Abstract
Purpose
Slow accrual to cancer clinical trials impedes the progress of effective new cancer treatments. Poor physician–patient communication has been identified as a key contributor to low trial accrual. Question prompt lists (QPLs) have demonstrated a significant promise in facilitating communication in general, surgical, and palliative oncology settings. These simple patient interventions have not been tested in the oncology clinical trial setting. We aimed to develop a targeted QPL for clinical trials (QPL-CT).
Method
Lung, breast, and prostate cancer patients who either had (trial experienced) or had not (trial naive) participated in a clinical trial were invited to join focus groups to help develop and explore the acceptability of a QPL-CT. Focus groups were audio-recorded and transcribed. A research team, including a qualitative data expert, analyzed these data to explore patients’ decision-making processes and views about the utility of the QPL-CT prompt to aid in trial decision making.
Results
Decision making was influenced by the outcome of patients’ comparative assessment of perceived risks versus benefits of a trial, and the level of trust patients had in their doctors’ recommendation about the trial. Severity of a patient’s disease influenced trial decision making only for trial-naive patients.
Conclusion
Although patients were likely to prefer a paternalistic decision-making style, they expressed valuation of the QPL as an aid to decision making. QPL-CT utility extended beyond the actual consultation to include roles both before and after the clinical trial discussion.
Keywords: Clinical trials, Question prompt lists, Physician–patient communication
Introduction
Slow accrual to cancer clinical trials has been identified as the greatest impediment to winning the “war on cancer” [1]. It is estimated that less than 5% of all adult cancer patients enter clinical trials [2-4]. Even lower rates of enrollment have been reported in underserved populations [5]. Poor physician–patient communication has been identified as a key contributor to low accrual [6-8]. Although identified as a significant challenge [9], oncologists need to communicate effectively the purpose, technical aspects, and implications of trials to patients [6, 10, 11], and to assist eligible patients in decision making. Moreover, patients commonly fail to understand and correctly recall the information they receive about trials they are asked to consider [12, 13].
Informed consent requires that patients understand the information that has been provided and be competent to weigh possible courses of action. Informed consent is intended to protect patient autonomy and ensure that patients have an active role in making treatment decisions [14-17]. However, the complex language and excessive detail of some clinical trial information statements and consent forms may confuse rather than enhance patient understanding [18]. Unfortunately, patients frequently do not understand the rationale for trials and may not recall that they are receiving treatment in a clinical trial [12, 13]. Although cancer patients typically express high informational needs regarding available treatment options, their preferences for decisional involvement are variable [19-23]. Unfortunately, the literature suggests that physicians are not effectively ascertaining patients’ informational or decision-making needs [24, 25]. To date, efforts to improve the quality of communication about informed consent have typically targeted improvement of physician communication, yet studies exploring the content of clinical trial discussions reveal that physicians remain dominant in these consultations [26-28]. Without appreciation of patients’ informational and decision-making needs, it is not surprising that informed consent and patient satisfaction with treatment decision making remain suboptimal [24, 25]. Thus, there is a need to understand more fully the patient-level factors that contribute to high quality informed consent regarding clinical trial participation.
Various methods have been described in the literature to assist doctors and patients in discussing clinical trials and aiding decision making including clinical trial-specific decision aids, educational videos, and communication skills training for oncologists. However, rigorous research in this area is in its infancy, and the efficacy of these methodologies is yet to be proven. Question prompt lists (QPLs) [29-32] have demonstrated a significant promise in aiding doctor–patient communication by promoting an active patient participation in their general, surgical, and palliative oncology consultations. QPLs consist of a written sample of questions separated into content categories (e.g., diagnosis and prognosis). Previous studies have shown that QPLs are useful in the general oncology setting as an aid to patient question asking, particularly regarding prognosis. In addition, patient outcomes are improved when patients ask questions and their oncologist endorses questions [29-31]. Salient questions will undoubtedly vary across oncology contexts, and therefore, researchers have developed QPLs with different question content for palliative and surgical oncology contexts [33, 34]. Investigators [33, 34] have used focus groups with patients in their target populations to develop new questions specific to these areas and then pilot tested these new QPLs in the clinical setting. The results of pilot testing have shown QPLs to be acceptable, understandable, and valued by patients [33, 34]. The first author (RB) previously collaborated to develop and conduct initial testing of a QPL that targeted patient information needs specific to informed consent to cancer clinical trials in an Australian oncology setting. Due to cultural and health system differences, the results of this preliminary work needed validation in the US setting.
Thus, to develop further a clinical trial-specific QPL by gathering a broad range of patient views, we conducted focus groups with two distinct groups of cancer patients, those who had participated (trial experienced) and those who had not participated (trial naive) in a clinical trial. Our goal was to explore patient views about their clinical trial information needs and decision-making processes, and obtain feedback about the utility and completeness of the previously developed QPL. In this paper, we present data about the role of the QPL in patients’ treatment decision making.
