Abstract
Background
Severe hypothermia (SH, 30 °C) increases the risk of pacing-induced ventricular fibrillation (PIVF) by enhancing spatially discordant alternans (SDA). Whether moderate hypothermia (MH, 33 °C), which is clinically used for therapeutic hypothermia, also facilitates SDA remains unclear. We hypothesized that MH attenuates SDA occurrence compared with that achieved by SH, and decreases the susceptibility of PIVF.
Methods
Using an optical mapping system, action potential duration (APD)/conduction velocity restitutions and thresholds of APD alternans were determined by S1 pacing in Langendorff-perfused isolated rabbit hearts. In the MH group (n = 7), S1 pacing was performed at baseline (37 °C), after 5-min MH, and after 5-min rewarming (37 °C). In the SH group (n = 9), pacing was also performed at baseline (37 °C), after 5-min SH, and after 5-min rewarming (37 °C). The thresholds of APD alternans were defined as the longest S1 pacing cycle length at which APD alternans were detected.
Results
Although the thresholds of APD alternans were not different between the MH (273 ± 46 ms) and the SH (300 ± 35 ms) (p = 0.281) groups, SDA threshold was shorter (at a faster heart rate) during MH (228 ± 33 ms) than that during SH (289 ± 42 ms) (p = 0.028). At APD alternans threshold, SH hearts showed more SDA than that during MH (SH: 7 hearts, MH: 2 hearts, p = 0.049). SDA could be induced in all 9 SH hearts (100%), while only 4 MH hearts (57%) had SDA (p = 0.029). The PIVF inducibility during SH (44 ± 53%) was higher than that during MH (0%) (p = 0.043).
Conclusions
Compared with SH, the MH group showed greater attenuation of SDA and decreased the susceptibility of PIVF. Therefore, MH is safer as a procedural guideline for use in clinical therapeutic hypothermia than SH.
Keywords: Cardiac alternans, Conduction velocity, Hypothermia, Optical mapping
INTRODUCTION
Therapeutic hypothermia (TH) has become the standard therapy for patients resuscitated from cardiac arrest due to ventricular fibrillation (VF) in that it improves neurological recovery and mortality.1,2 However, TH per se might be pro-arrhythmic because patients with accidental hypothermia (body temperature < 30 °C) typically present with cardiac arrest primarily due to VF.3,4 With a clinically recommended temperature for TH (32-34 °C),1,2 the incidence of ventricular arrhythmia was relatively low (~2.2%) in post-resuscitation patients undergoing TH.5,6 The mechanism by which moderate hypothermia (32-34 °C) decreases the incidence of ventricular arrhythmia while severe hypothermia (< 30 °C) increases arrhythmia remains unclear.
Piktel et al. used a canine left ventricular wedge preparation and found that cooling the heart to 26 °C significantly increased the transmural dispersion of repolarization (DOR) and ventricular arrhythmia susceptibility, while TH at 32 °C mildly increased the DOR and decreased ventricular arrhythmia.5 Harada et al. also showed that severe hypothermia (SH, 30 °C) enhanced wave breaks and regeneration of new spiral waves of VF which favored the maintenance of VF in a 2-dimensional ventricular preparation.7 During moderate hypothermia (MH, 33 °C), the VF spiral waves frequently collide and dissipate, leading to self-termination of VF.7 These data indicate possible mechanisms (DOR, wavefront characteristics) by which MH might be better able to hinder the susceptibility of VF compared to SH.
Cardiac alternans refers to beat-to-beat alternation of action potential duration (APD) which may be spatially concordant alternans (SCA), when all regions of tissue alternate are in phase, or spatially discordant alternans (SDA), when adjacent regions alternate are out of phase.8-10 SDA can facilitate the genesis of reentry arrhythmia, and is more arrhythmogenic than SCA.11-13 We have recently reported that SH (30 °C) increases the susceptibility to pacing-induced VF (PIVF) by promoting earlier onset of SDA.14 SDA during SH always precedes the onset of VF.14 Given the relatively low incidence of ventricular arrhythmia with MH (33 °C) in human and experimental studies,5,6 it is possible that MH might attenuate the occurrence of cardiac alternans, especially SDA, and contribute to a reduced incidence of ventricular arrhythmia. Furthermore, limited information is available regarding the properties of cardiac alternans during MH (33 °C).
In this study using Langendorff-perfused isolated rabbit hearts subjected to MH and SH involving an optical mapping system, we investigated the cardiac electrophysiological parameters, alternans properties, and the susceptibility to PIVF. Since the clinical guidelines recommended 32-34 °C as the target temperature for TH,1,2 we accordingly chose 33 °C as the MH temperature to be tested. Because 30 °C is the lowest temperature proven to be feasible in clinical practice,15-17 it was chosen as the SH temperature for purposes of this study. We hypothesized that MH would attenuate the occurrence of SDA to a greater extent compared with that achieved by SH, and would decrease the susceptibility to PIVF.
METHODS
The research protocol for this study was approved by the Institutional Animal Care and Use Committee of Taichung Veterans General Hospital.
Langendorff preparation of isolated rabbit hearts
New Zealand white rabbit (3.4 ± 0.4 kg, n = 16) hearts were excised under general anesthesia. The ascending aorta was cannulated and perfused with 37 °C Tyrode’s solution composed of (in mM): 125 NaCl, 4.5 KCl, 0.5 MgCl2, 24 NaHCO3, 1.8 NaH2PO4, 1.8 CaCl2, 5.5 glucose, and albumin (40 mg/L), respectively.18,19 Coronary perfusion pressure and flow rate were 60-65 mmHg and 35-45 mL/min. The hearts were perfused and superfused in a thermostatized tissue bath. Thereafter, a pseudo-electrocardiography was obtained with widely spaced bipoles to determine ventricular rhythm.20 The signals were digitized by an AxoScope with a sampling rate of 1 kHz.18-22 A pair of hook bipolar electrodes was inserted into the right ventricular outflow tract for ventricular pacing.
