across the blood–brain barrier (Pitas et al., 1987; Raffai and Weisgraber, 2003; Dietschy and Turley, 2004). Another mechanism of cholesterol excretion from the brain is through its conversion to 24S-hydroxycholesterol by the enzyme cholesterol 24-hydroxylase, belonging to the cytochrome P450 family (CYP46), which is expressed in the brain only by a subset of neurons, but not by astrocytes (Lund et al., 1999).
In the periphery, the transporter ABCA1 (ATP binding cassette-A1) is involved in HDL formation, as it transfers phospholipids and cholesterol from the cell membrane to extracellular lipid-poor apolipoproteins leading to the formation of nascent lipoproteins (Oram and Heinecke, 2005). Another transporter, ABCG1 (ATP binding cassette-G1) subsequently mediates cholesterol efflux to nascent lipoproteins, leading to the formation of spherical HDL (Vaughan and Oram, 2006). A similar mechanism has been shown to be present in the brain, where ABCA1 is essential for the generation of lipoproteins from astrocytes, and ABCG1 is involved in lipoprotein remodeling (Koldamova et al., 2003; Hirsch-Reinshagen et al., 2004; Wahrle et al., 2004; Yu et al., 2010).
We have previously shown that ethanol (25–100 mM) increases ABCA1 and ABCG1 levels, induces cholesterol efflux, and reduces cholesterol levels in primary rat astrocytes in culture (Guizzetti et al., 2007). Furthermore, we have also shown that the in vivo exposure to ethanol from postnatal day 4–7 increases ABCA1 protein levels in the rat cerebral cortex (Guizzetti et al., 2007). Ethanol also increases the release of lipoproteins from astrocytes (Chen et al., in preparation). Lipoproteins induce cholesterol efflux from neurons through the interaction with ABCG4 cholesterol transporters (Chen et al., 2013). We found that ABCG4 is not affected by ethanol in neurons and that ethanol-induced increase in cholesterol efflux from neurons is mediated exclusively by the increased availability of astrocyte-secreted lipoproteins (Chen et al., 2013). Because of the insensitivity of ABCG4 to the direct effects of ethanol, we did not investigate this cholesterol transporter in the present study.
Aim of the present study was to investigate whether prenatal alcohol exposure would alter ABCA1, ABCG1, cholesterol and phospholipid levels in brain of fetuses at the end of gestation. The neocortex was chosen as the brain region for our investigations, as it is prominently affected by prenatal ethanol exposure. Indeed, prenatal ethanol exposure decreases the number of cortical progenitor cells (leading to reduced number of neurons and astrocytes), by affecting their proliferation and survival, and by delaying the time and rate of cortical neuronal migration in the brain cortex (Miller, 1986; Miller and Potempa, 1990; Mooney and Miller, 2007). Supporting this hypothesis, a recent study found reduced levels of cholesterol in the cerebellum of newborn rats exposed to ethanol during gestation (Soscia et al., 2006).
MATERIALS AND METHODS
Materials
ABCA1 and ABCG1 antibodies were from Novus Biologicals (Littleton, CO, USA). Amplex Red Cholesterol Assay Kit was from Invitrogen (Carlsbad, CA, USA). Phospholipids C kit was from Wako Chemicals USA, Inc. (Richmond, VA, USA). The enzymatic kit for the measurement of ethanol levels was from Boehringer Mannheim/R-Biopharm. Time-pregnant Sprague-Dawley rats were purchased from Charles River (Wilmington, MA, USA). All other reagents were from Sigma (St Louis, MO, USA).
Prenatal chronic ethanol exposure
All animal procedures were carried out in accordance with Institutional Animal Care and Use Committee (IACUC) of the University of Washington on animal care guidelines for the care and use of laboratory animals (IACUC-approved protocol number 2077–12). Prenatal ethanol exposure was carried out as described by Dr Joanne Weinberg and her colleagues (Glavas et al., 2006, 2007). A high protein Lieber-DeCarli Rat Diet used for control and pair-fed animals, and a high protein Lieber-DeCarli Rat Ethanol Diet in which 36% calories are derived from ethanol used for ethanol treatments, were purchased from Dyets, Inc. (Bethlehem, PA, USA). These diets were designed to provide adequate nutrition to pregnant dams regardless of ethanol intake. Timed-pregnant Sprague-Dawley female (200 to 250 g) rats were randomly assigned to one of three treatment groups. Rats in the control (C) group received ad libitum (140 g) access to high protein liquid Lieber-DeCarli diet with maltose-dextrin isocalorically substituted for ethanol; ethanol (E)-treated rats received ad libitum (140 g) access to high protein liquid Lieber-DeCarli diet containing 36% ethanol-derived calories; pair-fed (PF) rats received high protein liquid Lieber-DeCarli diet with maltose-dextrin isocalorically substituted for ethanol, with each animal pair-fed the amount consumed by a pregnant rat in the E group the previous day (g/kg body weight on the same day of gestation), to control for nutritional effects of the reduced food intake that occurs with ethanol consumption. Fresh diets were provided daily approximately at the same time (5:00 PM). Daily food intake for each group was recorded.
The ethanol diet was introduced gradually during the first 3 days of treatments. On gestational day (GD) 6 ethanol-fed animals received 1/3 ethanol diet and 2/3 pair-fed diet. On GD 7 they received 2/3 ethanol diet and 1/3 pair-fed diet. From GD 8 to GD 21 animals received the full amount of the ethanol diet. On GD 21, pregnant rats were sacrificed and fetuses were removed and weighed. Maternal blood was collected by cardiac puncture at 6:00 AM from three additional animals for the determination of blood alcohol concentration (BAC). Serum was separated and ethanol concentrations were determined using a Boehringer Mannheim/R-Biopharm kit per manufacturer's protocol. Fresh neocortical tissue from male and female fetuses was harvested, snap-frozen in liquid nitrogen and stored at −80°C for protein and lipid extraction.
Brain tissue preparation
The cerebral neocortices were weighed and homogenized using a Bullet Blender as per manufacturer's optimized protocol. Right cerebral cortices were homogenized for 3 min (setting 6) in 200 μl homogenization buffer (150 mM NaCl, 50 mM Tris–HCl, pH 7.5); additional 400 µl of homogenization buffer were added and samples were homogenized for another 2 min (setting 3). Detergents (1% Nonidet P40, 0.5% Na- deoxycholate and 0.5% SDS) were added to the buffer after homogenization to avoid foam formation. The homogenates were then sonicated for three times 10 s each, rocked at 4°C for 40 min and stored at −80°C until analysis.
