Abstract
Two distinct subsets of γδ T cells that produce interleukin 17 (IL-17) (CD27− γδ T cells) or interferon-γ (IFN-γ) (CD27+ γδ T cells) develop in the mouse thymus, but the molecular determinants of their functional potential in the periphery remain unknown. Here we conducted a genome-wide characterization of the methylation patterns of histone H3, along with analysis of mRNA encoding transcription factors, to identify the regulatory frames of peripheral IFN-γ-producing or IL-17-producing γδ T cell subsets in vivo. We found that CD27+ γδ T cells were committed to the expression of Ifng but not Il17, whereas CD27− γδ T cells displayed permissive chromatin configurations at loci related to both the Th17 and Th1 subsets of helper T cells and differentiated into cells producing both IL-17 and IFN-γ in a tumor microenvironment.
γδ T cells have emerged as key providers of interleukin 17 (IL-17) in various models of infection, inflammation and autoimmunity1–6. Antibody-mediated or genetic depletion of γδ T cells greatly reduces disease severity in IL-17-driven models of chronic inflammation1–4,7. Those results notwithstanding, many reports have made a compelling case for γδ T cells as the main producers of interferon-γ (IFN-γ) in both mice and humans8, which has been a major foundation for clinical trials targeting these lymphocytes in cancer immunotherapy9. Given the dual ability of γδ cells to produce IL-17 and IFN-γ, published work has aimed to identify markers that associate with functional attributes of mouse γδ T cells10–13. Expression of the costimulatory receptor CD27 segregates IL-17-producing (CD27−) γδ T cells and IFN-γ-producing (CD27+) γδ T cells in both naive and Plasmodium-infected C57BL/6 mice10. Moreover, the chemokine receptor CCR6, which is expressed exclusively on CD27− γδ T cells, constitutes an additional marker for IL-17+ γδ T cells13,14.
Both CD27+ and CD27− γδ T cell subsets show spontaneous cytokine secretion after activation, in contrast to the delayed differentiation of conventional CD4+ T cells of the Th1 or Th17 subset of helper T cells15. That finding is highlighted by the observation that 30–40% of peripheral γδ T cells freshly isolated from naive mice produce either IL-17 or IFN-γ after 3 h of restimulation in vitro10. Those functionally mature γδ T cell subsets are also found in the thymus, as early as the embryonic stages of mouse development10–12,16. Moreover, the expression of genes linked to the production of IL-17 or IFN-γ segregates with particular γδ thymocyte subsets17. However, the epigenetic 'landscape' of γδ T cells, which, as for CD4+ T cells18,19, probably dynamically controls the expression of signature cytokine-encoding genes and their transcriptional regulators, remains unknown. Among other epigenetic mechanisms, methylation of histone H3 at Lys4 (H3K4) or Lys27 (H3K27) controls the accessibility of genes for the transcriptional machinery and thereby regulates cell fate21. Notably, the advent of deep (massive) sequencing, coupled to chromatin immunoprecipitation (ChIP), has allowed the genome-wide characterization of H3K4- and H3K27-methylation patterns in various cell types, including CD4+ helper T cells subsets that have differentiated in vitro18.
To gain insight into the epigenetic regulation of γδ T cell subsets, we conducted a genome-wide profiling of active dimethylated H3K4 (H3K4me2) and repressive trimethylated H3K4 (H3K4me2) modifications, complemented with analysis of additional chromatin marks (H3K36me3 and H3 acetylation) and transcriptional quantification of CD27+ γδ (γδ27+) T cell or CD27− γδ (γδ27−) T cell subsets isolated from secondary lymphoid organs and compared them with the histone modifications and mRNA abundance in Th1 or Th17 CD4+ T cells differentiated in vitro. Our results constitute a public resource describing the epigenomes of γδ27+ and γδ27− cells and their effect on key transcriptional regulators of differentiation and provide new insights on the peripheral functions of thymus-derived γδ T cell subsets.
RESULTS
Genome-wide analysis of H3 methylation in γδ T cell subsets
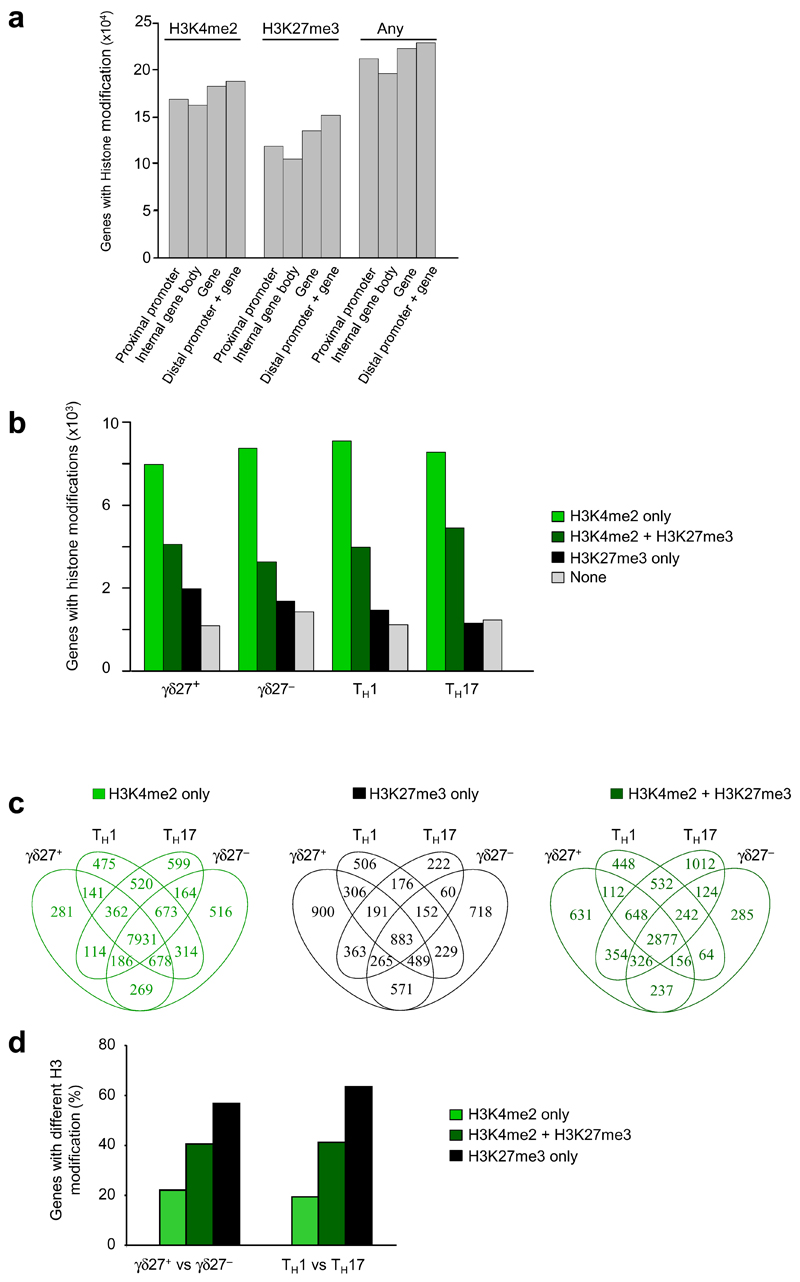
To obtain a global profiling of chromatin modifications in γδ T cell subsets (isolated from pooled lymph nodes and spleen), we performed ChIP with monoclonal antibody to H3K4me2 (anti-H3K4me2) and monoclonal anti-H3K27me3, followed by deep sequencing (ChIP-seq), of those cells. As reference, we differentiated CD4+ Th1 and Th17 subsets by standard in vitro protocols and subjected those to the same ChIP-seq analysis. This confirmed published observations of Th1-biased Ifng expression and H3K4me2 marks in the Ifng locus18, which contrasted with Il17a expression and H3K4me2 modifications in Th17 cells (Supplementary Fig. 1).
We subjected the ChIP-seq data to in-depth bioinformatics analysis. We used three different 'peak-calling' tools to detect enrichment of histone-modification density and assigned only peaks consistently retrieved by all three methods. We first examined the H3-methylation patterns, across the entire genome, in the total pool of T cell subsets under study: γδ27+ and γδ27− T cells, and Th1 and Th17 cells. This revealed that the vast majority (95%) of all H3-modified genes (in the total pool of T cell subsets) displayed the H3K4me2 or H3K27me3 marks in the promoter-proximal region (1 kilobase (kb) upstream and downstream of transcription start site), and we observed only a small increase in H3 modifications when we also considered the distal promoter region ( Fig. 1a). High proportions of H3-modified genes were associated with H3K4me2 alone (50%) or with both H3K4me2 or H3K27me3 marks (27%), with similar patterns observed across all four T cell subsets (Fig. 1b). A smaller fraction of H3-modified genes (<18%) displayed repressive H3K27me3 marks alone (Fig. 1b), with 4% (883 genes) of all H3-modified genes displaying only H3K27me3 marks concomitantly in all four T cell subsets (Fig. 1c). The quantitative analysis of the genes marked by H3K4me2 alone, H3K27me3 alone or both H3K4me2 and H3K27me3 revealed that from an epigenetic perspective, the γδ27+ and γδ27− T cell subsets generated in vivo were as distinct from each other as were the CD4+ Th1 and Th17 cells subsets polarized in vitro (Fig. 1d).
Figure 1.