Methods
Participants
Oncologists
Six medical oncologists from three services—breast, lung, and genitourinary (GU) oncology (two from each service)—at a comprehensive cancer center in New York City agreed to use their outpatient clinics to recruit study participants.
Patients
Participants were adult cancer patients who were treated at the participating cancer center. They were recruited consecutively during follow-up visits. All participants were classified as either “trial experienced” or “trial naive.” Exclusion criteria consisted of: (a) age less than 18 years, (b) not English speaking, and (c) cognitive or physical impairment rendering the patient incapable of providing informed consent to participate in this study.
Procedure
Each of the six participating oncologists and the principal investigator (RB) randomly selected ten patients who had participated in a clinical trial and ten who had not participated in a clinical trial in 12 months prior to the study period, from each oncologist’s patient lists. Our goal was to generate a list of 120 patients. As one oncologist had a small number of patients who participated in a trial, only five of his patients were selected. Thus, 115 eligible patients were identified. Of this total of 115, 55 had participated in a clinical trial, and 60 patients had not. From this available pool of 115 patients, we aimed to recruit between 24 and 32 patients (see sample size calculation below). The research assistant (RA) mailed a recruitment letter to patients that explained the study purpose, an invitation to attend a focus group, and a response form that patients could return via a prepaid envelope if they did not wish to be contacted about the study. The RA called those patients who had not returned a response form within 2 weeks of the initial mailing and discussed study participation. Interested patients gave verbal assent to participate and were scheduled for a focus group. Patients who attended the focus group were compensated $25 for their time and effort.
Focus group procedure
The focus groups were designed and conducted according to well-established methodologies [35, 36]. The investigators developed guides for each focus group containing a set of relevant topics and subsidiary questions. In the focus groups with patients who had participated in a clinical trial, we asked participants to answer questions retrospectively, i.e., as experienced participants. Patients who had not participated in a clinical trial were asked to answer questions prospectively, i.e., as trial naive. The focus group guide questions covered: needs for information when making trial decisions, the process of trial decision making, and the utility of the QPL (see Table 1 for sample questions).
Table 1.
Focus group probes
| Experience of the clinical trial |
| I’d like to talk about your experiences with the clinical trial you participated in. |
| Thinking back, |
| What were your thoughts and feelings about the clinical trial? |
| What were your impressions of the trial? |
| How did your experience meet your expectations? |
| Did you find it difficult to ask questions about the clinical trial? Why or why not? |
| Information needs about trials |
| Now I would like to talk with you about particular issues you wanted to know about before you joined the trial. |
| What were some of the issues you were interested in finding out about the clinical trial? |
| Did you find it difficult to get information about the trial? |
| Were you left with questions about the trial at the time you gave consent? |
| Thinking back, were there issues that you would have like to have been covered about the trial that came up for you after you had joined or once you completed the trial? |
| What are the important issues about clinical trials you would tell someone who was facing trial decision? |
| Here is the question prompt list. Please take a moment to read through it. |
| Completeness of the question prompt list (QPL) |
| Now that you have reviewed the question prompt list, I would like to find out about your impressions of the completeness of the list. |
| In what ways does the QPL provide a good coverage of the issues that you were interested in either before you joined the trial or after you joined? |
| What topics are not covered that in your experience would be good to include on the list? |
| Acceptability of the question prompt list |
| I am interested in your thoughts about whether you would have found the question prompt list useful when you were considering participating in the clinical trial. |
| Do you think you would have tried to use the QPL in your consultations where you were discussing a clinical trial with your oncologist? If you had used it how would you have found it useful? |
| If you do not think it would be useful, what are your reasons? |
| What kinds of issues do you think would have prevented you from using the QPL? |
| Ending/conclusion of focus group |
| Okay, we’ve covered all the issues that I wanted to discuss. Before we finish, I want to give you the opportunity to reflect on our discussion and share anything that you think is relevant to the question prompt list that didn’t come up during the conversation. |
| Again, thank you very much for your honesty and willingness to participate in this group. We have learned a lot today, and this will help us with helping patients’ communication with their doctors about clinical trials. If you’d like, please feel free to stay a bit if you have any questions about our research, or have any other comments you’d like to share. |
Immediately prior to the focus group, participants signed informed consent and completed a brief demographic questionnaire that included questions about age, gender, marital status, education level, occupation, nationality, first language, and whether the participant had previously participated in a clinical trial. Two authors, ES and RB, jointly moderated each focus group. The focus group guides were used as tools to moderate the discussions and allowed time for participants to discuss issues that were not included. Each focus group was audio-recorded and subsequently transcribed. The QPL was distributed during the focus group after a discussion of information needs and decision-making processes had occurred. The QPL is presented as Appendix 1.
