Abstract
Background
Although appl1 is overexpressed in many cancers, its status in gastric cancer (gc) is not known. In the present study, we used relevant pathologic and clinical data to investigate appl1 expression in patients with gc.
Methods
In 47 gc and 27 non-gc surgical specimens, immunohistochemistry was used to detect the expression of appl1, and reverse-transcriptase polymerase chain reaction (rt-pcr) was used to detect messenger rna (mrna). A scatterplot visualized the relationship between survival time and mrna expression in gc patients. The log-rank test and other survival statistics were used to determine the association of appl1 expression with the pathologic features of the cancer and clinical outcomes.
Results
In gc, appl1 was expressed in 28 of 47 specimens (59.6%), and in non-gc, it was expressed in 7 of 23 specimens (30.4%, p < 0.05). The expression of mrna in gc was 0.82 [95% confidence interval (ci): 0.78 to 0.86], and in non-gc, it was 0.73 (95% ci: 0.69 to 0.77; p < 0.05). Immunohistochemistry demonstrated that, in gc, appl1 expression was correlated with depth of infiltration (p = 0.005), lymph node metastasis (p = 0.017), and TNM stage (p = 0.022), but not with pathologic type (p = 0.41). Testing by rt-pcr demonstrated that, in gc, appl1 mrna expression was correlated with depth of infiltration (p = 0.042), lymph node metastasis (p = 0.031), and TNM stage (p = 0.04), but again, not with pathologic type (p = 0.98). The correlation coefficient between survival time and mrna expression was −0.83 (p < 0.01). Overexpression of appl1 protein (hazard ratio: 3.88; 95% ci: 1.07 to 14.09) and mrna (hazard ratio: 4.23; 95% ci: 3.09 to 15.11) was a risk factor for death in patients with gc.
Conclusions
Expression of appl1 is increased in gc. Overexpression is prognostic for a lethal outcome.
Keywords: appl1, gastric cancer, mrna, prognosis
INTRODUCTION
Gastric cancer (gc) is the second most common cancer worldwide1. In many countries in Asia, particularly China, gc is still a significant health issue even though its incidence has been decreasing annually since the mid-1990s2. At 30.1 per 100,000, the mortality rate in China is the highest in Asia3. Early diagnosis is more likely to result in a successful surgical intervention than is a diagnosis made when metastasis is present4,5. Unfortunately, early diagnosis presents difficulties6 because the “key” molecule or molecules for gc have not been identified.
The protein appl1 (adaptor protein containing ph domain, ptb domain, and leucine zipper motif 1) participates in the cell-signal pathway and interacts with follicle-stimulating hormone receptor7, dcc (“deleted in colorectal cancer”)8, Rab5a9, and Akt210. It has been shown that appl1 is overexpressed in some carcinomas such as lung cancer11, breast cancer12, and ovarian cancer9. However, the expression of appl1 in gc is unknown.
METHODS
Patients
The trial group consisted of patients with gc (27 men, 20 women; mean age: 57.7 ± 6.4 years) who underwent surgical gastric resection between January 2005 and December 2007 at the Chinese People’s Liberation Army (pla) 309 Hospital, Beijing, China. The patients had not received chemotherapy, radiotherapy, antibiotics, or nonsteroidal anti-inflammatory drugs before surgery. A control group (14 men, 9 women; mean age: 38.3 ± 5.8 years) also underwent gastric resection, but for noncancerous diseases, at the Chinese pla 309 Hospital.
All patients were reviewed annually after their operations. Duration of follow-up for both groups was 5 years. Should a patient’s survival duration exceed 5 years, that survival was treated as 5 years.
Ethics
All experimental procedures were approved by the ethics committee of Chinese pla 309 hospital in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients. Consent included the use of resected gastric samples for the study.