Genome-wide histone H3 methylation in subsets of γδ T cells and CD4+ helper T cells. (a) ChIP-seq quantification of genes associated with no histone modification (None), H3K4me3 or H3K27me3 alone or H3K4me3 or H3K27me3 together in the total pool of γδ27+ T cells, CCR6+ γδ27− γδ T cells and CD4+ Th1 and Th17 cells, in the following genomic regions: distal promoter (–4 kb to –1 kb upstream of the transcription start site) and gene (Dist prom + gene), proximal promoter (−1 kb to +1 kb around the transcription start site; Prox prom), internal gene body (+1 kb from the start site to end of gene; Int body) and the gene (proximal promoter + internal gene body; Gene).(b) ChIP-seq quantification of genes associated with histone modifications as in a in each of the four T cell subsets in a. (c) Overlap of genes associated with histone modifications in the four T cell subsets in a, presented as Venn diagrams. (d) Frequency of genes with differences in modification in γδ27+ T cells versus γδ27− T cells (left) or Th1 cells versus Th17 cells (right) among those with H3K4me2 or H3K27me3 modifications or both H3K4me2 and H3K27me3 modifications. Samples were analyzed a second time to ensure the technical reproducibility of ChIP-seq results; results were confirmed by ChIP-qPCR analysis of biological duplicates. Data are representative of QQ experiments (a), QQ experiments (b), QQ experiments (c) or QQ experiments (d).
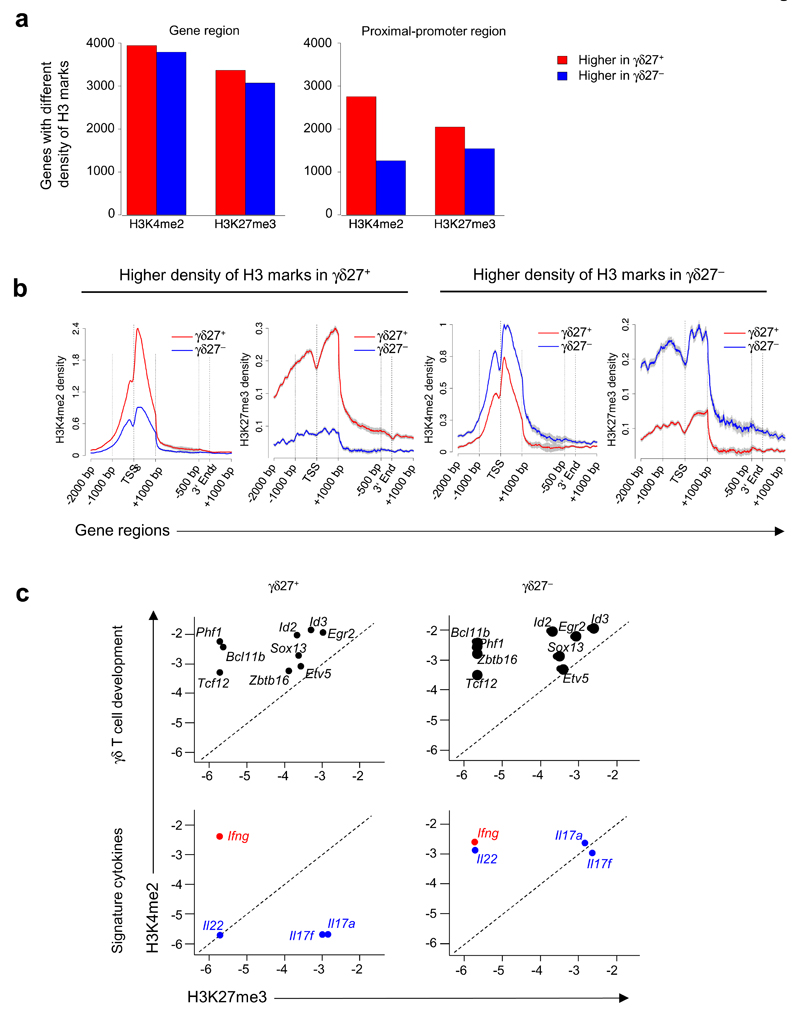
We next focused our analysis on the two γδ cell subsets and compared the H3-methylation densities of γδ27+ and γδ27− T cells. On the basis of quantitative algorithms, a total of 10,581 genes had a difference in the abundance of either H3K4me2 or H3K27me3 marks (Fig. 2a,b), which were located in the promoter-proximal region for 64% of all genes with a difference in H3 modification in γδ27+ T cells versus γδ27− T cells (Fig. 2a).
Figure 2:
Peripheral γδ27+ and γδ27− T cells display distinct genome-wide histone H3 methylation patterns (a) ChIP-seq quantification of genes associated with differences in H3K4me2 or H3K27me3 histone modifications in the full gene or the proximal promoter region (as defined in Fig. 1a) in peripheral γδ27+ and γδ27− T cells. (b) Histone-modification profiles of genes with a greater abundance of H3K4me2 or H3K27me3 in γδ27+ or γδ27− T cells; the 5´ and 3´ ends are 'unscaled' and the remainder of the gene is rescaled to 2 kb; long gray vertical lines correspond to gene regions along horizontal axes; gray shading indicates error bars. TSS, transcription start site. (c) Quantification (log10-transformed) of H3K4me2 and H3K27me3 modifications on genes linked to T cell development (top) or on signature cytokine genes (bottom), in γδ27+ or γδ27− T cells. Reproducibility and ChIP-qPCR, as in Figure 1. Data are representative of QQ experiments (a), QQ experiments (b; error bars, s.e.m.) or QQ experiments (c).
Selective inspection of the epigenetically regulated genes in γδ27+ and γδ27− T cell subsets indicated that genes linked to (γδ) T cell development (such as Bcl11b, Id3 or Etv5)17 displayed almost identical active histone marking in both subsets (Fig. 2c). In contrast, genes encoding effector cytokines (Il17a, Il17f and Il22) showed substantially more enrichment for permissive H3K4me2 marks in γδ27− T cells than in γδ27+ T cells (Fig. 2c). These epigenetic profiles suggested that γδ27+ and γδ27− T cells share an early developmental program but diverge during functional differentiation into cytokine-producing subsets.
Additional targets in the differentiation of γδ T cell subsets
We next examined the full H3K4me2-H3K27me3 epigenome of γδ27+ and γδ27− T cells (Supplementary Table 1) to identify genes encoding molecules involved in their differentiation. Il17a, Il17f and Il22 were among the genes with the greatest difference between the two subsets in H3 modification, and all showed more enrichment for active H3K4me2 marks in γδ27− T cells than in γδ27+ T cells (Table 1). We noted the same pattern for Ccr6 (which encodes the chemokine receptor CCR6) and Il1r1 and Il23r (which encode cytokine receptors) (Table 1), all known to be expressedv in γδ27− cells1,13,14,20. Notwithstanding those results, the 25 genes with the greatest difference in modification in γδ27+ T cells versus γδ27− T cells represented previously unknown targets for the development and function of γδ T cells (Table 1). Dock8 and Dkk3 displayed active H3K4me2 marks in γδ27− T cells but not in γδ27+ T cells. The guanine-exchange factor DOCK8 is a signaling adaptor that controls the survival and function of CD8+ T cells21 and activation of B cells22. Moreover, DOCK8 mutation in humans causes severe combined immunodeficiency associated with high susceptibility to infection21,22. DKK3 is a glycoprotein that modulates Wnt signaling and has a regulatory function in CD8+ T cells23.
Table 1.
