Abstract
Purpose
To assess survival following radical prostatectomy (RP), intensity modulated radiation therapy (IMRT) or conformal radiation therapy (CRT) versus no local therapy (NLT) for metastatic prostate cancer (MPCa), adjusting for patient comorbidity, androgen deprivation therapy (ADT) and other factors.
Materials and Methods
Men ≥66 with MPCa undergoing treatment by RP, IMRT, CRT or NLT identified from SEER-Medicare linked database (2004–2009). Multivariable Cox proportional hazards models, before and after inverse propensity score weighting, were used to assess all cause and PCa specific mortality. Competing risk regression analysis was used to assess PCa specific mortality.
Results
Among 4069 men with MPCa, RP (n=47), IMRT (n=88), CRT (n=107) were selected as local therapy versus NLT (n=3827). RP was associated with a 52% (HR: 0.48, 95% CI: 0.27–0.85) reduction in the risk of PCa specific mortality, after adjusting for socio-demographic, primary tumour characteristics, comorbidity, ADT and bone radiation within 6 months of diagnosis. IMRT was associated with a 62% (HR: 0.38, 95% CI: 0.24–0.61) reduction in the risk of PCa specific mortality, respectively. CRT was not associated with improved survival compared to NLT. Propensity score weighting yielded comparable results. Competing risk analysis revealed a 42% (SHR: 0.58, 95% CI: 0.35–0.95) and 57% (SHR: 0.43, 95% CI: 0.27–0.68) reduction in the risk of PCa specific mortality for RP and IMRT.
Conclusions
Local therapy with RP and IMRT, but not CRT, was associated with a survival benefit in MPC and warrants prospective evaluation in clinical trials
MeSH: Prostatic Neoplasm, Prostatectomy, Intensity-Modulated Radiotherapy, Conformal Radiotherapy, Cytoreduction Surgical Procedures, SEER Program
Introduction
The standard of care for metastatic prostate cancer (MPCa) is continuous androgen deprivation therapy (ADT)1,2. A secondary analysis of SWOG 8894 suggesting radical prostatectomy (RP) prior to MPCa was associated with a decreased risk of death implicated a potential role for local therapy3. Recent population based studies utilizing the Surveillance Epidemiology and End Results (SEER) database have demonstrated a potential survival benefit to RP in MPCa4–6.
Population-based studies have not assessed the role of intensity modulated radiation therapy (IMRT) or conformal radiation therapy (CRT) for local treatment in MPCa. Further, these studies have not investigated the differential utilization of androgen deprivation therapy (ADT) and patient comorbidity, which can dictate treatment selection and confound the relationship between treatment type and survival. To disentangle the relationship between these factors and survival we utilized the SEER-Medicare linked database to assess survival outcomes of RP, IMRT, CRT and no local treatment (NLT) for MPCa.
Materials and Methods
Study Subjects
The SEER registry captures 28% of the US population and contains information on patient demographics, tumour characteristics and choice of primary treatment modality7. Linkage to Medicare, which provides benefits to 97% of Americans aged ≥65 years, offers additional treatment data, including therapies administered in the outpatient setting such as ADT8,9.
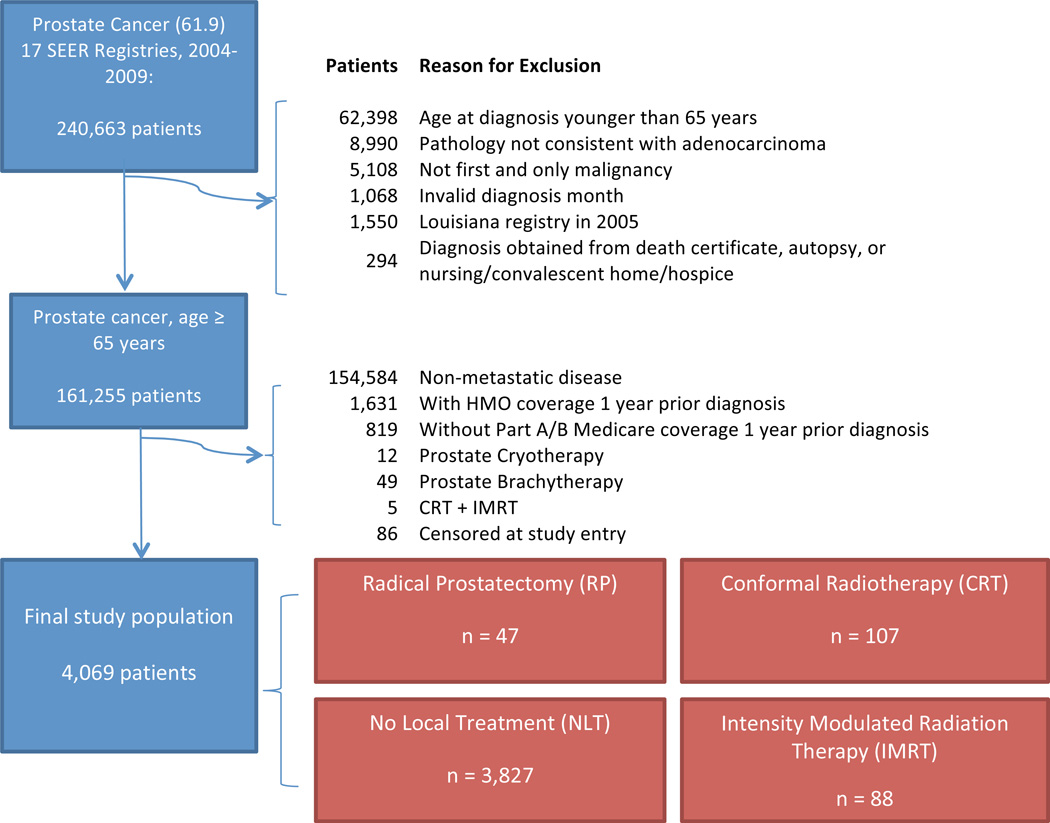
We identified a source population (N= 240,663) based on the International Classification of Diseases for Oncology (third edition, code 8140) of the prostate (site code 61.9) diagnosed between 2004–2009. Figure 1 details the exclusion process to optimize data reliability. MPCa was defined by radiographic and/or pathologic confirmation of metastatic cancer (SEER-collaborative stage) as per the American Joint Committee on Cancer (AJCC) Cancer Staging Manual 6th.
Figure 1.
Exclusion criteria utilized to derive the final study cohort from the SEER-Medicare linked database (2004–2009).