Immunohistochemistry
Dehydration
Specimens were cut to 4 μm thickness, placed in an incubator at 60°C for an hour, and then dewaxed in xylene for 20 minutes. The specimens were then dehydrated by inserting them into containers containing declining concentrations of ethanol (100%, then 95%, 85%, and 75%) for 5 minutes at each concentration. The specimens were then washed for 20 minutes in running water.
Washes
At the end of the dehydration process, the sections were boiled in edta (pH 9.0) for 3 minutes, then rinsed with distilled water, and subsequently washed in phosphate-buffered saline (pbs, pH 7.2) for 3 minutes. This procedure was repeated twice.
Antibody Staining Procedures
Every section was blocked with 5 μL endogenous peroxidase for 10 minutes at room temperature. After blocking, the slides were washed in pbs 3 times for 3 minutes each time. The slides were then further blocked with 5 μL nonimmune goat serum for 10 minutes at room temperature, followed again by a wash in pbs 3 times for 3 minutes each time.
The sections were then incubated with 50 μL appl1 antibody (SRP06369: Hufeng Bio-technology, Shanghai, P.R.C.) at room temperature for 60 minutes. After incubation, the slides were washed in pbs 3 times for 3 minutes each time. A second incubation with 50 μL polyperoxidase–anti-mouse/ rabbit immunoglobulin G (Invitrogen Bio-technology, Beijing, P.R.C.) at room temperature for 10 minutes was followed by a pbs wash that was repeated 3 times for 3 minutes each time. The final incubation consisted of 50 μL streptavidin–biotin–peroxidase (Invitrogen Bio-technology) at room temperature for 10 minutes. At the end of the procedure, the slides were again washed in pbs 3 times for 3 minutes each time.
Section Staining
Every section was then visualized with 100 μL diaminobenzidine stain (Shanghai Kaibo Bio-technology, Shanghai, P.R.C.) for 5 minutes. Afterwards, the slides were washed in running water. The sections were then counterstained with hematoxylin and progressively dehydrated with sequentially increasing ethanol solution (75%, followed by 85%, 95%, and 100%). At the end of the procedure, the slides were covered with resinene.
Reverse-Transcriptase Polymerase Chain Reaction
The appl1 and beta-actin primer sequences were designed using Software5.0 (Shenggong Bio-technology, Shanghai, P.R.C.):
- ■ appl1:
- 5′-CATCCAGAAAGAAACAACACCA-3′ (forward)
- 5′-CATTAAGGTATCCAGCCTTTCG-3′ (reverse)
- ■ Beta-actin:
- 5′-TGACGTGGACATCCGCAAAG-3′ (forward)
- 5′-CTGGAAGGTGGACAGCGAGG-3′ (reverse)
Gastric tissue was pulverized on ice and rna was extracted by the Trizol method (Boston Biomedical, Cambridge, MA, U.S.A.) according to the manufacturer’s instructions. The OneDrop OD-1000+ spectrophotometer (OneDrop, Nanjing, P.R.C.) was used to measure rna concentration and purity. The rna was then pretreated with rnase-free dnase and used for complementary dna synthesis primed with random hexamers by using PrimeScript RT Master Mix kits (Takara Bio-technology, Dalian, P.R.C.). The total mixture volume for reverse-transcriptase polymerase chain reaction (rt-pcr) was 25 μL. These steps were used: 95°C for 3 minutes for pre-denaturation, followed by 30 cycles that included denaturation at 94°C for 30 seconds, annealing at 56°C for 30 seconds, and extension at 72°C for 30 seconds. A final extension at 72°C for 10 minutes was followed by storage at 4°C.
Immunohistochemistry Scoring
Scoring for appl1 protein expression was performed using previously reported protocols13,14. The density scores ranged from 0 to 3: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. The percentage of stained cells ranged from 0 to 4: 0, fewer than 5%; 1, 5%–25%; 2, 26%–50%; 3, 51%–75%; and 4, more than 75%. The final score for a section was the density score multiplied by the percentage score. A result less than 8 was considered negative; a result of 8 or greater was considered positive. Each section was evaluated by 2 investigators blinded to the clinical status of the patient. The scores were accepted if both investigators agreed on the values. In the event of disagreement, a 3rd investigator acted as a tiebreaker to decide the result.