| Histone Mark |
Gene Symbol |
Fold Change |
γδ Subset |
Histone Mark |
Fold Change |
CD4 Subset |
|---|---|---|---|---|---|---|
| Top 25 miscellaneous | ||||||
| K4 | Gstcd | 67.74 | CD27- | |||
| K4 | Acyp2 | 61.71 | CD27- | |||
| K4 | Dock8 | 42.15 | CD27- | K27 | 13.57 | Th17 |
| K4 | Pdzd2 | 42.15 | CD27- | |||
| K4 | Wwox | 37.63 | CD27- | K27 | 10.89 | Th17 |
| K27 | 10.87 | Th1 | ||||
| K4 | 9.72 | Th17 | ||||
| K4 | Pla2g2d | 37.46 | CD27- | |||
| K4 | Clip1 | 37.20 | CD27+ | |||
| K4 | Dkk3 | 34.62 | CD27- | |||
| K4 | Kcnq5 | 33.12 | CD27- | K27 | 9.97 | Th17 |
| K4 | Ppp1r14c | 33.12 | CD27- | K27 | 16.39 | Th17 |
| K4 | 13.97 | Th17 | ||||
| K4 | Sgip1 | 30.10 | CD27- | |||
| K4 | Zp1 | 30.10 | CD27- | |||
| K4 | Hivep1 | 28.60 | CD27- | |||
| K4 | Slc17a4 | 28.60 | CD27- | |||
| K4 | Dcp1b | 27.90 | CD27+ | |||
| K4 | Crmp1 | 27.09 | CD27- | |||
| K4 | Psme4 | 27.09 | CD27- | |||
| K4 | Ptk2 | 27.09 | CD27- | |||
| K4 | Reps2 | 27.09 | CD27- | |||
| K4 | Sntb2 | 27.09 | CD27- | K27 | 15.08 | Th17 |
| K4 | Zranb3 | 27.09 | CD27- | |||
| K4 | Eif4g3 | 26.34 | CD27- | |||
| K4 | Myom1 | 25.59 | CD27- | |||
| K4 | Zfp408 | 25.25 | CD27+ | |||
| K4 | Cdon | 25.09 | CD27- | |||
| Receptor Activity | ||||||
| K4 | Trpm6 | 45.16 | CD27- | K27 | 16.97 | Th17 |
| K4 | Nrp2 | 37.20 | CD27+ | |||
| K4 | Il1r1 | 33.12 | CD27- | K4 | 10.69 | Th17 |
| K4 | Il17rd | 28.60 | CD27- | K27 | 15.08 | Th17 |
| K4 | Ntrk2 | 24.91 | CD27+ | K27 | 16.97 | Th17 |
| K4 | Fbn1 | 21.92 | CD27+ | K4 | 23.36 | Th1 |
| K4 | Pvrl1 | 20.57 | CD27- | K27 | 16.02 | Th17 |
| K4 | Ptprn2 | 19.57 | CD27- | |||
| K4 | Lrrn2 | 17.23 | CD27- | |||
| K4 | Gpr160 | 16.86 | CD27- | |||
| K4 | Grik1 | 16.18 | CD27- | K27 | 16.34 | Th17 |
| K4 | Gpr174 | 15.81 | CD27- | |||
| K4 | Itpr1 | 15.81 | CD27- | K27 | 25.14 | Th17 |
| K4 | Scarf1 | 14.30 | CD27- | K27 | 11.85 | Th17 |
| K4 | Ramp1 | 13.80 | CD27- | |||
| K4 | Ccr6 | 13.76 | CD27- | |||
| K4 | Xpr1 | 13.55 | CD27- | |||
| K4 | Ccr1 | 12.26 | CD27- | |||
| K4 | Ryk | 11.44 | CD27- | |||
| K4 | Ptprm | 10.63 | CD27+ | K27 | 14.61 | Th17 |
| K4 | Tnfrsf4 | 10.35 | CD27- | |||
| K4 | Vipr2 | 10.11 | CD27- | K4 | 9.93 | Th17 |
| K4 | Grik2 | 9.78 | CD27- | K27 | 12.57 | Th17 |
| K4 | Sorl1 | 9.78 | CD27- | |||
| K4 | Ncoa7 | 9.53 | CD27- | |||
| K4 | Stab1 | 9.53 | CD27- | |||
| K4 | Igf1r | 9.46 | CD27- | |||
| K4 | Flt1 | 9.43 | CD27+ | |||
| K4 | Cd44 | 9.41 | CD27- | |||
| K4 | Gpr98 | 9.41 | CD27- | |||
| K4 | Slamf1 | 9.28 | CD27- | |||
| K27 | Dner | 9.11 | CD27+ | K27 | 10.10 | Th17 |
| K4 | Ptpra | 9.03 | CD27- | |||
| K27 | Grm7 | 8.98 | CD27+ | K27 | 33.94 | Th17 |
| K4 | Ptpn5 | 8.92 | CD27+ | |||
| K4 | Il1r2 | 8.73 | CD27- | K27 | 10.77 | Th17 |
| K4 | Sema6a | 8.60 | CD27- | |||
| K4 | Ephb2 | 8.35 | CD27+ | |||
| K4 | Mtus1 | 8.28 | CD27- | K27 | 11.92 | Th17 |
| K4 | Il23r | 8.03 | CD27- | |||
| Cytokines | ||||||
| K4 | Il5 | 20.17 | CD27- | |||
| K4 | Il21 | 16.81 | CD27- | |||
| K4 | Il17a | 16.06 | CD27- | K4 | 11.89 | Th17 |
| K4 | Il17f | 13.25 | CD27- | K4 | 8.08 | Th17 |
| K4 | Il22 | 10.54 | CD27- | |||
| Signal Transduction | ||||||
| K4 | Tnk2 | 24.33 | CD27- | |||
| K4 | Brca1 | 20.07 | CD27- | |||
| K27 | Pde3b | 16.75 | CD27- | K27 | 30.16 | Th17 |
| K4 | Prkca | 15.05 | CD27- | K4 | 46.33 | Th1 |
| K4 | Tiam1 | 14.62 | CD27- | K27 | 15.08 | Th17 |
| K4 | Psd | 13.55 | CD27- | |||
| K4 | Wisp1 | 13.55 | CD27- | |||
| K4 | Optn | 13.17 | CD27- | K27 | 8.30 | Th17 |
| K4 | Txnl1 | 12.04 | CD27- | |||
| K4 | Baiap2l1 | 11.83 | CD27- | K27 | 12.57 | Th17 |
| K4 | Dapk1 | 11.21 | CD27+ | |||
| K4 | Fgd3 | 11.14 | CD27- | K4 | 8.76 | Th17 |
| K4 | Gpsm2 | 11.04 | CD27- | |||
| K4 | Irak3 | 11.04 | CD27- | |||
| K4 | Sh3gl3 | 11.04 | CD27- | |||
| K27 | Gna15 | 11.02 | CD27+ | K27 | 8.01 | Th17 |
| K27 | Pde8b | 10.61 | CD27+ | |||
| K4 | Mapk10 | 10.24 | CD27- | K4 | 9.45 | Th17 |
| K4 | Cradd | 10.16 | CD27- | K27 | 18.85 | Th17 |
| K4 | Bcar3 | 10.03 | CD27- | K27 | 11.31 | Th17 |
| K27 | Bre | 9.79 | CD27+ | |||
| K4 | Rasgrp3 | 9.78 | CD27- | |||
| K4 | Camk4 | 9.03 | CD27- | |||
| K4 | Nedd9 | 9.03 | CD27- | |||
| K4 | Camk2g | 8.66 | CD27- | K27 | 10.44 | Th17 |
| K4 | Rhot1 | 8.53 | CD27- | |||
| K4 | Pik3ca | 8.53 | CD27- | |||
| K4 | Elmo1 | 8.11 | CD27- | |||
| Transcription Factor Activity | ||||||
| K4 | Zbtb38 | 31.61 | CD27- | |||
| K4 | Tshz2 | 21.26 | CD27+ | K27 | 20.74 | Th17 |
| K4 | Stat5b | 18.06 | CD27- | |||
| K27 | Satb1 | 14.70 | CD27- | |||
| K4 | Arnt2 | 14.30 | CD27- | |||
| K27 | Mef2c | 13.47 | CD27+ | |||
| K4 | Mllt10 | 12.54 | CD27- | |||
| K4 | Dlx3 | 12.29 | CD27+ | |||
| K4 | Gabpb2 | 11.67 | CD27- | |||
| K4 | Vdr | 11.18 | CD27- | |||
| K4 | Atf6 | 11.04 | CD27- | |||
| K4 | Runx2 | 10.54 | CD27- | |||
| K4 | Tcf7l1 | 10.54 | CD27- | |||
| K4 | Pbx1 | 10.16 | CD27- | K27 | 25.77 | Th17 |
| K4 | Pbx3 | 10.16 | CD27- | K27 | 25.77 | Th17 |
| K27 | Clock | 9.38 | CD27+ | |||
| K4 | Lcor | 9.03 | CD27- | |||
| K4 | Rora | 9.03 | CD27- | K27 | 20.16 | Th1 |
| K27 | 15.08 | Th17 | ||||
| K4 | 11.70 | Th17 | ||||
| K4 | Erg | 8.80 | CD27+ | |||
| K4 | Foxn2 | 8.36 | CD27- | |||
| K4 | Arntl | 8.28 | CD27- | |||
| K4 | Nfatc2 | 8.28 | CD27- | |||
While our analysis revealed many signaling mediators and transcription factors of unknown function in γδ cells (or T cells in general), various candidates were among the proteins with receptor activity (Table 1): the costimulatory receptor SLAMF1 (CD150), which controls the development of natural killer (NK) T cells24 and promotes inflammation in a mouse model of colitis25; the scavenger receptor SCARF1, which recognizes and triggers innate immune responses to fungi26; EPHB2, which orchestrates the crosstalk between thymocytes and thymic epithelial cells and thus affects early T cell development27; Flt1, which binds vascular endothelial growh factor A and costimulates IFN-γ production28; and OX40 (TNFRSF4), a costimulator of CD4+ and CD8+ T cell, as well as NK and NKT cell function29, although its role in the activation of γδ T cells remains unclear. The only chemokine receptor–encoding gene other than Ccr6 with substantially different modification in γδ27− T cells versus γδ27+ T cells was Ccr1 (Table 1); CCR1 has been linked to the migration of macrophages and neutrophils30 but not T cells.
Six of those eight candidates also had a difference in H3K4me2 modification in thymic γδ27− T cells versus γδ27+ T cells (Supplementary Fig. 2). Except for Ccr1, those gene loci had a greater abundance of H3K4me2 marks in peripheral γδ T cell subsets than in thymic γδ T cell subsets (Supplementary Fig. 2). This suggested that the epigenetic segregation of the gene-expression programs of γδ27− and γδ27+ T cells starts in the thymus but is further consolidated as cells continue to mature in the periphery.