Outcome Measures, Treatment Categories and Covariates
The outcomes of interest were all cause mortality (ACM) and PCa specific mortality (PCSM). Survival time was determined from registry vital statistics from the date of diagnosis to the date of death, last known to be alive or last follow-up (December 2010), whichever occurred first. Patients receiving intensity-modulated radiotherapy (IMRT) or conformal radiotherapy (CRT) were identified from Medicare inpatient, outpatient, and carrier component files based on Current Procedural Terminology, Fourth Edition (CPT-4) codes as previously described using prostate diagnosis codes for treatment claims10. Patients with ≤15 treatment claims were excluded as this likely represented palliative radiation (e.g. bone) or treatment for local symptom control11. The practice pattern for palliative radiation varies, however, we selected a cut-off of 15, which represents the largest number of fractions reported from published randomized trials on palliative regimes12,13. We also identified patients who received EBRT to bone within 6 months of diagnosis as a marker of advanced disease. RP was defined using SEER surgery site codes 50 or 704. In order to assess possible discrepancies between SEER and Medicare data on treatment assignment14, we also identified patients that underwent RP using Medicare billing codes15. Accuracy of staging and treatment for each individual RP patient (n=47) was re-confirmed by directly contacting SEER registry directors for repeat patient-to-patient data reconfirmation. On review of 228 cases identified from SEER (2004–2010) as having metastatic PCa and receiving RP, 65% were confirmed as correct after registry audits, with individual registries varying from 45–100% with respect to accuracy of classification (Supplemental Figure 1). Patients receiving NLT for PCa never received RP, EBRT, brachytherapy10, or prostate cryotherapy (CPT-4 code 55873)16.
Covariates of interest included age at diagnosis (years, continuous), race (African American, Hispanic, Non-Hispanic White, Asian and Other/Unknown), marital status (single, married, unknown or other), year of diagnosis (categorical 2004–2009), pre-treatment PSA (highest recorded, continuous and categorical), Gleason score and clinical AJCC staging from registry data. Approximately 15% of patients had unknown PSA values. In order to ensure that missing PSA was non-informative, PSA was assessed as a categorical variable with an unknown category. PSA was also assessed as continuous variable after excluding unknown values, however, a sensitivity analysis showed comparable effect estimates (data not shown). Specifically, for Gleason score, we used the SEER Collaborative Stage Site-Specific Factor 6 grade variable, categorized as well (≤ 4) or moderately differentiated (5–6), intermediate (7) and poor (≥ 8) differentiated. A validated algorithm was used to derive the Charlson comorbidity index (CCI) from claims one year prior to the diagnosis of MPCa17. Lastly, androgen deprivation therapy (ADT) exposure was determined as previously reported18. Specifically, ADT exposure in this study included administration of GnRH agonists 3 months before to 12 months after diagnosis, or bilateral orchiectomy within 3 months of diagnosis.
Propensity Score Adjustment
In observational studies there can be significant bias introduced by inherent differences between patients based on treatment selection. In order to decrease the risk of biased estimates of treatment effect, we computed propensity scores by multinomial logistic regression with a four-level outcome variable (RP, IMRT, CRT or NLT) with predictor variables age at diagnosis, year of diagnosis, race, marital status, pre-treatment PSA (categorical), clinical tumour stage and grade, CCI, ADT use and bone radiation within 6 months of diagnosis. Propensity scores were then utilized for inverse propensity score weighted adjustment in the final cox proportional hazards models19.
Statistical Analysis
Differences between the distributions of socio-demographic and primary tumour factors according to RP, IMRT, CRT and NLT were examined using the Chi-square test. The hazard function of overall survival and PCa specific survival by treatment type was described using the Kaplan–Meier method. Cox proportional hazard models were fitted to assess the crude and adjusted hazard ratios (HRs) comparing RP, IMRT and CRT to NLT for ACM and PCSM. Covariates that were a priori deemed clinically important were mutually adjusted in multivariable models; the final adjusted model included registry, age at diagnosis, year of diagnosis, race, marital status, PSA, Gleason grade, AJCC T, N and M staging, CCI, and bone radiation within 6 months.
We hypothesized that ADT might modify the effect of treatment modality on survival, however, interaction (likelihood ratio test) was not significant (p=0.1) and ADT was included as a covariate in the final model. The proportional hazards assumption was satisfied in all variables except for ADT, where there was statistically significant interaction with time. Modeling ADT as a time-varying covariate did not significantly change the effect estimates (data not shown).
Given the possibility that Cox proportional hazard regression estimates of disease specific survival can overestimate risk, we also performed competing risk regression analysis to compute sub hazard ratios (SHR) as described by Fine and Gray20,21. All statistical analyses were performed using SAS version 9.4 (SAS Inc, Cary, NC, USA) and Stata S/E 12.1 (Stata Corporation, College Station, TX). A p-value < 0.05 was considered statistically significant.
Results
A total of 4069 cases with MPCa were identified as receiving RP (n = 47), IMRT (n=88), CRT (n=107) or NLT (n=3827). Total treatments by claim number for CRT (median: 23 [IQR: 19–30]) was less than for IMRT (median: 38 [IQR: 28–42], p<0.001). RP and IMRT groups were younger, had lower pre-treatment PSA, lower Gleason score, lower stage AJCC T and N stage compared to CRT and NLT (Table 1). The metastatic AJCC stage distribution between the treatment groups was relatively comparable. Additionally, RP and IMRT groups were less likely to receive ADT or bone radiation within 6 months of diagnosis (Table 1). The overall median follow up was 20 months (IQR: 10–36) with a total of 2872 total deaths (71%), of which 2058 (72%) deaths were attributable to PCa.
Table 1.
Socio-demographics, tumour characteristics, comorbidity, ADT use and receipt of bone radiation within 6 months among men with metastatic prostate cancer (N=4069) that received radical prostatectomy (RP), intensity modulated radiation therapy (IMRT), conformal radiation therapy (CRT), or No Local Treatment (NLT). Percentages are shown in parenthesis. One-way ANOVA (PSA and log-transformed age) and two-tailed chi-square tests were used to test the hypothesis that at least one of the proportions/distribution of covariates is different by treatment type. Note that 595 patients (~15%) have unknown PSA values and are not included in continuous description of PSA.
