Abstract
K+-Cl− cotransporters (KCCs) were originally characterized as regulators of red blood cell (RBC) volume. Since then, four distinct KCCs have been cloned, and their importance for volume regulation has been demonstrated in other cell types. Genetic models of certain KCCs, such as KCC3, and their inhibitory WNK-STE20/SPS1-related proline/alanine-rich kinase (SPAK) serine-threonine kinases, have demonstrated the evolutionary necessity of these molecules for nervous system cell volume regulation, structure, and function, and their involvement in neurological disease. The recent characterization of a swelling-activated dephosphorylation mechanism that potently stimulates the KCCs has pinpointed a potentially druggable switch of KCC activity. An improved understanding of WNK/SPAK-mediated KCC cell volume regulation in the nervous system might reveal novel avenues for the treatment of multiple neurological diseases.
Keywords: KCC2, KCC3, NKCC1, WNK-SPAK/OSR1, cell volume, cerebral edema
Cation-Cl− cotransporters in the brain: beyond NKCC1 and KCC2
Cation-chloride cotransporters (CCCs) are among the most medically relevant ion transporters in the human genome. Multiple members of this family [1], and their upstream regulators [i.e., the Kelch-like family member 3 (KLHL3)/Cullin 3 (CUL3)-WNK-SPAK/Oxidative stress response 1 (OSR1) kinase signaling pathway [2]], are mutated in human Mendelian disorders featuring brain or renal phenotypes resulting from impaired ion homeostasis. CCCs are also targets of several commonly used drugs utilized in clinical medicine, including hydrochlorothiazide, furosemide, and bumetanide [3]. CCCs are evolutionarily ancient, highly regulated secondary-active transporters utilizing cellular Na+ and/or K+ gradients to drive the transport of Cl− into, or out of, cells [4]. The N(K)CCs (including NCC and NKCC1/2) generally mediate net Cl− influx, whereas the four different K+-Cl− cotransporters (KCC1-4) mediate Cl− efflux in most physiological conditions [5].
Recent attention [6,7] has focused on the role of NKCC1 and KCC2 in the central nervous system (CNS) [8,9]. A postnatal upregulation of KCC2 activity is essential for establishing and maintaining the low intracellular concentration of Cl− ([Cl−]i) required for GABAA receptor (GABAAR)- and glycine receptor (GlyR)-mediated synaptic inhibition [6]. This so-called GABA ‘excitatory-inhibitory developmental sequence’ is dysregulated in multiple neurodevelopmental (e.g., autism [10]), psychiatric (e.g., schizophrenia [11] and anxiety [12]), and neurological diseases (e.g., neonatal seizures [13], spasticity [14], ammonia-induced myoclonic seizures [15], and neuropathic pain [16,17]). Given the role of NKCC1 and KCC2 in modulating inhibition, investigators and pharmaceutical companies are motivated to discover NKCC1 inhibitors and KCC2 activators that exhibit greater specificity and enhanced potency to facilitate neuronal Cl− extrusion for therapeutic benefit in these and other conditions [7].
In the context of the recent excitement surrounding KCC2 in the nervous system, it is interesting that K+-Cl− cotransport was first characterized as an N-ethylmaleimide (NEM)-sensitive, hypotonically activated, Cl−-driven, K+ efflux mechanism in human RBCs responsible for mediating regulatory volume decrease (RVD) to maintain cell volume in response to experimentally induced hypotonic cell swelling [18]. The so-called classical ‘volume-regulated’ or ‘swelling-activated’ KCCs that include KCC1/KCC3/KCC4, in contrast to KCC2 (which, however, is also activated by cell swelling [19]), are inactive in isotonic (nonswelling) conditions. Activation of K+-Cl− co-transport by NEM and hypotonicity-induced cell swelling had long been ascribed to the inhibition of protein kinase-mediated inhibitory phosphorylation, combined with the brisk activation of protein phosphatase 1 (PP1)-dependent dephosphorylation [20,21]. This mechanism of KCC swell activation has now been elucidated on the molecular level, and was shown to be mediated by inhibition of the Cl−-sensitive WNK kinases and their downstream substrate kinases (the SPAK/OSR1 kinases), which regulate the inhibitory phosphorylation of the KCCs on their C terminus [22].
Although swelling-sensitive K+-Cl− cotransport has been most extensively characterized in studies of RBC physiology and pathophysiology (reviewed in [23,24]), its role in cell volume regulation in the nervous system has not been explored in detail. This is surprising, because KCC3 and KCC4 are abundantly expressed in the nervous system [25–27], and knockouts of these KCCs and their kinase regulators exhibit striking phenotypes of the CNS and peripheral nervous system (PNS) due to dysregulated cell volume regulation [28–30]. Moreover, only mutations in KCC3, but not NKCC1 or KCC2, have been shown to cause a human Mendelian disease [Andermann syndrome, also known as agenesis of the corpus callosum with peripheral neuropathy (ACCPN); OMIM #218000] [31]. These firm genetic observations establish the necessity of the volume-regulated KCC pathway for proper development and function of the nervous system.
Here, we review KCC function in nervous system cell volume homeostasis by focusing on data derived from genetic models of the KCCs or their kinase regulators, and the physiological studies performed in these models. This subject is clinically relevant given the multiple neurological insults (e.g., trauma, tumor, infection, ischemic, and systemic electrolyte disturbances) that contribute to cerebral edema (i.e., ‘brain swelling’) [32]. Indeed, recent studies have uncovered a druggable kinase ‘switch’ controlling swell-regulated KCC activity that can activate the KCCs when pharmacologically inhibited [2,22]. Therefore, an improved understanding of the KCCs in nervous system cell volume regulation might provide novel treatment strategies for cerebral edema and other neurological disorders.
Cell volume regulation in the nervous system
Defense against significant changes in cell volume is required for cell function and survival [33]. Cell volume perturbations can result from changes in intracellular osmolarity (isosmotic volume stress) or extracellular osmotic pressure (anisosmotic volume stress). Isosmotic cell swelling, as occurs in cellular energetic failures such as ischemic stroke, results in increases in the intracellular concentration of Na+ ([Na+]i) and [Cl−]i. Anisosmotic cell swelling, as seen in acute hyponatremia from water intoxication or the syndrome of inappropriate antidiuretic hormone (SIADH), results in net water influx and decreases [Na+]i and [Cl−]i [6]. Both isosmotic and anisosmotic cell swelling in neurons, glia, and blood–brain barrier endothelial cells contribute to cerebral edema [34]. Due to the physical restriction imposed by the skull on the brain, cell volume regulation in cells of the nervous system is particularly important [35,36]. Brain swelling can cause increased intracranial pressure, uncal herniation, and brain death. Conversely, brain shrinkage, as seen in acute hypernatremia from dehydration or diabetes insipidus, can stretch cortical draining veins and cause extra-axial brain hemorrhage (i.e., subdural hematoma).
In neurons, transmembrane ionic gradients not only dictate osmotic pressure, but also impact membrane excitability. Therefore, volume changes in neurons resulting from extrinsic perturbation or intrinsic activity can affect intra- and extracellular ionic concentrations and have profound electrophysiological consequences [37,38]. In addition, cell volume changes in glial cells, such as astrocytes, which constitute the majority of brain mass and face constantly changing osmotic load stress as ‘sinks’ for K+ and other osmolytes, can significantly alter the physical and chemical properties of the extracellular space, and thereby indirectly alter the excitability of neurons. The differential responses of neurons and glia to isosmotic and anisosmotic perturbations reflect their distinct expression of ion transporters and aquaporin water channels (Box 1).
Box 1. Differential regulation of cell volume in neurons and glia.
Glia and neurons are differentially susceptible to isosmotic and anisosmotic perturbations, and use distinct mechanisms to maintain volume homeostasis. These differences are based on two key cellular properties: basal [Cl−]i and water permeability.
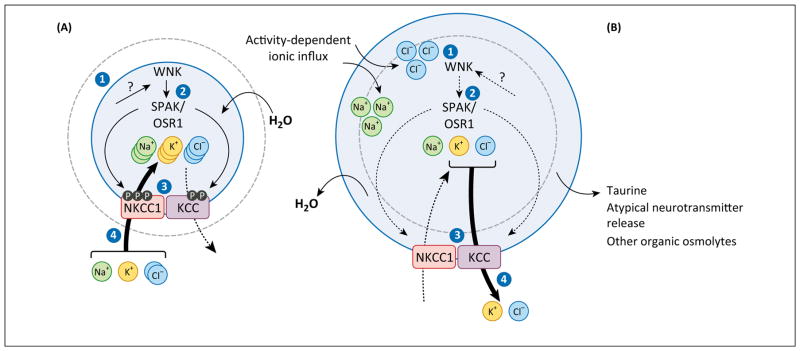
Cells of the nervous system differ in [Cl−]i owing to different expression patterns of KCCs and other Cl−-transporting molecules. Most mature CNS neurons have relatively low [Cl−]i (~4 mM, although with much variation) due to high activity of the Cl−-extruding KCC2 cotransporter, which is active in basal isosmotic conditions [19]. Low [Cl−]i is required for GABAAR- and GlyR-mediated hyper-polarizing synaptic inhibition, but limits the ability of mature neurons to utilize Cl−-dependent RVD to respond to cell swelling. Physiologic isosmotic challenges typically result from activity-dependent influx of ions, including Na+ and Cl−. This results in a transient increase in [Cl−]i that may enable KCC-mediated (Cl−-dependent) RVD in neurons under isosmotic stress. By contrast, CNS neurons under hyposmotic volume stress do not utilize KCC-mediated RVD due to their low [Cl−]i. Instead, these cells can utilize alternative pathways to decrease their cell volume, including organic anion efflux and atypical neuro-transmitter release [92]. Glia and neurons of the PNS have higher [Cl−]i due to elevated levels of NKCC1, absence of KCC2, and high expression of KCC3 (which is inhibited in isotonic conditions) [93,94]. As a consequence, these cells are more efficient in KCC3-mediated, Cl−-dependent volume regulation [95,96].