Optical mappings
By using a two-camera optical mapping system, epicardial activations in the anterior and posterior aspects of the hearts were simultaneously mapped.18,19 The hearts were stained with di-4-ANEPPS, and excited with 4 light-emitting diode modules (wavelength = 519 ± 20 nm).18,19 Induced fluorescence was collected by two image-intensified charge-coupled cameras (model CA D1-0128T). The optical signals were gathered at 3.85-ms sampling intervals, acquired from 128 × 128 sites simultaneously over a 30 × 30 mm2 area in each aspect of the heart.18,19 For each optical recording, data were acquired continuously for 3.85 seconds. To minimize motion artifacts, cytochalasin D (5 μM), an excitation-contraction uncoupler, was used.18,19 In a typical time-embedded phase portrait, the upstroke of the action potential corresponds to a phase ranging from -3/4 π to -1/4 π, represented by a light blue color (between dark blue and green).18,21
Induction of hypothermia (33 °C, 30 °C) and Rewarming (37 °C)
Two thermostatic systems were connected parallel to the Langendorff system.14,23 By controlling a switch between these two thermostatic systems, the temperature of perfusate and tissue bath could be switched to either 37 °C or TH (33 °C or 30 °C).14,23 To induce MH (33 °C), the thermostatic system was set at 33 °C. The superfusate was also quickly replaced with 33 °C Tyrode’s solution. When the tissue bath temperature reached 33 °C, an additional 5 min (stabilized at 33 °C) was used to ensure the homogeneity of tissue temperature, and thereafter the study protocol was started. The method to induce SH (30 °C) was the same, except that the thermostatic temperature was set at 30 °C. To re-warm (37 °C) the heart, the procedures were reversed.14,23
Study protocols
Protocol I: S1 pacing at baseline (37 °C), 5-min moderate hypothermia (MH, 33 °C), and 5-min rewarming (37 °C) (n = 7 hearts)
S1 pacing (2 × diastolic threshold) was used to determine the APD/conduction velocity (CV) restitutions, cardiac alternans properties, and vulnerability of PIVF at baseline, 5-min MH, and 5-min rewarming.14 APD and CV restitutions were determined using 11 different S1 pacing cycle lengths (PCLs: 400, 350, 300, 250, 200, 180, 160, 150, 140, 130 and 120 ms).14 For each pacing cycle length (PCL), an S1 pacing train was delivered for 15 seconds, and optical data were recorded at the end of the pacing train. If VF was induced and persisted for > 1 min after stopping S1 pacing, a defibrillation shock was delivered through a defibrillation coil.14,24 In this study, we used a fixed high-energy shock of 200 volts to ensure successful defibrillation for each VF episode. If a VF was induced and a defibrillation shock was given, the heart was allowed to rest for 3 min. Thereafter, the study protocol was continued.
Protocol II: S1 pacing at baseline (37 °C), 5-min severe hypothermia (SH, 30 °C), and 5-min rewarming (37 °C) (n = 9 hearts)
S1 pacing was used to determine the APD/CV restitutions, cardiac alternans properties, and vulnerability of PIVF at baseline, 5-min SH, and 5-min rewarming. This protocol was essentially the same as for protocol I, except that a SH was implemented instead of MH.
Data analysis
Construction of APD and CV restitution curves using S1 pacing method
The method of constructing APD and CV restitution curves has been reported elsewhere.20,21 Briefly, pixels at the center of the anterior and posterior surfaces of both ventricles (sites a through d, see Figure 1A) were selected to determine the APD70 (APD at 70% repolarization). APD restitution (APDR) curves of each heart were sampled and plotted from the 4 sites against different S1 PCLs.14 When APD alternans occurred during S1 pacing, the short and long APD70 were averaged.14 The epicardial CV was evaluated by dividing the distance between 2 epicardial points with the conduction time using depolarization isochronal maps (see Figure 1B, C). With the formula WL (wavelength, cm) = APD70 × CV, WL restitutions were obtained. The maximal slope of APDR for each heart is the mean of the maximal APDR slopes of the 4 sampling sites.14,21 “Maximal CV reduction”, which was defined as the difference of CV at the longest and the shortest S1 PCLs, was used to estimate the CV restitution (CVR, cm/s) at each sites.14,21 Spatial heterogeneity of CVRs was evaluated by comparing the differences in “Maximal CV reduction” among these 4 epicardial lines (see Figure 1B, C).14,21 APD70 dispersion was defined as the difference between the maximal and minimal APD70 from the entire mapped areas at a PCL.
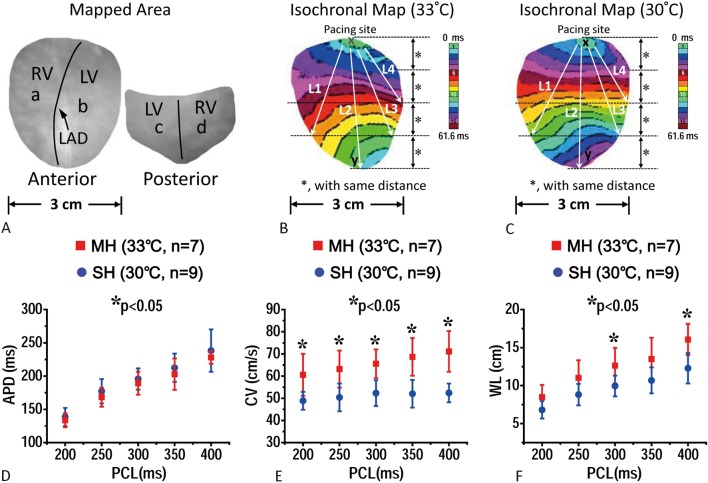
Figure 1.
(A) Optical mapped area of the hearts. Points a to d were areas for action potential duration (APD) restitution evaluation. LAD, left anterior descending artery; RV, right ventricle; LV, left ventricle. (B and C) isochronal maps of the hearts subjected to moderate hypothermia (MH, 33 °C) (B) and severe hypothermia (SH, 30 °C) (C). L1-4 lines were used for conduction velocity (CV) evaluation. D-F, effects of MH and SH on APD (D), CV (E), and wavelength (WL) (F) restitutions. PCL, pacing cycle length.