| RP | IMRT | CRT | NLT | p-value | |
|---|---|---|---|---|---|
| 47 | 88 | 107 | 3827 | ||
| Year of Diagnosis – N (%) | |||||
| 2004 | 6 (13) | 12 (14) | 21 (20) | 709 (19) | 0.2 |
| 2005 | 5 (11) | 9 (10) | 26 (24) | 692 (18) | |
| 2006 | 7 (15) | 14 (16) | 21 (20) | 667 (17) | |
| 2007 | 11 (23) | 21 (24) | 16 (15) | 619 (16) | |
| 2008 | 10 (21) | 18 (20) | 14 (13) | 580 (15) | |
| 2009 | 8 (17) | 14 (16) | 9 (8) | 560 (15) | |
| Age at Diagnosis | |||||
| Mean (SD) | 73.0 (6.0) | 74.2 (6.1) | 76.4 (6.3) | 78.2 (7.2) | < 0.001 |
| Race – N (%) | |||||
| NHW | 38 (81) | 75 (85) | 81 (76) | 2925 (76) | 0.5 |
| AA | 7 (15) | 9 (10) | 13 (12) | 608 (16) | |
| Hisp | 0 (0) | 2 (2) | 3 (3) | 95 (2) | |
| Asian | 2 (4) | 1 (1) | 6 (6) | 103 (3) | |
| Other/Unknown | 0 (0) | 1 (1) | 4 (4) | 96 (3) | |
| Marital Status – N (%) | |||||
| Single | 2 (4) | 10 (11) | 6 (6) | 408 (11) | 0.1 |
| Married | 35 (74) | 60 (68) | 68 (64) | 2248 (59) | |
| Separated/divorced/widowed/ domestic partners |
9 (19) | 12 (14) | 27 (25) | 933 (24) | |
| Unknown | 1 (2) | 6 (7) | 6 (6) | 238 (6) | |
| PSA – N (%) | |||||
| < 10 ng/ml | 25 (53) | 35 (40) | 9 (8) | 401 (10) | < 0.001 |
| 10–19 ng/ml | 6 (13) | 16 (18) | 20 (19) | 449 (12) | |
| 20–29 ng/ml | 3 (6) | 10 (11) | 9 (8) | 286 (7) | |
| > 30 ng/ml | 6 (13) | 17 (19) | 55 (51) | 2127 (56) | |
| Unknown | 7 (15) | 10 (11) | 14 (13) | 564 (15) | |
| PSA (Continuous) | |||||
| Mean (SD) | 181 (263) | 282 (338) | 531 (369) | 590 (380) | < 0.001 |
| Gleason Score – N (%) | |||||
| ≤6 | 5 (11) | 10 (11) | 8 (7) | 167 (4) | < 0.001 |
| 7 | 22 (47) | 24 (27) | 22 (21) | 569 (15) | |
| ≥8 | 19 (40) | 43 (49) | 59 (55) | 2042 (53) | |
| Unknown | 1 (2) | 11 (13) | 18 (17) | 1049 (27) | |
| T Stage – N (%) | |||||
| T1 | 0 (0) | 27 (31) | 31 (29) | 839 (22) | < 0.001 |
| T2 | 21 (45) | 36 (41) | 28 (26) | 1282 (33) | |
| T3 | 19 (40) | 10 (11) | 9 (8) | 298 (8) | |
| T4 | 6 (13) | 9 (10) | 17 (16) | 461 (12) | |
| Unknown | 1 (2) | 6 (7) | 22 (21) | 947 (25) | |
| N Stage – N (%) | |||||
| N0 | 34 (72) | 59 (67) | 63 (59) | 1930 (50) | < 0.001 |
| N1 | 10 (21) | 11 (13) | 15 (14) | 577 (15) | |
| NX | 3 (6) | 18 (20) | 29 (27) | 1320 (34) | |
| M Stage – N (%) | |||||
| M1a | 3 (6) | 4 (5) | 4 (4) | 190 (5) | 0.2 |
| M1b | 26 (55) | 65 (74) | 72 (67) | 2570 (67) | |
| M1c | 17 (36) | 16 (18) | 31 (29) | 922 (24) | |
| M1 NOS | 1 (2) | 3 (3) | 0 (0) | 145 (4) | |
|
Charlson Comorbidity Index – N (%) |
|||||
| 0 | 32 (68) | 60 (68) | 67 (63) | 2462 (64) | 0.9 |
| 1 | 9 (19) | 19 (22) | 25 (23) | 757 (20) | |
| 2 | 4 (9) | 4 (5) | 10 (9) | 331 (9) | |
| ≥3 | 2 (4) | 5 (6) | 5 (5) | 277 (7) | |
|
Androgen Deprivation Therapy – N (%) |
|||||
| None | 27 (57) | 30 (34) | 13 (12) | 1132 (30) | < 0.001 |
| Orchiectomy | 3 (6) | 0 (0) | 5 (5) | 331 (9) | |
| GnRH Agonist | 16 (34) | 56 (64) | 88 (82) | 2330 (61) | |
| Both | 1 (2) | 2 (2) | 1 (1) | 34 (1) | |
|
Bone Radiation Within 6 mo of Diagnosis – N (%) |
|||||
| No | 47 (100) | 86 (98) | 99 (93) | 3420 (89) | 0.005 |
| Yes | 0 (0) | 2 (2) | 8 (7) | 407 (11) |
RP and IMRT when compared to NLT were associated with 57% (HR: 0.43, 95% CI: 0.26–0.70) and 55% (HR: 0.45, 95% CI: 0.31–0.65) lower risk of ACM respectively, after adjusting for socio-demographic, primary tumour characteristics, CCI, ADT and bone radiation within 6 months of diagnosis (Table 2). The adjusted PCa specific mortality was 52% (HR: 0.48, 95% CI: 0.27–0.85) and 62% (HR: 0.38, 95% CI: 0.24–0.61) lower in patients undergoing RP and IMRT respectively, compared to NLT (Table 2). In contrast, CRT compared to NLT, was not associated with lower risk of death from prostate cancer (HR: 0.85, 95% CI: 0.64–1.14). IMRT and CRT as a combined category was associated with a decreased risk of PCSM (HR: 0.64, 95% CI: 0.50–0.82. Older age, higher PSA, more aggressive primary tumour pathology (AJCC Stage), increasing CCI and bone radiation within 6 months of diagnosis were independently associated with increase risk of PCSM. The 3-year overall survival rate was 73% for RP, 72% for IMRT, 37% for CRT, and 34% for NLT (Figure 2A). The 3-year disease specific survival rate was 79% for RP, 82% for IMRT, 49% for CRT, and 46% for NLT (Figure 2B).
Table 2.
