Abstract
A central tenet in support of research reproducibility is the ability to uniquely identify research resources, that is, reagents, tools, and materials that are used to perform experiments. However, current reporting practices for research resources are insufficient to identify the exact resources that are reported or to answer basic questions such as “How did other studies use resource X?” To address this issue, the Resource Identification Initiative was launched as a pilot project to improve the reporting standards for research resources in the methods sections of papers and thereby improve identifiability and scientific reproducibility. The pilot engaged over 25 biomedical journal editors from most major publishers, as well as scientists and funding officials. Authors were asked to include Research Resource Identifiers (RRIDs) in their manuscripts prior to publication for three resource types: antibodies, model organisms, and tools (i.e., software and databases). RRIDs are assigned by an authoritative database, for example, a model organism database for each type of resource. To make it easier for authors to obtain RRIDs, resources were aggregated from the appropriate databases and their RRIDs made available in a central web portal ( http://scicrunch.org/resources). RRIDs meet three key criteria: they are machine readable, free to generate and access, and are consistent across publishers and journals. The pilot was launched in February of 2014 and over 300 papers have appeared that report RRIDs. The number of journals participating has expanded from the original 25 to more than 40 with RRIDs appearing in 62 different journals to date. Here, we present an overview of the pilot project and its outcomes to date. We show that authors are able to identify resources and are supportive of the goals of the project. Identifiability of the resources post‐pilot showed a dramatic improvement for all three resource types, suggesting that the project has had a significant impact on identifiability of research resources.
Introduction
Research resources; defined here as the reagents, materials, and tools used to produce the findings of a study; are the cornerstone of biomedical research. However, as has long been bemoaned by database curators and investigated by Vasilevsky and colleagues, it is difficult to uniquely identify these resources in the scientific literature (Vasilevsky et al. 2013). This study found that researchers did not include sufficient detail for unique identification of several key research resources, including model organisms, cell lines, plasmids, knockdown reagents or antibodies. In most cases, authors provided insufficient metadata about the resource to conclusively identify the particular resource, for example, a nonunique set of attributes with no catalog or stock number. It should be noted that the authors were, generally speaking, following the reporting guidelines offered by the journals. Such guidelines traditionally state that authors should include the company name and city in which it was located for the resources used in the study. Further, even when uniquely identifying information was provided (e.g., a catalog number for a particular antibody), the vendor may have gone out of business, the particular product may no longer be available, or its catalog information may have changed. Given that in these cases a human cannot find which resources were used, an automated agent, such as a search engine or text mining tools will also not be able to identify the resources.
Because current practices for reporting research resources within the literature are inadequate, nonstandardized, and not optimized for machine‐readable access, it is currently very difficult to answer very basic questions about published studies such as “What studies used the transgenic mouse I am interested in?” These types of questions are of interest to the biomedical community, which relies on the published literature to identify appropriate reagents, troubleshoot experiments, and aggregate information about a particular organism or reagent to form hypotheses about mechanism and function. Such information is also critical to funding agencies that funded a research group to generate a particular tool or reagent; and the resource providers, both commercial and academic, who would like to be able to track the use of these resources in the literature. Beyond this basic utility, identification of the particular research resource used is an important component of scientific reproducibility or lack thereof.
The Resource Identification Initiative (RII) is laying the foundation of a system for reporting research resources in the biomedical literature that will support unique identification of research resources used within a particular study. The initiative is jointly led by the Neuroscience Information Framework (NIF; http://neuinfo.org) and the Oregon Health & Science University (OHSU) Library, data integration efforts occurring as part of the Monarch Initiative ( www.monarchinitiative.org), and with numerous community members through FORCE11, the Future of Research Communications and e‐Scholarship, which is a grassroots organization dedicated to transforming scholarly communication through technology. Since 2006, NIF has worked to identify research resources of relevance to neuroscience. The OHSU group has long‐standing ties to the model organism community, which maintains databases populated by curating the literature and contacting authors to add links between model organisms, reagents, and other data. In a 2011 workshop (see https://www.force11.org/node/4145) held under the auspices of the Linking Animal Models to Human Diseases (LAMHDI) consortium, various stakeholders from this community drafted recommendations for better reporting standards for animal models, genes, and key reagents.
The RII initiative was launched as a result of two planning meetings building off of the recommendations of the LAMHDI workshop. The first was held in 2012 at the Society for Neuroscience meeting with over 40 participants comprising editors, publishers and funders (sponsored by INCF; http://incf.org). This meeting outlined the problem of incomplete identification of research resources within papers, and the need for a computational solution for identifying and tracking them in the literature. Recognizing that any solution needed to work for both humans and machines, three broad requirements were identified: (1) the standard should be machine processable, that is, designed for search algorithms, in addition to human understanding; (2) the information should be available outside the paywall, so that search algorithms and humans have free access to the information across the biomedical literature; and (3) the standard should be uniform across publishers, to make uptake and usage easier for both human and machines.
A follow‐up workshop at the NIH ( https://www.force11.org/node/4857) was held in June of 2013 to gain agreement from this stakeholder group for the design of a pilot that would explore solutions for this problem. A working group, the Resource Identification Initiative, was established through FORCE11 comprised of publishers, journal editors, antibody manufacturers and distributors, biocurators, software tool developers, and foundations. Based upon agreements garnered at the June 2013 meeting, the RII designed a pilot project to test implementation of a system for authors submitting manuscripts to identify research resources through the use of a unique identifier, termed as Research Resource Identifier (RRID).
Pilot Project Overview
The pilot project limited its focus to three types of resources—primary antibodies, noncommercial software tools/databases, and model organisms. These were chosen because they are a major source of variation across experiments and are used broadly across biomedical research communities. For the purposes of this pilot, a critical aspect was that a relatively complete and authoritative central registry existed that could issue an accession number, as GenBank does for gene sequences. To gain broad agreement among publishers and editors who were concerned about the potential burden on authors and staff, it was agreed that participation in the pilot project would be voluntary for authors with participation not representing a condition of acceptance for publication. The pilot project was also designed to have minimal requirements for publishers such that modification of manuscript submission systems was not required.