Qualitative analysis plan
The research team reviewed and interpreted the data using thematic text analysis with an inductive, data-driven approach [37-39]. ATLAS.ti was used to manage the data coding [40]. Consistent with this method, each member independently developed codes to represent the underlying meaning of the text. The research team then met regularly to compare codes and achieve consensus. During these meetings, the team achieved consensus about code names and meanings and, through this process, developed a codebook. We ultimately created a codebook consisting of 90 descriptive codes. Our codes were descriptive in nature in that they each represented a description of distinct phenomena we identified through our coding process [39]. We identified a set of 27 codes that were most relevant to issues relating to clinical trial decision making and three codes within the category of assessment of the QPL description. We worked together in our consensus meetings to describe these recurrent themes and provide illustrative examples of each theme from our focus group data. Rigor in our qualitative analysis was derived from successive rounds of iterative consensus work among multiple team members who analyzed the focus group transcripts [41].
Sample size consideration
Based on established sample size recommendations for focus groups [35, 36] with the goal to achieve theoretical saturation (no new themes emerging), we aimed to recruit 11 patients each to four focus groups resulting in 44 potential participants. We estimated an attrition (no show) rate of approximately 20% leaving six to eight participants per group. Thus, our expected sample size was 24–32 participating patients.
The study received approval by the institutional review board at the participating institution.
Results
Participant characteristics
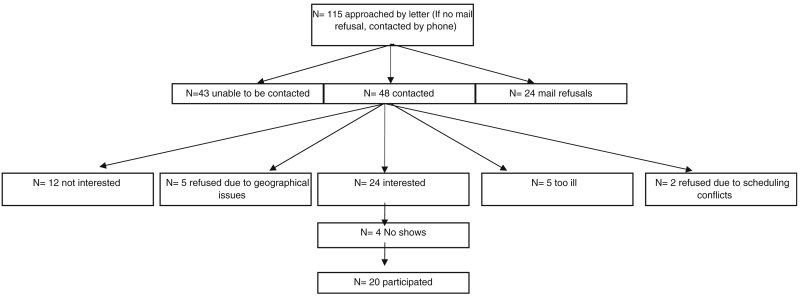
Twenty patients were recruited into four focus groups (two trial experienced and two trial naive). We recruited 9/55 trial-experienced patients and 11/60 trial-naive patients. An overview of our recruitment process of the 20 participants, including reasons for nonparticipation, is presented in Fig. 1. After four focus groups, no new themes emerged; therefore, as we had achieved our aim of theoretical saturation, recruitment ceased.
Fig. 1.
Sampling schema and flowchart of recruitment for question prompt list focus groups
Patients were mostly female (70%), with an average age of 60 years. Eight patients had been diagnosed with lung cancer, eight with breast cancer, and four with genitourinary cancer. Most patients (12/20) had advanced disease. Of nine trial-experienced patients, six had participated in a phase II trial and three in a phase I trial. Five of the 55 trial-experienced patients had participated in a phase III study; however, none agreed to participate in the focus groups (see Table 2).
Table 2.
Demographic and disease characteristics of patient sample
| Gender (n=20) | |
| Female | 14 (70%) |
| Male | 6 (30%) |
| Average age (n=20) | 60 years (range 36–83) |
| Education (n=20) | |
| Junior high school | 0 (0%) |
| Senior high school | 3 (15%) |
| High school equivalency General Educational Development (GED) |
2 (10%) |
| Technical degree | 0 (0%) |
| Junior college degree | 0 (0%) |
| Undergraduate degree | 5 (25%) |
| Higher degree (postgraduate) | 10 (50%) |
| Marital status (n=20) | |
| Single | 2 (10%) |
| Living together with partner | 0 (0%) |
| Married | 15 (75%) |
| Widowed | 2 (10%) |
| Divorced/separated | 1 (5%) |
| Other | 0 (0%) |
| Primary tumor site (n=20) | |
| GU oncology | 4 (20%) |
| Breast | 8 (40%) |
| Lung | 8 (40%) |
| Stage of disease (n=20) | |
| GU oncology (n=4) | |
| Stage I | 0 |
| Stage II | 1 |
| Stage III | 0 |
| Stage IV | 3 |
| Breast (n=8) | |
| Stage I | 2 |
| Stage II | 2 |
| Stage III | 4 |
| Stage IV | 0 |
| Lung (n=8) | |
| Stage I | 2 |
| Stage II | 1 |
| Stage III | 1 |
| Stage IV | 4 |
| Phase of trial (n=9) | |
| Phase I | 3 (33.3%) |
| Phase II | 5 (55.5%) |
| Phase III | 0 (0%) |
| Other | 1 (11.1%) |
Decision-making themes
Our qualitative analysis revealed that two factors predominantly influenced patient decision making regarding clinical trials—the outcome of patients’ comparative assessment of perceived risks versus benefits of a trial and the level of trust patients had in their doctors’ recommendation regarding whether a trial would be an appropriate medical option for them. The severity of a patient’s disease influenced trial decision making only for trial-naive patients. We then compared the similarities and differences between trial-experienced and trial-naive patients for each of these decision-making factors.
