Abstract
Cardiovascular disease is a major target for numerous experimental stem (progenitor) cell-based therapies. Mesenchymal stem cells (MSCs) from different sources confer regenerative effects in animal models of cardiovascular disease. Some of these investigations have proceeded into phase I and II clinical trials for limb ischemia, heart failure, and acute myocardial infarction. The rationale for MSC therapy is increasingly recognized on a secretion (paracrine) rather than differentiation mechanism. Recently, several groups have demonstrated that the “exosome” is a secreted agent mediating MSC therapeutic efficacy. Unlike cell therapy, exosomes have no risk of aneuploidy, and a lower rate of immune rejection following allogeneic administration. In this short review, we will focus on the potential of using this novel therapeutic modality for the treatment of cardiovascular disease, particularly acute myocardial infarction.
Keywords: Cardiovascular disease, Exosome, Mesenchymal
INTRODUCTION
Cardiovascular disease is the leading cause of mortality globally, and acute myocardial infarction (AMI) is the most common cause of heart failure. AMI triggers a series of cellular and molecular disturbances leading to apoptosis, necrosis and hypertrophy of cardiomyocytes, impaired neovascularization, myocardial fibrosis and inflammation, reduced contractility and subsequent pathological remodeling. Regenerative and reparative therapies could be particularly important for the treatment of cardiovascular disease, especially AMI.1
Mesenchymal stem cells (MSCs) are self-renewing, multipotent progenitors that can be isolated from various tissues. They have been and continue to be widely tested in clinical trials because of their multiple biological functions including multilineage differentiation, tissue repair, anti-inflammation, immunosuppression, and neuroprotection.2 The mechanisms underlying these biological functions were initially attributed to their homing to and engraftment in injured organs/tissues, and subsequent differentiation to repair and replace damaged cells. However, studies in animal models and humans have indicated that < 1% of transplanted MSCs localize to the target site.3 Instead, recent evidence4 indicates that transplanted MSCs secrete factors to reduce tissue injury and/or enhance tissue repair. Of even more recent origin, several groups have demonstrated the “exosome” as the secreted agent mediating MSC therapeutic efficacy.5,6 Compared with cell therapy, exosomes have no risk of aneuploidy and a lower rate of immune rejection following allogeneic administration.6 In this review we will focus on the current understanding of MSC biology and MSC mechanisms of action in the therapy of cardiovascular disease.
Mesenchymal stem cells for cardiac repair
Mesenchymal stem cells are self-renewing, multipotent progenitor cells with multilineage potential to differentiate into cell types of mesodermal origin such as adipocytes, osteocytes, and chondrocytes. While MSCs are most commonly isolated from bone marrow, they are also isolated from other tissues including peripheral blood, adipose tissue, placenta, amniotic fluid, umbilical cord blood and adventitia of vessel wall.7 Due to their accessibility and convenient expansion protocols, MSCs have been recognized as promising candidates for cellular therapy. However, a burgeoning interest in MSCs has led to the prudent question of whether MSCs isolated from different sources and expanded from various protocols are truly equivalent. To address this issue, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy developed certain minimal criteria to universally define human MSCs.8 The criteria include adherence to plastic, specific surface antigen expression (CD73+ CD90+ CD105+ CD34- CD45- CD11b- CD14- CD19- CD79a- HLA-DR-) as well as multipotent differential potential under standard in vitro differentiation conditions (Figure 1).
Figure 1.
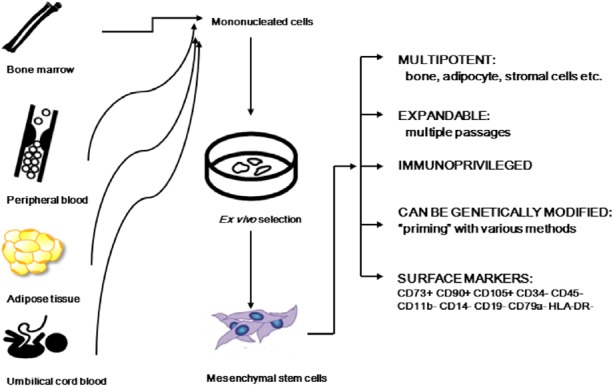
Mesenchymal stem cells (MSC). MSC can be isolated from the bone marrow, adipose tissue, placenta and umbilical cord blood. Some investigators have also described observing MSC in the peripheral blood. MSC can be expanded ex vivo, are multipotent and possess other favorable characteristics that make them suitable for cell therapy and myocardial repair (see text for details).