APD alternans and during S1 pacing
APD alternans was defined as a difference in APD70 on 2 consecutive beats of ≥ 3.85 ms during S1 pacing.9,14 The alternans threshold was defined as the longest S1 PCL at which APD alternans was detected.9,11,14,25 Spatially concordant alternans (SCA) was defined as APD alternation in phase spatially (i.e., for a given beat, the APD is either long or short everywhere in the tissue), whereas SDA was defined as alternation out of phase spatially (i.e., some regions of tissue alternate in a long-short-long pattern, while other regions simultaneously alternate in a short-long-short pattern).8,9,14 Therefore, the SDA threshold was defined as the longest S1 PCL at which SDA was detected.14
To determine the presence of SCA or SDA during APD alternans, APD difference maps were created by the difference in APD70 between 2 consecutive beats.14 APD difference maps appeared red if the differences were positive, and green if negative.9,14 Thus, during SCA, APD difference maps appeared all green on one beat and all red on the next beat. During SDA, red and green regions alternated, separated by a nodal line in which no alternans was present.14 By analyzing APD difference maps during SDA, the average numbers of nodal lines at each PCL were obtained.
Statistical analysis
Data are presented as mean ± SD. Wilcoxon rank sum test and Mann-Whitney U test were used to compare the data within and between groups. We used the analysis of variance (ANOVA) test to compare the means of three or more groups. Chi-square analysis was used to compare categorical data between and within groups. A probability value of p ≤ 0.05 was considered significant.
RESULTS
Effects of MH (33 °C) and SH (30 °C) on APD, CV, and WL restitutions
Effects of moderate hypothermia (MH, 33 °C) and severe hypothermia (SH, 30 °C) on APD, CV, and wavelength (WL) restitutions are summarized in Table 1 (MH) and Table 2 (SH). During MH and SH, APD70 increased, while CV decreased compared with the baseline levels (Table 1, Table 2). During MH, the WL was prolonged at the long PCL of 400 ms, while no changes of WL were observed at PCL ≤ 350 ms (Table 1). During SH, the WL was similar to the baseline levels at PCL ≥ 250 ms, while WL was shorter than baseline level at short PCL of ≤ 200 ms (Table 2). APD70, CV, and WL restitutions at rewarming were all similar to those at baseline. The APD70 during MH was not statistically different from those during SH at all PCLs (Figure 1A). However, CV during SH was significantly lower than that during MH (Figure 1B). The WL during MH was longer than those during SH at PCL of 400 (p = 0.02) and 300 ms (p = 0.03) (Figure 1C).
Table 1. Effects of moderate hypothermia (MH, 33 °C) on APD70, CV, and WL (n = 7).
| S1 PCL, ms | |||||||||
| 400 | 350 | 300 | 250 | 200 | 180 | 160 | 150 | 140 | |
| APD70, ms | |||||||||
| Baseline, 37 °C | 167 ± 8 | 162 ± 11 | 157 ± 8 | 148 ± 6 | 134 ± 6 | 123 ± 40 | 113 ± 4 | 107 ± 4 | 101 ± 3 |
| MH, 33 °C | 228 ± 10‡ | 203 ± 24‡ | 189 ± 17‡ | 168 ± 14‡ | 134 ± 11 | 124# | NA | NA | NA |
| Rewarming, 37 °C | 168 ± 9 | 166 ± 9 | 161 ± 6 | 149 ± 4 | 134 ± 10 | 122 ± 7 | 110 ± 8 | 100 ± 11 | 98 ± 8 |
| CV, cm/s | |||||||||
| Baseline, 37 °C | 79 ± 6 | 78 ± 6 | 76 ± 8 | 76 ± 7 | 74 ± 8 | 74 ± 7 | 72 ± 8 | 70 ± 9 | 68 ± 8 |
| MH, 33 °C | 71 ± 9‡ | 69 ± 9‡ | 66 ± 6‡ | 63 ± 8‡ | 61 ± 9‡ | 56# | NA | NA | NA |
| Rewarming, 37 °C | 79 ± 5 | 77 ± 6 | 76 ± 7 | 73 ± 7 | 71 ± 7 | 69 ± 8 | 69 ± 8 | 68 ± 7 | 68 ± 5 |
| WL, cm | |||||||||
| Baseline, 37 °C | 12.6 ± 2.1 | 12.2 ± 1.9 | 12.0 ± 1.7 | 11.3 ± 1.5 | 10.0 ± 1.4 | 9.2 ± 1.2 | 8.1 ± 1.1 | 7.5 ± 1.2 | 6.9 ± 0.9 |
| MH, 33 °C | 16.1 ± 2.0‡ | 13.5 ± 2.8 | 12.6 ± 2.3 | 11.0 ± 2.3 | 8.5 ± 1.6 | 6.2# | NA | NA | NA |
| Rewarming, 37 °C | 12.9 ± 1.3 | 12.6 ± 1.6 | 12.3 ± 1.5 | 11.0 ± 1.2 | 9.7 ± 1.2 | 8.6 ± 1.1 | 7.6 ± 1.1 | 6.6 ± 1.2 | 6.6 ± 0.9 |
APD, action potential duration; CV, conduction velocity; MH, moderate hypothermia; NA, not available; PCL, pacing cycle length; WL, wavelength.
* Data from hearts #2 and #4; # data from heart #4; † data from hearts #2, #3; ‡ p < 0.05, by Wilcoxon signed rank test when compared with baseline.
Table 2. Effects of severe hypothermia (SH, 30 °C) on APD70, CV, and WL (n = 9).