The pilot project was originally designed to run for 6 months, with each of the participating journals agreeing to participate for at least 3 months. The goal was to ensure a large enough sample to understand author behavior: could they and would they do the task, and to obtain sufficiently large participation to demonstrate the utility of RRIDs. Over the minimum 3‐month window, each partner journal would request authors to supply RRIDs in a standard format as a citation to indicate the use of antibodies, software and databases, and model organisms. To be as unambiguous as possible, authors were to include the RRIDs for resources that were utilized in the study and described in the text of the materials and methods, but not in the introduction or discussion sections where they might be mentioned in passing but not used in the study. The RRID syntax comprises an accession number assigned by the authoritative database with the prefix “RRID:” prepended (e.g., RRID:AB_2298772 for an antibody). We also requested that non‐open source journals include RRIDs in the keyword field, as this field is available for indexing in PubMed outside of paywalls. The journals were given flexibility for when and how they wanted to ask authors for these identifiers, namely, at time of submission, during review, or after acceptance. They were not required to modify their instructions to authors or their submission systems. The RII team would be responsible for preparing appropriate materials for requesting RRIDs and for establishing a central portal where these identifiers could be obtained. The RII team also agreed to establish a help desk to assist the authors if they encountered any difficulties.
The pilot project was designed to address four key questions. A set of evaluation criteria was designed for each question:
Participation: Would authors be willing to add resource identifiers to their publications and register new resources in the system? Participation was evaluated by examining the number of submissions to the participating journals, the rate of author participation in providing RRIDs, the number of new resources registered, and direct feedback from authors.
Performance: Could authors add these identifiers correctly or would additional editorial or staff oversight be necessary? Performance was measured by a quantitative analysis of RRID correctness by RII curators.
Identifiability: Would the use of RRIDs improve our ability to identify resources in the literature? Identifiability was measured both pre‐ and postpilot in the journals that participated.
Utility: Will RRID's be useful to the scientific community? Can the RRID's as constructed be used to identify all studies that use a particular research resource? To encourage the development of applications, the data set is being made freely available so that third parties can develop tools to work with RRIDs.
The pilot began in February 2014, with over 25 journals participating. Journals that sent a letter to authors at some stage of the review process included: Journal of Neuroscience, Brain and Behavior, Journal of Comparative Neurology, Brain Research, Experimental Neurology, F1000Research, PeerJ, Journal of Neuroscience Methods, Neurobiology of Disease, and the Frontiers group of journals. One journal, Neuroinformatics, chose to add the RRIDs to all manuscripts before asking authors to do this. Journals in the Elsevier and BMC groups were participants based upon updates to their instructions to authors. Because of the success of the project, it was subsequently extended and is still active as of this writing. The number of journals participating has expanded, and now includes PLoS Biology and PLoS Genetics as well as multiple immunology journals in the Elsevier family. A list of the participating journals is available on the Force 11 website ( https://www.force11.org/RII/SignUp).
One of the primary requirements of the pilot project was to make it as easy as possible for authors to obtain the appropriate identifiers and insert them correctly into their manuscripts. As noted above, the three research resources were chosen because each was covered by an authoritative database (Table 1) that assigned unique IDs and a standard set of metadata to each. However, as can be seen by the length of the list in Table 1, authors could potentially be required to visit several databases to obtain the appropriate identifiers.
Table 1.
Source databases and registries included in the RII portal
| Resource name | Resource content | Database Identifier |
|---|---|---|
| ZIRC, Zebrafish Resource Center | Zebrafish Stocks | RRID:nif‐0000‐00242 |
| ZFIN, Zebrafish Information Network | Zebrafish Nomenclature | RRID:nif‐0000‐21427 |
| RGD, Rat Genome Database | Rat | RRID:nif‐0000‐00134 |
| CGC, Caenorhabditis Genetics Center | Worm Stocks | RRID:nif‐0000‐00240 |
| WormBase | Worm Nomenclature | RRID:nif‐0000‐00053 |
| IMSR, International Mouse Strain Resource Center | Mouse Stocks | RRID:nif‐0000‐09876 |
| BDSC, Bloomington Drosophila Stock Center | Fly Stocks | RRID:nif‐0000‐00241 |
| MGI, Mouse Genome Informatics | Mouse Nomenclature | RRID:nif‐0000‐00096 |
| BCBC, Beta Cell Biology Consortium | Mouse stocks | RRID:nlx_144143 |
| antibodyregistry.org, Antibody Registry | Antibodies | RRID:nif‐0000‐07730 |
| SciCrunch Registry | Software Tools and Databases | RRID:nlx_144509 |
Each database has a weekly or monthly scheduled frequency of update and all new data is released weekly. If available, data from both model organism authorities is served, as well as the list of strains available via particular stock centers. In most cases the stock centers maintain a link between the genotype and the stock center animal identifier.
To simplify this process, we established a Resource Identification Portal based upon the SciCrunch platform, which leverages data aggregation performed by the DISCO aggregation engine (Marenco et al. 2014; http://scicrunch.org/resources; Fig. 1). The portal provides a unified query across different resource databases and displayed the results in a common format. The portal allows search on various facets such as resource name, catalog number, etc. There is a “cite this” link that provides the citation, as it should be reported in the paper. The citation generally includes not just the RRID, but a set of appropriate metadata that would identify the vendor and catalog number as well, for example: A polyclonal antibody against tyrosine hydroxylase (TH) (Chemicon, Cat. AB1542, RRID:AB_90755).
Figure 1.
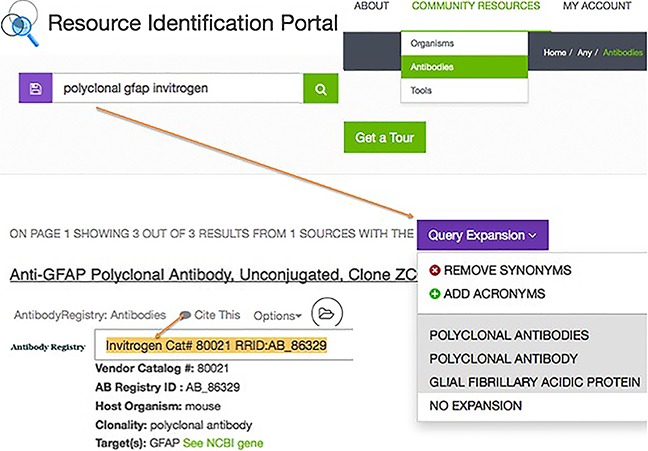
The Resource Identification Initiative portal containing citable Research Resource Identifiers (RRIDs). The workflow for authors is to visit http://scicrunch.org/resources, then select their resource type (see community resources box), type in search terms (note that the system attempts to expand known synonyms to improve search results) and open the “Cite This” dialog box. The dialog shown here displays the Invitrogen catalog number 80021 antibody with the RRID:AB_86329. The authors are asked to copy and paste this text into their methods section.