Western blot analysis
Protein levels in tissue homogenates were measured using the Bicinchoninic acid assay (BCA assay) according to the manufacturer's protocol; proteins (30 μg) were loaded on a 3–8% SDS–PAGE (ABCA1), or a 4–12% SDS–PAGE (ABCG1). Proteins were separated by electrophoresis and transferred to PVDF membrane. Membranes were then labeled with polyclonal antibodies against ABCA1 or ABCG1 followed by the appropriate horseradish peroxidase-conjugated IgG, and detected by chemiluminescence. Membranes were re-probed for β-actin to ensure equal loading. After detection, films were scanned and bands were quantified by densitometry and normalized to β-actin. In order to minimize errors and variability in the western blot results, ABCA1 and ABCG1 protein levels were quantified in three fetuses per sex per litter; the optical densities obtained from samples derived from fetuses of the same sex in the same litter were averaged to generate each individual value; a total of four litters were used.
Measurement of cholesterol mass
Total cholesterol content was measured using the Amplex Red assay kit (Molecular Probes) according to the manufacturer's protocol. The enzymatic assay used in these determinations can quantify both, free cholesterol and total cholesterol (by performing reactions in the presence and absence of cholesterol esterase, respectively); in this study we measured total cholesterol as it has been reported that essentially all cholesterol in the brain is unesterified (Turley et al., 1998; Quan et al., 2003; Dietschy and Turley, 2004). Brain tissue samples prepared as described above were diluted in 1× reaction buffer, and incubated with 150 μM Amplex Red reagent, 1 U/ml horseradish peroxidase, 1 U/ml cholesterol oxidase and 0.1 U/ml cholesterol esterase in final reaction volumes of 100 μl. Reactions were incubated at 37°C for 30 min and fluorescence was measured in a Fluorocount microplate reader (PACKard) (excitation 560 nm, emission 590 nm). A standard curve was simultaneously generated using a cholesterol standard provided with the kit. The levels of cholesterol were normalized to protein content.
Measurement of phospholipids
Choline-containing phospholipids were quantified in brain tissue homogenates prepared as described above using the kit Phospholipids C (Cat # 433–36201; Wako Pure Chemical Industries) according to the manufacturer's protocol. This kit is based on three enzymatic reactions: (a) choline-containing phospholipids, which include phosphatidylcholine (lecithin), lysophosphatidylcholine (lysolecithin) and ceramidephosphocholine (sphingomyelin), are hydrolyzed to choline and phosphatidic acid, choline and N-Acyl-sphingosylphosphate, and choline and lysophosphatidic acid, respectively, by the addition to the reaction mixture of phospholipase D; (b) choline is oxidized to betaine and hydrogen peroxide by the enzyme choline oxidase; (c) the hydrogen peroxide produced causes DAOS and 4aminoantipyrine, present in the reaction mixture, to undergo a quantitative oxidative condensation catalyzed by a peroxidase, producing a blue pigment. The amount of phospholipids in the sample is determined by measuring the absorbance of the blue color. Brain homogenate samples were supplemented with 0.47 U/ml phospholipase D, 250 U/ml choline oxidase, 4.2 units/ml peroxidase, 0.77 mmol/l N-ethyl-N-3, 5-dimethoxyaniline sodium salt, 0.24 mmol/l 4-aminoantipyrine and 3.9 units/ml ascorbate oxidase in final reaction volumes of 200 μl. Reactions were incubated at 37°C for 5 min and fluorescence was measured in a colorimeter reader (absorbance at 600 nm). A standard curve was simultaneously generated using a phospholipid standard provided with the kit. The levels of phospholipid were normalized to protein content.
Statistical analysis
Data depicted in the graphs represent the means (±SEM) for each group; individual determinations were the result of the average of three fetuses/gender/treatment; each treatment was carried out in 4–6 different litters (n = 4–6). Inter-group comparisons were made by ANOVA followed by the Newman–Keuls post hoc test.
RESULTS
Pregnant rats given the ethanol diet consumed significantly less (−33%) food than rats on an ad libitum ethanol-free liquid diet (76.5 ± 6.8 vs. 113.8 ± 6.8 g/kg/day; n = 6; P < 0.01; Table 1). As end-points measured in this study are highly dependent on the nutritional status of the animals (see also discussion), the pair-fed group (which consumed an average of 77.1 ± 4.3 g of food/kg/day) was considered the control group for direct comparisons with ethanol-fed animals. At the beginning of the treatment, on GD 6, maternal body weights did not differ among E, PF and C dams (Table 1). Rats in all three groups gained weight during gestation; however, at GDs 12, 18 and 21, E and PF dams weighed significantly less than C dams (P < 0.05), but did not differ from each other, suggesting an effect of reduced calorie intake, but not of ethanol, on maternal body mass. Pregnant dams in all three groups appeared healthy regardless of the lower weight gain. Ethanol intake of pregnant rats was consistent throughout gestation, averaging 13.5 ± 1.8, 13.4 ± 1.0 and 12.1 ± 2.8 g/kg body weight/day for Weeks 1, 2 and 3 of gestation, respectively (n = 6). BAC, measured in three additional animals, was 44.9 mM ± 6.5. Such BAC values are consistent with previous studies, in which upon similar treatments BAC ranged from 30 to 51 mM (Soscia et al., 2006; Glavas et al., 2007). It should also be noted that the BAC found in the present in vivo study is in the same range of ethanol concentrations (25–100 mM) previously found to affect cholesterol homeostasis in in vitro experiments (Guizzetti et al., 2007).
Table 1.
Pregnancy outcome and maternal and fetal body weights
| Pregnancy outcome variable | Ethanol | Pair-fed | Ad libitum |
|---|---|---|---|
| Control | |||
| Maternal death/illness | 0 | 0 | 0 |
| Spontaneous resorptions | 0 | 0 | 0 |
| Food consumed (g/Kg/day) | 76.5 ± 6.8# | 77.1 ± 4.3# | 113.8 ± 6.8 |
| Dam weight | |||
| GD6 (g) | 224.6 ± 17.7 | 218.6 ± 14.8 | 220.5 ± 11.3 |
| GD12 (g) | 243.5 ± 19.0 | 224.0 ± 14.0# | 258.9 ± 14.3 |
| GD 18 (g) | 280.2 ± 19.4# | 248.3 ± 14.04# | 305.4 ± 18.4 |
| GD21 (g) | 316.9 ± 26.5# | 302.7 ± 23.9# | 361.5 ± 20.6 |
| Litter size | 11 ± 3 | 12.7 ± 2.1 | 11 ± 3.8 |
| Fetus weight | |||
| Males | 4.98 ± 0.17# | 5.13 ± 0.26# | 5.84 ± 017 |
| Females | 4.53 ± 0.15*,# | 5.06 ± 0.28# | 5.59 ± 0.19 |
| Total | 4.74 ± 0.16*,# | 5.09 ± 0.27# | 5.71 ± 0.18 |
| Sex ratio (% male) | 47% | 53% | 50% |
Pregnant dams were fed with a high protein Lieber-DeCarli liquid diets containing ethanol (36% of caloric intake), pair-fed a isocaloric diet, or fed a isocaloric diet ad libitum beginning on gestation day 6 and continuing throughout pregnancy. Pregnant dams were weighed on gestational days (GD) 6, 12, 18 and 21. Fetuses were weighed on GD21. The data for maternal death and spontaneous abortion are reported as the number of occurrences. The other data are reported as group means ± SEM. Data were analyzed by one-way ANOVA followed by the Newman–Keuls post hoc test.