| S1 PCL, ms | |||||||||||
| 400 | 350 | 300 | 250 | 200 | 180 | 160 | 150 | 140 | 130 | 120 | |
| APD70, ms | |||||||||||
| Baseline, 37 °C | 161 ± 18 | 156 ± 15 | 154 ± 15 | 145 ± 13 | 129 ± 10 | 120 ± 8 | 109 ± 7 | 105 ± 9 | 95 ± 6 | 88 ± 9 | 84 ± 11* |
| SH, 30 °C | 238 ± 32‡ | 213 ± 21‡ | 196 ± 16‡ | 177 ± 18‡ | 139 ± 14 | 128 ± 16# | NA | NA | NA | NA | NA |
| Rewarming, 37 °C | 163 ± 13 | 155 ± 12 | 150 ± 11 | 141 ± 8 | 128 ± 7 | 120 ± 10 | 108 ± 10 | 100 ± 10 | 93 ± 11 | 85 ± 11 | 80 ± 10† |
| CV, cm/s | |||||||||||
| Baseline, 37 °C | 73 ± 3 | 72 ± 3 | 71 ± 3 | 71 ± 4 | 70 ± 3 | 69 ± 4 | 67 ± 4 | 66 ± 4 | 64 ± 4 | 61 ± 4 | 57 ± 5* |
| SH, 30 °C | 52 ± 4‡ | 52 ± 6‡ | 52 ± 6‡ | 50 ± 6‡ | 49 ± 4‡ | 45 ± 3# | NA | NA | NA | NA | NA |
| Rewarming, 37 °C | 72 ± 6 | 71 ± 7 | 70 ± 3 | 69 ± 6 | 68 ± 7 | 67 ± 5 | 65 ± 5 | 62 ± 4 | 58 ± 5 | 57 ± 5 | 53 ± 7† |
| WL, cm | |||||||||||
| Baseline, 37 °C | 11.6 ± 1.3 | 11.1 ± 1.2 | 10.9 ± 1.2 | 10.1 ± 0.8 | 9.0 ± 0.6 | 8.2 ± 0.6 | 7.1 ± 0.8 | 6.7 ± 0.8 | 5.9 ± 0.8 | 5.5 ± 0.8 | 4.9 ± 0.9* |
| SH, 30 °C | 12.3 ± 2.0 | 10.7 ± 1.7 | 10.0 ± 1.4 | 8.8 ± 1.4 | 6.8 ± 1.1‡ | 5.9 ± 1.0# | NA | NA | NA | NA | NA |
| Rewarming, 37 °C | 11.9 ± 1.4 | 11.6 ± 1.3 | 10.9 ± 1.4 | 10.1 ± 1.1 | 8.9 ± 1.2 | 8.0 ± 1.1 | 6.9 ± 1.1 | 6.1 ± 1.0 | 5.4 ± 1.0 | 4.9 ± 1.1 | 4.0 ± 1.0† |
APD, action potential duration; CV, conduction velocity; NA, not available; PCL, pacing cycle length; SH, severe hypothermia; WL, wavelength.
* Data from hearts #2, #3, #9; # data from hearts #1, #3, #4; † data from hearts #1-6; ‡ p < 0.05, by Wilcoxon signed rank test when compared with baseline.
Effects of MH (33 °C) and SH (30 °C) on APD dispersion and spatial heterogeneity of restitutions
APD dispersion
Figure 2 shows the APD70 dispersion at baseline, during hypothermia (MH, panel A; SH, panel B), and after rewarming. Hypothermia (MH and SH) increased the APD dispersion compared to those at baseline and rewarming (Figure 2). The APD dispersions during MH were similar to those during SH at these PCLs (i.e., at PCL 300 ms, MH: 36 ± 8 ms; SH: 40 ± 5 ms; p = 0.27).
Figure 2.
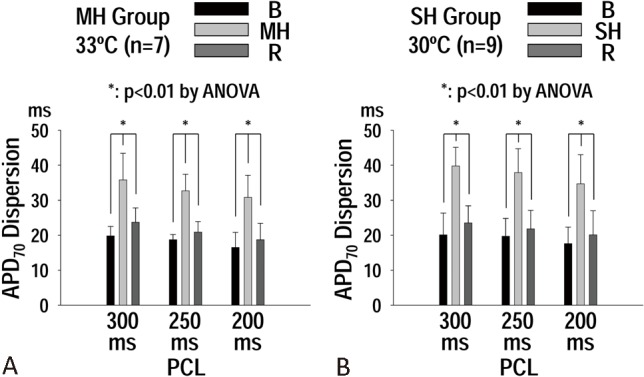
(A) Effect of moderate hypothermia (MH) on action potential duration (APD70) dispersions at S1 pacing cycle length (PCL) of 300, 250, and 200 ms. (B) effect of severe hypothermia (SH) on APD70 dispersions at S1 PCL of 300, 250, and 200 ms. Note that APD70 dispersion was increased with both MH and SH. p values were obtained by ANOVA test.
Maximal slope of APD restitutions and spatial heterogeneity of CV restitution
In the SH group, SH increased the maximal slope of APD restitution (APDR) curve from 1.60 ± 0.32 at baseline to 1.91 ± 0.23 during SH (p = 0.046) (Figure 3A). However, in the MH group, the maximal slope of the APDR curve was similar between baseline (1.65 ± 0.55) and during MH (1.38 ± 0.52) (p = 0.35). The maximal slope of APDR curve during SH (1.91 ± 0.23) was higher than that during MH (1.38 ± 0.52) (p = 0.029) (Figure 3A). The “Maximum CV reduction” along the 4 epicardial lines was significant in MH (16 ± 8, 15 ± 9, 7 ± 4, 8 ± 4 cm/s, lines 1-4, respectively, p = 0.026) and SH (13 ± 6, 7 ± 6, 5 ± 5, 6 ± 4 cm/s, lines 1-4, respectively, p = 0.016). The spatial heterogeneity of CV restitutions were not significant at baseline (MH group, p = 0.88; SH group, p = 0.37) and rewarming (MH group, p = 0.31; SH group, p = 0.31).
Figure 3.
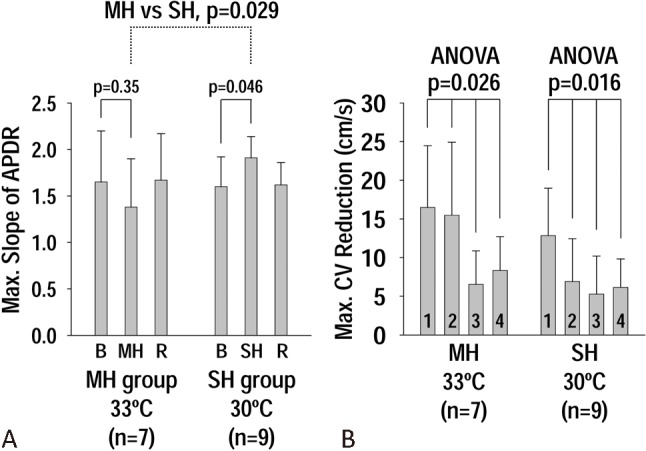
(A) Effects of moderate hypothermia (MH) and severe hypothermia (SH) on the maximal slope of action potential duration (APD) restitutions. Note that the maximal slope at SH is higher than that at MH. (B) effects of MH and SH on the spatial heterogeneity of conduction velocity (CV) restitutions. The heterogeneity is significant (p < 0.05) with both MH and SH.