Glia and PNS neurons are highly permeable to water because they express aquaporins (AQPs) and, therefore, are sensitive to changes in transmembrane osmolyte gradients. By contrast, CNS neurons do not express AQPs at high levels [97] and, thus, may be relatively impermeable to water under normal physiologic conditions [98]. However, in states of oxygen and/or glucose deprivation, or prolonged depolarization associated with increased extracellular K+, CNS neurons become more permeable to water via a variety of mechanisms, including the import of hydration shells with influxing ions, cytoskeletal changes [99], and the opening of unidentified non-AQP water channels [100]. Interestingly, CCCs themselves have been shown to transport water [101], although this finding is contested. The significance of CCC-mediated water transport is unclear, but is more likely to have a role in cells that express KCCs at a higher density than AQPs, such as neurons.
The physiology of cell volume regulation as it applies to eukaryotic cells in general has been reviewed in detail elsewhere [33], as well as in the nervous system in particular, although not recently [39,40]. Briefly, to defend against damaging changes to their volume, cells undergo RVD or regulatory volume increase (RVI), in response to cell swelling and cell shrinkage, respectively [41] (Figure 1). In response to acute cell swelling, RVD involves the activation of ion channels (including the recently cloned SWELL1 channel, of which Lrrc8a is a component [42,43]) and transporters (e.g., KCC3) that mediate the efflux of K+, Cl−, taurine, and other organic osmolytes [41]. By contrast, RVI utilizes inward Na+-K+-2Cl− cotransport via NKCC1, coupled activation of Na+-H+- and Cl−/HCO3− exchange, and the shrinkage activation of other Na+ and Cl− channels [41]. Crucial to the effective maintenance of cell volume is a molecular system of sensors that can detect changes in cell volume and intracellular ion content, transducers that integrate these signals to the plasma membrane, and the above-mentioned ion transport effectors that ultimately restore homeostasis.
Figure 1.
Cation-chloride cotransporters (CCCs) are key components of cell volume regulation. CCCs mediate transmembrane ion fluxes and can thereby increase or decrease cellular osmotic pressure. (A) Cell shrinkage (step 1) stimulates the WNK-STE20/SPS1-related proline/alanine-rich kinase (SPAK)/Oxidative stress response 1 (OSR1) kinase cascade (step 2), which triggers the phosphorylation of specific residues on both the N(K)CCs and the KCCs, causing their activation and inhibition, respectively (step 3). This net ionic influx of Na+, K+, and Cl−, coupled with obligatory water movement (step 4), restores cell volume in ‘regulatory volume increase’ (RVI). (B) Cell swelling (step 1) inhibits WNK-SPAK/OSR1 (step 2), and also stimulates protein phosphatases (such as PP1 and PP2A) that dephosphorylate N(K)CCs and the KCCs, causing their inhibition and activation, respectively (step 3). The resulting net ionic efflux of K+ and Cl−, coupled with obligatory water movement (step 4), restores cell volume in ‘regulatory volume decrease’ (RVD).
Swelling-sensitive KCCs in the nervous system
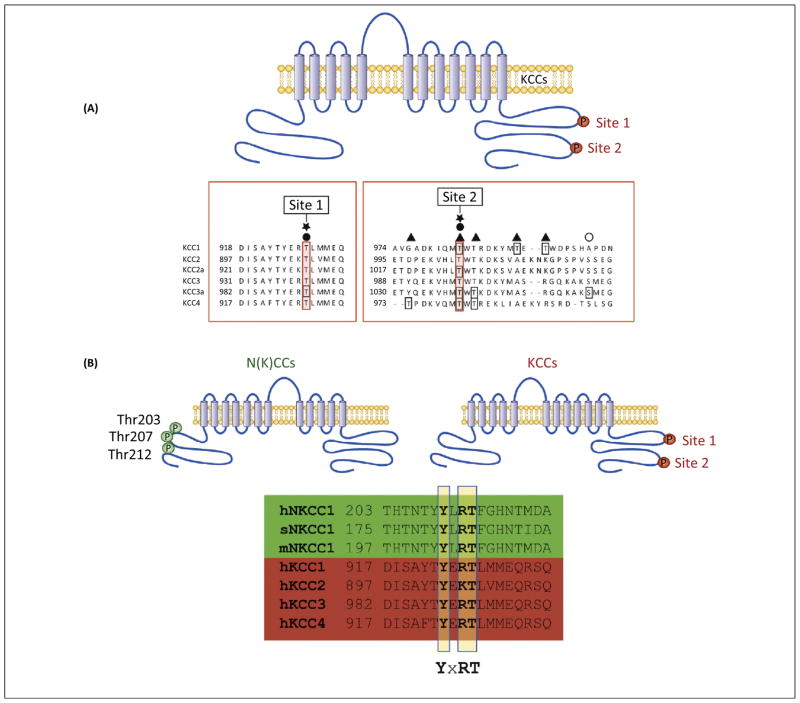
The structure, function, and regulation of the KCCs are highly conserved across evolution, consistent with their essential role in physiology [24]. Since human KCC1 was first cloned in 1996, four human genes encoding human KCCs (hKCC1–4) have been identified, which all have mouse and rat orthologs [19,25,44–48]. hKCCs share a similar membrane topology, including hydrophilic N- and C-terminal intracellular domains harboring multiple phosphoresidues important for transporter regulation in response to cell swelling. Notably, the key phosphorylation sites important for swelling activation of the KCCs are conserved in each KCC, including KCC2, and these motifs are also present in N(K)CCs, suggesting a mechanism of volume regulation mediated by the same (or similar) upstream kinase(s) (Figure 2).
Figure 2.
Conservation of phosphoregulatory sites in cation-chloride cotransporters (CCCs). (A) Two regulatory phosphosites in the cytoplasmic C terminus of KCCs, termed ‘site 1’ (Thr906 in KCC2, Thr991 in KCC3) and ‘site 2’ (Thr1007 in KCC2, Thr1048 in KCC3), are conserved in all KCC isoforms. Dephosphorylation of these sites, as occurs in hypotonic conditions, causes potent stimulation of these cotransporters. (B) The sequence context of a major regulatory site in the C terminus of the KCCs, Thr991 in KCC3 and Thr906 in KCC2, is conserved in the N terminus of human (at Thr212), shark, and mouse NKCC1 (hNKCC1, sNKCC1, and mNKCC1, respectively). This sequence homology suggests that these phosphoregulatory sites are targeted by a single kinase. The WNK-SPAK/OSR1 kinases are required for phosphorylation of both cotransporters at these sites, but reciprocally stimulate NKCC1 while inhibiting the KCCs.
The different KCCs exhibit unique biophysical and physiological properties, but are all volume sensitive; that is, their activities are regulated by cell swelling. However, the fact that KCCs are volume sensitive does not necessarily mean that they have a role in cell volume regulation per se in every cell type in which they are expressed. Indeed, most of what is known regarding the volume sensitivity of the KCCs is derived from heterologous expression systems, such as Xenopus oocytes (although the volume-sensitive kinase/phosphatase signaling molecules that regulate the KCCs in these amphibian cells, namely, the WNK-SPAK kinases, are also conserved and operative in most mammalian cells [49]). All KCCs are expressed in the nervous system, but KCC2, KCC3, and KCC4 appear to be the most physiologically relevant, given their neurological knockout phenotypes in mice [1].
Unlike the other KCCs, KCC2 is active in isotonic conditions [8,19,50], a property apparently conferred by a 73-residue amino acid sequence in the C-terminal domain of KCC2 that is absent in the other KCCs, the so-called ‘ISO domain’ [9]. This domain is present in both KCC2a and KCC2b isoforms. Notably, while exhibiting isotonic activity, KCC2 is also potently stimulated by hypotonicity in oocytes [19]. KCC3 also has at least two splice variants, KCC3a and KCC3b, which are encoded by alternate first exons [26] and differ by some 40 amino acids in their N termini. These splice variants are highly, but differentially, expressed in the nervous system, and may vary in their responses to hypotonicity. Human KCC3a is inactive in isotonic media but stimulated by hypotonicity [51,52]. KCC4 is also inactive in isotonicity, but is perhaps the KCC most strongly stimulated by hypotonicity, at least in oocytes [25]. Hypotonic stimulation of the KCCs is inhibited by calyculin A but not by okadaic acid, demonstrating a critical role for dephosphorylation by PP1, but not PP2A [53].