Assessment of perceived risks versus benefits of a trial
Overall, trial-experienced patients were more likely to regard clinical trials more positively than trial-naive patients, who tended to focus more on the potential risks from trials. Many trial-experienced patients expressed faith in the survival benefits of the trial treatment. These patients expressed a desire to “do whatever it is to survive” and were minimally concerned with potential negative side effects, financial costs, or the possibility that there might be a potential conflict of interest being industry-sponsored.
In contrast, trial-naive patients were more likely to have negative perceptions about clinical trials and would not be receptive to a clinical trial without concrete evidence about personal survival benefit. One trial-naive patient stated, “I don’t know if I would want a clinical study unless I really knew that maybe something good would come out of it. Otherwise I would feel as if it was just something they just wanted some data on.” Another trial-naive patient declined to participate in two trials that she was offered, as she was not convinced that the trial would be the best treatment option for her.
“So when I was asked to do two different clinical trials. And honestly my big question was ‘what’s in it for me…I didn’t want to be Saint Teresa or anybody. I really didn’t. I just said no if this isn’t like the best thing that’s gonna have a good outcome for me.’”
The primacy of trust in physician recommendations
Trust in their doctor’s recommendation regarding the benefit of a clinical trial was also central to trial decision making for both trial-experienced and trial-naive patients. Trial-experienced patients sought their doctors’ recommendation through two primary ways. First, many patients directly asked for their doctor’s expert recommendation and made their trial decision accordingly. Expert opinion was especially vital for patients who expressed feeling overwhelmed with the decision. One trial-experienced patient stated, “So I was trying to balance the risks and she’s the expert and I’m not…plus you’re so overwhelmed that you just want somebody to tell you what to do.” Second, some trial-experienced patients reported asking for a recommendation indirectly by using proxy questions. An example was asking whether the physician would recommend the trial to a close family member. Some trial-experienced patients reported that receiving a positive or negative answer to this proxy question could be a deal breaker in their decision making, as noted below.
“Because imagine what that would say if your doctor sat there and said no. If my wife were in your shoes I would not allow her in this trial…I mean I don’t know that I’d give it another thought.… It’s almost like that question ‘is this worth me going forward with?’”
Trial-naive patients indicated that, if they developed trust in their doctors’ medical judgment, they would be likely to follow the doctors’ guidance. One trial-naive patient reported, “I came here for only the best…If he came to me, my doctor, and said let’s do a clinical trial, I would do it. I trust him.”
Other trial-naive patients regarded the manner in which their physician presents the trial opportunity to them as central in engendering trust with the doctor’s recommendation. One patient stated that she would feel comfortable with her doctor’s judgment if the physician openly shared trial information and answered questions completely. Trial-naive patients differed in their views about the physician introducing a trial as a treatment option. Some patients would view their doctor as “looking out for” them if they suggested they consider a trial. On the other hand, one patient disliked that her own doctor presented her with a trial, as during standard treatment discussions, she had followed the doctor’s treatment recommendation and been a “loyal patient.” The trial decision-making process challenged her to make up her own mind about participation.
Severity of disease
As trial-naive patients were asked to discuss the factors that would influence their decision making if they were presented with the option to join a clinical trial, many stated that, if their disease were at a later stage or if they had exhausted other treatment options, they would be receptive to join a trial. Several of these trial-naive patients stated that they would be open to a trial if a trial was the last resort to improve their disease, or if they had already “gone through the arsenal” of other treatments. Severity of disease was not an important trial decision-making factor for trial-experienced patients.
Utility of the QPL for decision making
Patients viewed the QPL as a valuable tool to support their decision making regarding clinical trials. Patients divided the potential utility of the QPL into three categories: (a) preclinical trial consultation, (b) during a consultation, and (c) postconsultation.
Preconsultation benefits included the ability of the QPL to save patient’s time, in that they would not need to prepare questions on their own. The QPL presents patients with a menu of potential issues to discuss with a physician and thus prepares patients for a medical consultation. One trial-naive patient said of the questions in the QPL, “I found the questions themselves informed my thinking…the way they were phrased made me realize something or gave me information. And I would be very pleased to get that.” Some patients found the questions educational as they include trial-related terminology (e.g., randomized, blinded, response rate) that patients may not have known. One trial-naive patients noted that “the fact of even using the word, randomized, in here. It tells me something that I wouldn’t… may not have known.”
During consultation, benefits included enhanced the ability of patients to have meaningful discussions with physicians and helped patients use the limited time they had with their doctors to their best advantage. One trial-naive patient commented on the time aspect saying “I like the idea that if I had this (the QPL) in advance, I could just narrow it down to what’s really important to me and focus her and my energy because you do have a limited time period. So I think I would like this for everything.”