Several lines of evidence suggest that MSCs may not be subject to allogeneic rejection in human and animal models.9 First of all, MSCs are hypo-immunogenic, since they lack the expression of HLA class II and co-stimulatory molecules. Secondly, it has been shown that MSCs prevent a T cell response indirectly, through the modulation of dendritic cells, and by directly suppressing natural killer cells as well as CD8+ and CD4+ T cell function. Thirdly, MSCs induce a suppressive local microenvironment through the production of prostaglandins and interleukins.9 Another advantage of MSC is that they are easy to modify ex vivo using viral vectors. By overexpressing or silencing genes of interest, the functionality of MSC can be modulated.
Over the past 10 years, much of the research in cardiovascular regenerative therapies, both in animals and humans, has been mainly conducted using bone marrow (BM)-derived MSCs. Particularly, it has been demonstrated that the administration of BM-MSC can rescue damaged hearts and improve cardiac function in animal models of acute myocardial infarction (AMI) and multiple small-scale phase I and phase II clinical trials (Table 1). In a pilot study, 69 patients with acute myocardial infarction received percutaneous coronary intervention, and were randomized to receive intracoronary injection of autologous MSCs or standard saline as controls.10 There were no serious adverse events after MSC administration and the MSC-treated group showed significant improvements in left ventricle function, as compared to the control group. This was the first study to follow and detect the viability of MSCs and cardiac function with cardiac electromechanical mapping. The results indicated that MSCs were still viable 3 months after transplantation. Following this study, MSCs have been used to treat acute and chronic myocardial infarction patients, with significant improvements in heart functions, functional capacity, quality of life, and LV remodeling.10-19 Despite initial positive results indicating the safety of MSC transplantation and improved cardiac function, the differences in trial design, treatment methods, outcome evaluation, and cell isolation have prevented broader conclusions about safety from being reached prematurely. Furthermore, all of the aforementioned studies need to be interpreted cautiously and require long-term follow-up analysis to ensure that strategies for MCS cell therapy incorporate proper safeguard to maximize both treatment effectiveness and patient safety.
Table 1. Clinical trials of mesenchymal stem cell therapy for cardiovascular diseases.
| Reference | Disease | Phase | No. of patients | MSC source | Route | Primary endpoints |
| Chen et al.10 | AMI | I | 69 | Allo-BM | Intracoronary | Improved |
| Chen et al.11 | IHD | I | 46 | Allo-BM | Intracoronary | Improved |
| Katritsis et al.15 | AMI | I | 22 | Allo-BM | Intracoronary | Improved |
| Katritsis et al.14 | AMI | I | 5 | Allo-BM | Intracoronary | Improved |
| Yang et al.16 | AMI | I | 16 | Allo-BM | Intracoronary | Improved |
| Zeinaloo et al.17 | AMI | I | 1 | Allo-BM | Intracoronary | Improved |
| Hare et al.13 | AMI | I | 53 | Allo-BM | IV | Improved |
| Friis et al.12 | IHD | I | 31 | Auto-BM | IM | improved |
| Hare et al.18 | IHD | I/II | 30 | Atuo/allo-BM | IM | Improved |
| Houtgraaf et al.19 | IHD | I/II | 14 | ADRCs | Intracoronary | improved |
ADRCs, adipose tissue-derived regenerative cells; Allo-BM, allogenic bone marrow; AMI, acute myocardial infarction; Auto-BM, autologous bone marrow; IHD, ischemic heart disease; IM, intra-myocardial; IV, intravenous; MSCs, mesenchymal stem cells.