KCC3 is essential for nervous system cell volume regulation
Evidence that KCCs are important for cell volume maintenance in the nervous system has been derived from genetically modified worms and flies [47,54], transgenic or knockout mouse models [31], and human patients with inherited disease-causing mutations [28,31]. Delpire and colleagues first disrupted the KCC3-encoding Slc12a6 gene using homologous recombination in mice [31]. Slc12a6−/− but not Slc12a6−/+ mice exhibited weakness and incoordination of rear limbs by 2 weeks of age, correlating with significant axonal swelling accompanied by hypomyelination, demyelination, and fiber degeneration in the sciatic nerves. Serial morphometric analyses of sciatic nerves from various-aged Slc12a6−/− mice demonstrated that, at post-natal day 3, soon after peripheral myelination begins and KCC3 mRNA becomes detectable in peripheral nerve fibers, Schwann cells correctly segregated large-caliber axons and demonstrated early myelination indistinguishable from that in wild type (WT) counterparts [29]. However, the mean diameter of Slc12a6−/− axons was increased, whereas the proportion of completely myelinated, incompletely myelinated, and segregated but unmyelinated axons remained unchanged, suggesting that the axonal enlargement reflected cell swelling and not accelerated maturation. By postnatal day 8, nerve fibers demonstrated discontinuous loci of periaxonal fluid accumulation, suggesting impaired ion efflux from the periaxonal space. The combination of axonal swelling and impaired ionic clearance in sciatic nerves of Slc12a6−/− mice predicted a nerve conduction impairment, which was demonstrated in two independent studies [29,55]. A second KCC3-knockout mouse model was created by Boettger and colleagues [28], as well as a spontaneous mutation in the Jackson Laboratory ‘giant axonopathy’ (gaxp) mutant mouse strain [56]. Both mouse lines essentially phenocopy the neuropathy observed in the Slc12a6−/− mutant mouse.
In addition to the nerve and/or locomotor phenotype, adult Slc12a6−/− mice also exhibit several other phenotypes: reduced exploratory behavior, progressive deafness due to degeneration of cells of the inner ear that express KCC3 (Box 2), and impaired pre-pulse inhibition likely due to deafness [28,31]. Furthermore, in slice preparations of cerebellar Purkinje cells (which ordinarily express abundant KCC3), while both WT and KCC3-null cells exhibit swelling when placed in hypo-osmolar medium, after restoration of isotonic conditions, KCC3-null cells failed to normalize their volume, suggesting KCC3 dependence of RVD [28]. Additionally, gramicidin-perforated patch-clamp recordings of KCC3-null cerebellar Purkinje cells exhibited a reduced magnitude of GABA-induced hyperpolarization, suggesting high [Cl−]i. These mice also displayed a lower seizure threshold [28].
Box 2. KCC3 and KCC4 are required for inner ear structure and function.
KCC4, encoded by SLC12A7, is the KCC most potently activated by cell swelling [25] and is expressed in the kidney, brain, and inner ear [25]. KCC4-knockout mice are deaf, but otherwise apparently neurologically intact [30]. The inner ear of KCC4-knockout mice is indistinguishable from WT at P14, but loses almost all outer hair cells between P14 and P21, corresponding to profound hearing loss during this interval [30]. KCC4 is expressed on supporting cells of outer and inner hair cells in the organ of Corti, where it transports K+ released by outer hair cells into Deiters’ cells as part of a K+ recycling pathway that helps maintain the high [K+] of endolymph required for sensory hair cell function [30,102]. Hair cells likely degenerate in KCC4-knockout mice due to the disrupted ionic microenvironment caused by failure of supporting cells to properly handle K+.
Interestingly, similar to some forms of human deafness syndromes [102], KCC4-knockout mice also exhibit renal tubular acidosis [30]. KCC4 is expressed on the basolateral membranes of several tubular cell types, including α-intercalated cells. In KCC4-knockout mice, α-intercalated cells, but not principal or distal convoluted tubular cells, have significantly higher total [Cl−]i, suggesting an impairment of basolateral Cl− efflux [30]. Given that α-intercalated cells exchange intracellular Cl− for basolateral HCO3−, this may explain the acidosis and alkaline urine of KCC4-knockout mice.
Similar to KCC4, KCC3 is not only expressed in supporting cells of inner and outer hair cells, but is also found in type I and III fibrocytes underlying the stria vascularis, where it is thought to be involved in generating a K+ gradient within a fibrocyte gap junction system that drives K+ from type II fibrocytes (which lack KCC3) into type I and III fibrocytes [28]. Indeed, there is a significant loss of type I and III fibrocytes in KCC3-knockout mice, along with progressive degeneration of the entire organ of Corti, which causes significant hearing deficit by 1 year of age [28].
Mutations in human genes disrupting physiologic K+ (and Cl−) flow in the inner ear have been described that result in hereditary deafness [102]. Given the clear importance of K+ and Cl− homeostasis in the human inner ear, KCC4 may yet prove to represent a yet undiscovered form of hereditary deafness, or may contribute to age-related hearing loss. However, this remains speculative, because no disease-causing mutations in KCC4 have been identified thus far.
The notion that KCC3 may have an important role in regulating [Cl−]i (and, consequently, the response to GABA) is compelling and suggests links between neuronal excitability and cell volume homeostasis. The finding that KCC3-null neurons harbor elevated [Cl−]i and attenuated GABA-induced hyperpolarization indicates that KCC3 might have a dual role in the regulation of both cell volume and [Cl−]i. Swelling in neurons most commonly results from an activity-dependent influx of ions, particularly Na+ and Cl−. This activates RVD, which likely stimulates KCC3 and drives K+ and Cl− out of the cell, along with water. As Cl− is transported out of the cell, [Cl−]i falls and the Cl− reversal potential ECl becomes more negative. Consequently, when GABAARs are activated, there is a greater electrochemical drivefor Cl− to flow intothe cell, and the membrane potential hyperpolarizes. In this way, hyperactivity-induced neuronal swelling maypotentiate the hyperpolarizing action of GABA through KCC3-mediated Cl− efflux, which is also a component of RVD. The observed shift in ECl was smaller in KCC3−/− Purkinje neurons than in KCC2−/− Purkinje neurons [28], highlighting the importance of KCC2 in maintaining [Cl−]i in this cell type (and, according to other data [57], in many but not all other CNS neuronal cell types). It is important to point out that multiple KCC isoforms are often coexpressed in cells, raising the possibility that different KCCs interact through heterodimerization, and could possibly affect each other’s function (such as volume sensitivity). Heterologous expression studies have indeed shown physical interaction among different CCCs with functional dominant-negative effects [58–60].
The dual role of KCC3 points to its potential significance in pathophysiological states characterized by both cell swelling and neuronal hyperexcitability, particularly epilepsy (and especially status epilepticus). Several studies have indicated that neurons and glia in seizure foci undergo significant cellular swelling both before and during ictal activity as a result of ionic influx stimulated by severe persistent excitation [61]. Furthermore, unlike control cells that rapidly return to baseline volume upon removal of excitatory stimulus, neurons of the human epileptic hippocampus recover more slowly, exhibiting cell swelling that lasts up to an hour after hyperexcitation [62]. Thus, activation of RVD appears normal in hyperactive neurons, but is disrupted in epilepsy. In hyperactivity-induced RVD, KCC3 stimulation may serve dual roles of driving volume reduction and depleting [Cl−]i to potentiate GABA-induced inhibition, which may be beneficial in attenuating neuronal activity. Consequently, dysregulation of RVD, including KCC3, may not only impair cell volume maintenance, but also disrupt a potentially significant feedback mechanism that amplifies inhibitory signals in response to periods of hyperactivity. This hypothesis is consistent with the lower seizure threshold observed in KCC3-knockout mice and epilepsy in humans with loss-of-function mutations in KCC3 [28].
The peripheral axonal swelling and subsequent fiber degeneration in KCC3-null mice appear to reflect, at least in part, loss of KCC3-mediated RVD. However, whether these effects are driven by disrupted RVD in neurons or glia had remained unclear until two recent reports demonstrated that neuron-specific knockdown of KCC3 sufficed to recapitulate the neuropathology [63,64]. In the first study, CRE recombinase-mediated KCC3 deletion was driven by the synapsin promoter, thus targeting all neurons [63]. As with the global KCC3-knockout model, these mice had significantly impaired motor function in the rotarod and beam-task tests, but in contrast to Howard et al. [31], they also displayed increased spontaneous exploration in the open-field test. Both the global and neuron-specific knockouts also exhibited increased thresholds to inflammatory pain. Interestingly, only the global knockout experienced hearing deficiency in the startle response test, suggesting that the hearing loss phenotype is due to glial KCC3 activity (Box 2). Finally, gold chloride staining of midline callosal fibers revealed a significant decrease in the length of the corpus callosum in the global KCC3 knockout, and a smaller but still significant decrease in the length of the corpus callosum in neuron-specific KCC3 knockouts, but with no difference in the cortical plate length in any of these mice [63]. Global knockouts also exhibited decreased surface area of the anterior commissure and evidence of a shift in its position relative to the corpus callosum. Volumetric analysis of the corpus callosum using diffusion-weighted magnetic resonance imaging (MRI) confirmed a significant reduction of total volume in both mouse lines. Diffusion MRI tractography of fibers passing through the corpus callosum revealed reduced numbers of tracts passing through the external capsule (adjacent to the auditory cortex) in global but not in neuron-specific KCC3-knockout mice. Protein markers of corpus callosal embryonic development all appeared normal, suggesting KCC3 involvement in the maintenance, but not development, of callosal axons [63].