*P < 0.05 compared with pair-fed group.
#P < 0.05 compared with ad libitum control group (n = 6).
All subsequent determinations were carried out in GD 21 fetuses from C, E and PF pregnant rats. There were no significant differences among treatment groups with regard to litter size or number of dead fetuses (Table 1), similarly to what previously reported by others (Glavas et al., 2006). Body weights of the E and PF male and female fetuses were significantly lower than C fetuses at GD 21, indicating an effect of caloric restriction also on fetal growth. Furthermore, the average body weight of E fetuses was lower than that of PF fetuses, though this was due almost exclusively to an effect in female fetuses (Table 1). This latter finding confirms that prenatal alcohol exposure reduces fetal growth, a well-known characteristic of FAS.
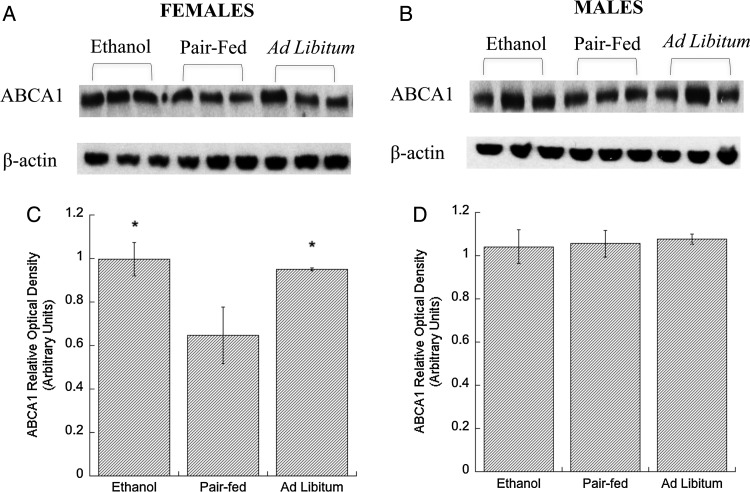
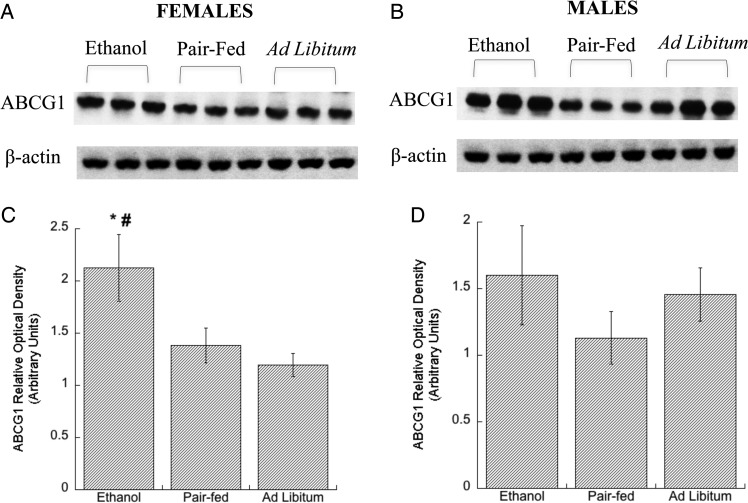
Western blot analysis of neocortices from E21 fetuses showed that ethanol feeding increased ABCA1 and ABGC1 levels in female rats (Figs 1A, C and 2A, C) when compared with the pair-fed groups. In the ad libitum control females, ABCA1 levels were comparable to those found after ethanol feeding and higher than in the brains of pair-fed females. In contrast, ABCG1 levels in the ad libitum control females were comparable to that of pair-fed control and lower than in the ethanol group, suggesting that, in female rats, brain ABCA1 levels are regulated by the nutritional status of the fetus, while ABCG1 transporters are not. No statistical differences between the three experimental groups were detected in male fetuses (Figs 1B, D and 2B, D). These findings confirmed our hypothesis that prenatal exposure of ethanol triggers an up-regulation of cholesterol transporters in the developing brain.
Fig. 1.
Effect of in utero ethanol exposure on ABCA1 protein levels in the neocortex. Pregnant rats were exposed to ethanol from GD 6 to GD 21 as described in Methods. ABCA1 protein levels in neocortical tissue lysates of E, PF and C male and female E21 rat fetuses were determined by western blot. Representative immunoblots from membranes labeled with ABCA1 (A, females, B, males), and β-actin (loading control; lower bands) antibodies are shown. Densitometric quantifications of ABCA1 levels in female (C) and male (D) fetuses are shown; the expression levels of ABCA1 were normalized to β–actin. Results show the mean (± SEM) of four independent measurements. Data were analyzed by one-way ANOVA followed by the Newman–Keuls post hoc test; *P < 0.05 vs. PF.
Fig. 2.
Effect of in utero ethanol exposure on ABCG1 protein levels in the neocortex. Pregnant rats were exposed to ethanol from GD 6 to GD 21 as described in Methods. Levels of ABCG1 protein in neocortical tissue lysates of E, PF and C male and female rat E21 fetuses were determined by western blot. Representative immunoblots from membranes labeled with ABCG1 (A, females, B, males), and β-actin (loading control; lower bands) antibodies are shown. Densitometric quantifications of ABCG1 levels in female (C) and male (D) fetuses are shown; the expression levels of ABCG1 were normalized to β-actin and. Results show the mean (± SEM) of four independent measurements. Data were analyzed by one-way ANOVA followed by the Newman–Keuls post hoc test; *P < 0.05 vs. PF; #P < 0.05 vs. Ad Libitum.