PCR Product Analysis
The appl1 and beta-actin pcr products (2 μL per sample) were electrophoresed in 1.5% agarose gel at 100 V for 60 minutes. After electrophoresis, a photo of the gel was taken, and the relative expressions of appl1 and beta-actin were analyzed using gel software (Gel-Pro analysis software, version 5.0: Media Cybernetics, Rockville, MD, U.S.A.).
Statistical Analysis
All statistics were analyzed using the IBM SPSS Statistics software application (version 19.0: IBM, Armonk, NY, U.S.A.). Chi-square tests, Fisher exact tests, and t-tests were used. The correlation between messenger rna (mrna) expression and survival time was also determined, and scatterplots were produced. Survival curves were constructed using the Kaplan–Meier method. Hazard ratios were calculated by log-rank test, with the prognostic outcome determined using Cox models. A p value less than 0.05 was considered statistically significant.
RESULTS
Immunohistochemistry
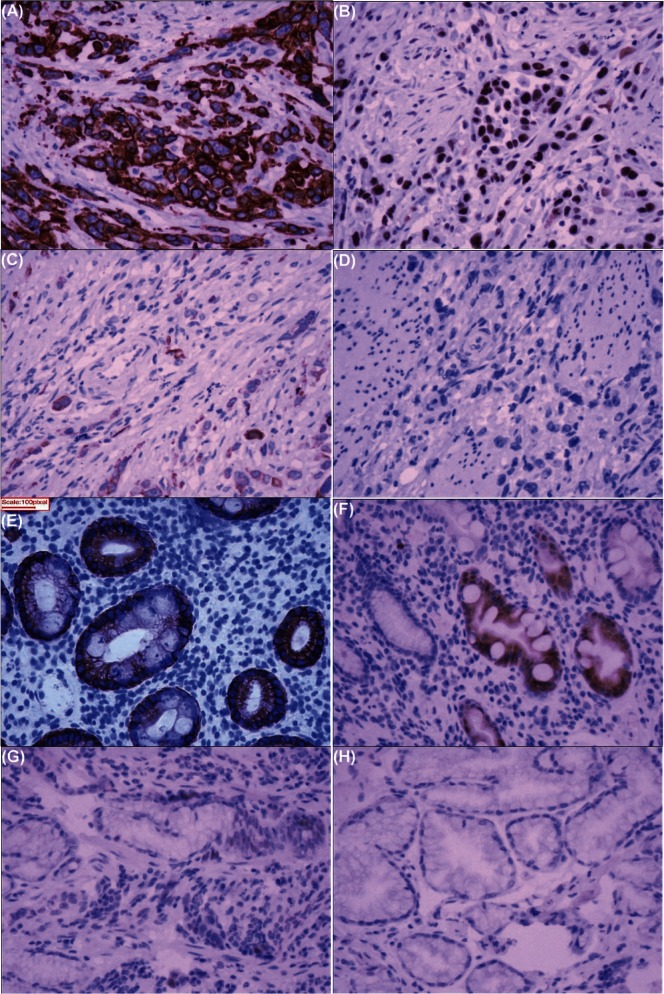
Staining for appl1 was found mostly in cytoplasm and the cell membrane. The colour was yellowish-brown. Of the 23 non-gc specimens, 7 were positive (30.4%); of the 47 gc specimens, 28 were positive (59.6%, p < 0.05; Table i, Figure 1).
TABLE I.
Expression of APPL1 by immunohistochemistry in the study groups
| Gastric cancer | APPL1 expression (n) | p Value | |
|---|---|---|---|
|
| |||
| Positive | Negative | ||
| Yes | 28 | 19 | 0.042 |
| No | 7 | 16 | |
FIGURE 1.