Patients also reported postconsultation benefits including that the QPL is a concrete takeaway that patients can review and be better prepared for a follow-up discussion. One trial-naive patient reported: “So if you had a piece of paper that outlines what it (the trial) is, and then you had these questions, then you could call back at a pre-determined time to talk to someone about it. And then it would help you make your decision, but I think these questions are very thoughtful and very good.” In addition, patient mentioned that seeing the question list might help them to remember the details of their clinical trial discussions with doctors.
Discussion
The aim of this study was to further develop a QPL for clinical trial discussions and assess its perceived utility to aid decision making using focus groups with trial-experienced and trial-naive patients. Cancer patients find it difficult to make decisions about clinical trials [42, 43]. Consistent with prior work, our results show that clinical trial decision making is primarily influenced by a trade-off between hope for personal treatment benefit and the risks of side effects [44], and by trust in the physician [45]. Most studies show that patient preferences for decision making vary with most well patients preferring a collaborative role [46-48], yet many sick patients prefer the doctor to make decisions on their behalf [49]. Most of our sample had stage 3 or 4 cancers and expressed a desire to make a trade-off guided by a trusted clinician who would steer them to an optimal treatment decision. Although shared decision making about treatment options is a well-accepted approach [46, 50, 51], paternalistic decision making was desired by our sample. Trial-naive patients indicated that they would choose a clinical trial only when all other options were exhausted. This is likely explained in part by these patients’ negative perceptions of trials in comparison with the trial-experienced patients. It may also be that trial-naive patients perceived that they had more available options than the experienced patients who opted for the trial.
For patients with advanced disease, decision making about whether to enroll in a clinical trial is complex. Patients’ decision-making processes may change as their illness progresses. Patients in our sample were enthusiastic about the QPL. Trial-experienced patients endorsed that it would have been helpful in their trial decision making. Trial-naive patients expressed a desire to use such a tool if they were faced with a choice about whether to join a trial. Much of the previous QPL, research has focused on the usefulness of a QPL within the cancer consultation as an aid to activate patient question asking [29, 30, 33, 34]. Focus group patients also noted the usefulness of the QPL outside of the consultation as a means of preparing for the consultation and as an education aid. Moreover, they expressed that the QPL could provoke patients to consider novel issues, gain salient information, be better informed for future trial discussions, and help make a trial decision. Future research could usefully measure the efficacy of the QPL to achieve these potential benefits.
These promising results suggest that a clinical trial QPL would be a valuable aid for patients facing difficult treatment decisions. Future research is underway to test the utility of the QPL in actual consultations, and to determine its impact on question asking and consultation communication. We will also assess the potential impact on decisions to accrue to a clinical trial.
Acknowledgments
This project was funded by the United States National Cancer Institute RO3 Small Grants Award—CA130598.
Appendix 1 Question prompt list
“I went into the consultation room and forgot every question I had.”
Introduction
People are often anxious when given the diagnosis of cancer and faced with making decisions about treatment. Being invited to join a clinical trial can make this process more difficult as there are extra options and concepts to think about. People come to the specialist to have the benefit of his/her knowledge, expertise and care. Often with the stress of the moment important questions can be forgotten.
The purpose of this question list is to
act as a prompt, if you so desire, in gaining relevant and important information about the clinical trial being considered and
assist you to make an informed decision regarding your treatment.
These questions have been developed after much discussion with patients who have been through the experience of participating in a clinical trial. They have been reviewed by oncologists and other health professionals who are involved in the care of people diagnosed with cancer faced with making a decision about trial participation. Your specialist will be pleased to answer any questions you may have and additional space has been included for you to jot down your own questions or concerns that you may wish to discuss in your consultation.
You may wish to use this prompt list during the first consultation where your specialist invites you to participate in a trial or you may choose to ask some of the questions at a later stage. Different people want different things at different times. You or your family may find it helpful as a reference, it is up to you. Please do not feel you should ask questions just because they are listed.
We have organised the list under headings. You may find that some are very relevant to you, and others are not.
If you have any further questions or comments on this brochure please do not hesitate to contact me, Dr Richard Brown at 646 888 0011.
Understanding my choices
What is the usual (standard) treatment for people in my situation?
Why are you offering me this particular trial? Does it ask an important question in cancer treatment?
Are there choices other than the trial and the standard treatment?
What other trials am I eligible for? What makes me eligible (or not)?
Finding out more information about this trial
-
5.
How can I learn more about the trial? Can I speak to someone who is already participating in this trial?
Understanding the trial’s purpose and background
-
6.
What is the purpose of this trial?
-
7.
What is already known about this treatment’s success?
-
8.
How does the treatment work?
Understanding the possible benefits
-
9.
What benefits could I possibly get if I join the trial?
-
10.
If I join this trial how might others benefit?
-
11.
Has the benefit of the new treatment already been proven in people like me?
-
12.
(If doctor describes response to treatment) What does response rate mean? How long would a response last?