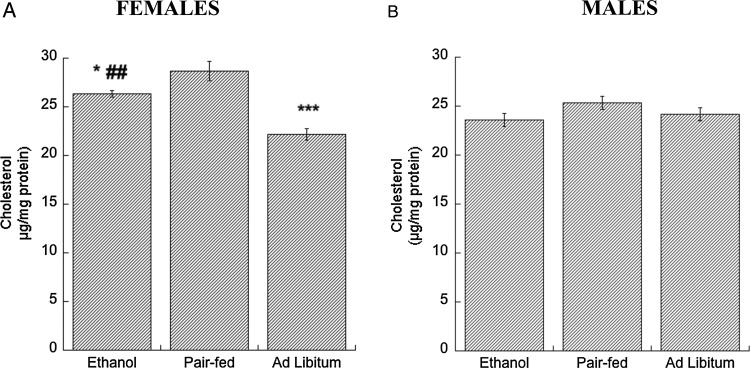
ABCA1 and ABCG1 are involved in the biogenesis and remodeling of brain lipoproteins. Increased lipoprotein availability increases cholesterol efflux from neurons and astrocytes (Guizzetti et al., 2007; Chen et al., 2013); lipoproteins represent one of the pathways of brain cholesterol clearance (Raffai and Weisgraber, 2003; Dietschy and Turley, 2004). We hypothesized that, similarly to what observed in vitro, increased ABCA1 and ABCG1 levels would decrease cholesterol content in the cortex of ethanol-exposed E21 fetuses. In agreement with this hypothesis, we found that brains from ethanol-treated female fetuses had significantly lower cholesterol levels than brains from female pair-fed fetuses (Fig. 3A). Furthermore, in agreement with the higher levels of ABCA1 present in ad libitum control females when compared with pair-fed animals (Fig. 1C), we also found that cholesterol levels were lower in ad libitum control rats (Fig. 3A). Cholesterol levels were not affected by ethanol treatments and feeding conditions in male fetuses (Fig. 3B).
Fig. 3.
Effect of in utero ethanol exposure on cholesterol levels in the neocortex. Pregnant rats were exposed to ethanol from GD 6 to GD 21 as described in Methods. Cholesterol content was measured in neocortex homogenates of E, PF and C female (A) and male (B) rat E21 fetuses using the Amplex Red Cholesterol assay kit. Results show the mean (± SEM) of four independent measurements. Data were analyzed by one-way ANOVA followed by the Newman–Keuls post hoc test; *P < 0.05; ***P < 0.001 vs. PF; ##P < 0.01 vs. Ad Libitum.
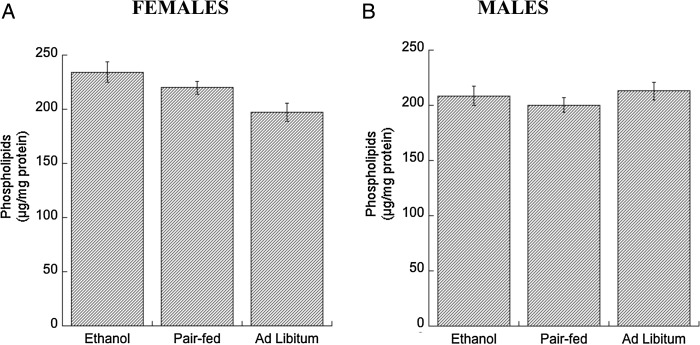
ABCA1 is also involved in phospholipid efflux (Oram and Heinecke, 2005); however, we found that phospholipid levels were not significantly changed in either ethanol-treated or ad libitum control male and female fetuses compared with pair-fed controls (Fig. 4A and B).
Fig. 4.
Effects of in utero ethanol exposure on phospholipid levels in the neocortex. Pregnant rats were exposed to ethanol from GD 6 to GD 21 as described in Methods. Phospholipid content was measured in neocortex homogenates of E, PF and C female (A) and male (B) rat E21 fetuses using the Phospholipid D assay kit (see Methods). Results show the mean (± SEM) of four independent measurements. Data were analyzed by one-way ANOVA followed by the Newman–Keuls post hoc test.
DISCUSSION
The observation that offspring of mothers who abuse alcohol during pregnancy are often diagnosed with FAS and have phenotypic features similar to those born with syndromes linked to genetic defects in cholesterol biosynthesis, such as SLOS, led us to formulate the hypothesis that one of the mechanisms underlying the developmental neurotoxicity of ethanol may involve interferences with cholesterol homeostasis (Guizzetti and Costa, 2005, 2007; Guizzetti et al., 2011). Cholesterol homeostasis in the CNS is indeed of relevance, as disruption of brain cholesterol homeostasis is associated with a number of neurodevelopmental conditions (Roux et al., 2000). Cholesterol plays a role in the activation of signal transduction pathways (including hedgehog, IGF and GDNF signaling) involved in brain development, and in glial cell proliferation, neuronal survival, neurite outgrowth and synapse formation (Langan and Slater, 1991; Michikawa and Yanagisawa, 1999; Bochelen et al., 2000; Mauch et al., 2001; Fan et al., 2002).
As major producers of cholesterol in the brain are glial cells, our initial studies focused on these cells cultured in vitro. We found that ethanol, at concentrations of 25–100 mM, increased cholesterol efflux from astrocytes, thereby significantly decreasing cellular cholesterol content (Guizzetti et al., 2007). This effect of ethanol was due to its ability to increase the expression and proper membrane localization of lipid transporters, such as ABCA1 and ABCG1 while cholesterol synthesis was unaffected (Guizzetti et al., 2007). Interestingly, various isomers of retinoic acid, which are also potent teratogens, caused a similar increase in ABCA1 and ABCG1 expression and in cholesterol efflux, and a decrease in cholesterol content in astrocytes (Guizzetti et al., 2007; Chen et al., 2011). Aim of the present study was to confirm the in vitro observations with ethanol in an in vivo situation. We had already shown that administration of ethanol (2, 4, 6 g/kg) from postnatal day 4–6 increased cortical levels of ABCA1 (Guizzetti et al., 2007). In the present study we investigated the effects on a prenatal exposure to alcohol on both transporters and on cholesterol and phospholipid levels.
Ethanol exposure from GD 6 to GD 21 significantly reduced the mean birth weight of female fetuses compared with the PF group (Table 1), indicating a specific effect of ethanol on body growth that was also reported in children affected by FAS; indeed, prenatal and/or postnatal growth retardation has been used in the diagnosis of FAS (Astley and Clarren, 2000). Some of the effects found in this study may be ascribed to a reduced caloric intake by ethanol-exposed dams; these effects can be easily identified in our model as two controls were introduced, an ad libitum-fed control and a pair-fed control. The average weight gain of the dams and mean birth weight of the fetuses from the PF group were significantly lower than the ad libitum group. Although pair-feeding is a necessary control for separating nutritional effects from those of the ethanol diet, it is essentially a treatment itself, as food-restriction may represent a maternal and fetal stressor (Weinberg, 1984).