Effects of MH (33 °C) and SH (30 °C) on the threshold of APD alternans and spatially discordant alternans
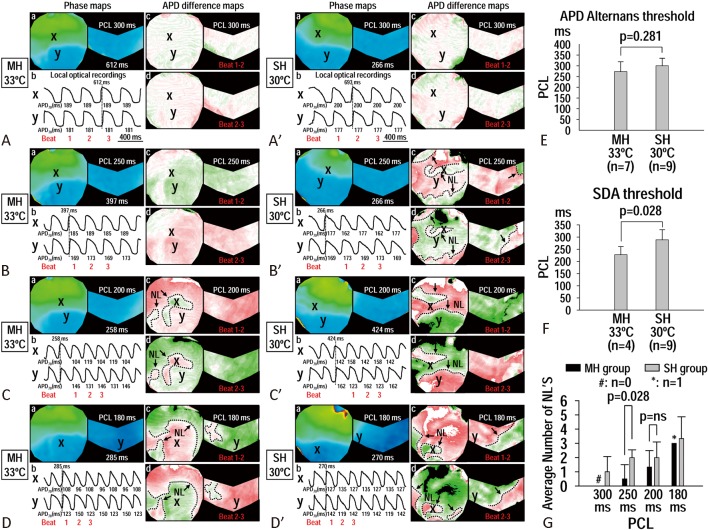
Figure 4 is an example showing the effects of MH and SH on the APD alternans property (Figure 4A-D, A′-D′). Although the thresholds of APD alternans were not different between MH (273 ± 46 ms) and SH (300 ± 35 ms) (p = 0.281) (Figure 4E), spatially discordant alternans (SDA) threshold during MH (228 ± 33 ms) was shorter (SDA occurs at faster heart rate) than that during SH (289 ± 42 ms) (p = 0.028) (Figure 4F). The number of nodal lines during MH (0.5 ± 1.0) was also lower than that during SH (2.0 ± 0.5) (p = 0.028) (Figure 4G).
Figure 4.
Effects of moderate hypothermia (MH) (A-D, heart #4 in MH group) and severe hypothermia (SH) (A′-D′, heart #3 in SH group) on action potential duration (APD) alternans by S1 pacing. During MH at pacing cycle length (PCL) of 300 ms, no APD alternans was observed (A). As the PCL decreased to 250 ms, APD alternans was observed (Ba-b), and the APD difference map showed spatially concordant alternans (SCA) (Bc-d). At 200 ms PCL (C), the APD alternans transformed to spatially discordant alternans (SDA) (Cb-d) with nodal lines formation (dash lines in Cc-d). As the PCL further decreased to 180 ms, SDA persisted, and the APD difference map became more complicated (Db-d). During SH at PCL of 300 ms, no APD alternans was observed (A′). As the PCL decreased to 250 ms, APD alternans was observed (B′a-b), and the APD difference map showed SDA with nodal lines formation (B′c-d). When the PCL decreased to 200 ms (C′) and 180 (D′) ms, SDA (C′c-d, D′c-d) persisted. (E) Effects of MH and SH on the threshold of APD alternans. F, effects of MH and SH on the threshold of SDA. G, effects of MH and SH on the number of nodal lines during SDA.
In the MH group at APD alternans threshold, the number of hearts with SDA was not increased by MH [1 out of the 7 MH hearts had SDA at baseline (B), 2 out of the 7 MH hearts had SDA at MH, and 1 out of the 7 MH hearts had SDA at rewarming (R), p = ns] (Figure 5A). However, in SH group at APD alternans threshold, SH significantly increased the number of hearts with SDA [7 out of the 9 SH hearts showed SDA at SH, while 0 and 1 out of the 9 SH hearts showed SDA at baseline (B) and rewarming (R), respectively, p < 0.001] (Figure 5A). Therefore, SH significantly increased the number of hearts with SDA compared with that achieved by MH at the APD alternans threshold (p = 0.049). Figure 5B showed the number of hearts with inducible SDA when all PCLs were included. With all PCLs included, SDA could be induced in 9 out of the 9 hearts (100%) during SH, while SDA was only observed in 4 out of the 7 hearts (57%) during MH (p = 0.029) (Figure 5B).
Figure 5.
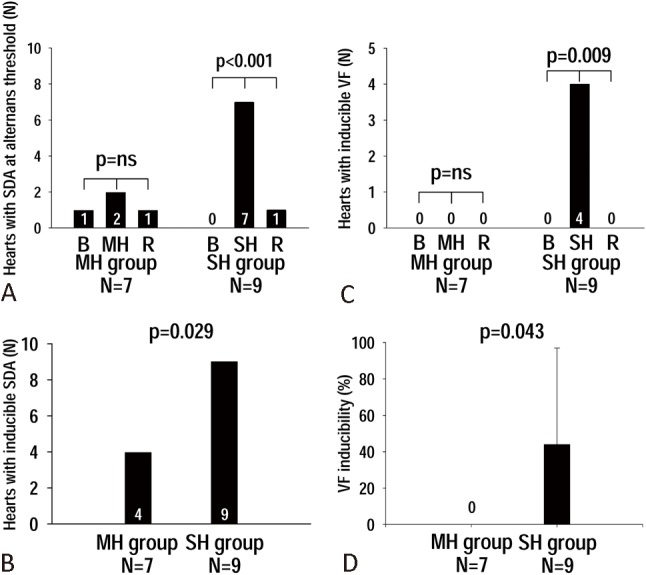
(A) The absolute heart number (N) with spatially discordant alternans (SDA) at action potential duration (APD) alternans threshold at baseline (B), during moderate hypothermia (MH) and severe hypothermia (SH), and at rewarming (R). (B) The number of hearts with inducible SDA throughout all pacing cycle lengths (PCLs) during MH and SH. (C) The number of hearts with inducible ventricular fibrillation (VF) at baseline (B), hypothermia (MH and SH), and rewarming (R). (D) The percentage of VF inducibility during MH and SH.
Spontaneous and pacing-induced VF during MH and SH
No spontaneous VF was observed during MH. However, we observed 1 episode of spontaneous VF at 2-min SH in SH group (Heart #7). This VF episode was successfully defibrillated by a 200-volt shock.