Immunohistochemical staining in resected specimens from patients with and without gastric cancer (GC). Yellowish-brown APPL1 staining was found mostly in cytoplasm and the cell membrane. (A) Strong, (B) moderate, (C) weak, and (D) lack of staining in specimens from patients with GC. (E) Strong, (F) moderate, (G) weak, and (H) lack of staining in specimens from patients without GC. 200× original magnification.
Reverse-Transcriptase Polymerase Chain Reaction
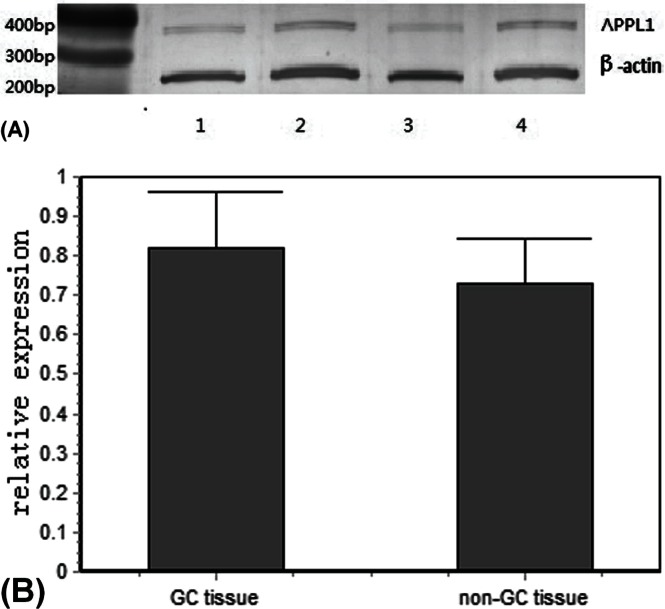
After appl1 mrna for gc and non-gc specimens was examined by rt-pcr, expression in gc specimens was 0.82 [95% confidence interval (ci): 0.78 to 0.86], and expression in non-gc specimens was 0.73 (95% ci: 0.69 to 0.77; p < 0.01; Table ii, Figure 2).
TABLE II.
Relative expression by RT-PCR of APPL1 messenger RNA in tissue from study patients
| Gastric cancer | Relative expression of APPL1 | p value | |
|---|---|---|---|
|
| |||
| Mean | 95% CI | ||
| Yes | 0.82 | 0.78 to 0.86 | 0.009 |
| No | 0.73 | 0.69 to 0.77 | |
RT-PCR = reverse-transcriptase polymerase chain reaction; CI = confidence interval.
FIGURE 2.
Relative expression of APPL1 messenger RNA in specimens from patients with and without gastric cancer (GC) [with: 0.82; 95% confidence interval (CI): 0.78 to 0.86; without: 0.73; 95% CI: 0.69 to 0.77]. (A) Reverse-transcriptase polymerase chain reaction: (1,3) non-GC specimens; (2,4) GC specimens. (B) Relative expression was higher in GC specimens than in non-GC specimens (p < 0.01).
Association of APPL1 Protein and mRNA Expression with Clinicopathologic Outcomes in GC
The expression of appl1 protein in gc specimens was correlated with depth of infiltration (p = 0.005), lymph node metastasis (p = 0.017), and TNM stage (p = 0.022), but not with pathologic type (p = 0.41). Expression of appl1 mrna in gc specimens was similarly correlated with depth of infiltration (p = 0.042), lymph node metastasis (p = 0.031), and TNM stage (p = 0.04), but not with pathologic type (p = 0.98). Age and sex were nonsignificant factors (p > 0.05, Table iii).
TABLE III.