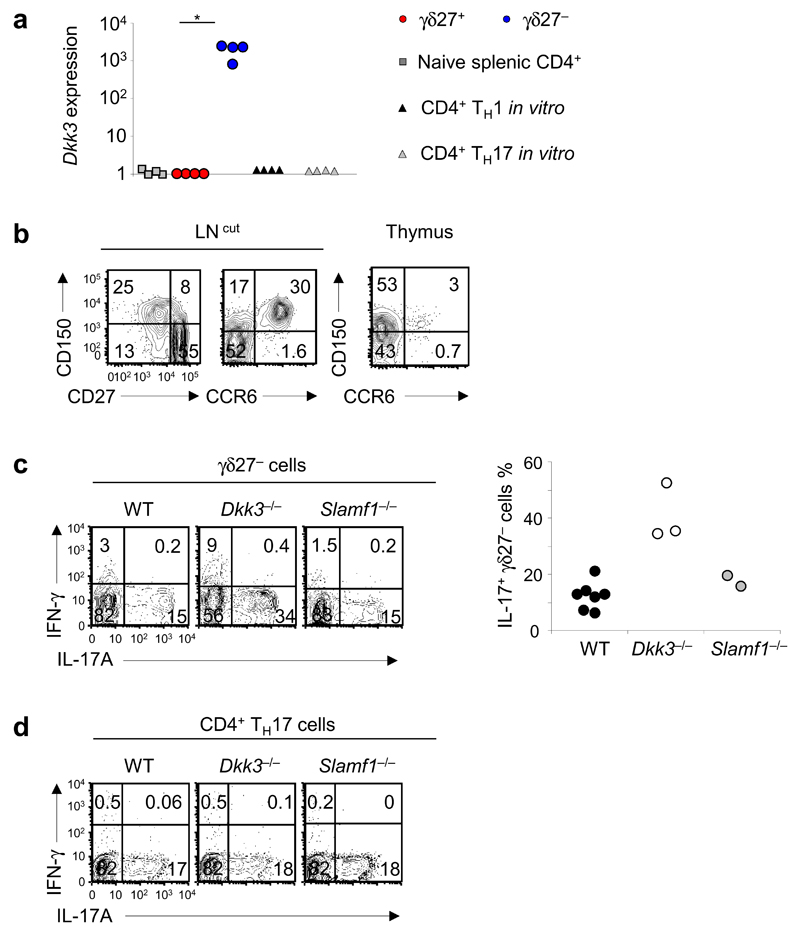
We did preliminary analysis of the expression of Dkk3 and Slamf1 on γδ T cells and assessed the effect of their deletion on the differentiation and function of γδ T cells. γδ27− T cells showed considerable enrichment for Dkk3 mRNA and SLAMF1 protein relative to their abundance in γδ27+ T cells (Fig. 3a, b). SLAMF1 was coexpressed with CCR6 in the thymus and in the periphery (Fig. 3b) and thus constitutes an additional marker for the CD27−CCR6+ γδ T cell subset10,13,14. Whereas we observed no difference between Slamf1−/− and wild-type mice in their production of L-17, Dkk3−/− mice displayed much greater frequencies of peripheral IL-17-producing γδ27− T cells than did wild-type mice (Fig. 3c). In contrast, IL-17 expression was normal in Dkk3−/− Th17 cells (Fig. 3d), and IFN-γ production was not altered in Dkk3−/− γδ27+ or Th1 cells (data not shown). These data suggested a role for DKK3 in selectively regulating IL-17 production in γδ27− T cells. Thus, this analysis has provided a set of potential additional regulators of the differentiation and activation of γδ T cells, some of which may also be shared with CD4+ helper T cells (Table 1).
Figure 3.
DKK3 and SLAMF1 are molecular determinants of γδ27− T cells. (a) Quantitative RT-PCR analysis of Dkk3 expression in ex vivo CD4+ T cells, γδ27+ T cells and γδ27− T cells (derived from peripheral T cells) and CD4+ Th1 and Th17 cells generated in vitro (all sorted from C57BL/6 mice); results are presented relative to those of Actb (control gene). *P < 0.05 (Mann-Whitney two-tailed test). (b) Surface expression of SLAMF1 (CD150), CD27 and CCR6 on total γδ T cells from cutaneous lymph nodes (LN (cut); left) and thymus (right), assessed by flow cytometry. Numbers in quadrants indicate percent cells in each throughout. (c) Intracellular staining of IFN-γ and IL-17A in γδ27− cells isolated from C57BL/6 wild-type (WT), Dkk3−/− and Slamf1−/− mice (left), and frequency of IL-17+ γδ27− T cells in those mice (right; n = 7 wild-type mice, 3 Dkk3−/− mice and 2 Slamf1−/− mice). (d) Intracellular staining of IFN-γ and IL-17A in Th17 cells differentiated in vitro from CD4+ T cells isolated from WT, Dkk3−/− and Slamf1−/− mice. Each symbol (a,c, right) represents an individual experiment. Data are from one experiment with cells pooled from four mice (a) or one experiment per mouse (c, right) or are representative of three independent experiments with two or three mice in each (b) or two independent experiments with for mice per strain in each (d).
Epigenetic control of cytokine expression in γδ T cell subsets
We next focused on the epigenetic regulation of genes encoding signature cytokines in the two γδ T cell subsets. In contrast to Il17a, IL17f or Il22 (Table 1), Ifng did not show a difference in H3 methylation in γδ27+ T cells versus γδ27− T cells (Supplementary Table 1). Individual ChIP-seq profiles showed that Il17a (Fig. 4a) and Il17f and Il22 (Supplementary Fig. 3a) accumulated many active H3K4me2 marks in γδ27− T cells but not in γδ27+ T cells, whereas the Ifng locus displayed H3K4me2 marks in both γδ cell subsets (Fig. 4a). ChIP followed by quantitative PCR (ChIP-qPCR) with primers for the respective promoters and other known regulatory conserved noncoding sequences31–33 confirmed accumulation of active H3K4me2 marks in the Ifng locus in both γδ27+ and γδ27− T cells in the periphery (Fig. 4b and Supplementary Fig. 3b,c) and in the thymus (Fig. 4c). The segregation of repressive H3K27me3 marks in γδ27+ T cells versus γδ27− T cells was less obvious at these loci by both ChIP-seq (Fig. 4a; Supplementary Fig. 3a) and ChIP-qPCR (Supplementary Fig. 4).
Figure 4.
Epigenetic and transcriptional patterning of Ifng and Il17a in γδ T cell subsets. (a) ChIP-seq analysis of H3K4me2 (green) and H3K27me3 (black) modifications on Ifng and Il17a in peripheral γδ27+ and γδ27− T cells; below, chromosome 10 (Chr 10; left) and chromosome 1 (Chr 1; right), showing the positions of (black boxes). (b,c) ChIP-qPCR analysis of H3K4me2 modifications on Ifng (promoter and conserved noncoding sequence –34 regions (CNS–34)) and Il17a (promoter and conserved noncoding sequences +5 regions) in peripheral (b) or thymic (c) γδ27+ and γδ27− T cells; results are presented relative to total H3. (d) ChIP-qPCR analysis of total acetylated histone H3 in the promoter regions of Ifng or IL17a in peripheral γδ27+ and γδ27− T cells (presented as in c). (e) Quantitative RT-PCR analysis of the expression of Ifng and Il17a in CD4+, γδ27+ and γδ27− T cells from pooled spleen and lymph nodes of C57BL/6 mice (e) and in CD4+CD8+ double-positive (DP) and CD4+ single-positive (SP) thymocytes of the αβ T cell lineage and CD25+CD27+ (γδ25+), CD25− γδ27+ and CD25− γδ27− thymocytes of the γδ T cell lineage (e), presented relative to Actb expression. Each symbol (e,f) represents an individual experiment. *P < 0.05 and **P < 0.001 (Mann-Whitney two-tailed test). Data are pooled from four to seven independent experiments with cells pooled from four mice in each (mean and s.d. in b–d).
We also investigated additional histone modifications that associate with gene expression. Acetylation of histone H3 (H3ac) has been linked to the expression of genes important for the differentiation of helper T cells31,34. ChIP-qPCR of γδ27+ and γδ27− T cells, with primers specific for the Ifng and Il17a promoters, showed the presence of H3ac marks at the Il17a locus exclusively in γδ27− cells, while these permissive marks were of almost identical abundance at the Ifng locus in both γδ27 T cell subsets (Fig. 4d).
To evaluate the effect of those histone-modification patterns on theexpression of genes encoding cytokines, we measured Ifng and Il17a mRNA by reverse transcription followed by quantitative PCR (quantitative RT-PCR). Consistent with the epigenetic data, Il17a had ˜700-fold higher expression in peripheral γδ27− T cells than in peripheral γδ27+ T cells, while Ifng had ˜10-fold higher expression in γδ27+ T cells than in γδ27− T cells (Fig. 4e). Moreover, Il17f and Il22 were also markedly enriched in γδ27− T cells (Supplementary Fig. 3d). We also found similar differences for thymic γδ T cell subsets, in which the difference in expression was ˜2-fold for Ifng and >200-fold for Il17a (Fig. 4f) and Il22 (data not shown). Of note, in the thymus, Ifng and Il17a transcripts were expressed only in γδ T cells and were not detectable in αβ thymocytes (Fig. 4f). Both Ifng and IL17 were expressed in CD25+ γδ thymocytes, which represent a common progenitor population for both γδ27− and γδ27+ T cells10, followed by further upregulation of Ifng in γδ27+ T cells, whereas Il17a expression was upregulated in γδ27− thymocytes (Fig. 4f). These data demonstrated that the expression of genes encoding signature cytokines in γδ T cells is epigenetically patterned in the thymus and is sustained in peripheral γδ T cell subsets and revealed a distinct degree of polarization toward Th1 or Th17 effector function among γδ T cell subsets.
Epigenetic patterning of Th1 versus Th17 factors in γδ T cells
We next analyzed our ChIP-seq data for (mostly transcription) factors that have been linked to Th1 and Th17 differentiation15 or, in some instances, specifically to the differentiation of γδ T cells35,36. Examination of individual ChIP-seq profiles (Fig. 5a) or their global activity (Fig. 5b) revealed Th17 polarization of γδ27− T cells but not of γδ27+, T cells, indicated by the accumulation of permissive H3K4me2 marks in genes encoding differentiation factors, such as Rorc, Rora or Batf (Fig. 5a,b), as well as in the cytokine receptor–encoding genes Il1r1 and Il23r1,20 and the chemokine receptor–encoding gene Ccr6 (ref. 13) (Supplementary Fig. 5a). Moreover, Rorc and Blk (Fig. 5a) and Maf and Irf4 (data not shown) displayed repressive H3K27me3 modifications in γδ27+ T cells but not in γδ27− T cells. In contrast, most genes encoding Th1-differentiation factors (such as Tbx21, Eomes and Hlx) were positively marked by H3K4me2 in both γδ T cell subsets, and some (Eomes and Hlx) also displayed substantial repressive H3K27me3 modification in both γδ T cell subsets (Fig. 5a). As reference, genes encoding molecules associated with cell survival, the development of γδ T cells or alternative T cell effector functions did not show a difference in H3 modification in the two γδ T cell subsets (Fig. 5b and data not shown).