Crude and adjusted probability of all cause and prostate cancer specific mortality after local treatment for metastatic prostate cancer. Hazard Ratios (HR) are adjusted for treatment group (NLT, CRT, IMRT and RP), age, year of diagnosis, race, marital status, PSA, Gleason score, AJCC staging (TNM), Charlson Comorbidity Index, androgen deprivation therapy, receipt of bone radiation within 6 months of diagnosis and registry. The model for prostate cancer specific mortality treats non-prostate cancer deaths as censored observations.
| Characteristic | N | All-Cause Mortality | P value | Prostate Cancer Specific Mortality |
P value |
|---|---|---|---|---|---|
| Adjusted HR (95% CI) | Adjusted HR (95% CI) | ||||
| Treatment | |||||
| NLT | 3827 | 1.0 (Ref) | 1.0 (Ref) | ||
| CRT | 107 | 0.90 (0.70–1.14) | 0.4 | 0.85 (0.64–1.14) | 0.3 |
| IMRT | 88 | 0.45 (0.31–0.65) | < 0.001 | 0.38 (0.24–0.61) | < 0.001 |
| RP | 47 | 0.43 (0.26–0.72) | 0.001 | 0.48 (0.27–0.85) | 0.01 |
| Age | |||||
| 5 yr. increment | 4069 | 1.17 (1.14–1.20) | < 0.001 | 1.12 (1.09–1.16) | < 0.001 |
| Year of Diagnosis | |||||
| 2004 | 748 | 1.0 (Ref) | 1.0 (Ref) | ||
| 2005 | 732 | 1.05 (0.93–1.18) | 0.4 | 1.07 (0.93–1.22) | 0.4 |
| 2006 | 709 | 0.98 (0.87–1.11) | 0.8 | 1.05 (0.91–1.20) | 0.5 |
| 2007 | 667 | 0.95 (0.84–1.08) | 0.4 | 0.90 (0.78–1.05) | 0.2 |
| 2008 | 622 | 0.98 (0.85–1.12) | 0.7 | 1.03 (0.88–1.21) | 0.7 |
| 2009 | 591 | 0.88 (0.75–1.04) | 0.1 | 0.87 (0.72–1.06) | 0.2 |
| Race | |||||
| NHW | 3,119 | 1.0 (Ref) | 1.0 (Ref) | ||
| AA | 637 | 0.97 (0.86–1.08) | 0.6 | 0.94 (0.83–1.08) | 0.4 |
| Hispanic | 100 | 1.11 (0.87–1.43) | 0.4 | 1.08 (0.80–1.47) | 0.6 |
| Asian | 112 | 0.79 (0.61–1.02) | 0.1 | 0.78 (0.57–1.06) | 0.1 |
| Other/Unknown | 101 | 0.89 (0.68–1.16) | 0.4 | 0.75 (0.54–1.05) | 0.1 |
| Marital Status | |||||
| Single | 426 | 1.0 (Ref) | 1.0 (Ref) | ||
| Married | 2411 | 0.72 (0.64–0.82) | < 0.001 | 0.77 (0.66–0.89) | < 0.001 |
| Separated/Divorced/Widowed | 981 | 0.80 (0.70–0.91) | 0.001 | 0.82 (0.70–0.96) | 0.01 |
| Unknown | 251 | 0.70 (0.57–0.84) | < 0.001 | 0.69 (0.55–0.88) | 0.002 |
| PSA | |||||
| < 10 ng/ml | 470 | 1.0 (Ref) | 1.0 (Ref) | ||
| 10–19 ng/ml | 491 | 1.08 (0.92–1.28) | 0.3 | 1.03 (0.84–1.25) | 0.8 |
| 20–29 ng/ml | 308 | 1.04 (0.87–1.25) | 0.7 | 1.04 (0.84–1.30) | 0.7 |
| 30+ ng/ml | 2205 | 1.25 (1.09–1.42) | 0.001 | 1.29 (1.10–1.51) | 0.002 |
| Unknown | 595 | 1.16 (0.99–1.35) | 0.07 | 1.10 (0.91–1.32) | 0.3 |
| Gleason Score | |||||
| ≤6 | 190 | 1.0 (Ref) | 1.0 (Ref) | ||
| 7 | 637 | 0.99 (0.81–1.22) | 0.9 | 1.12 (0.86–1.47) | 0.4 |
| ≥8 | 2163 | 1.39 (1.15–1.68) | 0.001 | 1.72 (1.35–2.21) | < 0.001 |
| Unknown | 1079 | 1.59 (1.30–1.94) | < 0.001 | 1.92 (1.48–2.49) | < 0.001 |
| T Stage | |||||
| T1 | 897 | 1.0 (Ref) | 1.0 (Ref) | ||
| T2 | 1367 | 1.09 (0.98–1.21) | 0.1 | 1.15 (1.01–1.31) | 0.03 |
| T3 | 336 | 1.10 (0.94–1.30) | 0.2 | 1.05 (0.87–1.28) | 0.6 |
| T4 | 493 | 1.30 (1.13–1.48) | < 0.001 | 1.35 (1.15–1.58) | < 0.001 |
| Unknown | 976 | 1.35 (1.19–1.54) | < 0.001 | 1.34 (1.14–1.57) | < 0.001 |
| N Stage | |||||
| N0 | 2086 | 1.0 (Ref) | 1.0 (Ref) | ||
| N1 | 613 | 1.15 (1.03–1.29) | 0.02 | 1.15 (1.01–1.32) | 0.04 |
| NX | 1370 | 1.07 (0.97–1.17) | 0.17 | 1.09 (0.98–1.21) | 0.13 |
| M Stage | |||||
| M1a | 201 | 1.0 (Ref) | 1.0 (Ref) | ||
| M1b | 2733 | 1.59 (1.29–1.94) | < 0.001 | 1.86 (1.44–2.40) | < 0.001 |
| M1c | 986 | 1.93 (1.57–2.39) | < 0.001 | 2.25 (1.72–2.93) | < 0.001 |
| M1 NOS | 149 | 1.69 (1.28–2.23) | < 0.001 | 1.97 (1.40–2.77) | < 0.001 |
|
Charlson Comorbidity Index |
|||||
| 0 | 2621 | 1.0 (Ref) | 1.0 (Ref) | ||
| 1 | 810 | 1.09 (0.99–1.20) | 0.09 | 1.04 (0.93–1.16) | 0.5 |
| 2 | 349 | 1.41 (1.24–1.61) | < 0.001 | 1.21 (1.03–1.43) | 0.02 |
| ≥3 | 289 | 1.85 (1.61–2.12) | < 0.001 | 1.51 (1.27–1.79) | < 0.001 |
|
Androgen Deprivation Therapy |
|||||
| None | 1202 | 1.0 (Ref) | 1.0 (Ref) | ||
| Orchiectomy | 339 | 0.82 (0.71–0.94) | 0.006 | 0.87 (0.74–1.02) | 0.09 |
| GnRH Agonist | 2490 | 0.68 (0.62–0.74) | < 0.001 | 0.72 (0.65–0.80) | < 0.001 |
| Both | 38 | 0.59 (0.40–0.87) | 0.007 | 0.67 (0.44–1.04) | 0.07 |
|
Bone Radiation Within 6 mo of Diagnosis |
|||||
| No | 3652 | 1.0 (Ref) | 1.0 (Ref) | ||
| Yes | 417 | 1.36 (1.21–1.53) | < 0.001 | 1.53 (1.34–1.75) | < 0.001 |
Figure 2.