Association of APPL1 protein and messenger RNA (mRNA) expression with clinicopathologic characteristics of gastric cancer
| Parameter | APPL1 protein | p Value | APPL1 mRNA | p Value | |
|---|---|---|---|---|---|
|
|
|
||||
| Positive | Negative | Mean | |||
| Sex | |||||
| Men | 18 | 9 | 0.395 | 0.81 | 0.489 |
| Women | 10 | 10 | 0.84 | ||
| Age | |||||
| <50 Years | 5 | 4 | 0.917 | 0.80 | 0.567 |
| ≥50 Years | 23 | 15 | 0.83 | ||
| Depth of infiltration | |||||
| Mucosa and submucosa | 1 | 4 | 0.005 | 0.73 | 0.042 |
| Muscle and subserosa | 8 | 11 | 0.79 | ||
| Extraserosal | 19 | 4 | 0.87 | ||
| TNM stage | |||||
| I | 1 | 3 | 0.022 | 0.73 | 0.040 |
| II | 4 | 8 | 0.78 | ||
| III | 8 | 5 | 0.84 | ||
| IV | 15 | 3 | 0.86 | ||
| Lymph node metastasis | |||||
| No | 8 | 13 | 0.017 | 0.76 | 0.031 |
| Yes | 20 | 6 | 0.85 | ||
| Pathologic type | |||||
| Adenocarcinoma | 16 | 6 | 0.410 | 0.81 | 0.981 |
| Unidentified carcinoma | 1 | 1 | 0.79 | ||
| Adenosquamous carcinoma | 9 | 9 | 0.83 | ||
| Carcinoid | 0 | 1 | 0.80 | ||
| Undifferentiated carcinoma | 2 | 2 | 0.79 | ||
Association of APPL1 Protein and mRNA Expression with Overall Survival in GC
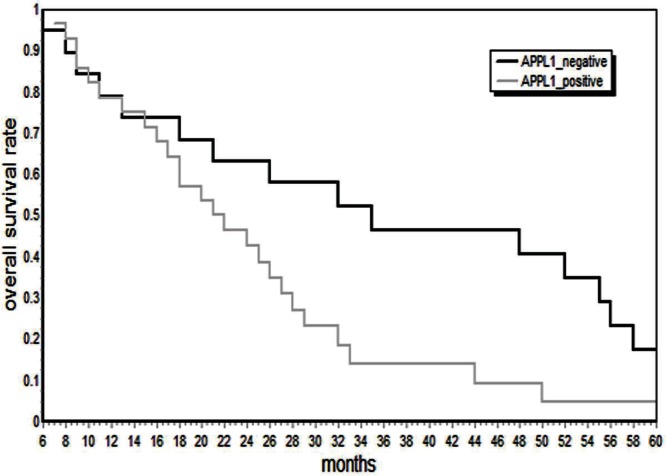
Using appl1 protein expression, patients with gc were divided into two groups: positive and negative. Survival duration in the positive group was 23.2 ± 11.2 months; in the negative group, it was 32.1 ± 18.2 months (p < 0.05, Figure 3, Table iv).
FIGURE 3.
Kaplan–Meier survival curves in for 47 patients with gastric cancer. Survival duration was shorter in the group positive for APPL1 than in the negative group (p < 0.05).
TABLE IV.
Association of APPL1 protein expression with overall survival gastric cancer (log-rank test)
| APPL1 expression | Unadjusted cases | Censored | Adjusted cases | Log-rank p value |
|---|---|---|---|---|
| No | 19 | 4a | 15 | 0.04 |
| Yes | 28 | 3b | 25 |
Treatment team lost contact with 1 patient at postoperative month 28; 3 patients survived for more than 5 years.
Treatment team lost contact with 2 patients at postoperative month 22 and month 31; 1 patient survived for more than 5 years.