Figure 5.
Permissive histone H3 methylation patterns associate with transcription of Th1- and Th17-related factors in γδ T cell subsets. (a) ChIP-seq analysis of H3K4me2 (green) and H3K27me3 (black) modifications on genes encoding Th1-related factors (Tbx21, Eomes, Hlx; left) and Th17-related factors (Rorc, Batf, Blk; right) in peripheral γδ27+ and γδ27− T cells (below plots, as in Fig 4a). (b) Quantification (log10-transformed) of gene-specific H3K4me2 modifications on genes in γδ27+ T cells and γδ27− T cells for genes grouped as Th1- and Th17- related factors (top left), genes linked to γδ T cell development (top right), genes associated with alternative effector cell types (bottom left) and housekeeping reference or survival genes (bottom right). (c) ChIP-qPCR analysis of H3K36me3 modifications on the transcription factor–encoding genes Tbx21, Eomes, and Rorc in γδ27+ and γδ27− T cells, presented relative to the abundance of total H3. (d) Quantitative RT-PCR analysis of genes encoding Th1-related factors (top) and Th17-related factors (bottom) on ex vivo CD4+ T cells and γδ27+ and γδ27− T cells (derived from peripheral T cells) and CD4+ Th1 and Th17 cells generated in vitro, presented relative to Actb expression. NS, non significant; *P < 0.05 (Mann-Whitney two-tailed test). (e) Expression of Th1- and Th17-associated genes (from data in d) in γδ27+ T cells versus γδ27− T cells (top) or CD4+ Th1 cells versus Th17 cells (bottom). Each symbol (d,e) represents an individual experiment. Data are pooled from five independent experiments with cells pooled from four mice in each (mean and s.d. in c).
To further document the epigenetic regulation of important transcription regulators in γδ T cell subsets, we analyzed the H3K36me3 marking of the Tbx21, Eomes and Rorc loci. H3K36me3 modifications, which accumulate at the 3′ end of genes, correlate with transcriptional activity in intron-containing genes37. The two γδ T cell subsets showed a difference of less than threefold in the H3K36me3 marking of Tbx21 or Eomes, whereas the Rorc locus had more than tenfold more H3K36me3 marking in γδ27− T cells than in γδ27+ T cells (Fig. 5c). The difference in the patterning of Th1 or Th17 factors in peripheral γδ T cell subsets extended to the transcriptional level, as quantitative RT-PCR analysis estimated the abundance of Rorc transcripts was ˜600-fold greater in γδ27− T cells than in γδ27+ T cells, whereas the abundance of Tbx21 and Eomes was only slightly (four- to sixfold) greater in γδ27+ T cells (Fig. 5d). Furthermore, H3K4me2 modifications of the Ifng promoter in γδ27+ T cells were similar in wild-type and Tbx21−/− mice (Supplementary Fig. 6), and Tbx21−/− mice had a significant proportion of IFN-γ-producing γδ T cells (Supplementary Fig. 7a, b. In contrast, Rorc−/− mice lacked the CCR6+ (γδ27−) T cell subset in both the thymus and spleen (Supplementary Fig. 7c) and did not express IL-17A in total γδ T cells (Supplementary Fig. 7d).
Although the expression of many Th1 factors versus that of Th17 factors segregated with the two peripheral γδ T cell subsets (Fig. 5e), the transcriptional polarization was much stronger for the Th17 program (Fig. 5d,e). That was consistent with the accumulation of repressive H3K27me3 marks at Th17-related loci, such as Rorc, Blk and Maf (Fig. 5a and data not shown), in γδ27+ T cells. Of note, the expression of Rorc, Rora and Maf was higher in γδ27− T cells ex vivo than in Th17 cells generated in vitro (Fig. 5d), and overall there was better segregation of Th17 transcription factors in γδ T cells than in CD4+ helper T cell subsets (Fig. 5e). These data documented distinct epigenetic patterning and transcriptional regulation of Th1 factors versus Th17 factors in γδ T cell subsets and showed that both γδ27+ and γδ27− T cells had Th1 factors epigenetically primed for expression.
Stable versus plastic differentiation of γδ T cell subsets
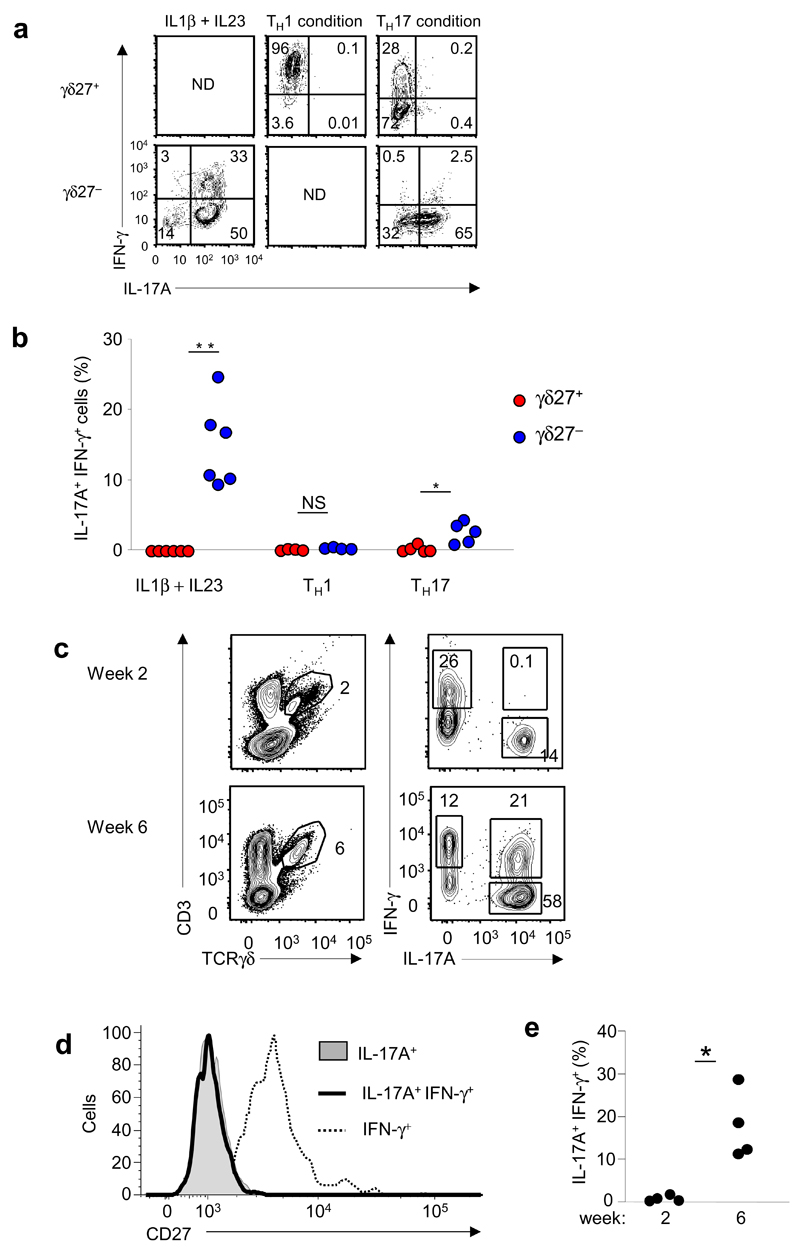
In line with the epigenetic status of Ifng and the genes encoding Th1 factors described above, γδ27− T cells stimulated in vitro are reported to produce both IL-17 and IFN-γ10. To further explore the conditions that trigger IFN-γ production in these cells, we established short-term cultures of highly purified γδ27− or γδ27+ T cells in cytokine-defined media. γδ27− T cells responded to IL-1β and IL-23 by acquiring IFN-γ production, which resulted in a sizeable population of cells producing both IL-17 and IFN-γ (Fig. 6a,b). The differentiation of γδ27− T cells into cells producing both IL-17 and IFN-γ was negligible under Th17-differentiation conditions (Fig. 6a,b), whereas Th1-differentiation conditions did not support the survival of γδ27− T cells (Supplementary Fig. 8). Furthermore, γδ27+ T cells stably and exclusively produced IFN-γ, even under Th17 conditions (Fig. 6a). Of note, the presence of IL-1β and IL-23 led to the death of γδ27+ cells (Supplementary Fig. 8), consistent with their lack of expression of the corresponding receptors (Supplementary Fig. 5).
Figure 6.
Differentiation of IL-17+ IFN-γ+ γδ27− cells in vitro and in vivo. (a) Intracellular staining of IFN-γ and IL-17A in γδ27+ and γδ27− T cells isolated from pooled spleen and lymph nodes and stimulated in vitro for 48 h in the presence of IL-1β plus IL-23 or standard Th1- or Th17-polarizing conditions. ND, not determined (lack of cell viability; Supplementary Fig. 8). (b) Frequency of IL-17+IFN-γ+ cells in the cultures in a, presented as a proportion of live cells. *P <0.05 and **P < 0.01 (Mann-Whitney two-tailed test). (c) Intracellular IFN-γ and IL-17A in total γδ T cells from peritoneal exudates obtained at 2 or 6 weeks of the development of ID8 tumors in C57BL/6 mice. Numbers adjacent to outlined areas indicate percent δγ T cells (right column) or IFN-γ+ γδ T cells (top left), IL-17+ γδ T cells (bottom right) or IL-17+IFN-γ+ γδ T cells (top right; all left column). (d) Overlay of the surface staining of CD27 on IL17+, IL-17+IFN-γ+ or IFN-γ+ γδ T cells from peritoneal exudates at 6 weeks of ID8 tumor development (in c). (e) Frequency of IL-17+ IFN-γ+ cells among CD27− γδ T cells (in c,d). Each symbol (b,e) represents an individual experiment. *P < 0.05 (Mann-Whitney two-tailed test). Data are representative of QQ experiments with four to six independent cultures per condition (a), QQ experiments (b), QQ experiments (c) or QQ experiments (d) or are from two independent experiments with four mice in each (e).