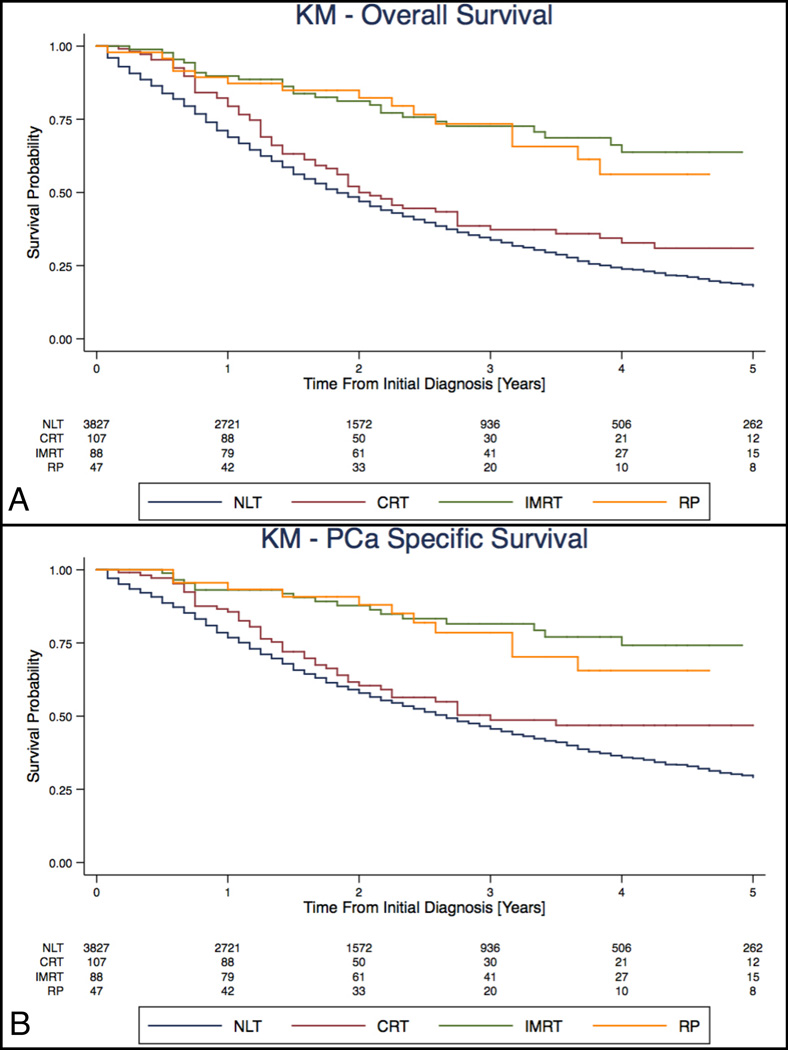
Kaplan-Meier survival curve of all cause mortality (A) and prostate-cancer specific mortality (B) in patients with metastatic prostate cancer treated by RP, IMRT, CRT or NLT. Curves have been adjusted for treatment group (NLT, CRT, IMRT and RP), age, year of diagnosis, marital status, PSA, Gleason score, AJCC staging (TNM), Charlson Comorbidity Index, androgen deprivation therapy, receipt of bone radiation within 6 months of diagnosis and registry.
Using Medicare billing codes, we identified 39 patients with MPCa as receiving RP. RP and IMRT when compared to NLT were associated with 66% (HR: 0.34, 95% CI: 0.15–0.76) and 62% (HR: 0.38, 95% CI: 0.24–0.61) adjusted lower risk of death from prostate cancer, respectively (data not shown).
Competing risk regression analysis showed that RP (SHR: 0.58, 95% CI: 0.35–0.95) and IMRT (SHR: 0.43, 95% CI: 0.27–0.68) were associated with decreased risk of PCSM compared to NLT (Table 3). Increasing age, PSA, Gleason score, more advanced primary tumour pathology (AJCC Stage), and bone radiation within 6 months of diagnosis were associated with PCSM.
Table 3.
Multivariable competing risk regression analysis by Fine and Gray method of patients receiving local therapy for metastatic prostate cancer. Sub Hazard Ratios (SHR) are reported after adjustment for treatment group (NLT, CRT, IMRT and RP), age, year of diagnosis, race, marital status, PSA, Gleason score, AJCC staging (TNM), Charlson Comorbidity Index, androgen deprivation therapy, receipt of bone radiation within 6 months of diagnosis and registry.