Understanding the possible risks
-
13.
What are the risks of taking the new treatment? Are there any long-term or permanent side effects from the treatment? Are there any serious or rare side effects that I should know about?
-
14.
Will there be side effects on the trial which I won’t get on the standard treatment? Are there different side effects depending on which arm I am randomised to receive?
-
15.
Whom can I call if something goes wrong?
-
16.
If I get a side effect or injury because of being in the trial, will I get compensation?
The differences between going on the trial and having the standard treatment
-
17.
If I enter the clinical trial, will it require me to have extra tests, to attend more clinics and will it cost me extra money? (extra parking, extra medication?)
-
18.
How often will I need to come in for treatment, and is that different from if I took the standard treatment?
-
19.
Will the treatment be given by experienced staff? Where will the treatment be given?
Understanding how the trial is being carried out
-
20.
Is the new treatment only available through joining in the trial?
-
21.
How long has the trial been going on? How many people will be studied and how many are on the trial already? Are there any concerns about the trial or treatment so far?
-
22.
Apart from the hospital staff, will other people have access to my medical records? Who? How will my confidentiality be protected?
-
23.
If the new treatment is beneficial, how can I get it (if I am not already on it)? How will I be informed of the results of the trial?
-
24.
How will the results of the trial be used?
Understanding randomization and blinding
-
25.
Is this trial randomised? What does that mean and why is it important?
-
26.
Will I know what treatment I am getting, or is this trial blinded? What does that mean and why is it important in this trial? Will I ever know what treatment I am getting?
Understanding possible conflicts of interest
-
27.
Are you in charge of the trial (the principal investigator)? If not, what’s your role in the trial?
-
28.
Is there a payment made by the trial sponsor/company to the hospital or to you as my doctor if I go on this trial? Could you tell me how much money and is this usual? How is the money spent?
Understanding my right to join or not to join the trial
-
29.
Will you still treat me if I decide not to go on the trial?
-
30.
Do I have time to think about whether to go on the trial (a day or two, or a week)? Will taking time to decide affect how well the treatment works?
-
31.
If I join the trial, but later change my mind, how can I stop? Will I be penalised in any way?
-
32.
If I join the trial will I be losing out on any new treatment opportunities (such as another trial or standard treatment later)?
Alternative Therapies
-
33.
Can I still have alternative therapies if I go on the trial (eg vitamins, herbal remedies, naturopathy, dietary changes)?
Footnotes
Conflict of interest No authors have a financial relationship with the project sponsors. The authors have primary control of the data that are available for review on request.
Contributor Information
Richard F. Brown, Department of Social and Behavioral Health, Virginia Commonwealth University School of Medicine, 1112 East Clay St, Richmond, VA 23298-0149, USA
Elyse Shuk, Department of Psychiatry and Behavioral Sciences, Memorial Sloan Kettering Cancer Center, 641 Lexington Ave, 7th Floor, New York, NY 10022, USA.
Natasha Leighl, Ontario Cancer Institute, Princess Margaret Hospital, 610 University Ave, Toronto, ON M5G 2M9, Canada.
Phyllis Butow, Centre for Medical Psychology and Evidence-based Decision-making, School of Psychology, University of Sydney, Brennan/McCallum Building (A18), Sydney, NSW 2006, Australia.
Jamie Ostroff, Department of Psychiatry and Behavioral Sciences, Memorial Sloan Kettering Cancer Center, 641 Lexington Ave, 7th Floor, New York, NY 10022, USA.
Shawna Edgerson, Department of Psychiatry and Behavioral Sciences, Memorial Sloan Kettering Cancer Center, 641 Lexington Ave, 7th Floor, New York, NY 10022, USA.
Martin Tattersall, Centre for Medical Psychology and Evidence-based Decision-making, School of Psychology, University of Sydney, Brennan/McCallum Building (A18), Sydney, NSW 2006, Australia; Department of Cancer Medicine, School of Medicine, University of Sydney, Blackburn Building, Sydney, NSW 2006, Australia.