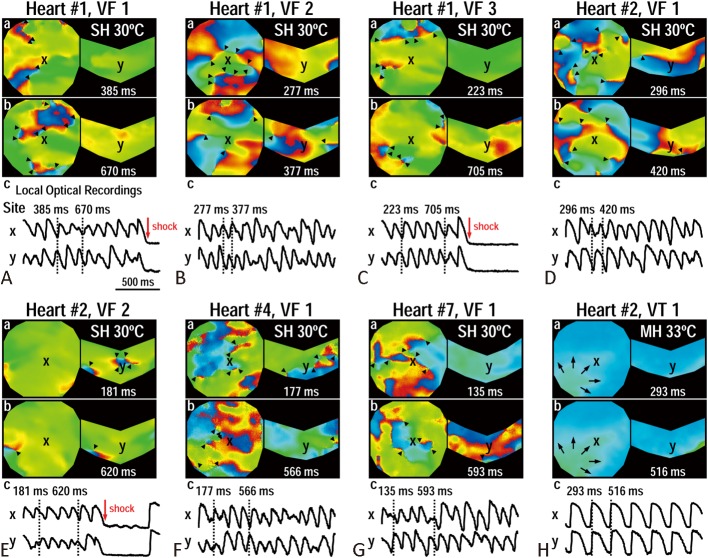
In the MH group, no VF was inducible at baseline (B), during MH, or at rewarming (R) (Figure 5C). Only 1 ventricular tachycardia (VT) episode was induced during MH. In the SH group, 7 pacing-induced VF episodes were observed in 4 out of the 9 SH hearts during SH, while no VF was inducible at baseline (B) or rewarming (R) (p = 0.009) (Figure 5C). The percentage of VF inducibility was also higher during SH (44 ± 53%) than that during MH (0%) (p = 0.043) (Figure 5D). Figure 6 shows the 7 VF episodes induced during SH (Figure 6A-G), and the only VT episode (Figure 6H) during MH. The successful rate of electric defibrillation was 100%. The VF wavefronts during SH (Figure 6A-G) showed multiple wavebreaks indicating an incessant character.
Figure 6.
Ventricular arrhythmias induced by S1 pacing during severe hypothermia (SH) (A-G) and moderate hypothermia (MH) (H). (A-C) Ventricular fibrillation (VF) episodes are from heart #1 in SH group. The VF episodes were induced by S1 pacing cycle length (PCL) of 200 (A), 180 (B), and 160 (C) ms, respectively. (D-E) VF episodes are from heart #2 in SH group, induced by S1 PCL of 200 (D) and 180 (E) ms. (F and G) VF episodes are from hearts #4, #7, respectively, in SH group. Both VF episodes were induced by PCL 180 ms. H, ventricular tachycardia (VT) episode is from heart #2 in MH group. This VT episode was induced by S1 PCL of 250 ms. Black triangles indicate phase singularities (a surrogate of wavebreaks) during VF.
DISCUSSION
The major findings of this study were as follows: (1) SDA threshold during MH was shorter (SDA at a faster heart rate) than that during SH, although the APD alternans thresholds were comparable; (2) Fewer MH hearts showed SDA than SH hearts at alternans threshold and when all PCLs were included; (3) Pro-arrhythmic parameters including CV slowing, maximal slope of APDR curve, and propagating WL were attenuated by MH than by SH, suggesting the role of an anti-arrhythmic substrate in MH; (4) The decreased SDA occurrence with MH was associated with a decrease in VF inducibility compared with that seen with SH. These findings indicate that MH decreases PIVF by attenuating the occurrence of SDA, and is a safer temperature than SH.
Myocardial substrate during MH (33 °C) and SH (30 °C)
In non-hibernating mammal hearts, including human, the heart usually goes into VF and cardiac arrest under extreme hypothermia (< 30 °C), suggesting that hypothermia creates an arrhythmogenic substrate for VF.3,4 This notion is supported by our previous study which showed that SH at 30 °C increases susceptibility to PIVF by enhancing conduction heterogeneity, increasing APD dispersion, and promoting wavebreaks of fibrillation conduction.14 However, clinical data showed that the incidence of ventricular arrhythmia was relatively low (~2.2%) in VF cardiac arrest patients undergoing TH at 32-34 °C.5,6 The mechanism underlying the weaker association of MH with ventricular arrhythmia and the stronger association of SH with ventricular arrhythmia remains unclear. Piktel et al. in a canine left ventricular preparation showed that severe hypothermia at 26 °C increased the transmural DOR and ventricular arrhythmia, whereas TH at 32 °C mildly increased the DOR and decreased ventricular arrhythmia.5 Harada et al. also showed that SH (30 °C) enhanced wavebreaks and regeneration of new spiral waves during VF favoring the maintenance of VF in 2-dimensional ventricular tissue.7 While using MH at 33 °C, the spiral waves of VF frequently collide and dissipate, leading to self-termination of VF.7 In the present study, we observed that APD dispersion over the entire epicardial surface was enhanced in both MH and SH compared with that at baseline (37 °C), which was similar to Piktel’s findings on transmural APD dispersion.5 However, we found that the pro-arrhythmic parameters including CV slowing, maximal slope of APDR curve, and propagating WL showed greater attenuation by MH than by SH, suggesting the role of an anti-arrhythmic substrate with MH. These changes in myocardial substrate with MH were associated with reduced VF episodes. Taken together, the myocardial substrate appears to be differentially remodeled depending on the degree of hypothermia. MH attenuates pro-arrhythmic parameters and hinders VF maintenance to a greater extent than SH. In fact, we observed that the susceptibility to PIVF in MH showed no differences among baseline (37 °C), MH, and rewarming (37 °C), indicating that MH is as safe as normothermia and rewarming. This finding might also explain the relatively low incidence of ventricular arrhythmia observed in post-resuscitation patients undergoing MH.