Finally, we sought to determine if the plasticity of γδ27− T cells was detectable in vivo. To investigate whether the systemic immune response to infections could drive the differentiation of IL-17+IFN-γ+ γδ T cells, we set up in vivo infection models based on four distinct types of microorganisms that elicit γδ T cell responses10,20: a parasite (Plasmodium berghei), a virus (murid herpes virus 4), a bacterium (Mycobacterium avium) and a fungus (Candida albicans). We isolated cells from spleen and lymph nodes at the peak of each γδ T cell response. However, we did not observe significant populations of IL-17+IFN-γ+ γδ T cells in these acute infection models Supplementary Fig. 9).
We reasoned that a strong local inflammatory response might be necessary for the differentiation of IL-17+IFN-γ+ γδ T cells in vivo. We therefore transplanted an ovarian cancer cell line (ID8) known to produce a highly inflammatory microenvironment38 into the peritoneal cavity of mice and monitored the growth of luciferase-positive ID8 tumor cells by bioimaging techniques38 (data not shown). We observed the accumulation of a sizeable population of IL-17+IFN-γ+ γδ T cells after tumor growth (at 6 weeks; Fig. 6c). Those IL-17+IFN-γ+ cells were γδ27−, unlike IFN-γ+ γδ T cells (Fig. 6d). In fact, IL-17+IFN-γ+ cells constituted up to 30% of the γδ27− T cell subset present in the tumor-bearing peritoneal cavity (Fig. 6e). In contrast, γδ27+ T cells remained exclusive producers of IFN-γ (Fig. 6d). These data demonstrated that the plasticity of γδ27− T cells, which is in contrast to the stable phenotype of the γδ27+ T cells, was triggered under specific local inflammatory conditions and may thus contribute to their function in vivo.
DISCUSSION
Epigenetic mechanisms ensure the autonomous maintenance of lineage phenotype in differentiated cells, even through mitotic divisions. Methylation patterns of active H3K4 and repressive H3K27 have been shown to affect the functional (in)stability of effector CD4+ helper T cell subsets18,32,39. Here we have described the epigenetic landscape of Th1- and Th17-related loci in γδ T cells freshly isolated from lymphoid organs. Through the use of genome-wide ChIP-seq analysis, we identified many potential previously unknown participants in the differentiation and activation of γδ T cell subsets. Notably, among the top genes selected on the basis of their differences in histone H3 marking in γδ cell subsets, only a minority (40 of 120) were also strongly biased between CD4+ helper T subsets. That suggested that different lineage-specific mechanisms of differentiation might operate in γδ T cells, as exemplified by DKK3.
DKK3 is a secreted glycoprotein that modulates Wnt signaling and whose expression is often epigenetically silenced in a variety of cancer cell types, which suggests a potential role as a tumor suppressor40. Although little is known about DKK3 functions in the immune system, it is reported to have a powerful regulatory function in CD8+ T cells23. DKK3 is specifically expressed in transgenic CD8+ T cells tolerized in the neonatal period through interactions with a self antigen expressed in keratinocytes (in a double transgenic mouse model) and was necessary and sufficient for maintenance of CD8+ T cell tolerance in this model. In particular, Dkk3−/− mice rejected autologous skin grafts and readily eradicated transplantable tumors23. Notably, published studies have suggested a general absence of Dkk3 expression on T cell subsets, except for a specific subset of long-term memory CD8+ T cells41. Our findings extend Dkk3 expression to the γδ27− T cell subset, while excluding it from γδ27+ T cells and CD4+ helper T subsets. Furthermore, Dkk3−/− γδ27− T cell populations showed enrichment for cells that produced IL-17, which selectively linked DKK3 to the functional differentiation of this γδ T cell subset.
Although the mechanisms of action of DKK3 remain unclear, it is widely regarded as an inhibitor of the Wnt signaling cascade40. Notably, the downstream (transcriptional) effectors of Wnt signaling, TCF1 and Lef, have been shown to inhibit the differentiation of IL-17-producingγδ T cells42. Moreover, in our ChIP-seq analyses, Tcf7 (which encodes TCF1) and Lef were biased toward γδ27+ T cells (albeit below the eightfold threshold than was the inclusive criteria we used here): Lef1 displayed a 6.4-fold enrichment for active H3K4me2 marks in γδ27+ T cells and 4.8-fold enrichment for repressive H3K27me3 marks in γδ27− T cells; and Tcf7 showed an 4.5-fold accumulation of H3K27me3 marks in γδ27− T cells. Given the increased production of IL-17 by Dkk3−/− γδ27− T cells, these data suggest crosstalk between DKK3 and Wnt signaling during the differentiation of γδ T cells that warrants further investigation.
As functional differentiation of γδ T cells can occur in the thymus10–12, the stability of cellular phenotypes in the periphery probably depends on the epigenetic patterning of key (master) transcription factors. Our data showed that Tbx21 and Eomes (which encode Th1-related transcription factors ) and Rorc and Batf (which encode Th17-related transcription factors ) were distinctively patterned (by histone H3 modifications) in the γδ27+ and γδ27− subsets, and this was associated with their mRNA expression. Notably, genetic deletion of Tbx21 or Rorc (data not shown) did not impair the epigenetic marking of the respective signature cytokine–encoding genes Ifng and Il17. That was consistent with studies showing that T-bet has very modest effect on the active enhancer repertoire of CD4+ Th1 cells43. That result notwithstanding, our data cannot exclude the possibility that histone patterning of Ifng or Il17 in γδ T cell subsets is controlled by unknown combinations of Th1- or Th17-related transcriptional regulators. Of note, members of the STAT family of transcription factors have a critical role in shaping the epigenetic landscape of Th1, Th2 and Th17 cells18,43,44. Although the role of STAT1 and STAT4 in the differentiation of IFN-γ+ γδ T cells requires further investigation, STAT3 is largely dispensable for the generation of IL-17-producing γδ T cells45. That is consistent with their independence on IL-6 signals46 and illustrates how the developmental programming of γδ T cells (in the thymus) follows rules distinct from those of CD4+ T cell differentiation after activation (by TCR and cytokine signals) in the periphery.
While our study here concentrated on intracellular (nuclear) mechanisms of differentiation, it is also important to consider the roles of extracellular cues. In particular, the innate cytokines IL-1β and IL-23 drive the selective population expansion of IL-17+ γδ T cells in infection20, autoimmunity1 and tumor47 models. Our data showed distinct epigenetic and transcriptional polarization of IL-1R1 and IL-23R in γδ27− T cells, which highlights the importance of these receptors in the biology of IL-17+ γδ T cells (but not IFN-γ+ γδ T cells). IL-1β and IL-23 also seemed to be key elements of the inflammatory milieu that triggered the plasticity of γδ27− T cell in vitro. In vivo, we detected that plasticity in the tumor microenvironment but not during systemic responses to infection. Notably, CD4+ T cells 'convert' from Th17 cells to Th1 cells under IL-23-dependent inflammatory conditions in experimental autoimmune encephalomyelitis but not during acute cutaneous infection with Candida albicans48, while in the same conditions, γδ T cells remained producers of IL-17 and did not acquire IFN-γ expression48. However, this does not indicate that microorganisms cannot trigger plasticity of γδ27− T cell, as it has been observed in gut-associated (mesenteric) lymph nodes after oral infection with Listeria49. Instead, we think this highlights the importance of the local inflammatory environment for the acquisition of IFN-γ expression by γδ27− T cells.
The epigenomic data on γδ T cell subsets presented here provides a framework for the analysis and interpretation of the functions of γδ27+ and γδ27− T cells in the periphery. In particular, we found that γδ27− T cells that acquired the ability to produce IL-17 during thymic development were nonetheless endowed with functional plasticity that allowed them to also produce IFN-γ under local inflammatory conditions. It will now be important to determine the specific roles of IL-17+IFN-γ+ γδ cells in vivo models of infection, cancer or autoimmunity. These cells have been observed in the central nervous system of mice suffering experimental autoimmune encephalomyelitis (but not in healthy control mice)50 and may thus contribute to the pathogenic role of γδ T cells in this1 and other disease models.
ONLINE METHODS
Mice
All mice used were adults 6–12 weeks of age. C57BL/6, Tcra−/−, Rorc−/− and Tbx21−/− mice were from Jackson Laboratories. Slamf1−/− and Dkk3−/− mice were provided by C. Terhorst and B. Arnold, respectively. Mice were bred and maintained in the specific pathogen–free animal facilities of Instituto de Medicina Molecular (Lisbon, Portugal) or Queen Mary London University (London, UK). All experiments involving animals were done in compliance with the relevant laws and institutional guidelines and were approved by the ethics committees of Instituto de Medicina Molecular and Blizard Institute.
Cell sorting or analysis by flow cytometry
For cell surface staining, single-cell suspensions were incubated for 15 min on ice with anti-FcγR (2.4G2; BD Pharmingen), and then for 30 min with saturating concentrations of the appropriate monoclonal antibody. The following monoclonal antibodies were used (BD Pharmingen or eBiosciences): eFluor 450–anti-CD4 (RM4-5), fluorescein isothiocyanate– or phycoerythrin–anti-TCRγδ (GL3), peridinin chlorophyll protein–cyanine 5.5–anti-CD3ε (145.2C11), phycoerythrin–indotricarbocyanine–anti-CD27 (LG.7F9), Alexa Fluor 647–anti-CCR6 (29-2L17), allophycocyanin–anti-CD25 (PC61 5.3), allophycocyanin–eFluor 780–anti-CD8 (53-6.7). For sorting of T cell subsets, lymphoid organs were pooled from four mice (quantitative RT-PCR) or six mice (ChIP-qPCR or ChIP-seq). Cells were sorted on FACSAria III (BD Biosciences) or were analyzed on a FACSFortessa or FACSCalibur (BD Biosciences) and data were analyzed with FlowJo software (Tree Star).