Prenatal alcohol exposure increased ABCA1 and ABCG1 levels in the neocortex of GD 21 female rat fetuses, and decreased cholesterol levels, thus confirming in vivo our previous in vitro findings (Guizzetti et al., 2007; Chen et al., 2013). Of relevance is also the fact that these changes were observed at BAC of 45 mM, in the same range (25–100 mM) found to exert similar effects in vitro (Guizzetti et al., 2007). These findings suggest that the decrease in cholesterol content observed in ethanol-treated fetuses may be mediated by the increase in cholesterol transporters ABCA1 and ABCG1, involved in the biogenesis of lipoproteins in astrocytes. Increased availability of lipoproteins further increases cholesterol efflux from astrocytes and neurons (Guizzetti et al., 2007; Chen et al., 2013). The passage of brain lipoproteins into the blood stream through the blood–brain barrier represents one of the two pathways of brain cholesterol clearance (Raffai and Weisgraber, 2003; Dietschy and Turley, 2004).
Using an enzymatic method to quantify cholesterol we found that the cerebral neocortex of fetuses at GD 21 contains ∼25 µg cholesterol/mg protein corresponding to 2 mg/g tissue (ww) and 5.1 µmol/g tissue (ww). Several studies investigated cholesterol content using standard lipid analytical methods in lambs, mice and rats of different ages and found that cholesterol levels increase with the maturation of the brain especially during the process of myelination. In one study cholesterol levels in the whole mouse brain are reported to be 1.5 mg/g tissue (ww) at 1 week, 6.0 mg/g tissue (ww) at 3 weeks, 8.5 mg/g tissue (ww) at 10 weeks and 10.6 mg/g tissue (ww) mg at 25 weeks (Quan et al., 2003). Cholesterol content in the cerebrum in 1-week-old animals is ∼5 mg/g tissue (ww) (Quan et al., 2003). Another study found that cholesterol content in the whole brain of Longs-Evans rats at gestational day 22 is 7.1 µmol/g tissue (ww), while at postnatal day 35 is 36.4 µmol/g tissue (ww) (Jurevics et al., 1997). Taken together these reports indicate that previously published cholesterol levels obtained using standard analytical methods provided similar quantitative results, when the developmental stage of animals is kept in consideration, to the method used in this study therefore validating our results.
With regard to the effect of prenatal alcohol exposure on cholesterol levels, one study found that the cholesterol content (measured by standard analytical method) in postnatal day 20 rats whose mothers were fed ethanol during gestation and lactation was reduced (Duffy et al., 1991), while a more recent study showed that ethanol decreases cholesterol content (measured by an enzymatic method) in the cerebellum of newborn rats prenatally exposed to ethanol (Soscia et al., 2006). On the other hand, it was recently reported that prenatal alcohol exposure increases cholesterol levels in adult male rats (Barcelo-Coblijn et al., 2013).
As ABCA1 is also a phospholipid transporter (Oram and Heinecke, 2005), we also investigated whether ethanol would alter phospholipid levels in the neocortex; however, this was not the case (Fig. 4), possibly because of a compensatory increase in phospholipid biosynthesis stimulated by ethanol-induced ABCA1 up-regulation.
We measured phospholipid levels using an enzymatic assay (see ‘Methods’) and found ∼200 µg/mg of phospholipids in our samples, corresponding to 16 µmol/g tissue (ww). To validate these results we compared them to values of phospholipids measured in rodent brains using standard analytical methods. In one study it was reported that ∼37 µmol/g tissue (ww) was found in the cerebrum of 21 day-old Charles Foster rats (Lalitha et al., 1988). In another study 70.8 µmol/g tissue (ww) phospholipids were found in the brain of 25 week-old Sprague-Dawley rats (Barcelo-Coblijn et al., 2013). Furthermore, an age-dependent increase in the content of phospholipid phosphorus was reported in 10 and 20 day-old Wistar rats (Duffy et al., 1991). Evidence that phospholipid content increases during brain development derives also from studies in guinea pig during fetal development. A progressive increase in phosphoatidylcholine and phosphatidylethanolamine was observed in guinea pig fetuses at gestational days 25, 35, 40 and 68 (Burdge and Postle, 1995). Together these observations suggest that brain phospholipid content increases during brain development and that the levels of phospholipids we found using an enzymatic method in fetuses at gestational day 21 are about half of the amount of phospholipids found at postnatal day 21 and a fourth of the phospholipids present in the brain of 25 week-old rats. Therefore the measurement of phospholipids carried out by an enzymatic assay appears to be in the order of magnitude of what reported by others using standard analytical methods and in line with the notion that phospholipid content increases during brain maturation. The method used in this study quantifies the amount of choline after phospholipase D hydrolysis; this approach is very specific since free choline is not present in mammalian cells where 95% of the pool of total choline is present as phosphatidylcholine, while the remaining 5% includes choline, phosphocholine, glycerophosphocholine, CDP-choline and acetylcholine (Li and Vance, 2008).
A novel and interesting finding of the present study was that the increase in ABCA1 and ABCG1 levels and the decrease in cholesterol content were only seen in female fetuses. These sex-specific effects may be due to genetic differences between males and females. In support to this possibility, several behavioral and biochemical sex differences have been reported in rats prenatally exposed to alcohol (Weinberg et al., 2008; Hellemans et al., 2010; Verma et al., 2010; Uban et al., 2013). Another possibility is that, at GD 21, male and female brains are at a slightly different stage of development and for this reason they respond differently to alcohol insult. In support to this hypothesis we have reported that when male rats are exposed to ethanol neonatally between postnatal day 4 and 9 (which correspond to the third trimester of human gestation), we observed an increase in ABCA1 protein levels (Guizzetti et al., 2007), suggesting that cholesterol and ABCG1 may also be affected by ethanol when male rats are exposed to ethanol later in development. Further studies in an animal model mimicking alcohol exposure during the third trimester of human gestation will test this hypothesis.
Also of interest is the finding that the ad libitum female controls displayed lower cholesterol content and higher ABCA1 levels in comparison to the PF group. This suggests that in addition to exposure to developmental neurotoxicants such as ethanol, cholesterol and cholesterol transporters are also regulated by nutritional factors, particularly in females. While changes in ABCA1 and cholesterol levels induced by nutritional factors represent a physiological adaptation, the effects of ethanol represent a pathological condition that may exacerbate the effects of poor nutrition on the developing brain.
Interestingly, cholesterol levels in the ad libitum control female fetuses were significantly lower also than the levels found in ethanol-treated female fetuses, while ABCA1 levels were not different in the two conditions and ABCG1 levels were significantly lower in ad libitum control than in ethanol-exposed female fetuses. It is possible that, besides the upregulation of ABCA1, inhibition of cholesterol synthesis also contributes to the reduction of cholesterol levels when the liquid diet is available ad libitum therefore leading to a further decline in cholesterol content.