Methods
SciCrunch was built based on the extensible Neuroscience Information Framework platform described previously (Gardner et al. 2008; Marenco et al. 2014; RRID:nif‐0000‐25673), and the portal infrastructure for RII was developed under an award from NIDDK to create a dkNET portal (RRID:nlx_153866), while the customization of the portal was done by Monarch staff. The data is aggregated from the SciCrunch tool registry, the antibody registry, as well as the model organism community databases and stock centers (Table 1). The data infrastructure allows curators to keep indexes synchronized with the source databases by using an automated crawling engine and new data are released on a weekly basis. All open data from each of these databases is available to download from the source sites, where update frequencies are listed.
The journal editors were provided with recommended instructions to authors (the instructions to authors are available here: https://www.force11.org/node/4856). For antibodies, we only required authors to identify primary antibodies and not secondary or tertiary complexes. For software tools and databases, we focused on freely available and generally publicly funded noncommercial tools. For model organisms, we focused on the five commonly used organisms: mouse, rat, zebrafish, fruit fly, and worm. Authors were asked to insert the correct citation for the resource into the text of the materials and methods section and in the keywords. A help desk was established by the RII working group that provided help if an author encountered difficulty. In most cases, requests were handled in less than 24 h.
If a resource was not found via the portal, authors were given the option to submit the resource to obtain an identifier. For antibodies and software/databases, which are found in databases maintained within the NIF, submission was handled through the Resource Identification Portal. For model organisms, the author was referred to the authoritative model organism database for their organism (RGD, MGI, ZFIN, Wormbase, or Flybase). All new submissions were curated by their respective databases and the data was pulled back into the RII portal weekly so that authors could see their newly registered resources in approximately a week.
To evaluate the aims of the pilot project, we tracked the use of RRIDs in published papers and journals. We performed an in depth analysis of the first 100 papers found through Google Scholar that reported RRID's. For each paper, we examined the methods section to determine the correct usage (i.e., if the RRID pointed to the correct resource), the syntactic correctness (i.e., if the author reported the RRID using the correct syntax), and the identifiability of the three resource types. The total number of research resources reported in the first 100 papers reporting RRIDs was determined by manual inspection of each paper by two independent curators. A Google Scholar alert was used to track all new papers that contained an RRID, using the search “RRID:”. Each of the first 100 papers was downloaded and examined for the snippets of text surrounding research resources (in the methods or data use sections).
Curation workflow to determine correct usage of reported RRIDs
To determine if the RRIDs were reported correctly for the three resource types, the following criteria were applied.
A resource was considered correct if resource reported an RRID and that RRID pointed to the correct resource in the RII portal. This determination was made both by manual search of the RII portal and via the SciCrunch resolving service for each reported RRID (for example, https://scicrunch.org/resolver/RRID:AB_262044).
A resource was considered incorrect if the reported RRID pointed to a different or nonexising resource in the RII portal or SciCrunch resolving service.
A resource was considered to have the correct syntax if the resource reference contained an RRID, and the RRID was formatted correctly, had no missing characters or other typos.
Curation workflow for identifiability of the three resource types
To determine if the three resource types were identifiable in the journal articles that reported RRIDs (postpilot), and in articles from the same journals before the pilot started. To select the prepilot articles, articles were selected by performing a PubMed search filtered for each journal and using the first five publications returned that contained the relevant resource types from approximately January–March 2013. The following criteria were applied: Resources (primary antibodies, organisms, and noncommercial tools) were considered identifiable if they contained an accurate RRID or by using the same specific resource identification criteria as described in Vasilevsky et al. (2013). Noncommercial software and databases that were not previously analyzed were considered identifiable if they contained the correct RRID or reported the manufacturer and version number for that tool. Note, we distinguished commercially produced for‐profit software from public or individually produced software (noncommercial).
Statistical analysis for identifiability of the three resources
Since the data was binomial in that each resource was either identifiable or not, we used a binomial confidence interval strategy for calculating upper and lower 95% confidence intervals (CI) ( http://www.danielsoper.com/statcalc3/calc.aspx?id=85, RRID:SCR_013827). Error bars for the corresponding 95% CI are displayed on the graphs. Statistical significance was determined by calculating the z‐score.
Results
The first RRID's began appearing in the literature in April of 2014. Although the first paper was identified through PubMed, the majority of papers were found via Google Scholar by searching for “RRID”. Google Scholar, unlike PubMed, appears to search the full text of articles, as it returns snippets of text from the materials and methods containing the RRID's (e.g., Fig. 2). A search in PubMed returns very few papers, indicating that most journals were not including the RRIDs outside of the paywall. As these papers start to appear in PubMed Central, where full text search is possible, we anticipate that more papers utilizing RRIDs will be identifiable through the National Library of Medicine. Google Scholar possesses the advantage in that it obtains papers without an embargo period and makes them available for search immediately at the time of publication. In this manuscript, we therefore present analysis based upon Google Scholar.
Figure 2.
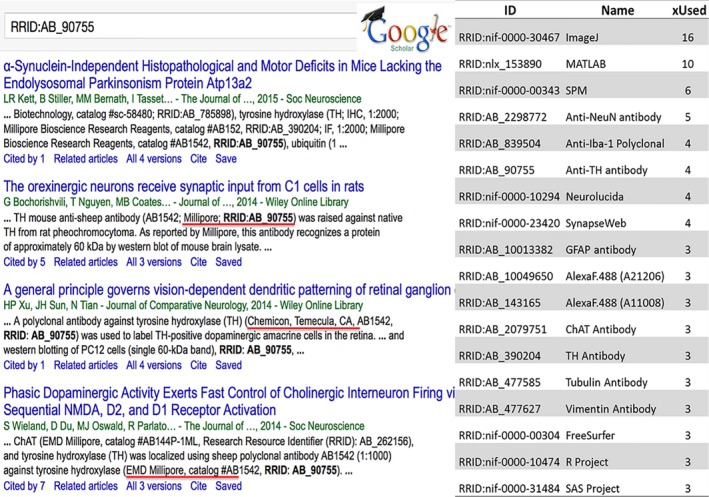
RRIDs found in the published literature. (A) Google Scholar result for the anti‐tyrosine hydroxylase antibody RRID (9/2014; http://scholar.google.com/scholar?q=RRID:AB_90755). (B) The most frequently reported RRIDs in the first 100 papers, by number of papers using the identifier. All data is available in Supplementary Table and all identifiers can be accessed in Google Scholar (see also Table S1).
Search via Google Scholar reveals that the RRID prefix is not a unique string, but is an acronym for several entities, mostly commonly the Renal Risk in Derby clinical study (e.g., McIntyre et al. 2012). To return, examples of RRIDs requires the use of additional filters, for example, restricting search to the years 2014 and later. The combination of the RRID prefix with the resource accession number is unique, however, in that searching for a particular RRID, for example RRID:AB_90755 returns only papers that use this research resource (Fig. 2).