References
- 1.Kolata G. New York Times. New York Times Co; New York: 2009. Forty years’ war: lack of study volunteers hobbles cancer fight. [Google Scholar]
- 2.Ellis P, et al. Accrual to clinical trials in breast cancer; Annual Scientific Meeting of the Clinical Oncological Society of Australia; Brisbane, Australia. 1996. [Google Scholar]
- 3.Avis NE, et al. Factors associated with participation in breast cancer clinical trials. J Clin Oncol. 2006;24(12):1860–1867. doi: 10.1200/JCO.2005.03.8976. [DOI] [PubMed] [Google Scholar]
- 4.Lara PN, et al. Prospective evaluation of clinical trial accrual patterns: identifying potential barriers to enrolment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 5.Robinson JM, Trochim WMK. An examination of community members’, researchers“ and health professionals” perceptions of barriers to minority participation in medical research: an application of concept mapping. Ethn Health. 2007;12(5):521–539. doi: 10.1080/13557850701616987. [DOI] [PubMed] [Google Scholar]
- 6.Lee JY, Breaux SR. Accrual of radiotherapy patients to clinical trials. Cancer. 1983;52:1014–1016. doi: 10.1002/1097-0142(19830915)52:6<1014::aid-cncr2820520614>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Martin JF, Henderson WG, Zacharski LG. Accrual of patients into a multi hospital cancer clinical trial and its implications on planning future studies. Am J Clin Oncol. 1984;7:173–182. doi: 10.1097/00000421-198404000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Mills EJ, et al. Barriers to participation in clinical trials of cancer: a meta analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7(2):141–148. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 9.Fallowfield L. Can we improve the professional personal fulfilment of doctors in cancer medicine. Br J Cancer. 1995;71:1132–1133. doi: 10.1038/bjc.1995.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallowfield L, Ratcliffe D, Souhami RL. Clinicians’ attitudes to clinical trials of cancer therapy. Eur J Cancer. 1997;33:2221–2229. doi: 10.1016/s0959-8049(97)00253-0. [DOI] [PubMed] [Google Scholar]
- 11.Hall A. In: The role of effective communication in obtaining informed consent in medical research. Loyal L, Tobias JS, editors. BMJ Books; London: 2001. pp. 290–298. [Google Scholar]
- 12.Benson A, Pregle J, Bean J. Oncologists reluctance to accrue patients onto clinical trials. J Clin Oncol. 1991;9:2067–2075. doi: 10.1200/JCO.1991.9.11.2067. [DOI] [PubMed] [Google Scholar]
- 13.Penman D, Holland J, Bahna G. Informed consent for investigational chemotherapy; patients’ and physicians’ perceptions. J Clin Oncol. 1984;2:849–855. doi: 10.1200/JCO.1984.2.7.849. [DOI] [PubMed] [Google Scholar]
- 14.Cahn CH. Consent in psychiatry. The position of the Canadian Psychiatric Association. Can J Psychiatry. 1980;25:78–84. doi: 10.1177/070674378002500115. [DOI] [PubMed] [Google Scholar]
- 15.Finklestein D, Karsh-Smith M, Faden R. Informed consent in medical ethics. Arch Ophthalmol. 1993;111:324–326. doi: 10.1001/archopht.1993.01090030042034. [DOI] [PubMed] [Google Scholar]
- 16.Kirby MD. Informed consent: what does it mean. J Med Ethics. 1983;9:69–75. doi: 10.1136/jme.9.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebacqz K, Levine RJ. Respect for persons and informed consent to participate in cancer research. J Clin Res. 1977;25:101–107. [PubMed] [Google Scholar]
- 18.Grossman SA, Piantadosi S, Cohavey C. Are informed consent forms that describe clinical oncology research protocols readable by most patients and their families? J Clin Oncol. 1994;12:2211–2215. doi: 10.1200/JCO.1994.12.10.2211. [DOI] [PubMed] [Google Scholar]
- 19.Cassileth BR, et al. Information and participation preferences among cancer patients. Ann Intern Med. 1980;92:832–836. doi: 10.7326/0003-4819-92-6-832. [DOI] [PubMed] [Google Scholar]
- 20.Blanchard CG, et al. Information and decision making preferences of hospitalised cancer patients. Soc Sci Med. 1988;27(11):1139–1145. doi: 10.1016/0277-9536(88)90343-7. [DOI] [PubMed] [Google Scholar]
- 21.Whelan T, Mohide EA, Willan AR. The supportive care needs of newly diagnosed cancer patients attending a regional cancer centre. Cancer. 1997;80:1518–1524. doi: 10.1002/(sici)1097-0142(19971015)80:8<1518::aid-cncr21>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins V, Fallowfield L, Saul J. Information needs of cancer patients: results from a large study in UK cancer centres. Br J Cancer. 2001;84:48–51. doi: 10.1054/bjoc.2000.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleissig A, Jenkins VA, Fallowfield L. Results of an intervention study to improve communication about randomised clinical trials of cancer therapy. Eur J Cancer. 2000;37:322–331. doi: 10.1016/s0959-8049(00)00415-9. [DOI] [PubMed] [Google Scholar]
- 24.Strull WM, Lo B, Charles G. Do patients want to participate in medical decision making? J Am Med Assoc. 1984;252(21):2990–2994. [PubMed] [Google Scholar]
- 25.Bilodeau BA, Degner LF. Information needs, sources of information, and decisional roles in women with breast cancer. Oncol Nurs Forum. 1996;23(4):691–696. [PubMed] [Google Scholar]