For intracellular cytokine staining, cells sorted by flow cytometry were stimulated for 4 h at 37 °C with PMA (phorbol 12-myristate 13-acetate; 50 ng/mL) and ionomycin (1 μg/mL) (Sigma) with 10 μg/mL brefeldin A (Sigma) added during the final 2 h. Cells were stained for the appropriate cell surface markers, then were fixed for 15 min at 4 °C with 2% paraformaldehyde, permeabilized for 30 min at room temperature with 0.5% saponin and 0.1% FCS in 2 mM EDTA-PBS in the presence of 2.4G2 (BD Pharmingen) and finally incubated for 1 h at room temperature with fluorescein isothiocyanate–anti-IL-17 (17B7) and allophycocyanin–anti-IFN-γ (XMG1.2).
In vitro cell stimulation and polarization
CD27+ and CD27- γδ T cells were sorted by flow cytometry and subjected to various stimulation conditions for 48 h; CD4+ T cell–polarization cultures were maintained for 6 d. For Th1 culture conditions, cells were activated with plate-bound monoclonal antibody (mAb) to CD3ε and soluble mAb to CD28 (37.51) (1 μg/ml) in the presence of IL-12 (5 ng/ml) and neutralizing mAb to IL-4 (11B11) (10 μg/ml). For Th17 culture conditions, cells were activated with plate-bound anti-CD3ε, and soluble anti-CD28 and TGF-β (2 ng/ml), IL-1β (10 ng/ml), IL-6 (20 ng/ml), IL-21 (100 ng/ml), IL-23 (10 ng/ml) and neutralizing anti-IFN-γ (10 μg/ml) were added to the medium. Alternatively, cells were incubated on plate-bound anti-CD3ε (145.2C11) (5 μg/ml), in the presence or absence of mouse IL-7 (10 ng/ml) or IL-1β plus IL-23 (both at 10 ng/ml).
ChIP-seq and ChIP-qPCR
Cells were sorted by flow cytometry from the thymus or pooled spleen and lymph nodes for ChIP. The following antibodies were used: antibody to histone H3 (ab1791; Abcam), antibody to H3K36me3 (ab9050; Abcam), , antibody to acetylated histone H3 (06-599; Millipore), antibody to H3K4me2 (07-030; Millipore) and antibody to H3k27me3 (07-449; Millipore).
Cells (1 × 105 to 1 × 106) were crosslinked with formaldehyde and nuclei were isolated and sonicated with a Sanyo Soniprep 150 at an amplitude of 10 μm with 17 bursts of 10 s, which resulted in chromatin fragments 200–400 bp in length. Chromatin was immunoprecipitated as described51. The immunoprecipitated DNA released from crosslinked proteins was extracted with a QiaQuick kit in accordance with the manufacturer's instructions (Qiagen).
Deep sequencing was done at the GeneCore facility of the European Molecular Biology Laboratory. At least 1 ng of immunoprecipitated DNA was used for library preparation according to the Illumina protocol.
Alternatively, candidate genes were analyzed by ChIP-qPCR (primers, Supplementary Table 2).
The occupancy of the immunoprecipitated protein at each DNA site was estimated as follows: 2(Cspecific − CtotalH3) where, Cspecific and C totalH3 are threshold cycles of PCR of DNA from specific immunoprecipitation or total H3 immunoprecipitation, respectively.
ChIP-seq data analysis
High-throughput sequencing 'reads' were aligned to the reference mouse (mm9) genome with Bowtie software for the alignment of short DNA sequences52. 'Read' quality was assessed with the FastQC quality-control tool for high throughput sequence data (Babraham Bioinformatics). 'Reads' with bad-quality scores, 'reads' not uniquely mapped and PCR duplicates (identical coordinates) were filtered out. The SAMtools utility for storing large nucleotide sequence alignments and manipulating alignments53 and BEDtools software for the comparison of genomic features54 were used for filtering steps and file format conversion. Three different peak-calling tools (MACS55,56, SICER57 and RSEG58) were used for the detection of enrichment of histone modifications density. The recommended settings for histone modifications were used for these analyses, and only peaks that were consistently detected by the three methods were considered. Regions with different histone-modification densities in two samples were identified with the submodule of SICER57, with a false-discovery rate of 0.05 and absolute change in density >1.5-fold.
Regions with enrichment were then assigned to known genes of the UCSC Genome Browser, including a 1-kilobase region upstream of transcription start site (mm959).
For quantitative calculation and profiles of histone modifications for all genes, uniquely mapped 'reads' were extended in the 3′ direction to reach 150 nt with the Pyicos deep-sequencing analysis tool60. Only 'read' counts that overlapped histone-enriched regions identified above were considered. The quantitative results per gene were log10-transformed. For genes without enriched regions, the amount was defined as 0.
For average profiles across genes with differences in histone densities, a 'metagene' profile was plotted for each gene group. Genes were aligned at the first and last nucleotides of the annotated transcripts and sequencing tags were scaled as follows: the 5′ end (2 kb upstream of the transcription start site to 1 kb downstream) and the 3′ end (500 bp upstream of the poly(A) site to 1 kb downstream) were unscaled and averaged in a 10-bp window, and the remainder of the gene was scaled to 200 bins of equal size so that all genes seem to have the same length (2 kb). Individual profiles were produced with a window of 5 bp. All profiles were plotted on a normalized reads-per-million basis. The processed data were plotted and visualized using software of the R project for statistical computing61.
Real-time PCR
Cell populations were sorted by flow cytometry and mRNA was prepared from those with a High Pure RNA Isolation kit (Roche). Random oligonucleotides (Invitrogen) and MMLV reverse transcriptase (Promega) were used for reverse transcription for 1 h at 42 °C. SYBR or TaqMan probe chemistry on an ABI ViiA7 cycler (Applied Biosystems) was used for quantification of specific cDNA species relative to that of Actb . The cycling threshold (Ct) for the target gene was subtracted from that of Actb and the relative amount was calculated as 2−ΔCt. Primers were designed with Primer Express software (Applied Biosystems) and their sequences were as follows: Actb forward, CGTGAAAAGATGACCCAGATCA, and reverse, TGGTACGACCAGAGGCATACAG; Blk forward, TTATGTGCCCAGCAACTTTGTG, and reverse, AAGGCACCTTTATTGCTCTCACTCT; Eomes forward, CGTTCACCCAGAATCTCCTAACA, and reverse, TGCAGCCTCGGTTGGTATTT; Hlx forward, AGCTCCAACCCAAGAAATTCTGT, and reverse, GCTTGTATGTCTGTGGCATGGT; probe, ACACATTTCCAGGTCCCTATGCTGTGCTC; Ifng forward, TCTTCTTGGATATCTGGAGGAACTG, and reverse, GAGATAATCTGGCTCTGCAGGATT; Il17a forward, CCAGAAGGCCCTCAGACTACCT, and reverse, TCCCTCCGCATTGACACA; Il17f forward, CAACCAAAACCAGGGCATTT, and reverse, ACTGGGCCTCAGCGATCTCT; Il22 forward, GTGCCTTTCCTGACCAAACT, and reverse, CTGTCTCCTTCAGCCTTCTG; Il23r forward, TCAGTGCTACAATCTTCAGAGGACA, and reverse, GCCAAGAAGACCATTCCCGA; Maf forward, AGCAGGTAGACCACCTCAAGCA, and reverse, GAGTCCCTTGGGTACATGAAAAATT; Rora forward, TCCCCTACTGTTCCTTCACCAA, and reverse, GGAAGGTCTGCCACGTTATCTG; Rorc forward, GTCCAGACAGCCACTGCATTC, and reverse, TGCGCTGCCGTAGAAGGT; Runx3 forward, ACTGGCGCTGCAACAAGAC, and reverse, GGCCCACGAATCGAAGGT; and Tbx21 forward, CACACACGTCTTTACTTTCCAAGAGA, and reverse, CACTCGTATCAACAGATGCGTACAT
Tumor transplantation
ID8 ovarian cancer cells (provided by K. Roby) were injected intraperitoneally into C57BL/6 female mice (5 × 106 cells per mouse). Tumor growth was monitored as described38. Peritoneal exudate cells were collected 2–6 weeks after tumor transplantation, then were stimulated in vitro for 4 h with PMA and ionomycin and stained intracellularly for IL-17 and IFN-γ.
Infection
Mice were infected intraperitoneally with 1 × 106 red blood cells infected with Plasmodium berghei ANKA with transgenic expression of GFP (provided by A. Pamplona) and were monitored as previously described10. Cells from the spleen and lymph nodes were collected for analysis after 3 d.
Mice were infected intraperitoneally with 1 × 106 plaque-forming units of murid herpes virus 4 (provided by P. Simas), and cells from the spleen and lymph nodes were collected after 14 d.
Mice were infected intravenously with 1 × 106 colony-forming units of Mycobacterium avium strain 2447 (provided by M. Correia-Neves). Cells from the spleen and lymph nodes were collected 17 d after infection. Infection was confirmed by serial dilutions of homogenized spleen plated onto Middlebrook 7H10 agar.
Mice were infected intravenously with 1 × 105 live Candida albicans yeast strain WT-SC 3314 (provided by C. Reis e Sousa). Cells from the spleen and lymph nodes were collected 7 d after infection. Infection was confirmed by serial dilutions of homogenized kidney suspension plated onto yeast extract peptone dextrose agar medium.