The first 100 papers were published in 16 journals and included 562 RRIDs reported by authors. The bulk of the identifiers (490) came from two journals, the Journal of Comparative Neurology (JCN) and the Journal of Neuroscience, as these two journals were first to participate both starting the pilot in early February of 2014.
Outcome #1: Participation
As of March 1, 2015 there were 312 papers published with at least one RRID, from 44 unique journals (Table S1 shows the updated list of journals and a count for each) indicating that hundreds of authors have participated in the pilot project even though it is voluntary. Table 2 shows the different mechanisms and timing of contact for authors by different journals. Informal feedback from the editors and authors via the help requests and other correspondence indicates that authors who are attempting to find RRIDs are supportive of the aims of the project and readily able to find the correct RRIDs.
Table 2.
Journal practices in contacting authors
| Journal | Number authors contacted during submission (mechanism) | Number authors contacted during review (mechanism) | Number authors contacted during acceptance (mechanism) | Participation rate |
|---|---|---|---|---|
| Journal of Neuroscience | 1175 (letter to author) | 163 (letter to author) | 25 (letter to author) | ~12% |
| Journal of Comparative Neurology | (direct author assist) | (direct author assist) | (direct author assist) | >90% |
| Brain and Behavior | ~100 (letter to author) | ~25% | ||
| Neuroinformatics | (staff looks up RRIDs) | 100% | ||
| F1000 Research | ~50 (letter to author) | ~12% | ||
| Brain Research | 671 (letter to author) | 1% | ||
| Journal of Neuroscience Methods | 314 (letter to author) | 1% | ||
| Neurobiology of Disease | 291 (letter to author) | 3% | ||
| Experimental Neurology | 297 (letter to author) | 3% |
Different journals chose to contact authors at different stages of the publishing cycle and assist in the addition of RRIDs via different mechanisms. The participation rate was by far the lowest with only instructions to authors; these journals are not included in this table (for example BMC) and had <1% participation rates. When authors were asked by a blanket mailing containing instructions, participation rates ranged between 1 and 15%. Participation was very high if the editorial staff asked authors directly or suggested identifiers for their manuscript. Note that in some cases only an approximation could be made by the participating journals.
One journal, the Journal of Neuroscience, sent authors letters asking authors for their participation during different periods of the publication cycle. There did not appear to be a more advantageous time for the correspondence. The Journal of Comparative Neurology directly assisted authors during different periods of the publication cycle and had excellent participation. The high rate of compliance is likely due to the direct assistance, but also to the publication of an editorial to support awareness (Bandrowski et al. 2014) and a long‐standing history of antibody identification back to 2006. Neuroinformatics has section for tools, and several papers incorporated RRIDs even prior to staff looking them up. The Journal of Comparative Neurology also has such a section and antibodies.
Authors were willing to add resources to the registries if they were not available. We analyzed the statistics for the Antibody Registry and SciCrunch Tool Registries, as we had programmatic access to these. Since the project began, over 200,000 antibodies from vendors, both solicited and unsolicited and at least 200 from individual authors were added to the Antibody Registry (antibodyregistry.org). In cases where antibodies are sold by government‐led projects such as NeuroMab from UC Davis, antibody identifiers have been included in the antibody manufacturer's website. Many of the additions were secondary antibodies, which were not part of the pilot project, but authors felt that they should also be identified. In one representative example, Jackson ImmunoResearch was contacted by several authors and subsequently submitted their full catalog to the Antibody Registry, allowing authors to report RRIDs for their secondary antibodies. Additionally, there were over 100 software tools and databases registered. Many were for common commercial statistical tools (e.g., SPSS, Graphpad), technically out of scope for the pilot project, but authors did not make the distinction between commercial and noncommercial tools. A comparison of new resources added versus those reported in the first 100 papers, indicates that the Registries already listed the majority of research resources in each of these categories, as the number of new resources added for this set represented only ~10% of the total reported resources.
Figure 2 shows the most common tools identified by RRID in papers from the first 100 papers. Commercial tools such as MATLAB, SAS, and GraphPad were cited along with noncommercial tools such as ImageJ and FreeSurfer. The most common antibody was the anti‐NeuN antibody from Millipore, now Merk. These same resource identifiers have continued to be very highly cited in subsequent papers, with ImageJ cited in 42 papers and the NeuN antibody cited in 8 papers (Google Scholar March 17, 2015).
Outcome #2: Performance
A major concern of the publishers and editors was whether or not authors could retrieve RRID's correctly and whether significant editorial oversight would be necessary for quality control (see workshop outcome documents at https://www.force11.org/node/4857).
To determine if authors were correctly reporting RRIDs, we analyzed the reported RRIDs, and determined if they pointed to the correct resources in the RII portal, by comparing the metadata and RRID reported for each resource using the resolving service (e.g., see https://scicrunch.org/resolver/RRID:AB_262044) or by querying the portal. Overall, 96% (538/562) of the RRIDs reported by authors were correct (i.e., the RRID pointed to the correct resource). More specifically, 96% of antibodies (413/429), 87% of organisms (48/55), and 99% of tools (77/78) were correctly reported (Fig. 3).
Figure 3.
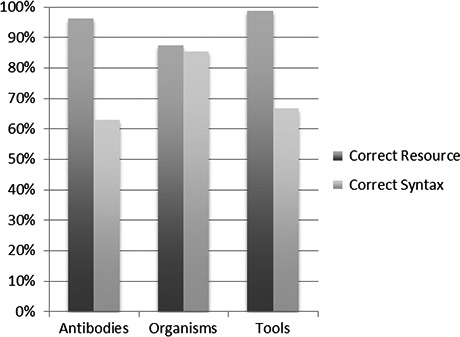
Percent correctly reported RRIDs. The percentage of resources that reported an RRID that pointed to the correct resource and with the correct syntax for each resource type is shown. The total number of resources for each type during the postpilot is: primary antibodies, n = 429; organisms, n = 55; noncommercial tools, n = 78.
Inspection of the 16 errors in reporting RRIDs for antibodies (4% error rate), showed that three errors were copy/paste mistakes where authors mixed up the combination of catalog number and identifier for resources used in their paper; three errors resulted from identifiers missing a digit at the end of the ID (e.g., “Swant, catalog #6B3, RRID: AB_1000032” should have been labeled RRID: AB_10000320); and one error involved reporting a reference PMID instead of the resource identifier. The apparent cause of the other antibody errors was not possible to determine. For organisms, seven errors were made (13% error rate). All of these errors involved mice for which authors used the appropriate gene or allele identifier from Mouse Genome Informatics (MGI), rather than the stock number or genotype identifying the organism. It should be noted that MGI as of 2015 can search the genotypes, but at the time of the pilot project, the search was limited to alleles, thus it stands to reason that authors went to MGI as opposed to the SciCrunch portal to identify resources. The fewest errors were made in identifying software tools and databases, with only one mistake from a total of 78 (1% error rate). The mistake was made as the author apparently used an antibody identifier instead of a tool identifier.