Cardiac alternans and PIVF during MH (33 °C) and SH (30 °C)
Cardiac alternans has been reported to be a good marker in predicting sudden cardiac death in clinical patients and experimental arrhythmic situation, including myocardial ischemia, heart failure, and hypothermia.14,26,27 As cardiac alternans occurs, it may manifest as either SDA or SCA, separated by a nodal line where no alternans is observed.8-10 SDA can facilitate the genesis of reentry arrhythmia at the nodal line where the spatial APD gradient is the steepest, and more arrhythmogenic than SCA.11-13 We have recently reported that SH (30 °C) increases susceptibility to PIVF by promoting the onset of SDA.14 These SDA episodes during SH always precede the onset of VF (see Figure 5B in reference 14),14 suggesting that SDA plays an important role in transforming SDA to VF during SH.14 This hypothesis is supported by results reported by Egorov et al. showing that hibernating mammals are more resistant to VF formation during SH than non-hibernating animals (i.e., human, rabbit) by impeding SDA occrrence.28 In this study, we found that MH decreased the PCL needed to induce SDA (SDA occurs at a faster heart rate), reduced the number of hearts with SDA, and decreased the number of pro-arrhythmic nodal lines, suggesting that MH is more resistant to SDA than SH. The decreased SDA episodes during MH were associated with decreased VF episodes. In fact, no VF (only 1 VT episode) was induced during MH in this study.
The mechanism of greater attenuation of SDA onset by MH than by SH remains unclear. Dynamic changes in both APD and CV restitution contribute to the formation of SDA. A steep APD restitution curve (maximal slope > 1) and CV restitution curve (due to incomplete Na channel recovery) are essential for converting SCA to SDA.8,28,29 That is, as the amplitude of APD alternans grows large by rapid pacing, it can engage the sloped region of CV restitution curve and converts SCA into SDA.8,28,29 Hypothermia has been reported to delay recovery of Na channels from inactivation in a temperature-dependent manner, and to increase the steepness of CV alternans.30,31 In this study, in addition to the decreased inactivation of Na channels (less CV slowing) with MH, MH reduces the maximal slope of APD restitution to a greater extent than SH. The attenuation of CV and APD restitution by MH might contribute to less SDA and reduced subsequent likelihood of ventricular arrhythmia.
Intracellular calcium (Cai) overload resulting from either increased calcium leakage from sarcoplasmic reticulum (SR) or reduced calcium re-uptake by SR Ca2+ ATPase (SERCA) might cause Cai to alternate and convert SCA to SDA.8 Hypothermia has been reported to increase the open probability of cardiac SR Ca-release channels and reduces SERCA activity, favoring the occurrence of SDA.32,33 The effect of calcium dynamics in mediating SDA occurrence was not explored in this study. Further study is needed to elucidate the role of calcium dynamics in SDA during hypothermia.
Structural remodeling and cardiac alternans during MH and SH
SDA has been reported during ischemia, in which cell-to-cell coupling is impaired, suggesting that inter-cellular uncoupling is an important mechanism of SDA.25,34 In mammals, myocardial cell-to-cell coupling is mediated by gap junction (GJ) constructed from connexin 43 (Cx43) proteins.23 We have previously found that hypothermia caused a prompt structural remodeling in Cx43 GJ.23 Furthermore, SH hearts showed a greater extent in total-Cx43 lateralization (anatomical remodeling) and non-phosphorylated Cx43 downregulation (biochemical remodeling), compared to MH hearts.23 The differences in structural remodeling between MH and SH might contribute to the different alternans properties with different temperature. However, the individual impact of hypothermia-induced GJ remodeling to cardiac alternans properties remains to be explored.
Limitation
In this model, cardiac alternans properties were evaluated with short-duration (5 min) MH or SH. Whether a longer duration (12-24 h) of TH (compliant with clinical guidelines) might lead to a greater difference in cardiac alternans properties warrants further investigation. Cai overload might drive Cai to alternate and convert SCA to SDA, leading to VF formation. Additionally, we did not map Cai in this study. Further study is needed to determine whether Ca dynamics contribute to SDA and subsequent VF in MH and SH.
CONCLUSIONS
Compared with SH, MH decreases susceptibility to PIVF by attenuating the occurrence of SDA. The favorable electrophysiological properties of MH, such as the reduced likelihood of inducing SDA, indicate that MH is safer for clinical TH than SH.
Acknowledgments
This study was supported by grants from the National Science Council (100-2314-B-075A-008-MY3 and 102-2314-B-075A-009-MY2), Taipei, Taiwan; Taichung Veterans General Hospital (TCVGH-1013107C, TCVGH-1023106C, TCVGH-1033103C and TCVGH-1033105C), Taichung, Taiwan; and National Chiao Tung University (VGHUST103-G5-5-2), Hsinchu, Taiwan.
REFERENCES
- 1.Nolan JP, Morley PT, Vanden Hoek TL, et al. Therapeutic hypothermia after cardiac arrest:an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;108:118–121. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 2.Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care:2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S768–S786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 3.Mouritzen CV, Andersen MN. Mechanisms of ventricular fibrillation during hypothermia. Relative changes in myocardial refractory period and conduction velocity. J Thorac Cardiovasc Surg. 1966;51:579–584. [PubMed] [Google Scholar]
- 4.Badeer H. Ventricular fibrillation in hypothermia; a review of factors favoring fibrillation in hypothermia with and without cardiac surgery. J Thorac Surg. 1958;35:265–273. [PubMed] [Google Scholar]
- 5.Piktel JS, Jeyaraj D, Said TH, et al. Enhanced dispersion of repolarization explains increased arrhythmogenesis in severe versus therapeutic hypothermia. Circ Arrhythm Electrophysiol. 2011;4:79–86. doi: 10.1161/CIRCEP.110.958355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 7.Harada M, Honjo H, Yamazaki M, et al. Moderate hypothermia increases the chance of spiral wave collision in favor of self-termination of ventricular tachycardia/fibrillation. Am J Physiol Heart Circ Physiol. 2008;294:H1896–H1905. doi: 10.1152/ajpheart.00986.2007. [DOI] [PubMed] [Google Scholar]