We hypothesize that cholesterol levels are physiologically regulated by factors, such as brain cholesterol availability, that may be modulated by the diet. The nutritional status of pair-fed animals is different from the nutritional status of ad libitum liquid diet fed animals. Indeed, the pair-fed animals received the same amount of diet ingested by animals in the ethanol group. Since ethanol-treated animals eat less than control animals (as shown in Table 1, rats in the ethanol and pair-fed group eat ∼35% less diet than animals in the ad libitum group and the weight of dams in the pair-fed and ethanol groups is significantly lower than the weight of dams in the ad libitum control group on gestational days 18 and 21), we hypothesize that the availability of nutrients and cholesterol, such as under the ad libitum conditions, triggers a physiological homeostatic mechanism that reduces cholesterol content through the upregulation of ABCA1 transporter. On the other hand, under conditions in which the availability of dietary cholesterol is restricted, the developing brain prevents cholesterol loss by down-regulating ABCA1 levels. However, ethanol disrupts this equilibrium by upregulating ABCA1 and ABCG1 levels and reducing cholesterol levels under the conditions of low nutrient availability. The hypothesized interaction of ethanol with the nutritional status of the fetus suggested by our results is in line with the observation that an extraordinarily high prevalence of FAS and FASD has been reported in very poor regions of South Africa in which malnutrition is widespread (May et al., 2000).
Although not fully characterized, there are at least two pathways by which cholesterol exits the brain. One pathway is through the conversion of cholesterol to 24-S-hydroxycholesterol, a blood–brain barrier-permeable cholesterol metabolite, by the neuronal specific enzyme cholesterol 24-hydroxylase (or CYP46) (Lund et al., 1999; Dietschy and Turley, 2004). The other pathway involves the passage of brain lipoproteins into the blood stream, likely mediated by lipoprotein transporters or receptors present in the endothelial cells of the blood–brain barrier (Pitas et al., 1987; Raffai and Weisgraber, 2003; Dietschy and Turley, 2004). The conversion of cholesterol to 24-S-hydroxycholesterol is considered the main pathway of cholesterol clearance in the normal, adult brain, accounting for about 64% of cholesterol clearance. However, CYP46 is expressed only in a subset of neurons in the brain. Furthermore, CYP46 and, consequently, 24-S-hydroxycholesterol levels are low at birth and very slowly increase to reach the maximal levels only at about 4 weeks of age (Lund et al., 1999; Dietschy and Turley, 2004). Cholesterol clearance by the lipoprotein pathway through the blood–brain barrier is likely to play a major role during brain development when CYP46 levels are low and the blood–brain barrier not completely formed, particularly for removing cholesterol from neurons non-expressing CYP46 and glial cells.
It has been reported that astrocytes-released lipoproteins interact with members of the LDL receptor family expressed in neurons to induce synaptogenesis and axonal growth after axotomy and to prevent neuronal apoptosis induced by growth factor withdrawal (Mauch et al., 2001; Hayashi et al., 2004, 2009; Matsuo et al., 2011). The effects of astrocytes-released lipoproteins on synaptogenesis have been attributed to cholesterol (Mauch et al., 2001), on neuronal survival to the signaling pathway activated by the binding (but not uptake) of lipoproteins to the low-density lipoprotein receptor-related protein-1 (Hayashi et al., 2004, 2009) and on axonal growth to sphingomyelin (Matsuo et al., 2011). It has also been reported that, under specific conditions, such as after nerve injury, lipoprotein receptors are upregulated, likely to allow for the uptake of cholesterol at the damaged axonal terminal (Boyles et al., 1989). In a similar fashion, an upregulation of lipoprotein receptors at the axonal terminals may occur during synaptogenesis to provide cholesterol necessary to the formation of synaptic structures. It should however be pointed out that several members of the low-density lipoprotein receptors found in the brain do not have a lipoprotein uptake function, but instead activate signal transduction pathways important during brain development (Herz, 2001).
On the other hand, our group and others have shown that HDL and astrocyte-released lipoproteins act as cholesterol acceptors and extract cholesterol from astrocytes and neurons through their interaction with ABCA1, ABCG1 and ABCG4 transporters (Michikawa et al., 2000; Guizzetti et al., 2007; Kim et al., 2007; Chen et al., 2013). This role of HDL-like proteins in the brain is similar to the well-characterized HDL-mediated removal of excessive cellular cholesterol in the periphery known as reverse cholesterol transport (Oram and Heinecke, 2005). Indeed, lipid-poor, HDL-like lipoproteins released by astrocytes extract lipids and cholesterol from brain cells, including other glial cells and neurons, and are remodeled into bigger lipoproteins that are spherical and richer in lipids and are found in the cerebrospinal fluid (LaDu et al., 1998; Yu et al., 2010). We also observed that protracted exposures to lipoproteins induce continuous cholesterol efflux form neurons, which is initially compensated by increased cholesterol synthesis but that later leads to reduced levels of cholesterol in these cells (Chen et al., unpublished).
Further studies are necessary to establish the relative contribution of these two pathways to cholesterol homeostasis in neurons and to fully understand the role of brain lipoproteins (Wang and Eckel, 2014). Our results showing higher cholesterol transporter levels and lower cholesterol levels after prenatal alcohol exposure and under conditions of high nutrient availability are consistent with the role of ABC cholesterol transporters as regulators of brain cholesterol levels.
In conclusion, we have shown that prenatal alcohol exposure up-regulates ABCA1 and ABCG1 cholesterol transporters and reduces the levels of cholesterol in the neocortex of GD 21 female fetuses. These effects may contribute to the developmental effects of ethanol, as cholesterol plays a major role in brain development.
Funding
This study was supported in part by grants AA017180 and AA021876 from the National Institute of Alcoholism and Alcohol Abuse.
Conflict of interest statement
None declared.
Acknowledgements
We thank Dr Joanne Weinberg for her advice on the in vivo prenatal ethanol exposure model.