| Characteristic | N | Adjusted SHR (95% CI) | P value |
|---|---|---|---|
| Treatment | |||
| NLT | 3827 | 1.0 (Ref) | |
| CRT | 107 | 0.87 (0.65–1.18) | 0.4 |
| IMRT | 88 | 0.43 (0.27–0.68) | < 0.001 |
| RP | 47 | 0.58 (0.35–0.95) | 0.03 |
| Age Group | |||
| 5 yr. increment | 4069 | 1.05 (1.02–1.08) | 0.003 |
| Year of Diagnosis | |||
| 2004 | 748 | 1.0 (Ref) | |
| 2005 | 732 | 1.06 (0.92–1.21) | 0.4 |
| 2006 | 709 | 1.04 (0.91–1.19) | 0.5 |
| 2007 | 667 | 0.83 (0.72–0.97) | 0.02 |
| 2008 | 622 | 0.94 (0.81–1.10) | 0.5 |
| 2009 | 591 | 0.73 (0.60–0.88) | 0.001 |
| Race | |||
| NHW | 3,119 | 1.0 (Ref) | |
| AA | 637 | 0.97 (0.85–1.11) | 0.7 |
| Hispanic | 100 | 0.96 (0.71–1.29) | 0.8 |
| Asian | 112 | 0.82 (0.59–1.15) | 0.3 |
| Other/Unknown | 101 | 0.71 (0.50–1.03) | 0.07 |
| Marital Status | |||
| Single | 426 | 1.0 (Ref) | |
| Married | 2411 | 0.88 (0.76–1.02) | 0.1 |
| Separated/divorced/widowed | 981 | 0.88 (0.75–1.04) | 0.1 |
| Unknown | 251 | 0.77 (0.61–0.98) | 0.04 |
| PSA | |||
| < 10 ng/ml | 470 | 1.0 (Ref) | |
| 10–19 ng/ml | 491 | 1.01 (0.83–1.23) | 0.9 |
| 20–29 ng/ml | 308 | 1.06 (0.86–1.31) | 0.6 |
| 30+ ng/ml | 2205 | 1.26 (1.08–1.48) | 0.003 |
| Unknown | 595 | 1.07 (0.89–1.29) | 0.5 |
| Gleason Score | |||
| ≤6 | 190 | 1.0 (Ref) | |
| 7 | 637 | 1.14 (0.88–1.47) | 0.3 |
| ≥8 | 2163 | 1.66 (1.32–2.10) | < 0.001 |
| Unknown | 1079 | 1.73 (1.35–2.22) | < 0.001 |
| T Stage | |||
| T1 | 897 | 1.0 (Ref) | |
| T2 | 1367 | 1.16 (1.02–1.31) | 0.02 |
| T3 | 336 | 0.97 (0.80–1.16) | 0.7 |
| T4 | 493 | 1.25 (1.07–1.46) | 0.005 |
| Unknown | 976 | 1.23 (1.05–1.44) | 0.009 |
| N Stage | |||
| N0 | 2086 | 1.0 (Ref) | |
| N1 | 613 | 1.13 (0.98–1.29) | 0.08 |
| NX | 1370 | 1.07 (0.96–1.19) | 0.2 |
| M Stage | |||
| M1a | 201 | 1.0 (Ref) | |
| M1b | 2733 | 1.76 (1.37–2.25) | < 0.001 |
| M1c | 986 | 1.93 (1.49–2.51) | < 0.001 |
| M1 NOS | 149 | 1.82 (1.29–2.56) | 0.001 |
| Charlson Comorbidity Index | |||
| 0 | 2621 | 1.0 (Ref) | |
| 1 | 810 | 1 (0.89–1.12) | > 0.9 |
| 2 | 349 | 1.01 (0.85–1.19) | > 0.9 |
| ≥3 | 289 | 1.06 (0.89–1.27) | 0.5 |
|
Androgen Deprivation Therapy |
|||
| None | 1202 | 1.0 (Ref) | |
| Orchiectomy | 339 | 1.01 (0.85–1.19) | > 0.9 |
| GnRH Agonist | 2490 | 0.87 (0.78–0.97) | 0.01 |
| Both | 38 | 0.91 (0.59–1.40) | 0.7 |
|
Bone Radiation Within 6 mo of Diagnosis |
|||
| No | 3652 | 1.0 (Ref) | |
| Yes | 417 | 1.54 (1.34–1.77) | < 0.001 |
After propensity score adjustment, RP compared to NLT was associated with a 45% lower risk of PCSM (HR: 0.55, 95% CI: 0.30–1.02), although not statistically significant (Table 4). IMRT was associated with a 53% decreased risk of PCSM (HR: 0.47, 95% CI: 0.31–0.72). There was no statistically significant evidence of interaction between local treatment type and CCI, PSA, metastatic stage, ADT exposure, age and bone radiation within 6-months with respect to ACM and PCSM. As these variables are of clinical interest, exploratory analyses were undertaken by relevant subsets, although limited in sample size in several groups. RP was associated with improved PCSM (HR: 0.07, 95% CI: 0.02–0.23) in the subset of patients with PSA ≤ 20, whereas the same protective association was not observed in those with PSA > 20. A consistent pattern was not observed after subsets by age, Charlson comorbidity index, metastatic stage and by ADT exposure.
Table 4.
Inverse propensity score weight adjusted probability of all cause mortality and prostate cancer specific mortality in patients with metastatic prostate cancer at diagnosis. Results are shown for full study cohort and after stratification by age, Charlson Comorbidity Index, PSA level, metastatic stage and androgen deprivation therapy exposure. Patients with unknown PSA levels or metastatic stage were excluded for stratified analysis.
| All-Cause Mortality | P value | Prostate Cancer Specific Mortality |
||||
|---|---|---|---|---|---|---|
| Treatment Type | N | Adjusted HR (95% CI) | Adjusted HR (95% CI) | P value | ||
| Non-Stratified | NLT | 3827 | 1.0 (Ref) | 1.0 (Ref) | ||
| CRT | 107 | 1.01 (0.73–1.39) | 0.9 | 0.97 (0.66–1.43) | 0.9 | |
| IMRT | 88 | 0.57 (0.41–0.79) | 0.001 | 0.47 (0.31–0.72) | 0.001 | |
| RP | 47 | 0.42 (0.18–0.96) | 0.04 | 0.55 (0.30–1.02) | 0.057 | |
|
Stratified Analyses |
||||||
| AGE | ||||||
| ≤ 75 | NLT | 1400 | 1.0 (Ref) | 1.0 (Ref) | ||
| CRT | 49 | 1.15 (0.72–1.86) | 0.6 | 1.26 (0.72–2.20) | 0.4 | |
| IMRT | 54 | 0.57 (0.37–0.88) | 0.01 | 0.44 (0.25–0.76) | 0.004 | |
| RP | 35 | 0.76 (0.36–1.61) | 0.5 | 1.12 (0.56–2.22) | 0.8 | |
| > 75 | NLT | 2427 | 1.0 (Ref) | 1.0 (Ref) | ||
| CRT | 58 | 1.41 (0.96–2.08) | 0.08 | 1.25 (0.80–1.97) | 0.3 | |
| IMRT | 34 | 0.54 (0.33–0.89) | 0.02 | 0.41 (0.22–0.78) | 0.006 | |
| RP | 12 | 0.36 (0.11–1.20) | 0.10 | 0.38 (0.16–0.89) | 0.03 | |
| Charlson Comorbidity Index | ||||||
| < 2 | NLT | 3219 | 1.0 (Ref) | 1.0 (Ref) | ||
| CRT | 92 | 0.99 (0.72–1.36) | > 0.9 | 0.98 (0.66–1.44) | > 0.9 | |
| IMRT | 79 | 0.41 (0.24–0.70) | 0.001 | 0.34 (0.17–0.66) | 0.002 | |
| RP | 41 | 0.61 (0.26–1.46) | 0.3 | 0.60 (0.28–1.29) | 0.19 | |
| ≥ 2 | NLT | 608 | 1.0 (Ref) | 1.0 (Ref) | ||
| CRT | 15 | 1.34 (0.35–5.13) | 0.7 | 0.82 (0.25–2.63) | 0.7 | |
| IMRT | 9 | 0.61 (0.26–1.39) | 0.2 | 0.62 (0.15–2.63) | 0.5 | |
| RP | 6 | 0.37 (0.16–0.83) | 0.02 | 0.36 (0.15–0.88) | 0.03 | |
| PSA | ||||||
| ≤ 20 | NLT | 850 | 1.0 (Ref) | 1.0 (Ref) | ||
| CRT | 29 | 0.85 (0.47–1.55) | 0.6 | 1.14 (0.56–2.31) | 0.7 | |
| IMRT | 51 | 0.61 (0.32–1.17) | 0.1 | 0.40 (0.15–1.09) | 0.07 | |
| RP | 31 | 0.07 (0.02–0.24) | < 0.001 | 0.07 (0.02–0.23) | < 0.001 | |
| > 20 | NLT | 2413 | 1.0 (Ref) | 1.0 (Ref) | ||
| CRT | 64 | 1.50 (1.03–2.18) | 0.03 | 1.29 (0.81–2.06) | 0.3 | |
| IMRT | 27 | 0.55 (0.35–0.86) | 0.009 | 0.44 (0.27–0.74) | 0.002 | |
| RP | 9 | 0.69 (0.22–2.14) | 0.5 | 0.62 (0.29–1.30) | 0.2 | |
|
Metastatic Stage |
||||||
| M1a + M1b | NLT | 2760 | 1.0 (Ref) | 1.0 (Ref) | ||
| CRT | 76 | 1.43 (0.97–2.12) | 0.07 | 1.56 (1.03–2.35) | 0.04 | |