The use of a unique string to retrieve RRIDs is aided by a common syntax. In our analysis of RRIDs we also noted whether or not the RRID was correctly formed. In 66% (369/562) of cases, the RRID was reported with the correct syntax, 63% of antibodies, 85% of organisms, and 67% of tools were formatted correctly (Fig. 3). The most common variant was the addition of extra spaces (RRID:AB_90755 vs. RRID: AB_90755), with 67% (129/193) of the minor corrections being due to an extra space. Other common variants were failure to include the RRID prefix, using various symbols or spaces in the identifier, or splitting up the RRID prefix and identifier in a table. Authors did not create RRIDs for resources they were either unable to find, or were not in the portal in 142 cases, which constitutes an overall 20% false negative rate (36/465 reported antibodies were false negatives 8%, 84/139 reported organisms were false negatives 60%, and 22/101 tools 22% were false negatives). In other words, authors included RRIDs for the appropriate resource in over 80% of cases.
Outcome #3 Identifiability
An outcome of this study was to determine if the use of RRIDs in the literature increased the identifiability of research resources. As shown in Figure 4, when authors were asked by their editors to provide RRIDs, regardless of their compliance with the RII project, the identifiability of research resources significantly increased. We calculated the percentage of identifiable research resources in the same journals, just before the pilot project and after. The reporting of research resources prepilot was consistent with findings from the 2013 study (Vasilevsky et al. 2013), in that roughly 50–60% were found to be identifiable. But, when asked by their editors, researchers used identifying information in 80–90% of research resources, showing that they presumably had the data available, but did not put it into their papers unless prompted by communication from the editors.
Figure 4.
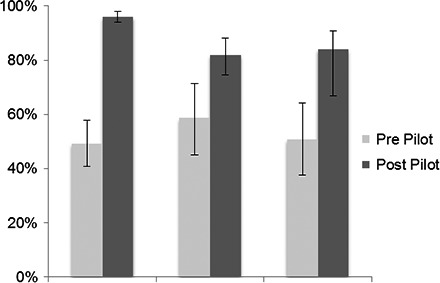
Pre‐ and postpilot identifiability. Resources (primary antibodies, organisms, and tools) were considered identifiable if they contained an accurate RRID or by using the same criteria as described in (Vasilevsky et al. 2013). For tools (software and databases, which were not previously analyzed), these resources were considered identifiable if they contained an RRID or reported the manufacturer and version number. The total number of resources for each type is: primary antibodies prepilot, n = 140; primary antibodies postpilot, n = 465; organisms prepilot, n = 58; organisms postpilot, n = 139; noncommercial tools prepilot, n = 59; noncommercial tools postpilot, n = 101. The y‐axis is the average percent identifiable for each resource type. Variation from this average is shown by the bars: error bars indicate upper and lower 95% confidence intervals. Asterisks indicate significant difference by a z‐score greater than 1.96.
Outcome #4 Utility
Machine processability
The ability to search all studies that used a particular research resource was a prime motivation for this pilot. The current project had a loose definition of “machine processable” because we did not want to impose any requirements on the publishers to modify their journal submission system for a pilot. Thus, we opted to craft RRIDs as unique, indexable alphanumeric strings based upon authoritative sources that could support use of web search engines to return papers using a particular research resource. We specifically asked authors to assess resources mentioned in the materials and methods section where they would normally provide identifying information because we wanted to track actual use of the resource and not just mentions.
For individual RRIDs, the approach was highly successful as is illustrated by the ability to type a particular RRID into three search engines for the biomedical literature: Google Scholar, PubMed, and Science Direct and retrieve appropriate papers, for example, RRID:AB_90755 or AB_2298772 (for Google Scholar see Fig. 2). It is important to note that each of these systems will come back with different results because each search tool has different types of data about each paper. For example, ScienceDirect has a good full text search of all Elsevier content, but it does not search other publisher's content. Both PubMed and Scopus search only the abstracts and return a subset of articles where authors followed instructions to add RRIDs to the keywords, but not those that are only in the methods section. Google Scholar is the most comprehensive as it appears to search full text and brings back papers that are both published and unpublished (usually these are accepted for publication, but not yet indexed by PubMed). An analysis performed in October 2014 showed varying results from each search engine: Google Scholar returned 315 results (from 2014, 174 are true RRIDs), and ScienceDirect returned 18 (from 2014, 3 are RRIDs). PubMed revealed 23 papers that contained RRIDs (from 2014, all identify the resource identification initiative identifiers). Scopus returned 48 documents (from 2014, 18 are RRIDs).
To promote the development of thirdrd party tools around RRID's, we created a resolver service for RRIDs using SciCrunch. Typing http://scicrunch.com/resolver/RRID:AB_90755, will resolve to a landing page with metadata on a particular entity. The resolving service allows applications to make use of RRIDs to, for example, enhance articles with RRIDs by providing additional information about the entity and link to relevant articles and resources. For instance, Elsevier has released their antibody application, which displays antibody metadata in the right hand side panel, next to the article (see Fig. 5 for a screenshot below for (MacLaren et al. 2015): http://www.sciencedirect.com/science/article/pii/S0306452214008458). The reader can browse through antibodies referred to in the article, view complete records in antibodyregistry.org, and access additional information via direct links to GenBank, ZFIN, and other relevant databases. The application also recommends three most relevant articles published in Elsevier journals that refer to the same antibody. The application is freely available on ScienceDirect.
Figure 5.