The second conditional mouse model was crossed to several CRE lines, including a Schwann cell-specific CRE and parvalbumin CRE [64]. Absence of a neurodegenerative phenotype in the desert hedgehog-CRE line revealed little participation of Schwann cells in the development of the disease. By contrast, neurodegeneration and locomotor phenotypes were observed in mice with parvalbumin-driven deletion of KCC3 [64]. Parvalbumin is expressed not only in CNS interneurons, but also in large sensory neurons transmitting proprioceptive signals from peripheral tissues to CNS. Interestingly, immunostaining of dorsal root ganglions (DRGs) isolated from WT mice, parvalbumin-driven KCC3-knockout mice, and global KCC3-knockout mice revealed that neurodegeneration occurs in neurons that express parvalbumin, confirming a key role for these cells in the development of the neuropathy. While the locomotion phenotype measured on the accelerated rotarod was highly significant, it was less severe than that of the global knockout, suggesting contributing roles of additional cell types, possiblyincluding motorneurons [64]. A strong phenotype of increased activity in an open-field chamber, observed in synapsin-KCC3-knockout mice [63], was also noted in parvalbumin-KCC3-knockout mice, although failing to reach statistical significance. However, because KCC3 is expressed in CNS inhibitory interneurons [65], a subset of which also express parvalbumin, the biological importance of these observations should not be minimized. The absence of KCC3 in these interneurons might reduce inhibitory output to pyramidal neurons, increasing pyramidal neuronal activity.
KCC3 is required for normal structure and function of the human nervous system
Molecular genetics has also established the necessity of KCC3-mediated cell volume regulation in humans. Loss-of-function mutations in KCC3 have been identified as the cause of agenesis of the corpus callosum associated with peripheral neuropathy [ACCPN or hereditary motor and sensory neuropathy/absent corpus collosum (HSMN/ACC)], a severe sensorimotor neuropathy associated with mental retardation, dysmorphic features, and complete or partial agenesis of the corpus callosum [66]. ACCPN is transmitted in an autosomal recessive fashion and is found at high frequency in Quebec, Canada [67]. Given that the ACCPN critical genomic region included the SLC12A6 gene encoding KCC3, Howard et al. screened KCC3 for mutations in individuals with the disorder [31]. Four distinct protein-truncating mutations in KCC3 were identified. In the French-Canadian population, two mutations were found: a deletion in exon 18 resulting in a frame shift that truncates the open reading frame (Thr813fsX), and another frameshift (Phe529fsX532) in exon 11 in an individual heterozygous for Thr813fsX. In two Canadian families of non-French ancestry, two additional mutations (Arg1011X and Arg675X in exon 22 and 15, respectively) were described. Additional loss-of-function mutations in KCC3 have subsequently been reported in patients with ACCPN [68–71]. Importantly, neuropathological examination of patients with ACCPN shows axonal swelling and hypomyelination, consistent with findings from mouse KCC3-knockout models [68]. Together, these data revealed the necessity of KCC3 in the development and/or maintenance of both the human CNS and PNS, likely via effects on neuronal cell volume homeostasis and/or the closely linked regulation of [Cl−]i.
While disruption of mouse KCC3 impaired the ability of neurons and kidney cells to volume regulate under hypo-osmotic conditions [28], Lauf et al. showed no effect on the basic properties (e.g., osmotic fragility) of RBCs isolated from patients with ACCPN [72]. This observation is in line with the absence of an RBC phenotype in the unstressed KCC3-knockout mice. Given that several KCC isoforms are expressed in RBCs [73], the disruption of one isoform might not be enough to generate a strong phenotype in unstressed mice. This was demonstrated in double-knockout KCC3−/−, KCC1−/− mice, whose RBCs showed evidence of aberrant volume regulation, including increased mean corpuscular volume and increased susceptibility to osmotic lysis [74]. However, K+ influxes measured in RBCs from patients with ACCPN responded differently to biochemical stressors, such as NEM or Mg removal, suggesting a compromised function of the ‘KCC-heteromeric complex’ [72].
Human disease mutations in SLC12A6 disrupt KCC3 function by two mechanisms. Most pathogenic truncating mutations disrupt the KCC3 C terminus, the interaction site of a potential regulatory kinase. Oocytes expressing the exon 18 C-terminal truncation (KCC3Q) exhibit aberrant swelling-activated Cl−-dependent uptake of 86Rb+, despite normal protein N-glycosylation and surface membrane localization, suggesting an important regulatory role for the truncated C terminus [31]. Using a yeast two-hybrid approach, Salin-Cantegrel et al. showed that the C-terminal domain of KCC3 directly interacts with brain-specific creatine kinase (CK-B), and that pharmacological inhibition of CK-B reduces the activity of KCC3 in Xenopus oocyte functional assays [75]. Rinehart et al. identified two phosphorylation sites in the C-terminal domain, Thr991 and Thr1048, which are dephosphorylated to stimulate KCC3 activity in response to hypotonic stress [76]. Absence of these regulatory sites precludes KCC3 activation and could be the major mechanism of KCC3 inactivation in SLC12A6 disease mutations. The molecular identity of kinase and phosphatase regulators of KCC3 is an ongoing area of study.
A second mechanism by which SLC12A6 mutations impair KCC3 activity is through disruption of normal KCC3 intracellular transit and membrane localization. One ACCPN mutation, KCC3 R207C, significantly decreases plasma membrane localization of KCC3. This misfolding mutation results in KCC3 retention in the endoplasmic reticulum [77]. Curcumin, which has been shown to stimulate the release of misfolded transmembrane proteins from the endoplasmic reticulum, partially rescues this transport deficit in cells expressing the R207C mutant [77].
The Cl−-sensitive WNK-SPAK kinase complex: master regulator of KCC and N[K]CC volume sensitivity
Until recently, the mechanisms regulating KCC swelling sensitivity were not defined on the molecular level. While cell swelling is a quasi-immediate phenomenon due to the water permeability of a cell membrane, there is a significant time delay in the activation of K+-Cl− cotransport [20]. This delay is temperature dependent, indicating that swelling activation is an energy and/or metabolic-dependent process. In fact, based on kinetic arguments and the use of phosphatase inhibitors, early studies demonstrated that K+-Cl− cotransport activation was mediated via time-dependent inhibition of protein kinases. Jennings et al. initially proposed that phosphorylation inhibits the KCCs, while dephosphorylation has an opposite effect [21,24,78–81]. Early physiological studies using radiotracer flux assays and relatively nonspecific kinase and phosphatase inhibitors illustrated a powerful mechanism that reciprocally regulates cellular N[K]CCs and KCCs through a coordinated system of serine-threonine protein kinase phosphorylation and protein phosphatase-mediated dephosphorylation [24,82].
Phosphorylation, stimulated by cell shrinkage, intracellular Cl− depletion, and protein phosphatase inhibitors, activates NCC/NKCC1/NKCC2, but inhibits the KCCs [83]. Serine-threonine dephosphorylation, triggered by cell swelling, intracellular Cl− accumulation, and protein phosphatases, exerts the opposite effects, stimulating the KCCs. This inverse regulation of Na+- and K+-driven CCCs by the same signals, and likely by the same kinase-phosphatase pathway, ensures tight coordination of cellular Cl− influx and efflux, and avoids unnecessary consumption of cellular ATP stores (Figure 2). The importance of this phosphoregulatory mechanism is emphasized by its strong evolutionary conservation [84] (Box 3).
Box 3. Evolutionary importance of the WNK-SPAK-KCC pathway for cell volume regulation in neurons and glia.
Phylogenetic analysis suggests a single ancestral CCC gene in Archaea underwent multiple gene duplications and gene-loss events to generate the varied distribution and functional specialization of the CCCs [102]. Evolution of KCCs, which first appeared in basal metazoan taxa, such as sponges, likely enabled fast hyperpolarizing neurotransmission via their establishment of low neuronal [Cl−]i [103]. The evolution of organismal multicellularity likely necessitated the ability to precisely regulate the ionic and osmotic composition of fluid compartments separated by epithelia. Accordingly, phylogenetic analysis suggests that the interaction between WNK kinase and the SPAK/OSR1 kinases, which enabled their development into a Cl−-and volume-sensing signaling pathway, first evolved in the early metazoan lineage [84]. Indeed, WNK kinases are expressed in most (probably all) multicellular organisms, with one ortholog in Drosophila melanogaster and Caenorhabditis elegans, and at least nine orthologs in Arabidopsis thaliana [104]. The earliest WNK kinase homolog was identified in the fungus Phycomyces, but is absent from the yeast Saccharomyces. SPAK is similarly conserved, and is closely related to OSR1; both are mammalian homologs to yeast Ste20p serine/threonine kinases [105]. SPAK likely arose from gene duplication of OSR1, because only late vertebrates contain both genes [106]. The C. elegans OSR1 ortholog GCK3 negatively regulates the swelling-activated Cl− channel CLH-3b, and is located downstream of WNK1 kinase in a volume-sensing signaling pathway [84].