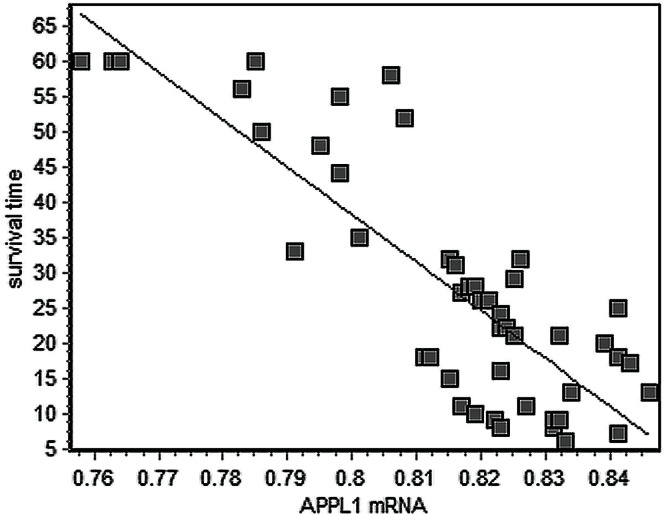
The correlation coefficient for scatterplot of survival time versus appl1 mrna for the 47 patients with gc was −0.83 (p < 0.01, Figure 4).
FIGURE 4.
Scatterplot of survival time against APPL1 messenger RNA for 47 patients with gastric cancer. The correlation coefficient was −0.83 (p < 0.01).
Analysis of GC Prognosis
A Cox model showed that expression of appl1 (protein hr: 3.88; 95% ci: 1.07 to 14.09; mrna hr: 4.23; 95% ci: 3.09 to 15.11), TNM stage (hr: 1.82; 95% ci: 1.03 to 3.24), depth of infiltration (hr: 2.75; 95% ci: 1.20 to 6.21), and lymph node metastasis (hr: 3.07; 95% ci: 1.24 to 7.61) were risk factors. Age, sex, and pathologic type were not independent risk factors in gc patients (Table v).
TABLE V.
Cox model analysis for patients with gastric cancer
| Parameter | HR | 95% CI |
|---|---|---|
| APPL1 protein | 3.88 | 1.07 to 14.09 |
| APPL1 messenger RNA | 4.23 | 3.09 to 15.11 |
| Depth of infiltration | 2.75 | 1.22 to 6.21 |
| Sex | 0.81 | 0.40 to 1.64 |
| Lymph node metastasis | 3.07 | 1.24 to 7.61 |
| Pathologic type | 0.92 | 0.65 to 1.31 |
| TNM stage | 1.82 | 1.03 to 3.24 |
| Age | 0.47 | 0.18 to 1.22 |
HR = hazard ratio; CI = confidence interval.
DISCUSSION
The mechanism of gc involves the interaction of many genes. It has been found that the MMP915, COX216, c-Myc17 genes are upregulated, and the CDKN2A (formerly p16)18 and TP5319 genes are downregulated. Here, we focused on changes in appl1 expression in gc and the relationships of appl1 and associated clinicopathologic characteristics with clinical prognosis.
In our study, appl1 protein and mrna were expressed in both gc and non-gc specimens. However, the expression of appl1 protein in gc specimens was, at 59.6%, significantly higher than it was in non-gc specimens (30.4%, p = 0.042). Expression of appl1 mrna was also higher in gc than in non-gc specimens (p = 0.009). Those results were similar to findings previously reported in prostate cancer20 and breast cancer12.
The Akt pathway is important to the occurrence of gc21. Experiments have shown that appl1 enhances Akt phosphorylation by competitive-inhibition against TAS2R13 (formerly TRB3)22,23. Concurrently, appl1 actives the Akt pathway by acting as a scaffold protein that interacts with GIPC1, one of GIPC family members24. After phosphorylation, Akt phosphorylates the activated Cdc42-associated kinase 1, induces reactions in cells, and promotes tumour growth25. The activation of glycogen synthase kinase 3β, which depends on the Akt pathway, impedes cell apoptosis in gc26. Activated nuclear factor κB plays a critical role in angiogenesis, which is involved in carcinogenesis27, and appl1 regulates the basal activity of nuclear factor κB by modulating the stability of nuclear factor κB–inducing kinase28.