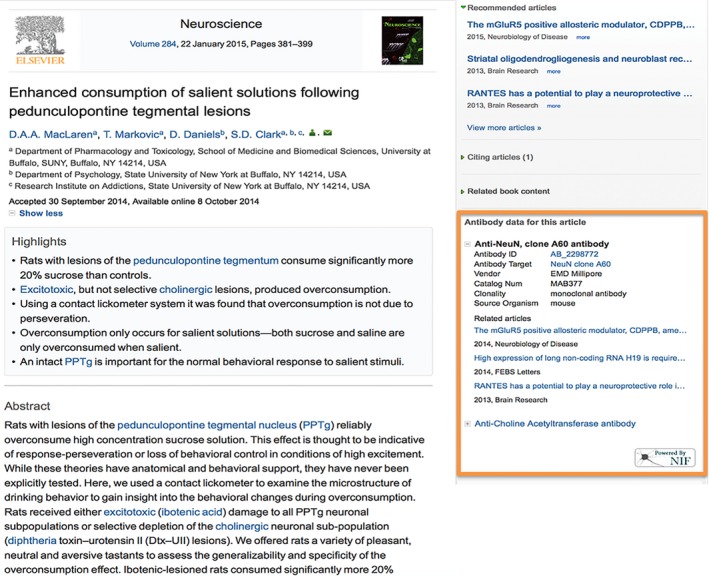
An exemplar third‐party application using the RRID resolving service. The “Antibody data for this article” application developed by Elsevier enhances articles on ScienceDirect. The application is available in 211 articles in 19 journals (more information can be found at: http://www.elsevier.com/about/content-innovation/antibodies).
Publication practices
Non‐open access journals were asked to add RRIDs to publication keywords, but our initial findings suggest that this practice was not being consistently followed. Only 23 papers of 41 total (as of Oct 20, 2014) were accessible in PubMed. Additionally, it should be noted that in two cases, identifiers were removed at typesetting after the initial online version of the manuscript was published with the RRIDs. These identifiers were removed not only from the manuscript, but also from PubMed keywords. Although this was reversed when noted by the working group, this demonstrates that successful implementation requires knowledge of the RRIDs and agreement by the publishers at all steps.
Discussion
The pilot RRID project has been highly successful in demonstrating the utility of a system to aid in identification of antibodies, software and databases, and model organisms in the biomedical literature. We showed that authors were willing to adopt new styles of citation for research resources that promoted more accurate identification of research resources used in a study, and that were more amenable to machine‐based identification. To date, RRIDs have appeared in over 400 papers from 60 journals. With one exception (the Journal of Neuroscience), journals have continued their request for RRIDs beyond the initial 3‐month pilot project and new journals have signed up beyond the initial set that started the project. We believe that the success of the project was due to the extensive preplanning that involved the publishers and the editors, the limited scope of the initial request, and the recognized need by researchers for better and more useful reporting standards for research resources.
The load on curation staff with participating journals has been minimal and the initial portal prototype appears reasonable for the majority of authors to find their resource identifiers. With >10,000 searches in the RII portal, there were approximately 100 help questions. Many of these questions were about scope, that is, whether a particular research resource should be identified, others were for assistance in finding a resource or guidance in adding a resource not yet contained in the community authorities. While this is not a large number, it is also not insignificant, particularly as the project expands, and certainly points out the need for specific help functions.
Given the relative completeness of the registries and the rapid advance of machine learning‐based techniques for entity recognition, we can envision a semiautomated system that assists the author in supplying correct IDs. We have already improved our ability to detect digital research resources in the literature using machine learning (Ozyurt et al., unpubl. data, PLoSOne). In this system, machine learning is used to identify software tools and databases in text, and compare the information to Registry listings. The development of such functions would allow the development of recommender systems for authors and automated fact checkers for journal staff.
Why unique identifiers?
Unique identifiers serve as a primary key for identifying a given research resource and providing the ability for search engines to parse them is paramount. Unique identifiers enable disambiguation of entities with similar labels. The ID should not point to two different entities and needs to be persistent, that is, they need to outlive the entity itself. They also need to be at least minimally machine processable. While many authors supplied identifying information like the catalog number for an antibody supplied by the vendor, or the official strain nomenclature supplied by the IMSR for a mouse, neither of these served the required functions. A catalog number is not a unique identifier, but rather a useful way for vendors to identify their products. If different vendors sell the same antibody, it will have different catalog numbers. If the same antibody is sold in different aliquots, it may have different catalog numbers. When the antibody is no longer available, the catalog number may disappear, or in some cases it may be reassigned to another antibody. All of these features are undesirable in an identifier system. The Antibody Registry, in contrast, was specifically designed to supply useful and stable identifiers for antibodies and not as a commercial source of antibodies. Similarly, the strain nomenclature developed by the Jackson Laboratory, with its superscripts and special characters, is useful for human curators to identify a particular strain, but causes hiccups in most search engines because of all of the special characters. We believe that a well‐curated registry is essential to the success of such a system because of the necessity of these two functions, which currently cannot be replaced with a simple uncurated registration service. For example, we found in the registries we maintain, both software or antibodies, that authors sometimes register an entity that is found by a curator to be a duplicate.
Reporting of RRIDs
When considering accuracy and syntax, the majority of the issues were due to minor syntax errors (33% of RRIDs had a syntax error), and a minority of the resources (4%) was incorrectly reported. The data suggest authors are able to find the correct RRID for their resource, but the higher syntax error rate indicates a need for an improved process for reporting the RRIDs in the manuscripts. The typesetting may cause some of the syntax issues, for example, spaces may be introduced, especially when the RRID is at the end of a line. Additionally, these types of syntax errors are resolvable by the resolver, so they do not pose an issue for the machine readability.
Authors included RRIDs for the appropriate resources in 80% of the papers. This analysis did not allow us to determine if authors did not report RRIDs because the resource was not available in the RII portal at the time, or if they failed to include the RRID for another reason.
The analysis for this pilot project focused on primary antibodies and noncommercial tools, however, many authors included RRIDs for secondary antibodies and commercial tools, such as MATLAB or SAS. While this was out of scope for this analysis, this indicates that authors are willing and eager to provide RRIDs for additional research types, not just those included in this pilot project.
In two papers, authors reported RRIDs for resources that were not used as part of the study, but rather were discussed in the introduction or discussion sections. A goal of this study is to enable one to determine the usage of a particular resource, as reported in the published literature. For example, one could query Google Scholar for all the papers that report a particular RRID to get a sense of how frequently that resource appears in publications. Therefore, it is important that only resources that are used in a study are assigned an RRID. This should be further clarified in the instructions to authors.
Which identifiers?
There are many types of and formats of identifiers in use today (e.g., DOIs, URIs, ARCs), each with varying amounts of associated infrastructure and use in different communities. For this project, we elected to use simple alphanumeric strings and a common syntax in the form of accession numbers issued by the authoritative community‐based registries. We relied on each registry to impose the uniqueness constraint at the level of the entity, for example, ensuring that there was only one mouse genotype per unique ID, and to ensure standard metadata by curating each entry. The reuse of authoritative accessions with the RRID prefix provides maximal flexibility and interoperability and minimal ID churn, while also provisioning for resource identification.