Paracrine effects of MSCs
Paracrine secretion of MSCs was reported more than 15 years ago when Haynesworth et al. reported that MSCs synthesize and secrete a broad spectrum of growth factors and cytokines such as vascular endothelial growth factor, fibroblast growth factors, monocyte chemoattractant protein-1, hepatocyte growth factor, insulin-like growth factor 1, stromal cell-derived factor 1 and thrombopoietin, which exert effects on cells in their vicinity.20 Many of these factors have also been demonstrated to exert beneficial effects on the heart, including neovascularization, attenuation of ventricular remodeling and increased angiogenesis.21,22 In 2006, Gnecchi et al.23 demonstrated that either intramyocardial injection of culture medium conditioned by MSCs overexpressing the Akt (protein kinase B) gene (Akt-MSCs) or Akt-MSCs reduced infarct size in a rodent model of AMI to the same extent. This provided the first direct evidence that cellular secretion could be cardioprotective through paracrine effects.
WHAT IS AN “EXOSOME”?
The secretion of nanovesicles during maturation of sheep reticulocytes was discovered in the 1980s.24 These vesicles were named exosomes and thought to be necessary to remove unneeded proteins from cells. Thereafter, many cell types were found to secrete exosomes, including B and T cells, dendritic cells, cancer cells, stem cells, and endothelial cells. Exosomes are one of several groups of secreted vesicles, which also include microvesicles, ectosomes, membrane particles, exosome-like vesicles or apoptotic bodies.25 Typically, exosomes have a defined diameter of 40-100 nm with a density of 1.13-1.19 g/mL in a sucrose solution, and can be sedimented by centrifugation at 100,000 g. Exosomes from different cellular sources share an evolutionarily conserved set of protein molecules including membrane transport and fusion proteins (GTPases, annexins, and flotillin), tetraspanins (CD81, CD63, CD9), proteins involved in multivesicular body biogenesis (Alix and TSG101), as well as lipid-related proteins and phospholipases.26 But they also carry proteins and RNAsunique to their cell source and the pathophysiological states of the cell source. In addition to protein delivery, exosomes transport various kinds of mRNAs and miRNAs with the potential to alter the fate of recipient cells.27 The list of proteins and RNAs reported to be present in exosomes are freely accessible at ExoCarta (http://www.exocarta.org) or Vesiclepedia (http://www.microvesicles.org).
PROPERTIES OF EXOSOMES DERIVED FROM MSCs
MSC-derived exosomes were first investigated in 2010 by Lai et al.28 in a mouse model of myocardial ischemia/reperfusion injury, and were thereafter tested in several disease models. MSC-derived exosomes express not only the conserved surface markers of exosomes, such as CD9 and CD81, but also some adhesion molecules, including CD29, CD44 and CD73, which are expressed on the membrane of MSCs. As a consequence of the close association between the composition of exosomes and physiological or pathological states of the secreting cells, exosomes are good sentinels of cellular health and pathology, and have become an attractive source of disease biomarkers.29 MSCs may also exert some biological effects on other cells through secretion of miRNAs in exosomes. Pretreating MSC conditioned medium (CM) with RNase abolishes its renal protective effect completely30 in an animal model of acute kidney injury. Accordingly, the two possible mechanisms of paracrine effects of MSCs are summarized in Figure 2.
Figure 2.
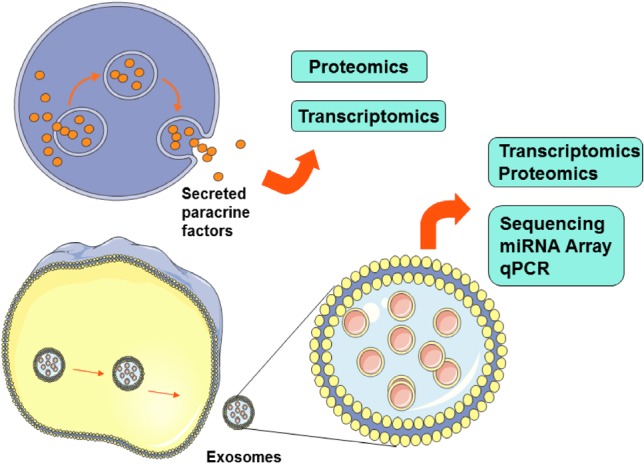
Paracrine effects of mesenchymal stem cells.