Drosophila has proven to be a valuable model for investigating the WNK-SPAK-CCC pathway in vivo. Drosophila has only one WNK kinase, one SPAK/OSR1 ortholog (fray), one NKCC ortholog (ncc69), and one KCC ortholog (kcc or kazachoc), thereby eliminating redundancy and allowing for facile genetic manipulation [54,107]. Null mutants of fray have severe nerve swelling and degeneration due to failure of glial cells to correctly ensheath axons [108], a phenotype that bears a striking resemblance to Slc12a6 (KCC3)-null mice [29]. Rescue of the null fray mutant phenotype by rat SPAK suggests functional homology, and Drosophila ncc69 is regulated by Fray, just as NKCC1 is regulated by SPAK [54]. Knockdown of kcc or ncc69 in glia, and kcc (but not ncc69) knockdown in neurons correlates with larval and adult cell volume abnormalities and adult seizure sensitivity [107]. These data demonstrate a highly conserved system of volume regulation involving the WNK-SPAK-CCC pathway, and suggest glial contributions to seizure susceptibility via cell volume regulation and associated effects on the volume and ion content (particularly of K+) of the extracellular space [107].
Recent work indicates that the phosphorylation status of two threonine residues that are conserved in all KCC isoforms, termed ‘site 1’ (Thr906 in KCC2; Thr991 in KCC3) and ‘site 2’ (Thr1007 in KCC2; Thr1048 in KCC3) are the prime regulators of KCC volume sensitivity (Figure 2). Hypotonic high K+ conditions that activate KCC isoforms (and inhibit the N[K]CCs) induce a rapid and robust dephosphorylation of site 1 and site 2 [22]. Dual mutation of site 1 and site 2 to alanine (Ala) in the KCCs (preventing phosphorylation), results in constitutively active KCCs with >25-fold greater activity compared with WT KCCs in similar conditions, defining the most potent known activation mechanism of the KCCs thus far [22,76].
Small interfering (si)-RNA-mediated knockdown of WNK1 in model cell systems partially suppresses the phosphorylation of site 1 and site 2 in KCC3 in vitro [76]. Consistent with a role for WNK kinase isoforms in regulating KCC activity, overexpression of WNK isoforms (including WNK2 and WNK3) inhibits the KCCs, whereas dominant-negative WNK3 overexpression uniquely stimulates KCC activity >100-fold [85]. The WNK kinase substrates SPAK/OSR1 serine-threonine kinases, in the presence of the Cab39/MO25 regulatory subunit, promote inhibition of all KCC isoforms by directly phosphorylating site 2, and STOCK1S-50699, a WNK-SPAK/OSR1 pathway inhibitor, suppresses KCC phosphorylation with similar potency [22]. The SPAK/OSR1 kinase pathway was originally discovered when these two Ste20 kinases were shown to physically interact with the N terminus of KCC3 in a yeast two-hybrid screen, and was subsequently found to interact with the N terminus of other CCCs [83,86]. Evidence links the binding of the kinases to phosphorylation of these sites, eliciting NKCC1 activation and KCC inhibition [87]. SPAK/OSR1 kinases are required for KCC site 2 phosphorylation, because the endogenous phosphorylation of all KCC isoforms (including KCC2) at site 2 is abolished in embryonic stem cells lacking SPAK/OSR1 activity. Accordingly, these cells exhibit elevated basal activity of Cl−-dependent, furosemide-sensitive 86Rb+ flux consistent with KCC activation [22].
Of note, mutant KCC2 with Ala substitutions at positions Thr906 and Thr1007 (corresponding to Thr991 and Thr1048 in KCC3a) exhibited a tenfold increase in activity compared with WT KCC2 in HEK-293 cells in standard isotonic conditions, suggesting the importance of these phosphorylation sites for maximal KCC2 activation [76]. Interestingly, in the developing brain, the extent of dephosphorylation of KCC2 at Thr906 parallels its activity level: KCC2 phosphorylation is almost completely absent in adult brain, in which KCC2 activity is maximal, but robustly phosphorylated in the developing mouse brain at P0, when KCC2 is less active [76]. This compelling result suggests that developmental changes in KCC2 phosphorylation at Thr906 and/or Thr1007, perhaps mediated by postnatal changes in the expression and/or activity of WNK kinase or the SPAK/OSR1 kinases, could be involved in modulating KCC2 activity and, therefore, neuronal [Cl−]i during the GABA excitatory-inhibitory sequence.
Modulation of KCC3 swell activation mechanisms to counter pathological brain swelling
Blocking KCC3 Thr991/Thr1048 inhibitory phosphorylation is a potent mechanism of stimulating KCC activity. Could this mechanism be pharmacologically exploited to protect against isosmotic cell swelling in certain disease states, such as cerebral edema associated with ischemic stroke, epilepsy, or tumors? Interestingly, the sequence context of the KCC phosphorylation site 1 is also conserved in the amino terminus of NKCC1, suggesting a coordinated but opposite regulation of NKCCs and KCCs by a single kinase complex (Figure 2). Indeed, WNK1 kinase phosphorylates and activates SPAK (and OSR1), which in turn promotes the phosphorylation of both NKCC1 and the KCCs, but does so with reciprocal effect, stimulating NKCC1 but inhibiting KCC [2,7,22,76]. Inhibiting the WNK-SPAK kinases, which phosphorylate KCC site 2 directly, and are necessary for both site 1 and site 2 phosphorylation [22], might have the added benefit of concurrently decreasing the stimulatory phosphorylation of NKCC1 at Thr212/Thr217, which is required for NKCC1 activity [88] and is pathologically upregulated in ischemic edema [7]. This simultaneous reduction of NKCC1-mediated ion influx and activation of KCC3-mediated ion efflux seems ideally suited to counter pathological cell swelling in isosmotic swelling conditions, which results from channel- and carrier-mediated increases in cellular ionic load [34].
Importantly, the potent KCC-activating switch in KCC1/3/4 responsible for swelling activation of the transporter is also conserved in KCC2, suggesting an important but yet unrecognized function of this motif in KCC2. Although perhaps not essential for neuronal cell volume homeostasis, this phospho-motif in KCC2 might have been retained during evolution to finely modulate KCC2 activity in response to physiological demand or to restore homeostasis in response to perturbation. The brisk and significant decrease in inhibitory phosphorylation at Thr906 in mouse KCC2 brain during embryonic development suggests an important role for phosphorylation at this residue [76]. Could neuronal [Cl−]i increases that accompany energy failure during stroke, or intense neuronal activity, both of which are accompanied by increases in neuronal cell volume, be compensated by increases in KCC2 activity achieved by promoting maximal Thr906/Thr1007 dephosphorylation? This is an important issue worthy of future investigation with clinical implications.
Exploiting KCC swell activation mechanisms for neurological diseases not associated with cell swelling
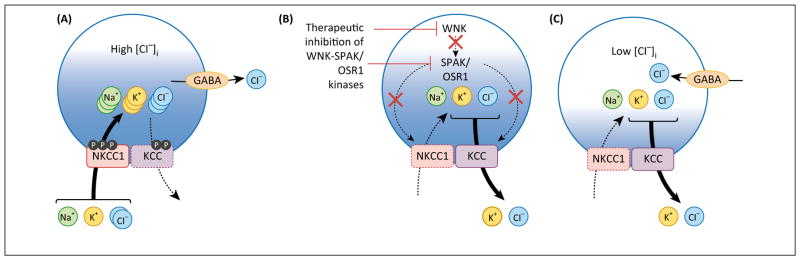
Might it also be possible to exploit the biochemical mechanisms of NKCC1 and KCC2 swelling regulation for therapeutic benefit in neurological diseases not associated with cell swelling? Multiple diseases, including seizures, neuropathic pain, autism, schizophrenia, spasticity, and others, feature more depolarized values of EGABA, and hyperexcitability of GABAergic neurons and circuits, due to pathologically elevated neuronal [Cl−]i resulting from increases in NKCC1 activity, or decreases in KCC2 activity [6,7]. Other therapeutic efforts to date have focused on targeting NKCC1 and KCC2 individually with low potency, relatively unspecific diuretics that have suboptimal CNS penetration, although novel KCC2 activators currently in development show promise [1]. However, since the CCCs work in concert with one another and with other Cl− channels to achieve homeostasis, it is unknown whether targeting one CCC (e.g., NKCC1) might be compensated for by the activity of other CCCs. Inhibition of WNK-SPAK kinases might be expected to inhibit both NKCC1-mediated Cl− loading and stimulate KCC2-mediated Cl− extrusion by decreasing their stimulatory and inhibitory phosphorylation, respectively (Figure 3). Therefore, WNK-SPAK inhibition might be more efficacious than either poorly CNS-penetrant loop diuretics (bumetanide, see [89]) or KCC2 activators [1].
Figure 3.