A frequent question regarding the RRID is why we did not use a DOI as a unique identifier instead of the Registry Accession number. Part of the reason was cultural: researchers were used to supplying accession numbers for Genbank, Gene Expression Omnibus, Protein Data Bank, etc. and understand this requirement. Part of the reason is practical: unlike DOIs, accession numbers are already available for most of the research resources to be identified in this pilot and did not require special infrastructure to resolve or cost to issue. Part of reason is also philosophical: DOIs are for digital objects, such as individual articles, that live on the web and need to be resolvable. A DOI resolves to a particular article that is self‐contained—it is the object. In contrast, an antibody does not exist on the web, but is an independent entity that has data about it scattered across various articles. There is no single digital record that is the antibody; there are documents and data about the entity. We note that in our community we also do not use DOIs to identify people, but rather an ORCID, which serves the same purpose as the RRID.
A case could be made for using DOIs to identify particular software tools and databases, as they are digital objects. As discussed in the next section, our preference is that DOIs be used to identify the particular instance used, for example, the version of data or software and any supporting workflows, and that the RRID be used to identify the entity or project referenced. Thus, the RRID would be used to identify the Protein Databank, and a PDB identifier or a DOI used to reference the specific data from the PDB. However, we believe that as the RRID system is adopted, each community should set appropriate identifier systems. The RRID syntax is meant to be simple and generic and could, in theory, work with any existing authoritative identifier system.
How granular should RRIDs be?
RRIDs are meant to identify research resources at a fairly high level of granularity. At some of the planning meetings, there was a push for more granular information, for example, lot and/or batch numbers for antibodies. We recognize that this level of granularity is likely an important factor in determining how a given reagent performs (Slotta et al. 2014). In our analysis by Vasilevsky et al. (2013) and in our experience using text mining, the biggest problem is not that authors were not supplying lot numbers, but that they are not even supplying the minimal identifying information such as catalog numbers. Given that the catalog numbers themselves do not serve as stable identifiers because antibodies are bought and sold and redistributed by many vendors, we elected to tackle the problem of identifying the root antibody first, that is, a particular clone for a monoclonal antibody or a type of polyclonal antibody produced by particular protocol. To illustrate the problem, consider the study by Slotta et al. (2014) that provided an analysis of the performance of antibodies to NF‐κB‐subunit p65, as a follow‐up to a similar study by Herkenham et al. (2011). Both studies performed specificity tests on a variety of antibodies and, as is common, did not produce concordant results on all of them. Slotta originally generated the antibody now commonly known as MAB3026 (AB_2178887) and provided its provenance: “It was transferred to Boehringer Mannheim as Clone 12H11, resold to Roche and finally bought by Chemicon, and it is now sold as MAB3026.” They then speculate that a mutation may have crept in at some point that altered the specificity of the antibody. However, the discrepancies may also be attributed to the additional testing of the antibody in new conditions, revealing problems that had not been apparent during the initial applications. The authoritative Antibody Registry identifier (and therefore the RRID) for this antibody combines these different representations together so that all references to this antibody can be tracked. Authors are encouraged in the citation format to include details about the particular instance of this antibody, namely, the vendor from which the antibody was purchased and the catalog, batch, and lot numbers. However, we did not want to overload the ID system to require assignment of these different lot numbers different RRIDs and maintain the mappings. We were also concerned that this would grossly decrease compliance.
For organisms, all of the authors “errors” were due to the allele being reported, but not the organism stock or genotype. The allele ID is not sufficient for identifying the animal used as the same allele may be inserted into different mice of various backgrounds and with other alleles, and therefore will have different phenotypic characteristics. It should also be noted that authors consulting the MGI database (up to October 2014), which maintains the authoritative mouse nomenclature, would be able to search for a MGI identifiers for genes and alleles, but not genotypes. This shows that authors likely went to MGI to obtain their identifiers rather than searching the RII portal, but were not able to find the genotype information and substituted the allele ID. MGI now searches the genotype information for all mice suggesting that authors of newer papers can now also find the genotype information more easily at MGI and a tutorial for how to obtain a genotype identifier from MGI is now posted on the RII portal pages. Support for genotype identification, and therefore RRIDs, is planned to also be provided by a new Monarch Initiative phenotyping tool for submission of genotype–phenotype data to journals and model organism databases.
For tools (software and databases), we elected to identify the root entity and not a granular citation of a particular software version or database. Our main goal in the case of software tools and databases was to track broad patterns of utilization of these resources (e.g., how many times NeuroMorpho.org was used) and not particular versions. More complete practices for citing software and data sets are emerging from recent efforts like the Joint Declaration of Data Citation principles ( https://www.force11.org/datacitation), the W3C HCLS dataset description ( http://tiny.cc/hcls-datadesc), the software discovery index ( http://softwarediscoveryindex.org/), and many others. These groups are exploring more complete reporting standards for the individual instances (versions, workflows, virtual machines) that can be used to reproduce the findings. We note that the goal of using RRIDs for software tools was to determine participation rates for authors identifying these resources using the easiest possible solution, with the longer term goal including more robust versioning and archival software practices that would support reproducibility.
What are the next steps?
The RII is a grass‐roots effort that took advantage of existing investments by the NIH to solve a problem without extensive new infrastructure. The RII is continuing to run and has expanded beyond the initial participants. We believe that the growth of the initiative indicates that it fills a need not currently met by our existing practices and infrastructure.
Should RRIDs be adopted broadly across all of biomedicine? We would argue yes, the RRID syntax should become the standard for reporting on usage of research resources. We have shown that the requirements for this type of broad adoption are the availability of comprehensive and authoritative registries for the appropriate entities, a centralized portal or services that aggregate these registries into a single search, and the willingness of a community including journals and publishers to support this type of reporting. More sophisticated services can be built to improve and automate authoring and editorial oversight, but these are not required. The solution is therefore accessible both to large commercial publishers and smaller community‐ or society‐based journals.
If RRIDs were to be broadly adopted tomorrow, what are the outstanding issues regarding implementation and scalability? The first issue is one of scope. The current RII focused on three types of research resources that were broadly used and a known source of variability within experiments. Should all research resources be similarly identified, that is, every chemical, instrument, etc.? We think such an approach would be clumsy and difficult to implement. We can imagine a future where all reagents and tools are bar coded and scanned as they are used in a study. However, as long as humans are responsible for supplying identifiers, we think that the effort should focus on certain types of known problematic entities for which better metadata and ability to query across papers is required. Given the recent problems associated with certain cell lines, for example, these are obvious candidates (ICLAC 2015). The advantage of the current system is that it allows communities who have taken the steps to aggregate and organize resources that are of use to them to agree to include the RRID syntax and single entry point.