Statistical analysis
A two-tailed non-parametric Mann-Whitney test was used for statistical analysis. P values of <0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank V. Benes for technical assistance with the ChIP-seq experiments; J. Ribot, S. de Almeida and N. Gonçalves-Sousa for technical advice; H. Kulbe, F. Balkwill and R. Thompson for help with the ID8 tumor model; M. J. Nunes and E. Rodrigues for help with ChIP procedures; and M. Soares, A. Vieira and S. Marques for help with cell sorting; A. Hayday, L. Lefrançois and M. Saraiva for discussions; B. Arnold (Deutsches Krebsforschungszentrum, Heidelberg) and C. Niehrs for Dkk3-deficient mice; C. Terhorst (Harvard Medical School) and B. van Driel () for Slamf1-deficient mice; K. Roby (University of Kansas) for ID8 ovarian cancer cells; A. Pamplona (IMM) Plasmodium berghei ANKA with transgenic expression of GFP; P. Simas (IMM) for murid herpes virus 4; M. Correia-Neves (Universidade do Minho) for Mycobacterium avium strain 244.; C. Reis e Sousa (The London Research Institute) for Candida albicans yeast strain WT-SC 3314; and the staff of the Animal and Flow Cytometry facilities of our institutes for experimental assistance. Supported by the European Research Council (StG_260352 to B.S.-S.), the Wellcome Trust (D.J.P.), the European Molecular Biology Organization (B.S.-S.), Fundação para a Ciência e Tecnologia (K.S., A.R.G. and M.R.) and the Universidade do Porto (M.R.).
Footnotes
AUTHOR CONTRIBUTIONS
N.S. planned and did experiments in Figures 1,2,4,5; K.S. planned and did experiments in Figures 3–6; A.R.G. planned and did experiments in Figures 1,2; M.R. planned and did experiments in Figure 6; A.Q.G. planned and did experiments in Figures 4,5; D.J.P. contributed to designing the study and writing the manuscript; A.Q.G. helped to design and supervise the study; and B.S.-S. designed and supervised the study and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Petermann F, et al. γδ T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pantelyushin S, et al. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J Clin Invest. 2012;122:2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shichita T, et al. Pivotal role of cerebral interleukin-17 producing γδ T cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 5.Cai Y, et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection . J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 7.Bonneville M, O'Brien RL, Born WK. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 8.Yin Z, et al. T-Bet expression and failure of GATA-3 cross-regulation lead to default production of IFN- γ by γδ T cells. J Immunol. 2002;168:1566–1571. doi: 10.4049/jimmunol.168.4.1566. [DOI] [PubMed] [Google Scholar]
- 9.Gomes AQ, Martins DS, Silva-Santos B. Targeting γδ T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res. 2010;70:10024–10027. doi: 10.1158/0008-5472.CAN-10-3236. [DOI] [PubMed] [Google Scholar]
- 10.Ribot JC, et al. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17-producing γδ T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen KD, et al. Thymic selection determines γδ T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon γ. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibata K, et al. Identification of CD25+ γδ T cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol. 2008;181:5940–5947. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 13.Haas JD, et al. CCR6 and NK1.1 distinguish between IL-17A and IFN- γ-producing γδ effector T cells. Eur J Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 14.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17 producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas JD, et al. IL-17 mediated negative feedback restricts development of IL-17-producing γδ T cells to an embryonic wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Narayan K, et al. Intrathymic programming of effector fates in three molecularly distinct γδ T cell subtypes. Nat Immunol. 2012;13:511–518. doi: 10.1038/ni.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei G, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirahara K, et al. Helper T-cell differentiation and plasticity: insights from epigenetics. Immunology. 2011;134:235–245. doi: 10.1111/j.1365-2567.2011.03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribot JC, et al. Cutting edge: adaptive versus innate receptor signals selectively control the pool sizes of murine IFN-γ- or IL-17 producing γδ T cells upon infection. J Immunol. 2010;185:6421–6425. doi: 10.4049/jimmunol.1002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randall KL, et al. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. 2011;208:2305–2320. doi: 10.1084/jem.20110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabara HH, et al. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat Immunol. 2012;13:612–620. doi: 10.1038/ni.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papatriantafyllou M, et al. Dickkopf-3, an immune modulator in peripheral CD8 T-cell tolerance. Proc Natl Acad Sci USA. 2012;109:1631–1636. doi: 10.1073/pnas.1115980109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griewank K, et al. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Driel B, et al. Signaling lymphocyte activation molecule regulates development of colitis in mice. Gastroenterology. 2012;143:1544–1554. doi: 10.1053/j.gastro.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Means TK, et al. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J Exp Med. 2009;206:637–653. doi: 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alfaro D, et al. EphrinB1-EphB signaling regulates thymocyte-epithelium interactions involved in functional T cell development. Eur J Immunol. 2007;37:2596–2605. doi: 10.1002/eji.200737097. [DOI] [PubMed] [Google Scholar]
- 28.Basu A, et al. Cutting edge: Vascular endothelial growth factor-mediated signaling in human CD45RO+CD4+ T cells promotes Akt and ERK activation and costimulates IFN-γ production. J Immunol. 2010;184:545–549. doi: 10.4049/jimmunol.0900397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furuichi K, et al. Chemokine receptor CCR1 regulates inflammatory cell infiltration after renal ischemia-reperfusion injury. J Immunol. 2008;181:8670–8676. doi: 10.4049/jimmunol.181.12.8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akimzhanov AM, Yang XO, Dong C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J Biol Chem. 2007;282:5969–5972. doi: 10.1074/jbc.C600322200. [DOI] [PubMed] [Google Scholar]
- 32.Mukasa R, et al. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoenborn JR, et al. Comprehensive epigenetic profiling identifies multiple distal regulatory elements directing transcription of the gene encoding interferon-γ. Nat Immunol. 2007;8:732–742. doi: 10.1038/ni1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, et al. Transcription of il17 and il17f is controlled by conserved noncoding sequence 2. Immunity. 2012;36:23–31. doi: 10.1016/j.immuni.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turchinovich G, Hayday AC. Skint-1 identifies a common molecular mechanism for the development of interferon- γ-secreting versus interleukin-17-secreting γδ T cells. Immunity. 2011;35:59–68. doi: 10.1016/j.immuni.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Laird RM, Laky K, Hayes SM. Unexpected role for the B cell-specific Src family kinase B lymphoid kinase in the development of IL-17-producing γδ T cells. J Immunol. 2010;185:6518–6527. doi: 10.4049/jimmunol.1002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Almeida SF, et al. Splicing enhances recruitment of methyltransferase HYPB/Setd2 and methylation of histone H3 Lys36. Nat Struct Mol Biol. 2011;18:977–983. doi: 10.1038/nsmb.2123. [DOI] [PubMed] [Google Scholar]
- 38.Charles KA, et al. The tumor-promoting actions of TNF-α involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veeck J, Dahl E. Targeting the Wnt pathway in cancer: the emerging role of Dickkopf-3. Biochim Biophys Acta. 2012;1825:18–28. doi: 10.1016/j.bbcan.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 42.Malhotra N, et al. A network of high-mobility group box transcription factors programs innate interleukin-17 production. Immunity. 2013;38:681–693. doi: 10.1016/j.immuni.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vahedi G, et al. STATs shape the active enhancer landscape of T cell populations. Cell. 2012;151:981–993. doi: 10.1016/j.cell.2012.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Durant L, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shibata K, et al. Notch-Hes1 pathway is required for the development of IL-17 producing γδ T cells. Blood. 2011;118:586–593. doi: 10.1182/blood-2011-02-334995. [DOI] [PubMed] [Google Scholar]
- 46.Lochner M, et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+Foxp3+RORγt+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carmi Y, et al. Microenvironment-derived IL-1 and IL-17 interact in the control of lung metastasis. J Immunol. 2011;186:3462–3471. doi: 10.4049/jimmunol.1002901. [DOI] [PubMed] [Google Scholar]
- 48.Hirota K, et al. Fate mapping of IL-17 producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheridan BS, et al. γδ T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity. 39:184–195. doi: 10.1016/j.immuni.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reynolds JM, Martinez GJ, Chung Y, Dong C. Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci USA. 2012;109:13064–13069. doi: 10.1073/pnas.1120585109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnekenburger M, Talaska G, Puga A. Chromium cross-links histone deacetylase 1-DNA methyltransferase 1 complexes to chromatin, inhibiting histone-remodeling marks critical for transcriptional activation. Mol Cell Biol. 2007;27:7089–7101. doi: 10.1128/MCB.00838-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feng J, Liu T, Zhang Y. in Curr Protoc Bioinformatics. 2011;Ch Unit 2.14 doi: 10.1002/0471250953.bi0214s34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zang C, et al. A clustering approach for identification of enriched domains from histone modification ChIP-Seq data. Bioinformatics. 2009;25:1952–1958. doi: 10.1093/bioinformatics/btp340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song Q, Smith AD. Identifying dispersed epigenomic domains from ChIP-Seq data. Bioinformatics. 2011;27:870–871. doi: 10.1093/bioinformatics/btr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rhead B, et al. The UCSC Genome Browser database: update 2010. Nucleic Acids Res. 2010;38:D613–D619. doi: 10.1093/nar/gkp939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Althammer S, Gonzalez-Vallinas J, Ballare C, Beato M, Eyras E. Pyicos: a versatile toolkit for the analysis of high-throughput sequencing data. Bioinformatics. 2011;27:3333–3340. doi: 10.1093/bioinformatics/btr570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The R Development Core Team. R Foundation for Statistical Computing. Vienna, Austria: 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.