Exploiting genetic conservation of swelling-regulated K+-Cl− cotransporters (KCC) activation for therapeutic restoration of ionotropic inhibition. The regulatory mechanisms of the WNK-STE20/SPS1-related proline/alanine-rich kinase (SPAK) kinases on KCC3 are conserved in KCC2, and suggest that targeting this motif in KCC2 is relevant for neurological diseases beyond those related to problems in cell volume control. (A) Several neurological disease are associated with a pathological increase in activity of NKCC1 and/or decrease in activity of KCC2, including temporal lobe epilepsy, neonatal seizures, neuropathic pain, schizophrenia, and motor spasticity. This imbalance results in influx of Cl−, which raises [Cl−]i and disrupts GABAA receptor (GABAAR)-mediated fast hyperpolarizing currents. (B) The WNK-SPAK/Oxidative stress response 1 (OSR1) kinases phosphorylate both NKCC1 and the KCCs, which reciprocally stimulate NKCC1 and inhibit KCC. Inhibition of WNK-SPAK/OSR1 kinases would promote dephosphorylation of both NKCC1 and the KCCs, resulting in inhibition and activation of these molecules, respectively. Activation of KCCs and inhibition of NKCC1 would result in net efflux of K and Cl−. (C) Reciprocal activation of KCC and inhibition of NKCC1 via therapeutic inhibition of the WNK-SPAK/OSR1 kinases resulting in net KCl efflux would deplete [Cl−]i. This renormalization of neuronal [Cl−]i homeostasis is a therapeutic strategy to restore normal GABAergic signaling in the several neuropsychiatric disorders in which it is disrupted.
Interestingly, Piala et al. recently found that Cl− binds directly to the catalytic site of WNK1, thereby stabilizing the inactive conformation of WNK1 and preventing kinase autophosphorylation and activation [90]. Mutagenesis of this site rendered WNK1 less sensitive to Cl−-mediated inhibition of autophosphorylation, suggesting that WNK1 functions as a Cl− sensor. Thus, WNK kinases may not only be effector kinases that work through the SPAK/OSR1 kinases to phosphoregulate CCC activity, but also function as the sensors that detect changes in intracellular Cl− (and potentially cell volume) [90]. Note that the activities of SPAK and OSR1, measured in vitro, are also sensitive to changes in Cl− concentration [91]. These data suggest that inhibiting these molecules would also block feedback mechanisms activated to counter the effects of targeting NKCC1 or the KCCs alone. Therefore, modulation of the Cl−-dependent functional plasticity of GABAARs by coincident NKCC1 inhibition and KCC2 stimulation via WNK-SPAK kinase antagonism may be a tenable method of restoring ionotropic inhibition for a range of disorders, including neurodevelopmental diseases, such as neonatal seizures, autism, and schizophrenia, and adult disorders, such as neuropathic pain, temporal lobe epilepsy, and spasticity [2] (Figure 3). These hypotheses will be rich subjects for future basic and translational investigation.
Concluding remarks
In neuroscience, considerable attention has been rightly paid to KCC2, which mediates Cl− efflux in isotonic conditions and is required for the establishment of GABAergic hyperpolarizing synaptic inhibition. However, genetic models of KCC3 and KCC4, and their upstream inhibitory WNK-SPAK kinases, have demonstrated the necessity of swelling-regulated K-Cl cotransport for nervous system development and cell volume regulation, and their involvement in neurological disease. The recent discovery and characterization of an activating dephosphorylation mechanism of the KCCs has pinpointed a druggable switch of KCC function that could be pharmacologically exploited to therapeutically facilitate ionic efflux in neurological diseases. More work is needed to explore the role of the KCCs, including KCC2, in nervous system cell volume regulation. Work on these topics is clinically relevant and may reveal novel insights to help understand potential links between cell volume regulation and neuronal excitability.
References
- 1.Gagnon KB, Delpire E. Physiology of SLC12 transporters: lessons from inherited human genetic mutations and genetically engineered mouse knockouts. Am J Physiol. 2013;304:C693–C714. doi: 10.1152/ajpcell.00350.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi DR, et al. The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci Signal. 2014;7:re3. doi: 10.1126/scisignal.2005365. [DOI] [PubMed] [Google Scholar]
- 3.Markadieu N, Delpire E. Physiology and pathophysiology of SLC12A1/2 transporters. Pflugers Arch. 2014;466:91–105. doi: 10.1007/s00424-013-1370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev. 2005;85:423–493. doi: 10.1152/physrev.00011.2004. [DOI] [PubMed] [Google Scholar]
- 5.Arroyo JP, et al. The SLC12 family of electroneutral cation-coupled chloride cotransporters. Mol Aspects Med. 2013;34:288–298. doi: 10.1016/j.mam.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Kaila K, et al. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci. 2014;15:637–654. doi: 10.1038/nrn3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahle KT, et al. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol. 2008;4:490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- 8.Payne JA. Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]oregulation. Am J Physiol. 1997;273:C1516–C1525. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- 9.Mercado A, et al. A C-terminal domain in KCC2 confers constitutive K+-Cl-cotransport. J Biol Chem. 2006;281:1016–1026. doi: 10.1074/jbc.M509972200. [DOI] [PubMed] [Google Scholar]
- 10.Cellot G, Cherubini E. GABAergic signaling as therapeutic target for autism spectrum disorders. Front Pediatrics. 2014;2:70. doi: 10.3389/fped.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arion D, Lewis DA. Altered expression of regulators of the cortical chloride transporters NKCC1 and KCC2 in schizophrenia. Arch Gen Psychiatry. 2011;68:21–31. doi: 10.1001/archgenpsychiatry.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tornberg J, et al. Behavioural phenotypes of hypomorphic KCC2-deficient mice. Eur J Neurosci. 2005;21:1327–1337. doi: 10.1111/j.1460-9568.2005.03959.x. [DOI] [PubMed] [Google Scholar]
- 13.Khanna A, et al. Limitations of current GABA agonists in neonatal seizures: towards GABA modulation via the targeting of neuronal Cl-transport. Front Neurol. 2013;4:78. doi: 10.3389/fneur.2013.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulenguez P, et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med. 2010;16:302–307. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- 15.Rangroo Thrane V, et al. Ammonia triggers neuronal disinhibition and seizures by impairing astrocyte potassium buffering. Nat Med. 2013;19:1643–1648. doi: 10.1038/nm.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coull JA, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 17.Kahle KT, et al. Therapeutic restoration of spinal inhibition via druggable enhancement of potassium-chloride cotransporter kcc2-mediated chloride extrusion in peripheral neuropathic pain. JAMA Neurol. 2014;71:640–645. doi: 10.1001/jamaneurol.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauf PK, Theg BE. A chloride dependent K+ flux induced by N-ethylmaleimide in genetically low K+ sheep and goat erythrocytes. Biochem Biophys Res Commun. 1980;92:1422–1428. doi: 10.1016/0006-291x(80)90445-3. [DOI] [PubMed] [Google Scholar]
- 19.Song L, et al. Molecular, functional, and genomic characterization of human KCC2, the neuronal K-Cl cotransporter. Brain Res Mol Brain Res. 2002;103:91–105. doi: 10.1016/s0169-328x(02)00190-0. [DOI] [PubMed] [Google Scholar]
- 20.Jennings ML, al-Rohil N. Kinetics of activation and inactivation of swelling-stimulated K+/Cl− transport. The volume-sensitive parameter is the rate constant for inactivation. J Gen Physiol. 1990;95:1021–1040. doi: 10.1085/jgp.95.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jennings ML, Schulz RK. Okadaic acid inhibition of KCl cotransport. Evidence that protein dephosphorylation is necessary for activation of transport by either cell swelling or N-ethylmaleimide. J Gen Physiol. 1991;97:799–817. doi: 10.1085/jgp.97.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de los Heros P, et al. The WNK-regulated SPAK/OSR1 kinases directly phosphorylate and inhibit the K+-Cl− co-transporters. Biochem J. 2014;458:559–573. doi: 10.1042/BJ20131478. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Lauf PK, Adragna N. Properties and membrane transport mechanisms of erythrocytesIn. In: Lang F, Föller M, editors. Erythrocytes: Physiology and Pathophysiology. Imperial College Press; 2012. pp. 57–228. [Google Scholar]
- 24.Adragna NC, et al. Regulation of K-Cl cotransport: from function to genes. J Membr Biol. 2004;201:109–137. doi: 10.1007/s00232-004-0695-6. [DOI] [PubMed] [Google Scholar]
- 25.Mount DB, et al. Cloning and characterization of KCC3 and KCC4, new members of the cation-chloride cotransporter gene family. J Biol Chem. 1999;274:16355–16362. doi: 10.1074/jbc.274.23.16355. [DOI] [PubMed] [Google Scholar]