The second issue is governance. We deliberately designed a decentralized system that gives control of issuing identifiers to multiple authorities. Such a model requires some governance, in the form of willingness of the authorities to maintain the integrity of any identifiers and links and implementation of a policy regarding entities that are no longer available. We would also need some governance to ensure that multiple, uncoordinated authorities are not issuing IDs for the same research resource and that the IDs assigned to each entity are unique. The latter constraint is handled by the centralized aggregation service currently provided by SciCrunch, however, it may be handled by other services in the future. Further, the RRID project promotes consistent citation of research resources at a first‐ level identifiability. We believe that more granular reporting standards can and should work hand in hand with the RRIDs, and could be coordinated with the authoritative communities, for example, versioned software releases in GitHub.
Some of these governance issues are necessarily interdependent on issues relating to sustainability. As we increase participation among journals and resource providers, it would make sense to spread the cost of maintenance and development. One thing to consider is that resolution services can provide advertising for resource providers as third‐party applications are developed to connect people to resources in different contexts (such as in the Elsevier application described above). We would conjecture that as the number and types of these applications increase the need to contribute and therefore help sustain resource registries will become increasingly advantageous.
We believe that the RRID project lays an important foundation for creating a type of “universal product code” (UPC) to help alert the scientific community when issues are raised about key research resources. Reagents and tools are not perfect and problems can arise, as the resources themselves can have issues as they are tested across various paradigms and systems. Even when a resource initially performed well, due to spontaneous mutations in biological resources and interactions between particular software tools and platforms, problems can arise over time. For example, two recent papers have published extensive tests showing that common antibodies for NF‐κB show nonspecificity under some circumstances (Listwak et al. 2013; Slotta et al. 2014). Many of these antibodies are extensively used in the literature, but readers of a particular article have no way of knowing that concerns have been raised. We have similar examples with software tools (Gronenschild et al. 2012), data sets (Button et al. 2013; Hupé 2015), and genetically modified animals (Cone et al. 2013). We have an infrastructure in place, CrossMark, to alert readers of a particular article that an addendum or erratum has been posted. The RRID system can serve as the basis for a similar system for research resources.
Supporting information
Table S1. The goal of this doc is to keep track of publications that have reported RRIDs and cross check the RRID, to ensure the authors are using the correct ID.
Acknowledgments
We thank J. Maunsell for his effort in getting this effort going and helpful comments on the paper, as well as the many other editors who wrote letters to their authors and the staff at various publishers and many other community projects that wrote blogs and press releases about this initiative. We also thank NIH and INCF for providing the venues and vision for this pilot project, specifically Dr. J. Pollock, who drove the workshop and saw early the need to bring this particular group together around a central goal of better identification of research resources. We thank ZFIN, who recognized the need for an RRID system and provided inspiration for this project. We also thank L. Ponting for her help with curating the drosophila strains.
Bandrowski A., Brush M., Grethe J. S., Haendel M. A., Kennedy D. N., Hill S., Hof P. R., Martone M. E., Pols M., Tan S. C., Washington N., Zudilova‐Seinstra E., Vasilevsky N., RINL Resource Identification Initiative . The Resource Identification Initiative: a cultural shift in publishing, Brain and Behavior, 2016; 6(1), e00417, doi: 10.1002/brb3.417
This article is simultaneously published in Journal of Comparative Neurology (DOI: 10.1002/cne.23913), F1000Research (DOI: 10.12688/f1000research.6555.2), and Neuroinformatics (DOI: 10.1007/s12021‐015‐9284‐3).
References
- Bandrowski, A. , Tan S., and Hof P. R.. 2014. Promoting research resource identification at JCN. J. Comp. Neurol. 522:1707. [DOI] [PubMed] [Google Scholar]
- Button, K. S. , Ioannidis J. P., Mokrysz C., Nosek B. A., Flint J., Robinson E. S., et al. 2013. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14:365–76. [DOI] [PubMed] [Google Scholar]
- Cone, A. C. , Ambrosi C., Scemes E., Martone M. E., and Sosinsky G. E.. 2013. A comparative antibody analysis of pannexin1 expression in four rat brain regions reveals varying subcellular localizations. Front. Pharmacol. 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner, D. , Akil H., Ascoli G. A., Bowden D. M., Bug W., Donohue D. E., et al. 2008. Essen DC Van, RW Williams. The neuroscience information framework: a data and knowledge environment for neuroscience. Neuroinformatics 6:149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronenschild, E. H. , Habets P., Jacobs H. I., Mengelers R., and Rozendaal N.. 2012. Os J van, M Marcelis. The effects of FreeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS ONE 7:e38234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham, M. , Rathore P., Brown P., and Listwak S. J.. 2011. Cautionary notes on the use of NF‐κB p65 and p50 antibodies for CNS studies. J. Neuroinflammation 8:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupé, J. M. 2015. Statistical inferences under the Null hypothesis: common mistakes and pitfalls in neuroimaging studies. Front. Neurosci. 9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICLAC . 2015. Cell line cross‐contamination: WSU‐CLL is a known derivative of REH and is unsuitable as a model for chronic lymphocytic leukaemia. Leuk. Res. 38:999–1001. [DOI] [PubMed] [Google Scholar]
- Listwak, S. J. , Rathore P., and Herkenham M.. 2013. Minimal NF‐κB activity in neurons. Neuroscience 250:282–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren, D. A. , Markovic T., Daniels D., and Clark S. D.. 2015. Enhanced consumption of salient solutions following pedunculopontine tegmental lesions. Neuroscience 284:381–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenco, L. N. , Wang R., Bandrowski A. E., Grethe J. S., Shepherd G. M., and Miller P. L.. 2014. Extending the NIF DISCO framework to automate complex workflow: coordinating the harvest and integration of data from diverse neuroscience information resources. Front. Neuroinform. 8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre, N. J. , Fluck R., McIntyre C., and Taal M.. 2012. Treatment needs and diagnosis awareness in primary care patients with chronic kidney disease. Br. J. Gen. Pract. 62:e227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotta, C. , Müller J., Tran L., Hauser S., Widera D., Kaltschmidt B., et al. 2014. An investigation of the specificity of research antibodies against NF‐κB‐subunit p65. J. Histochem. Cytochem. 62:157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilevsky, N. A. , Brush M. H., Paddock H., Ponting L., Tripathy S. J., Larocca G. M., et al. 2013. On the reproducibility of science: unique identification of research resources in the biomedical literature. PeerJ 1:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The goal of this doc is to keep track of publications that have reported RRIDs and cross check the RRID, to ensure the authors are using the correct ID.