REFERENCES
- Astley SJ, Clarren SK. Diagnosing the full spectrum of fetal alcohol-exposed individuals: introducing the 4-digit diagnostic code. Alcohol Alcohol. 2000;35:400–10. doi: 10.1093/alcalc/35.4.400. [DOI] [PubMed] [Google Scholar]
- Barcelo-Coblijn G, Wold LE, Ren J, et al. Prenatal ethanol exposure increases brain cholesterol content in adult rats. Lipids. 2013;48:1059–68. doi: 10.1007/s11745-013-3821-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochelen D, Langley K, Adamczyk M, et al. 7beta-hydroxysterol is cytotoxic to neonatal rat astrocytes in primary culture when cAMP levels are increased. J Neurosci Res. 2000;62:99–111. doi: 10.1002/1097-4547(20001001)62:1<99::AID-JNR11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Boyles JK, Zoellner CD, Anderson LJ, et al. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J Clin Invest. 1989;83:1015–31. doi: 10.1172/JCI113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdge GC, Postle AD. Phospholipid molecular species composition of developing fetal guinea pig brain. Lipids. 1995;30:719–24. doi: 10.1007/BF02537798. [DOI] [PubMed] [Google Scholar]
- Chen J, Costa LG, Guizzetti M. Retinoic Acid isomers up-regulate ATP binding cassette A1 and g1 and cholesterol efflux in rat astrocytes: implications for their therapeutic and teratogenic effects. J Pharmacol Exp Ther. 2011;338:870–8. doi: 10.1124/jpet.111.182196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang X, Kusumo H, et al. Cholesterol efflux is differentially regulated in neurons and astrocytes: implications for brain cholesterol homeostasis. Biochim Biophys Acta. 2013;1831:263–75. doi: 10.1016/j.bbalip.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarren SK, Alvord EC, Jr, Sumi SM, et al. Brain malformations related to prenatal exposure to ethanol. J Pediatr. 1978;92:64–7. doi: 10.1016/s0022-3476(78)80072-9. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Jr, Shiota K. Teratogenesis of holoprosencephaly. Am J Med Genet. 2002;109:1–15. doi: 10.1002/ajmg.10258. [DOI] [PubMed] [Google Scholar]
- Cooper MK, Wassif CA, Krakowiak PA, et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33:508–13. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- Dehart DB, Lanoue L, Tint GS, et al. Pathogenesis of malformations in a rodent model for Smith-Lemli-Opitz syndrome. Am J Med Genet. 1997;68:328–37. doi: 10.1002/(sici)1096-8628(19970131)68:3<328::aid-ajmg15>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12:105–12. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–97. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48:757–67. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy O, Menez JF, Floch HH, et al. Changes in whole brain membranes of rats following pre- and post-natal exposure to ethanol. Alcohol Alcohol. 1991;26:605–13. doi: 10.1093/oxfordjournals.alcalc.a045164. [DOI] [PubMed] [Google Scholar]
- Famy C, Streissguth AP, Unis AS. Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am J Psychiatry. 1998;155:552–4. doi: 10.1176/ajp.155.4.552. [DOI] [PubMed] [Google Scholar]
- Fan QW, Yu W, Gong JS, et al. Cholesterol-dependent modulation of dendrite outgrowth and microtubule stability in cultured neurons. J Neurochem. 2002;80:178–90. doi: 10.1046/j.0022-3042.2001.00686.x. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Yu WK, Weinberg J. Effects of mineralocorticoid and glucocorticoid receptor blockade on hypothalamic-pituitary-adrenal function in female rats prenatally exposed to ethanol. Alcohol Clin Exp Res. 2006;30:1916–24. doi: 10.1111/j.1530-0277.2006.00236.x. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Ellis L, Yu WK, et al. Effects of prenatal ethanol exposure on basal limbic-hypothalamic-pituitary-adrenal regulation: role of corticosterone. Alcohol Clin Exp Res. 2007;31:1598–610. doi: 10.1111/j.1530-0277.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa LG. Disruption of cholesterol homeostasis in the developing brain as a potential mechanism contributing to the developmental neurotoxicity of ethanol: an hypothesis. Med Hypotheses. 2005;64:563–7. doi: 10.1016/j.mehy.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa LG. Cholesterol homeostasis in the developing brain: a possible new target for ethanol. Hum Exp Toxicol. 2007;26:355–60. doi: 10.1177/0960327107078412. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Chen J, Oram JF, et al. Ethanol induces cholesterol efflux and up-regulates ATP-binding cassette cholesterol transporters in fetal astrocytes. J Biol Chem. 2007;282:18740–9. doi: 10.1074/jbc.M702398200. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Chen J, Costa LG. Disruption of cholesterol homeostasis in developmental neurotoxicity. In: Gupta RC, editor. Reproductive and Developmental Toxicology. London: Academic Press; 2011. pp. 855–62. [Google Scholar]
- Hayashi H, Campenot RB, Vance DE, et al. Glial lipoproteins stimulate axon growth of central nervous system neurons in compartmented cultures. J Biol Chem. 2004;279:14009–15. doi: 10.1074/jbc.M313828200. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Campenot RB, Vance DE, et al. Protection of neurons from apoptosis by apolipoprotein E-containing lipoproteins does not require lipoprotein uptake and involves activation of phospholipase Cgamma1 and inhibition of calcineurin. J Biol Chem. 2009;284:29605–13. doi: 10.1074/jbc.M109.039560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, et al. Prenatal alcohol exposure and chronic mild stress differentially alter depressive- and anxiety-like behaviors in male and female offspring. Alcohol Clin Exp Res. 2010;34:633–45. doi: 10.1111/j.1530-0277.2009.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J. The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron. 2001;29:571–81. doi: 10.1016/s0896-6273(01)00234-3. [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Zhou S, Burgess BL, et al. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J Biol Chem. 2004;279:41197–207. doi: 10.1074/jbc.M407962200. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Roelink H. The role of cholesterol in Shh signaling and teratogen-induced holoprosencephaly. Cell Mol Life Sci. 2000;57:1709–19. doi: 10.1007/PL00000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurevics HA, Kidwai FZ, Morell P. Sources of cholesterol during development of the rat fetus and fetal organs. J Lipid Res. 1997;38:723–33. [PubMed] [Google Scholar]
- Kim WS, Suryo Rahmanto A, Kamili A, et al. Role of ABCG1 and ABCA1 in regulation of neuronal cholesterol efflux to apolipoprotein-E discs and suppression of amyloid-beta peptide generation. J Biol Chem. 2007;282:2851–61. doi: 10.1074/jbc.M607831200. [DOI] [PubMed] [Google Scholar]
- Koldamova RP, Lefterov IM, Ikonomovic MD, et al. 22R-hydroxycholesterol and 9-cis-retinoic acid induce ATP-binding cassette transporter A1 expression and cholesterol efflux in brain cells and decrease amyloid beta secretion. J Biol Chem. 2003;278:13244–56. doi: 10.1074/jbc.M300044200. [DOI] [PubMed] [Google Scholar]