Possible refinement of mesenchymal stem cells therapy for cardiac repair
In a porcine model of myocardial ischemia and reperfusion (MI/R) injury, intravenous and intracoronary MSC-CM treatment reduced the infarct size by approximately 50% when administered just prior to reperfusion.31 Size fractionation studies have demonstrated that the active component is a large complex of 50-200 nm in diameter. Electron microscopy showed that these complexes are phospholipid vesicles. They contain co-immunoprecipitating exosome-associated proteins CD81, CD9, and Alix, and can be purified with a hydrodynamic radius of 55-65 nm by size exclusion fractionation in HPLC. Purified exosomes administered to a mouse MI/R injury model revealed that MSCs mediate their cardioprotective paracrine effect by exosome secretion.28 These results suggest that exosomes are a highly efficacious therapeutic agent that neutralizes MI/R injury and an effective adjuvant to complement current reperfusion therapy. It is therefore postulated that exosomes may participate in many biochemical and cellular activities and correct various ischemia-induced cascades. The efficacy of MSC exosomes in reducing reperfusion injury and long term preservation of cardiac function and geometry in animal models of AMI28,32-35 therefore provides a compelling rationale for its translation into a pharmaceutical drug to the adjunct of conventional revascularization therapy for AMI.
The refinement of MSC therapy from a cell- to a secretion-based process offers several advantages as it translates the therapeutic agent from a living to non-living agent and obliterates the burdensome task of preserving cell viability and function during manufacture, storage and delivery to patient. As a result, cellular secretions are more amenable to development as an “off-the-shelf” therapeutic that can be delivered to patients in a timely manner. They also mitigate the safety risks inherent in administering large amount of viable cells such as the risk of occlusion in microvasculature or unregulated growth.
However, there are still some concerns about the use of exosomes for the treatment of MI/R injury. Many of the proteins in exosomes are enzymes, and the enzyme-based therapeutic activities may be activated by the release of injury-associated substrates. Resolution of the microenvironment would reduce the release of these substrates, which would influence enzymatic activities. Consequently, the efficacy of exosome-based therapeutics may be responsive to, but also limited by, the disease-precipitating microenvironment. At last, based on the proteomic and genomic complexities of exosomes, their probable mechanisms and exact compositions need further investigation. Ultimately, the collection protocol must me standardized to match the needs of different studies because the protein and RNA components are not always the same in exosomes obtained from CM of MSCs cultured for different growth periods.
CONCLUSIONS
Identifying exosomes as the major player mediating the therapeutic and paracrine effects of MSCs provides a rationale for refining MSC-based therapy from a cellular to a non-cellular one. Although an exosome-based therapy could potentially reduce the complexities in the manufacturing and usage of a cell-based agent, the clinical development of exosome as a “first-in-class” drug presents unique but highly tractable manufacturing and regulatory challenges.
Acknowledgments
This article is supported by a grant from Feng-Yuan Hospital (FYH103-002).