- 26.Pearson MM, et al. Localization of the K(+)-Cl(−) cotransporter, KCC3, in the central and peripheral nervous systems: expression in the choroid plexus, large neurons and white matter tracts. Neuroscience. 2001;103:481–491. doi: 10.1016/s0306-4522(00)00567-4. [DOI] [PubMed] [Google Scholar]
- 27.Karadsheh MF, et al. Localization of the KCC4 potassium-chloride cotransporter in the nervous system. Neuroscience. 2004;123:381–391. doi: 10.1016/j.neuroscience.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Boettger T, et al. Loss of K-Cl co-transporter KCC3 causes deafness, neurodegeneration and reduced seizure threshold. EMBO J. 2003;22:5422–5434. doi: 10.1093/emboj/cdg519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byun N, Delpire E. Axonal and periaxonal swelling precede peripheral neurodegeneration in KCC3 knockout mice. Neurobiol Dis. 2007;28:39–51. doi: 10.1016/j.nbd.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boettger T, et al. Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature. 2002;416:874–878. doi: 10.1038/416874a. [DOI] [PubMed] [Google Scholar]
- 31.Howard HC, et al. The K-Cl cotransporter KCC3 is mutant in a severe peripheral neuropathy associated with agenesis of the corpus callosum. Nat Genet. 2002;32:384–392. doi: 10.1038/ng1002. [DOI] [PubMed] [Google Scholar]
- 32.Kahle KT, et al. Molecular mechanisms of ischemic cerebral edema: role of electroneutral ion transport. Physiology. 2009;24:257–265. doi: 10.1152/physiol.00015.2009. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann EK, et al. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 34.Simard JM, et al. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McManus ML, et al. Regulation of cell volume in health and disease. N Engl J Med. 1995;333:1260–1266. doi: 10.1056/NEJM199511093331906. [DOI] [PubMed] [Google Scholar]
- 36.Jackson PS, Madsen JR. Cerebral edema, cell volume regulation, and the role of ion channels in organic osmolyte transport. Pediat Neurosurg. 1997;27:279–285. doi: 10.1159/000121271. [DOI] [PubMed] [Google Scholar]
- 37.Pasantes-Morales H, et al. Signaling events during swelling and regulatory volume decrease. Neurochem Res. 2000;25:1301–1314. doi: 10.1023/a:1007652330703. [DOI] [PubMed] [Google Scholar]
- 38.Pasantes-Morales H, Tuz K. Volume changes in neurons: hyperexcitability and neuronal death. Contrib Nephrol. 2006;152:221–240. doi: 10.1159/000096326. [DOI] [PubMed] [Google Scholar]
- 39.Strange K. Regulation of solute and water balance and cell volume in the central nervous system. J Am Soc Nephrol. 1992;3:12–27. doi: 10.1681/ASN.V3112. [DOI] [PubMed] [Google Scholar]
- 40.Pasantes-Morales H. Volume regulation in brain cells: Cellular and molecular mechanisms. Metab Brain Dis. 1996;11:187–204. doi: 10.1007/BF02237957. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen SF, et al. Osmosensory mechanisms in cellular and systemic volume regulation. J Am Soc Nephrol. 2011;22:1587–1597. doi: 10.1681/ASN.2010121284. [DOI] [PubMed] [Google Scholar]
- 42.Qiu Z, et al. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell. 2014;157:447–458. doi: 10.1016/j.cell.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voss FK, et al. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science. 2014;344:634–638. doi: 10.1126/science.1252826. [DOI] [PubMed] [Google Scholar]
- 44.Race JE, et al. Molecular cloning and functional characterization of KCC3, a new K-Cl cotransporter. Am J Physiol. 1999;277:C1210–C1219. doi: 10.1152/ajpcell.1999.277.6.C1210. [DOI] [PubMed] [Google Scholar]
- 45.Gillen CM, et al. Molecular cloning and functional expression of the K-Cl cotransporter from rabbit, rat, and human. A new member of the cation-chloride cotransporter family. J Biol Chem. 1996;271:16237–16244. doi: 10.1074/jbc.271.27.16237. [DOI] [PubMed] [Google Scholar]
- 46.Hiki K, et al. Cloning, characterization, and chromosomal location of a novel human K+-Cl− cotransporter. J Biol Chem. 1999;274:10661–10667. doi: 10.1074/jbc.274.15.10661. [DOI] [PubMed] [Google Scholar]
- 47.Holtzman EJ, et al. Cloning, characterization, and gene organization of K-Cl cotransporter from pig and human kidney and C. elegans. Am J Physiol. 1998;275:F550–F564. doi: 10.1152/ajprenal.1998.275.4.F550. [DOI] [PubMed] [Google Scholar]
- 48.Pellegrino CM, et al. Molecular identification and expression of erythroid K:Cl cotransporter in human and mouse erythroleukemic cells. Blood Cells Mol Dis. 1998;24:31–40. doi: 10.1006/bcmd.1998.0168. [DOI] [PubMed] [Google Scholar]
- 49.Strange K, et al. Ste20-type kinases: evolutionarily conserved regulators of ion transport and cell volume. Physiology. 2006;21:61–68. doi: 10.1152/physiol.00139.2005. [DOI] [PubMed] [Google Scholar]
- 50.Strange K, et al. Dependence of KCC2 K-Cl cotransporter activity on a conserved carboxy terminus tyrosine residue. Am J Physiol Cell Physiol. 2000;279:C860–C867. doi: 10.1152/ajpcell.2000.279.3.C860. [DOI] [PubMed] [Google Scholar]
- 51.Bergeron MJ, et al. Ammonium transport and pH regulation by K+-Cl− cotransporters. Am J Physiol Ren Physiol. 2003;285:F68–F78. doi: 10.1152/ajprenal.00032.2003. [DOI] [PubMed] [Google Scholar]
- 52.Mercado A, et al. Functional characterization of two alternative isoforms of the KCC3 K–Cl cotransporter. FASEB J. 2002;16:A58. [Google Scholar]
- 53.Mercado A, et al. Functional comparison of the K+-Cl− cotransporters KCC1 and KCC4. J Biol Chem. 2000;275:30326–30334. doi: 10.1074/jbc.M003112200. [DOI] [PubMed] [Google Scholar]
- 54.Leiserson WM, et al. Drosophila glia use a conserved cotransporter mechanism to regulate extracellular volume. Glia. 2011;59:320–332. doi: 10.1002/glia.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun YT, et al. Deficiency of electroneutral K+–Cl− cotransporter 3 causes a disruption in impulse propagation along peripheral nerves. Glia. 2010;58:1544–1552. doi: 10.1002/glia.21028. [DOI] [PubMed] [Google Scholar]
- 56.Jiao Y, et al. A deletion mutation in Slc12a6 is associated with neuromuscular disease in gaxp mice. Genomics. 2008;91:407–414. doi: 10.1016/j.ygeno.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seja P, et al. Raising cytosolic Cl− in cerebellar granule cells affects their excitability and vestibulo-ocular learning. EMBO J. 2012;31:1217–1230. doi: 10.1038/emboj.2011.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casula S, et al. A dominant negative mutant of the KCC1 K-Cl cotransporter both N-and C-terminal cytoplasmic domains are required for K-Cl cotransport activity. J Biol Chem. 2001;276:41870–41878. doi: 10.1074/jbc.M107155200. [DOI] [PubMed] [Google Scholar]
- 59.Ding J, et al. A trafficking-deficient mutant of KCC3 reveals dominant-negative effects on K-Cl cotransport function. PLoS ONE. 2013;8:e61112. doi: 10.1371/journal.pone.0061112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simard CF, et al. Homooligomeric and heterooligomeric associations between K+-Cl− cotransporter isoforms and between K+-Cl− and Na+-K+-Cl− cotransporters. J Biol Chem. 2007;282:18083–18093. doi: 10.1074/jbc.M607811200. [DOI] [PubMed] [Google Scholar]
- 61.Olsson T, et al. Cell swelling, seizures and spreading depression: an impedance study. Neuroscience. 2006;140:505–515. doi: 10.1016/j.neuroscience.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 62.Isokawa M. N-methyl-D-aspartic acid-induced and Ca-dependent neuronal swelling and its retardation by brain-derived neurotrophic factor in the epileptic hippocampus. Neuroscience. 2005;131:801–812. doi: 10.1016/j.neuroscience.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 63.Shekarabi M, et al. Loss of neuronal potassium/chloride cotransporter 3 (KCC3) is responsible for the degenerative phenotype in a conditional mouse model of hereditary motor and sensory neuropathy associated with agenesis of the corpus callosum. J Neurosci. 2012;32:3865–3876. doi: 10.1523/JNEUROSCI.3679-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ding J, Delpire E. Deletion of KCC3 in parvalbumin neurons leads to locomotor deficit in a conditional mouse model of peripheral neuropathy associated with agenesis of the corpus callosum. Behav Brain Res. 2014;274:128–136. doi: 10.1016/j.bbr.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shekarabi M, et al. Cellular expression of the K+–Cl− cotransporter KCC3 in the central nervous system of mouse. Brain Res. 2011;1374:15–26. doi: 10.1016/j.brainres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 66.Mathieu J, et al. Motor and sensory neuropathies with or without agenesis of the corpus callosum: a radiological study of 64 cases. Can J Neurol Sci. 1990;17:103–108. (in French) [PubMed] [Google Scholar]
- 67.Hauser E, et al. Occurrence of Andermann syndrome out of French Canada: agenesis of the corpus callosum with neuronopathy. Neuropediatrics. 1993;24:107–110. doi: 10.1055/s-2008-1071524. [DOI] [PubMed] [Google Scholar]