- LaDu MJ, Gilligan SM, Lukens JR, et al. Nascent astrocyte particles differ from lipoproteins in CSF. J Neurochem. 1998;70:2070–81. doi: 10.1046/j.1471-4159.1998.70052070.x. [DOI] [PubMed] [Google Scholar]
- Lalitha T, Kumar K, Ramakrishnan CV, et al. Effect of maternal alcohol consumption on the lipid composition of CNS in the offspring. J Neurochem. 1988;50:1346–51. doi: 10.1111/j.1471-4159.1988.tb03014.x. [DOI] [PubMed] [Google Scholar]
- Langan TJ, Slater MC. Quiescent astroglia in long-term primary cultures re-enter the cell cycle and require a non-sterol isoprenoid in late G1. Brain Res. 1991;548:9–17. doi: 10.1016/0006-8993(91)91099-m. [DOI] [PubMed] [Google Scholar]
- Lanoue L, Dehart DB, Hinsdale ME, et al. Limb, genital, CNS, and facial malformations result from gene/environment-induced cholesterol deficiency: further evidence for a link to sonic hedgehog. Am J Med Genet. 1997;73:24–31. [PubMed] [Google Scholar]
- Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. J Lipid Res. 2008;49:1187–94. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- Lund EG, Guileyardo JM, Russell DW. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc Natl Acad Sci USA. 1999;96:7238–43. doi: 10.1073/pnas.96.13.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti E, Bovolenta P. Sonic hedgehog in CNS development: one signal, multiple outputs. Trends Neurosci. 2002;25:89–96. doi: 10.1016/s0166-2236(02)02062-3. [DOI] [PubMed] [Google Scholar]
- Matsuo M, Campenot RB, Vance DE, et al. Involvement of low-density lipoprotein receptor-related protein and ABCG1 in stimulation of axonal extension by apoE-containing lipoproteins. Biochim Biophys Acta. 2011;1811:31–8. doi: 10.1016/j.bbalip.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–7. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- May PA, Brooke L, Gossage JP, et al. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am J Public Health. 2000;90:1905–12. doi: 10.2105/ajph.90.12.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michikawa M, Yanagisawa K. Inhibition of cholesterol production but not of nonsterol isoprenoid products induces neuronal cell death. J Neurochem. 1999;72:2278–85. doi: 10.1046/j.1471-4159.1999.0722278.x. [DOI] [PubMed] [Google Scholar]
- Michikawa M, Fan QW, Isobe I, et al. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J Neurochem. 2000;74:1008–16. doi: 10.1046/j.1471-4159.2000.0741008.x. [DOI] [PubMed] [Google Scholar]
- Miller MW. Effects of alcohol on the generation and migration of cerebral cortical neurons. Science. 1986;233:1308–11. doi: 10.1126/science.3749878. [DOI] [PubMed] [Google Scholar]
- Miller MW, Potempa G. Numbers of neurons and glia in mature rat somatosensory cortex: effects of prenatal exposure to ethanol. J Comp Neurol. 1990;293:92–102. doi: 10.1002/cne.902930108. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Miller MW. Nerve growth factor neuroprotection of ethanol-induced neuronal death in rat cerebral cortex is age dependent. Neuroscience. 2007;149:372–81. doi: 10.1016/j.neuroscience.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowaczyk MJ, Whelan DT, Heshka TW, et al. Smith-Lemli-Opitz syndrome: a treatable inherited error of metabolism causing mental retardation. Can Med Assoc J. 1999;161:165–70. [PMC free article] [PubMed] [Google Scholar]
- Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85:1343–72. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- Pitas RE, Boyles JK, Lee SH, et al. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J Biol Chem. 1987;262:14352–60. [PubMed] [Google Scholar]
- Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–9. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- Quan G, Xie C, Dietschy JM, et al. Ontogenesis and regulation of cholesterol metabolism in the central nervous system of the mouse. Brain Res Dev Brain Res. 2003;146:87–98. doi: 10.1016/j.devbrainres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Raffai RL, Weisgraber KH. Cholesterol: from heart attacks to Alzheimer's disease. J Lipid Res. 2003;44:1423–30. doi: 10.1194/jlr.R300007-JLR200. [DOI] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux C, Wolf C, Mulliez N, et al. Role of cholesterol in embryonic development. Am J Clin Nutr. 2000;71:1270–9. doi: 10.1093/ajcn/71.5.1270s. [DOI] [PubMed] [Google Scholar]
- Soscia SJ, Tong M, Xu XJ, et al. Chronic gestational exposure to ethanol causes insulin and IGF resistance and impairs acetylcholine homeostasis in the brain. Cell Mol Life Sci. 2006;63:2039–56. doi: 10.1007/s00018-006-6208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, O'Malley K. Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Semin Clin Neuropsychiatry. 2000;5:177–90. doi: 10.1053/scnp.2000.6729. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Landesman-Dwyer S, Martin JC, et al. Teratogenic effects of alcohol in humans and laboratory animals. Science. 1980;209:353–61. doi: 10.1126/science.6992275. [DOI] [PubMed] [Google Scholar]
- Turley SD, Burns DK, Rosenfeld CR, et al. Brain does not utilize low density lipoprotein-cholesterol during fetal and neonatal development in the sheep. J Lipid Res. 1996;37:1953–61. [PubMed] [Google Scholar]
- Turley SD, Burns DK, Dietschy JM. Preferential utilization of newly synthesized cholesterol for brain growth in neonatal lambs. Am J Physiol. 1998;274:E1099–105. doi: 10.1152/ajpendo.1998.274.6.E1099. [DOI] [PubMed] [Google Scholar]
- Uban KA, Comeau WL, Ellis LA, et al. Basal regulation of HPA and dopamine systems is altered differentially in males and females by prenatal alcohol exposure and chronic variable stress. Psychoneuroendocrinology. 2013;38:1953–66. doi: 10.1016/j.psyneuen.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, Oram JF. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res. 2006;47:2433–43. doi: 10.1194/jlr.M600218-JLR200. [DOI] [PubMed] [Google Scholar]
- Verma P, Hellemans KG, Choi FY, et al. Circadian phase and sex effects on depressive/anxiety-like behaviors and HPA axis responses to acute stress. Physiol Behav. 2010;99:276–85. doi: 10.1016/j.physbeh.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, et al. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem. 2004;279:40987–93. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- Wang H, Eckel RH. What are lipoproteins doing in the brain? Trends Endocrinol Metab. 2014;25:8–14. doi: 10.1016/j.tem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. Nutritional issues in perinatal alcohol exposure. Neurobehav Toxicol Teratol. 1984;6:261–9. [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, et al. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–88. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Youmans KL, LaDu MJ. Proposed mechanism for lipoprotein remodelling in the brain. Biochim Biophys Acta. 2010;1801:819–23. doi: 10.1016/j.bbalip.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]