- 8.Weiss JN, Karma A, Shiferaw Y, et al. From pulsus to pulseless:the saga of cardiac alternans. Circ Res. 2006;98:1244–1253. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 9.de Diego C, Pai RK, Dave AS, et al. Spatially discordant alternans in cardiomyocyte monolayers. Am J Physiol Heart Circ Physiol. 2008;294:H1417–H1425. doi: 10.1152/ajpheart.01233.2007. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi H, Shiferaw Y, Sato D, et al. Dynamic origin of spatially discordant alternans in cardiac tissue. Biophys J. 2007;92:448–460. doi: 10.1529/biophysj.106.091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pastore JM, Girouard SD, Laurita KR, et al. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–1394. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
- 12.Qu Z, Garfinkel A, Chen PS, Weiss JN. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation. 2000;102:1664–1670. doi: 10.1161/01.cir.102.14.1664. [DOI] [PubMed] [Google Scholar]
- 13.Choi BR, Jang W, Salama G. Spatially discordant voltage alternans cause wavebreaks in ventricular fibrillation. Heart Rhythm. 2007;4:1057–1068. doi: 10.1016/j.hrthm.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh YC, Lin SF, Lin TC, et al. Therapeutic hypothermia (30 degrees C) enhances arrhythmogenic substrates,including spatially discordant alternans,and facilitates pacing-induced ventricular fibrillation in isolated rabbit hearts. Circ J. 2009;73:2214–2222. doi: 10.1253/circj.cj-09-0432. [DOI] [PubMed] [Google Scholar]
- 15.Marion DW, Penrod LE, Kelsey SF, et al. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336:540–546. doi: 10.1056/NEJM199702203360803. [DOI] [PubMed] [Google Scholar]
- 16.Hale SL, Kloner RA. Myocardial hypothermia: a potential therapeutic technique for acute regional myocardial ischemia. J Cardiovasc Electrophysiol. 1999;10:405–413. doi: 10.1111/j.1540-8167.1999.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 17.Clifton GL, Jiang JY, Lyeth BG, et al. Marked protection by moderate hypothermia after experimental traumatic brain injury. J Cereb Blood Flow Metab. 1991;11:114–121. doi: 10.1038/jcbfm.1991.13. [DOI] [PubMed] [Google Scholar]
- 18.Wu TJ, Lin SF, Hsieh YC, et al. Early recurrence of ventricular fibrillation after successful defibrillation during prolonged global ischemia in isolated rabbit hearts. J Cardiovasc Electrophysiol. 2008;19:203–210. doi: 10.1111/j.1540-8167.2007.00979.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu TJ, Lin SF, Hsieh YC, et al. Ventricular fibrillation during no-flow global ischemia in isolated rabbit hearts. J Cardiovasc Electrophysiol. 2006;17:1112–1120. doi: 10.1111/j.1540-8167.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh YC, Horng TL, Lin SF, et al. d,l-sotalol at therapeutic concentrations facilitates the occurrence of long-lasting non-stationary reentry during ventricular fibrillation in isolated rabbit hearts. Circ J. 2009;73:39–47. doi: 10.1253/circj.cj-08-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu TJ, Lin SF, Weiss JN, et al. Two types of ventricular fibrillation in isolated rabbit hearts: importance of excitability and action potential duration restitution. Circulation. 2002;106:1859–1866. doi: 10.1161/01.cir.0000031334.49170.fb. [DOI] [PubMed] [Google Scholar]
- 22.Wu TJ, Lin SF, Baher A, et al. Mother rotors and the mechanisms of D600-induced type 2 ventricular fibrillation. Circulation. 2004;110:2110–2118. doi: 10.1161/01.CIR.0000143834.51102.91. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh YC, Yeh HI, Lin SF, et al. Short-duration therapeutic hypothermia causes prompt connexin 43 gap junction remodeling in isolated rabbit hearts. Circ J. 2011;75:1706–1716. doi: 10.1253/circj.cj-10-1001. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh YC, Chang PC, Hsueh CH, et al. Apamin-sensitive potassium current modulates action potential duration restitution and arrhythmogenesis of failing rabbit ventricles. Circ Arrhythm Electrophysiol. 2013;6:410–418. doi: 10.1161/CIRCEP.111.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pastore JM, Rosenbaum DS. Role of structural barriers in the mechanism of alternans-induced reentry. Circ Res. 2000;87:1157–1163. doi: 10.1161/01.res.87.12.1157. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum DS, Jackson LE, Smith JM, et al. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330:235–241. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- 27.Kjolbye AL, Dikshteyn M, Eloff BC, et al. Maintenance of intercellular coupling by the antiarrhythmic peptide rotigaptide suppresses arrhythmogenic discordant alternans. Am J Physiol Heart Circ Physiol. 2008;294:H41–H49. doi: 10.1152/ajpheart.01089.2006. [DOI] [PubMed] [Google Scholar]
- 28.Egorov YV, Glukhov AV, Efimov IR, Rosenshtraukh LV. Hypothermia-induced spatially discordant action potential duration alternans and arrhythmogenesis in nonhibernating versus hibernating mammals. Am J Physiol Heart Circ Physiol. 2012;303:H1035–H1046. doi: 10.1152/ajpheart.00786.2011. [DOI] [PubMed] [Google Scholar]
- 29.Nolasco JB, Dahlen RW. A graphic method for the study of alternation in cardiac action potentials. J Appl Physiol. 1968;25:191–196. doi: 10.1152/jappl.1968.25.2.191. [DOI] [PubMed] [Google Scholar]
- 30.Dudel J, Rüdel R. Voltage and time dependence of excitatory sodium current in cooled sheep Purkinje fibres. Pflugers Arch. 1970;315:136–158. doi: 10.1007/BF00586657. [DOI] [PubMed] [Google Scholar]
- 31.Liu B, Arlock P, Wohlfart B, Johansson BW. Temperature effects on the Na and Ca currents in rat and hedgehog ventricular muscle. Cryobiology. 1991;28:96–104. doi: 10.1016/0011-2240(91)90011-c. [DOI] [PubMed] [Google Scholar]
- 32.Sitsapesan R, Montgomery RA, MacLeod KT, Williams AJ. Sheep cardiac sarcoplasmic reticulum calcium-release channels: modification of conductance and gating by temperature. J Physiol. 1991;434:469–488. doi: 10.1113/jphysiol.1991.sp018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puglisi JL, Bassani RA, Bassani JW, et al. Temperature and relative contributions of Ca transport systems in cardiac myocyte relaxation. Am J Physiol. 1996;270:H1772–H1778. doi: 10.1152/ajpheart.1996.270.5.H1772. [DOI] [PubMed] [Google Scholar]
- 34.Dekker LR, Rademaker H, Vermeulen JT, et al. Cellular uncoupling during ischemia in hypertrophied and failing rabbit ventricular myocardium:effects of preconditioning. Circulation. 1998;97:1724–1730. doi: 10.1161/01.cir.97.17.1724. [DOI] [PubMed] [Google Scholar]