- 68.Dupre N, et al. Hereditary motor and sensory neuropathy with agenesis of the corpus callosum. Ann Neurol. 2003;54:9–18. doi: 10.1002/ana.77777. [DOI] [PubMed] [Google Scholar]
- 69.Uyanik G, et al. Novel truncating and missense mutations of the KCC3 gene associated with Andermann syndrome. Neurology. 2006;66:1044–1048. doi: 10.1212/01.wnl.0000204181.31175.8b. [DOI] [PubMed] [Google Scholar]
- 70.Salin-Cantegrel A, et al. Distal truncation of KCC3 in non-French Canadian HMSN/ACC families. Neurology. 2007;69:1350–1355. doi: 10.1212/01.wnl.0000291779.35643.15. [DOI] [PubMed] [Google Scholar]
- 71.Rudnik-Schoneborn S, et al. Andermann syndrome can be a phenocopy of hereditary motor and sensory neuropathy: report of a discordant sibship with a compound heterozygous mutation of the KCC3 gene. Neuropediatrics. 2009;40:129–133. doi: 10.1055/s-0029-1234084. [DOI] [PubMed] [Google Scholar]
- 72.Lauf P, et al. K-Cl cotransport in red blood cells from patients with KCC3 isoform mutants. Biochem Cell Biol. 2006;84:1034–1044. doi: 10.1139/o06-203. [DOI] [PubMed] [Google Scholar]
- 73.Lauf PK, et al. K-Cl co-transport: immunocytochemical and functional evidence for more than one KCC isoform in high K and low K sheep erythrocytes. Comp Biochem Physiol A: Mol Integr Physiol. 2001;130:499–509. doi: 10.1016/s1095-6433(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 74.Rust MB, et al. Disruption of erythroid K-Cl cotransporters alters erythrocyte volume and partially rescues erythrocyte dehydration in SAD mice. J Clin Invest. 2007;117:1708–1717. doi: 10.1172/JCI30630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salin-Cantegrel A, et al. HMSN/ACC truncation mutations disrupt brain-type creatine kinase-dependant activation of K+/Cl− co-transporter 3. Hum Mol Genet. 2008;17:2703–2711. doi: 10.1093/hmg/ddn172. [DOI] [PubMed] [Google Scholar]
- 76.Rinehart J, et al. Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell. 2009;138:525–536. doi: 10.1016/j.cell.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salin-Cantegrel A, et al. Transit defect of potassium-chloride co-transporter 3 is a major pathogenic mechanism in hereditary motor and sensory neuropathy with agenesis of the corpus callosum. J Biol Chem. 2011;286:28456–28465. doi: 10.1074/jbc.M111.226894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dunham PB, Ellory JC. Stimulation of the sodium-potassium pump by trypsin in low potassium type erythrocytes of goats. J Physiol. 1980;301:25–37. doi: 10.1113/jphysiol.1980.sp013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Altamirano AA, et al. Vanadate and fluoride effects on Na+-K+-Cl− cotransport in squid giant axon. Am J Physiol. 1988;254:C582–C586. doi: 10.1152/ajpcell.1988.254.4.C582. [DOI] [PubMed] [Google Scholar]
- 80.Lytle C, Forbush B., 3rd Regulatory phosphorylation of the secretory Na-K-Cl cotransporter: modulation by cytoplasmic Cl. Am J Physiol. 1996;270:C437–C448. doi: 10.1152/ajpcell.1996.270.2.C437. [DOI] [PubMed] [Google Scholar]
- 81.Haas M, Forbush B., III The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol. 2000;62:515–534. doi: 10.1146/annurev.physiol.62.1.515. [DOI] [PubMed] [Google Scholar]
- 82.Adragna NC, et al. Signal transduction mechanisms of K+-Cl− cotransport regulation and relationship to disease. Acta Physiol. 2006;187:125–139. doi: 10.1111/j.1748-1716.2006.01560.x. [DOI] [PubMed] [Google Scholar]
- 83.Piechotta K, et al. Cation chloride cotransporters interact with the stress-related kinases ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1) J Biol Chem. 2002;277:50812–50819. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- 84.Choe KP, Strange K. Evolutionarily conserved WNK and Ste20 kinases are essential for acute volume recovery and survival after hypertonic shrinkage in Caenorhabditis elegans. Am J Physiol Cell Physiol. 2007;293:C915–C927. doi: 10.1152/ajpcell.00126.2007. [DOI] [PubMed] [Google Scholar]
- 85.de Los Heros P, et al. WNK3 bypasses the tonicity requirement for K-Cl cotransporter activation via a phosphatase-dependent pathway. Proc Natl Acad Sci USA. 2006;103:1976–1981. doi: 10.1073/pnas.0510947103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Piechotta K, et al. Characterization of the interaction of the stress kinase SPAK with the Na+-K+-2Cl− cotransporter in the nervous system: evidence for a scaffolding role of the kinase. J Biol Chem. 2003;278:52848–52856. doi: 10.1074/jbc.M309436200. [DOI] [PubMed] [Google Scholar]
- 87.Gagnon KB, et al. A single binding motif is required for SPAK activation of the Na-K-2Cl cotransporter. Cell Physiol Biochem. 2007;20:131–142. doi: 10.1159/000104161. [DOI] [PubMed] [Google Scholar]
- 88.Kahle KT, et al. WNK protein kinases modulate cellular Cl− flux by altering the phosphorylation state of the Na-K-Cl and K-Cl cotransporters. Physiology. 2006;21:326–335. doi: 10.1152/physiol.00015.2006. [DOI] [PubMed] [Google Scholar]
- 89.Tyzio R, et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343:675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- 90.Piala AT, et al. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal. 2014;7:ra41. doi: 10.1126/scisignal.2005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gagnon KB, et al. Characterization of SPAK and OSR1, regulatory kinases of the Na-K-2Cl cotransporter. Mol Cell Biol. 2006;26:689–698. doi: 10.1128/MCB.26.2.689-698.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lang F. Swelling-activated release of excitatory’amino acids in the brain: relevance for pathophysiology. Contrib Nephrol. 1998;23:24m257. doi: 10.1159/000059916. [DOI] [PubMed] [Google Scholar]
- 93.Payne JA, et al. Molecular characterization of a putative K-Cl cotransporter in rat brain: a neuronal-specific isoform. J Biol Chem. 1996;271:16245–16252. doi: 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- 94.Alvarez-Leefmans FJ, et al. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol. 1988;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shields SD, et al. Anatomical and functional analysis of aquaporin 1, a water channel in primary afferent neurons. Pain. 2007;131:8–20. doi: 10.1016/j.pain.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 96.Gagnon KB, et al. Volume sensitivity of cation-Cl− cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol. 2006;290:C134–C142. doi: 10.1152/ajpcell.00037.2005. [DOI] [PubMed] [Google Scholar]
- 97.Badaut J, et al. Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 2002;22:367–378. doi: 10.1097/00004647-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 98.Andrew RD, et al. Physiological evidence that pyramidal neurons lack functional water channels. Cereb Cortex. 2007;17:787–802. doi: 10.1093/cercor/bhk032. [DOI] [PubMed] [Google Scholar]
- 99.Gisselsson LL, et al. Actin redistribution underlies the sparing effect of mild hypothermia on dendritic spine morphology after in vitro ischemia. J Cereb Blood Flow Metab. 2005;25:1346–1355. doi: 10.1038/sj.jcbfm.9600131. [DOI] [PubMed] [Google Scholar]
- 100.Anderson TR, et al. Blocking the anoxic depolarization protects without functional compromise following simulated stroke in cortical brain slices. J Neurophysiol. 2005;93:963–979. doi: 10.1152/jn.00654.2004. [DOI] [PubMed] [Google Scholar]
- 101.MacAulay N, et al. Water transport in the brain: role of cotransporters. Neuroscience. 2004;129:1031–1044. doi: 10.1016/j.neuroscience.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 102.Lang F, et al. Functional significance of channels and transporters expressed in the inner ear and kidney. Am J Physiol Cell Physiol. 2007;293:C1187–C1208. doi: 10.1152/ajpcell.00024.2007. [DOI] [PubMed] [Google Scholar]
- 103.Hartmann AM, et al. Evolution of the cation chloride cotransporter family: ancient origins, gene-losses, and subfunctionalization through duplication. Mol Biol Evol. 2013;31:434–447. doi: 10.1093/molbev/mst225. [DOI] [PubMed] [Google Scholar]
- 104.Verissimo F, Jordan P. WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene. 2001;20:5562–5569. doi: 10.1038/sj.onc.1204726. [DOI] [PubMed] [Google Scholar]
- 105.Johnston AM, et al. SPAK, a STE20/SPS1-related kinase that activates the p38 pathway. Oncogene. 2000;19:4290–4297. doi: 10.1038/sj.onc.1203784. [DOI] [PubMed] [Google Scholar]
- 106.Delpire E, Gagnon KB. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J. 2008;409:321–331. doi: 10.1042/BJ20071324. [DOI] [PubMed] [Google Scholar]
- 107.Rusan ZM, et al. Modeling glial contributions to seizures and epileptogenesis: cation–chloride cotransporters in Drosophila melanogaster. PLoS ONE. 2014;9:e101117. doi: 10.1371/journal.pone.0101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leiserson WM, et al. Fray, a Drosophila serine/threonine kinase homologous to mammalian PASK, is required for axonal ensheathment. Neuron. 2000;28:793–806. doi: 10.1016/s0896-6273(00)00154-9. [DOI] [PubMed] [Google Scholar]