| IMRT | 69 | 0.69 (0.47–1.00) | 0.051 | 0.60 (0.37–0.98) | 0.04 | |
| RP | 29 | 0.73 (0.27–1.93) | 0.5 | 0.64 (0.32–1.30) | 0.2 | |
| M1c | NLT | 922 | 1.0 (Ref) | 1.0 (Ref) | ||
| CRT | 31 | 0.90 (0.57–1.44) | 0.7 | 0.75 (0.40–1.41) | 0.4 | |
| IMRT | 16 | 0.48 (0.22–1.01) | 0.054 | 0.37 (0.16–0.85) | 0.02 | |
| RP | 17 | 0.33 (0.13–0.82) | 0.017 | 0.34 (0.12–0.95) | 0.04 | |
| Androgen Deprivation Therapy | ||||||
| No | NLT | 1132 | 1.0 (Ref) | 1.0 (Ref) | ||
| CRT | 13 | 1.14 (0.62–2.11) | 0.7 | 1.34 (0.67–2.66) | 0.4 | |
| IMRT | 30 | 0.60 (0.34–1.08) | 0.09 | 0.54 (0.23–1.27) | 0.2 | |
| RP | 27 | 0.78 (0.30–2.04) | 0.6 | 0.83 (0.31–2.27) | 0.7 | |
| Yes | NLT | 2695 | 1.0 (Ref) | 1.0 (Ref) | ||
| CRT | 94 | 1.21 (0.86–1.71) | 0.3 | 1.10 (0.75–1.61) | 0.6 | |
| IMRT | 58 | 0.55 (0.37–0.82) | 0.003 | 0.43 (0.25–0.72) | 0.002 | |
| RP | 20 | 0.47 (0.24–0.92) | 0.03 | 0.82 (0.45–1.50) | 0.5 | |
Discussion
To our knowledge, this is the first population-based study examining the outcomes of RP in comparison to two modalities of external beam radiation therapy (IMRT and CRT) or no local treatment in MPCa. Additionally, in contrast to past studies4,5, we adjusted for important confounders of survival in the metastatic setting by using billing derived patient comorbidity, receipt of ADT and early (<6 month) bone radiation as a marker of advanced disease. After accounting for these and conventional risk factors, RP and IMRT were associated with a 52% and 62% reduction in the risk of PCa specific mortality, respectively. Similar results were seen after propensity score adjustment and competing risk analysis.
Our results remain consistent with earlier SEER based analyses, which also suggested a benefit to RP and brachytherapy4,5. The observation that IMRT but not CRT was associated with improved ACM and PSCM may be indicative that patients receiving CRT have more advanced tumour burden, worse tumour biology and higher comorbidity that are inadequately measured and controlled for by the variables we have utilized in this retrospective study. In contemporary practice, CRT may be viewed as non-definitive therapy, used in the setting of MPCa for local symptom control, wherein lower doses and treatments are delivered11. Consistent with this, we observed a nearly two-fold lower number of treatment claims for CRT compared to IMRT.
The SEER-Medicare database provides important claims derived patient variables, however, there are important limitations. This cohort consists of men > 65 years of age and hence these results may not be broadly generalizable. Further, errors in coding can occur in large databases like SEER and can be more problematic in studies involving small study samples22. However, in this study, men undergoing RP were all individually confirmed to have the correct staging and treatment by registry audits. Nonetheless, while the same protective effect of RP was observed when treatment was classified by Medicare billing codes, the discrepancy between SEER and Medicare highlights the need for prospective evaluation and caution related to accuracy of stage and treatment classification. Other limitation of the data include uncertainty regarding radiation doses and whether radiation was indeed delivered to the prostate and not elsewhere (e.g. bone). Further, critical variables including imaging results (e.g bone scan), lab values (e.g. PSA response to ADT) and baseline pain scores, which are necessary to define the metastatic burden, were not available. Moreover, the receipt of docetaxel based chemotherapy, immunotherapy or novel androgen receptor pathway targeted agents after the development of castrate-resistant PCa is unknown and can differentially impact survival if one group receives aggressive treatment. Taken together, selection bias may be driving the conclusions about RP and IMRT, reflecting residual confounding due to the lack critical variables that can be measured, but also concerns about the reliability and quality of measurements, such as comorbidity from claims based data23. Despite accounting for the receipt of early bone radiation as a marker of advanced disease, the most important selection bias remains metastatic burden. It remains possible that the survival benefit observed for RP and IMRT is purely on the basis of having less or slowly progressing metastatic deposits than patients whom underwent no local therapy. Despite these limitations, the consistency in findings for ACM and PCSM using traditional multivariable, propensity-weighted and competing risk analyses warrants further investigation.
Adoption of local treatment in MPCa must be judicious as the treatments themselves increase the risk of surgical morbidity and can be detrimental to health related quality of life. Recent data for RP in the setting of MPCa supports its feasibility with acceptable functional outcomes as well as decreased need for percutaneous or surgical interventions for local tumour growth24. The mechanism and underlying tumour biology that explains a potential oncologic benefit remains unknown, however, there are several hypotheses. First, eradication of the primary tumour eliminates the source of cytokine signalling that prepares niches for eventual sites of metastases and promotes their growth25. Second, the primary tumour may remain a source of circulating tumour cells that are capable of “self-seeding” the primary organ26. Lastly, local therapy may eradicate self-renewing progenitor cells persisting after ADT which have been shown to have a immature luminal, androgen receptor low phenotype and are capable of propagating adenocarcinoma27. Moving forward, at the very least, tissue banking RP specimens after ADT may facilitate studies of tumour and progenitor cell biology28 including the use of high throughput genomic and transcriptome analyses to improve patient prognosis and eventually develop targeted therapy.