- 26.Butow PN, et al. A randomized trial of a consultation skills training to improve communication about cancer treatment options and clinical trials: interim analysis using Australian and New Zealand data. Psycho-oncol. 2006;14((2):Supplement S38) [Google Scholar]
- 27.Brown RF, et al. Developing ethical strategies to assist oncologists in seeking informed consent to cancer clinical trials. Soc Sci Med. 2004;58:379–390. doi: 10.1016/s0277-9536(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 28.Brown RF, et al. Seeking informed consent to cancer clinical trials: describing current practice. Soc Sci Med. 2004;58(12):2445–2457. doi: 10.1016/j.socscimed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Brown RF, et al. Promoting patient participation in the cancer consultation; evaluation of a prompt sheet and coaching in question asking. Br J Cancer. 1999;80(1/2):242–248. doi: 10.1038/sj.bjc.6690346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown RF, et al. Promoting patient participation and shortening cancer consultations: a randomised trial. Br J Cancer. 2001;85:1273–1279. doi: 10.1054/bjoc.2001.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butow PN, et al. Patient involvement in the cancer consultation: evaluation of a question prompt sheet. Ann Oncol. 1994;5:199–204. doi: 10.1093/oxfordjournals.annonc.a058793. [DOI] [PubMed] [Google Scholar]
- 32.Roter D. Patient participation in the patient-provider interaction: the effects of patient question asking on the quality of interaction, satisfaction and compliance. Health Educ Monogr. 1977;5:281–315. doi: 10.1177/109019817700500402. [DOI] [PubMed] [Google Scholar]
- 33.Clayton J, et al. Asking questions can help: development and preliminary evaluation of a question prompt list for palliative care patients. Br J Cancer. 2003;89:2069–2077. doi: 10.1038/sj.bjc.6601380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McJannett M, et al. Asking questions can help: development of a question prompt list for cancer patients seeing a surgeon. Eur J Cancer Prev. 2003;12:397–405. doi: 10.1097/00008469-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Morgan D. Focus groups as qualitative research. Sage Publications; Thousand Oaks, CA: 1988. [Google Scholar]
- 36.Krueger R, Casey M. Focus groups: a practical guide for applied research. Sage Publications; Thousand Oaks: 2000. [Google Scholar]
- 37.Bernard HR, Ryan GW. Text analysis: qualitative and quantitative methods. In: Bernard HR, editor. Handbook of methods in cultural anthropology. Sage; Thousand Oaks: CA: 1998. [Google Scholar]
- 38.Creswell J. Qualitative inquiry and research design: choosing among five traditions. Sage Publications; Thousand Oaks, CA: 1988. [Google Scholar]
- 39.Miles MV, Huberman MA. Qualitative data analysis: a sourcebook of new methods. Sage; London: 1984. [Google Scholar]
- 40.Lewis RB. A comprehensive review of two leading qualitative data analysis packages. Cultural Anthropology Methods. 1998;10:41–47. [Google Scholar]
- 41.Morse JM, et al. Verification strategies for establishing reliability and validity in qualitative research. Int J Qual Stud Educ. 2002;1:1–19. [Google Scholar]
- 42.Flynn KE, et al. Decisional conflict among patients who accept or decline participation in phase I clinical trials. JERHRE. 2008;3(3):69–77. doi: 10.1525/jer.2008.3.3.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stryker JE, et al. Understanding the decisions of cancer clinical trial participants to enter research studies: factors associated with informed consent, patient satisfaction and decisional regret. Patient Educ Couns. 2006;63:104–109. doi: 10.1016/j.pec.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Cox AC, Fallowfield LJ, Jenkins VA. Communication and the informed consent process in phase I trials: a review of the literature. Support Care Cancer. 2006;14:303–309. doi: 10.1007/s00520-005-0916-2. [DOI] [PubMed] [Google Scholar]
- 45.Eggly S, et al. Oncologists’ recommendations of clinical trial participation to patients. Patient Educ Couns. 2008;70(1):143–148. doi: 10.1016/j.pec.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Soc Sci Med. 1997;44(5):681–692. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 47.Evans RG. Strained mercy: the economics of Canadian health care. Butterworths; Toronto: 1984. [Google Scholar]
- 48.Gattellari M, Butow PN, Tattersall MH. Sharing decisions in cancer care. Soc Sci Med. 2001;52(12):1865–1878. doi: 10.1016/s0277-9536(00)00303-8. [DOI] [PubMed] [Google Scholar]
- 49.Degner LF, Sloan JA. Decision making during serious illness: what role do cancer patients really want to play? J Clin Epidemiol. 1992;45:941–950. doi: 10.1016/0895-4356(92)90110-9. [DOI] [PubMed] [Google Scholar]
- 50.Charles C, Gafni A, Wheelan T. Decision making in the physician–patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651–661. doi: 10.1016/s0277-9536(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 51.Kaplan SH, et al. Characteristics of physicians with participatory decision making styles. Ann Intern Med. 1996;124:497–504. doi: 10.7326/0003-4819-124-5-199603010-00007. [DOI] [PubMed] [Google Scholar]