REFERENCES
- 1.Wollert KC, Drexler H. Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nat Rev Cardiol. 2010;7:204–215. doi: 10.1038/nrcardio.2010.1. [DOI] [PubMed] [Google Scholar]
- 2.Pfister O, Della Verde G, Liao R, Kuster GM. Regenerative therapy for cardiovascular disease. Translational research. Transl Res. 2014;163:307–320. doi: 10.1016/j.trsl.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Phinney DG, Prockop DJ. Concise review:mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 4.Chin SP, Poey AC, Wong CY, et al. Intramyocardial and intracoronary autologous bone marrow-derived mesenchymal stromal cell treatment in chronic severe dilated cardiomyopathy. Cytotherapy. 2011;13:814–821. doi: 10.3109/14653249.2011.574118. [DOI] [PubMed] [Google Scholar]
- 5.Akyurekli C, Le Y, Richardson RB, et al. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Rev. 2014 doi: 10.1007/s12015-014-9545-9. [DOI] [PubMed] [Google Scholar]
- 6.Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–4157. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim EJ, Kim N, Cho SG. The potential use of mesenchymal stem cells in hematopoietic stem cell transplantation. Exp Mol Med. 2013;45:e2. doi: 10.1038/emm.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 9.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 10.Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Liu Z, Tian N, et al. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol. 2006;18:552–556. [PubMed] [Google Scholar]
- 12.Friis T, Haack-Sorensen M, Mathiasen AB, et al. Mesenchymal stromal cell derived endothelial progenitor treatment in patients with refractory angina. Scand Cardiovasc J. 2011;45:161–168. doi: 10.3109/14017431.2011.569571. [DOI] [PubMed] [Google Scholar]
- 13.Hare JM, Traverse JH, Henry TD, et al. A randomized,double-blind,placebo-controlled,dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katritsis DG, Sotiropoulou P, Giazitzoglou E, et al. Electrophysiological effects of intracoronary transplantation of autologous mesenchymal and endothelial progenitor cells. Europace. 2007;9:167–171. doi: 10.1093/europace/eul184. [DOI] [PubMed] [Google Scholar]
- 15.Katritsis DG, Sotiropoulou PA, Karvouni E, et al. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheterization and cardiovascular interventions. Catheter Cardiovasc Interv. 2005;65:321–329. doi: 10.1002/ccd.20406. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Zhang F, Ma W, et al. A novel approach to transplanting bone marrow stem cells to repair human myocardial infarction:delivery via a noninfarct-relative artery. Cardiovasc Ther. 2010;28:380–385. doi: 10.1111/j.1755-5922.2009.00116.x. [DOI] [PubMed] [Google Scholar]
- 17.Zeinaloo A, Zanjani KS, Bagheri MM, et al. Intracoronary administration of autologous mesenchymal stem cells in a critically ill patient with dilated cardiomyopathy. Pediatr Transplant. 2011;15:E183–E186. doi: 10.1111/j.1399-3046.2010.01366.x. [DOI] [PubMed] [Google Scholar]
- 18.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houtgraaf JH, den Dekker WK, van Dalen BM, et al. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59:539–540. doi: 10.1016/j.jacc.2011.09.065. [DOI] [PubMed] [Google Scholar]
- 20.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro:effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 22.Min JY, Sullivan MF, Yang Y, et al. Significant improvement of heart function by cotransplantation of human mesenchymal stem cells and fetal cardiomyocytes in postinfarcted pigs. Ann Thorac Surg. 2002;74:1568–1575. doi: 10.1016/s0003-4975(02)03952-8. [DOI] [PubMed] [Google Scholar]
- 23.Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 24.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 26.Chaput N, Thery C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33:419–440. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- 27.Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Duijvesz D, Luider T, Bangma CH, Jenster G. Exosomes as biomarker treasure chests for prostate cancer. Eur Urol. 2011;59:823–831. doi: 10.1016/j.eururo.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 30.Reis LA, Borges FT, Simões MJ, et al. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PloS One. 2012;7:e44092. doi: 10.1371/journal.pone.0044092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome:a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6:481–492. doi: 10.2217/rme.11.35. [DOI] [PubMed] [Google Scholar]
- 32.Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Lai RC, Arslan F, Tan SS, et al. Derivation and characterization of human fetal MSCs: an alternative cell source for large-scale production of cardioprotective microparticles. J Mol Cell Cardiol. 2010;48:1215–1224. doi: 10.1016/j.yjmcc.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Timmers L, Lim SK, Arslan F, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1:129–137. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Timmers L, Lim SK, Hoefer IE, et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011;6:206–214. doi: 10.1016/j.scr.2011.01.001. [DOI] [PubMed] [Google Scholar]