Conclusions
Local therapy with RP or IMRT but not CRT compared to no local treatment was associated with decreased risk of all cause and prostate cancer specific mortality, after accounting for patient comorbidity, ADT exposure and receipt of early palliative bone radiation. These results should be viewed as hypothesis generating as the lack of information on metastatic disease burden is a critical caveat in this analysis. Future prospective trials are crucial and must aim to access health related quality of life as well as oncological benefits to local therapy.
Supplementary Material
Distribution of patients correctly coded with respect to metastatic stage and receipt of radical prostatectomy sorted by individual SEER registry (SEER 2004–2010 Dataset).
Acknowledgments
The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumour registries in the creation of the SEER-Medicare database and for their generous assistance in individually auditing cases for this study. A.E.K. received support from grant 5T32 ES013678 from the National Institute of Environmental Health Sciences
Funding: None
Standard Abbreviations
- MPCa
Metastatic Prostate Cancer
- RP
Radical Prostatectomy
- IMRT
Intensity Modulated Radiation Therapy
- CRT
Conformal Radiation Therapy
- NTL
No Local Therapy
- CCI
Charlson Comorbidity Index
- ADT
Androgen Deprivation Therapy
- ACM
All Cause Mortality
- PCSM
Prostate Cancer Specific Mortality
- SEER
Surveillance Epidemiology and End Results
- HR
Hazard Ratio
- SHR
Sub hazard Ratio
References
- 1.Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N. Engl. J. Med. 2013;368:1314–1325. doi: 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. Journal of Clinical Oncology. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 3.Thompson IM, Tangen C, Basler J, et al. Impact of previous local treatment for prostate cancer on subsequent metastatic disease. JURO. 2002;168:1008–1012. doi: 10.1016/S0022-5347(05)64562-4. [DOI] [PubMed] [Google Scholar]
- 4.Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur. Urol. 2014;65:1058–1066. doi: 10.1016/j.eururo.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Antwi S, Everson TM. Prognostic impact of definitive local therapy of the primary tumor in men with metastatic prostate cancer at diagnosis: A population-based, propensity score analysis. Cancer Epidemiol. 2014;38:435–441. doi: 10.1016/j.canep.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Fossati N, Trinh Q-D, Sammon J, et al. Identifying Optimal Candidates for Local Treatment of the Primary Tumor Among Patients Diagnosed with Metastatic Prostate Cancer: A SEER-based Study. Eur. Urol. 2014 doi: 10.1016/j.eururo.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 7.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol. Biomarkers Prev. 1999;8:1117–1121. [PubMed] [Google Scholar]
- 8.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40 doi: 10.1097/01.MLR.0000020942.47004.03. IV–3–18. [DOI] [PubMed] [Google Scholar]
- 9.Fleming ST, Hamilton AS, Sabatino SA, et al. Treatment Patterns for Prostate Cancer: Comparison of Medicare Claims Data to Medical Record Review. Med Care. 2012 doi: 10.1097/MLR.0b013e318277eba5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldin GH, Sheets NC, Meyer A-M, et al. Comparative effectiveness of intensity-modulated radiotherapy and conventional conformal radiotherapy in the treatment of prostate cancer after radical prostatectomy. JAMA Intern Med. 2013;173:1136–1143. doi: 10.1001/jamainternmed.2013.1020. [DOI] [PubMed] [Google Scholar]
- 11.Cameron MG, Kersten C, Guren MG, et al. Palliative pelvic radiotherapy of symptomatic incurable prostate cancer - a systematic review. Radiother Oncol. 2014;110:55–60. doi: 10.1016/j.radonc.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Wu JS-Y, Wong R, Johnston M, et al. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int. J. Radiat. Oncol. Biol. Phys. 2003;55:594–605. doi: 10.1016/s0360-3016(02)04147-0. [DOI] [PubMed] [Google Scholar]
- 13.Ellsworth SG, Alcorn SR, Hales RK, et al. Patterns of care among patients receiving radiation therapy for bone metastases at a large academic institution. Int. J. Radiat. Oncol. Biol. Phys. 2014;89:1100–1105. doi: 10.1016/j.ijrobp.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noone A-M, Lund JL, Mariotto A, et al. Comparison of SEER Treatment Data With Medicare Claims. Med Care. 2014 doi: 10.1097/MLR.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009;302:1557–1564. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 16.Roberts CB, Jang TL, Shao Y-H, et al. Treatment profile and complications associated with cryotherapy for localized prostate cancer: a population-based study. Prostate Cancer Prostatic Dis. 2011;14:313–319. doi: 10.1038/pcan.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 18.Shahinian VB, Kuo Y-F, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N. Engl. J. Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry SD, Ngo L, Samelson EJ, et al. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58:783–787. doi: 10.1111/j.1532-5415.2010.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 22.Nguyen MM, Gill IS. Coded tumor size may be unreliable for small metastatic renal cancers in the Surveillance, Epidemiology, and End Results dataset. Urology. 2010;75:266–270. doi: 10.1016/j.urology.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Stattin P, Loeb S. “To measure is to know. If you cannot measure it, you cannot improve it”: statistical modeling cannot compensate for unmeasured bias. Eur. Urol. 2014;65:701–703. doi: 10.1016/j.eururo.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 24.Heidenreich A, Pfister D, Porres D. Cytoreductive Radical Prostatectomy in Patients with prostate cancer and low volume skeletal metastases - results of a feasibility and case-control study. J. Urol. 2014 doi: 10.1016/j.juro.2014.09.089. [DOI] [PubMed] [Google Scholar]
- 25.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat. Rev. Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim M-Y, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoyanova T, Cooper AR, Drake JM, et al. Prostate cancer originating in basal cells progresses to adenocarcinoma propagated by luminal-like cells. Proc. Natl. Acad. Sci. U.S.A. 2013;110:20111–20116. doi: 10.1073/pnas.1320565110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tzelepi V, Efstathiou E, Wen S, et al. Persistent, biologically meaningful prostate cancer after 1 year of androgen ablation and docetaxel treatment. Journal of Clinical Oncology. 2011;29:2574–2581. doi: 10.1200/JCO.2010.33.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of patients correctly coded with respect to metastatic stage and receipt of radical prostatectomy sorted by individual SEER registry (SEER 2004–2010 Dataset).