The greater the expression of appl1, the deeper the infiltration by tumour. In specimens from patients with lymph node metastasis, appl1 expression was 76.9%; it was 38.1% in patients with no lymph node metastasis (p = 0.017). That observation suggests that expression of appl1 has a role in tumour infiltration and metastasis. By changing the phosphorylation site of RAF1, appl1 activates the mapk/erk cell-signal pathway29. The mapk/erk pathway promotes tumour cell proliferation and suppresses tumour cell apoptosis30. The mapk/erk pathway activates many oncogenes—for example, COX231. The activated oncogenes can induce angiogenesis with consequent infiltration and metastasis32 and can inhibit apoptosis by inhibiting TP5333. Kim et al. found that RAB5A (formerly RAB5) acting through appl1 mediation induced dysfunction of endosomes34. Recent studies showed that activation of RAB5A was a significant event in maintaining the dynamics of focal cellular adhesions, influencing not only cell migration, but also tumour cell invasion35.
In the present study, we found that survival duration was shorter in the group of patients positive for the appl1 protein than in those who were negative (23.2 ± 11.2 months vs. 32.1 ± 18.2 months, p < 0.05). We also found that higher levels of appl1 mrna expression were associated with shorter survival duration. A Cox model showed that appl1 expression was a risk factor for death. A systematic review and meta-analysis showed that higher levels of epidermal growth factor receptor predict for a poor prognosis in gc patients36. Lee et al.37 found that overexpression of appl1 enhances the stability of epidermal growth factor receptor in HeLa cells; appl1 depletion by small interfering rna reduces the epidermal growth factor receptor concentration.
In vitro studies have revealed that, in pancreatic cancer, intracellular expression of appl1 is upregulated after irradiation. Depletion of appl1 protein by small interfering rna enhances the sensitivity of cancer cells to X-rays, leading to an increased number of residual dna double-strand breaks38. Whether targeting appl1 protein would have the same effect in gc cells remains an area of investigation.
SUMMARY
In the present study, expression of appl1 protein and mrna were upregulated in gc. The expression of appl1 in gc was found to be statistically associated with depth of infiltration, lymph node metastasis, and TNM stage, but not with pathologic type. Overexpression of appl1 predicts poor prognosis for gc patients, making appl1 a novel molecular marker for gc.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (no. 81101852). We thank Professor Jin Bo and Dr. Anthony E. Yeo for their help with the manuscript.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Goh KL. Changing trends in gastrointestinal disease in the Asia–Pacific region. J Dig Dis. 2007;8:179–85. doi: 10.1111/j.1751-2980.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 3.Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20:4483–90. doi: 10.3748/wjg.v20.i16.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu LC, Shao YY, Hsu CH, et al. Metastasectomy of Kruken-berg tumors may be associated with survival benefits in patients with metastatic gastric cancer. Anticancer Res. 2012;32:3397–401. [PubMed] [Google Scholar]
- 5.Aizawa M, Nashimoto A, Yabusaki H, Nakagawa S, Matsuki A. Clinical benefit of surgical management for gastric cancer with synchronous liver metastasis. Hepatogastroenterology. 2014;61:1439–45. [PubMed] [Google Scholar]
- 6.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Bio-markers Prev. 2014;23:700–13. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas RM, Nechamen CA, Mazurkiewicz JE, Ulloa-Aguirre A, Dias JA. The adapter protein appl1 links fsh receptor to inositol 1,4,5-trisphosphate production and is implicated in intracellular Ca2+ mobilization. Endocrinology. 2011;152:1691–701. doi: 10.1210/en.2010-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Yao F, Wu R, et al. Mediation of the dcc apoptotic signal by dip13 alpha. J Biol Chem. 2002;277:26281–5. doi: 10.1074/jbc.M204679200. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Z, Liu XF, Wu HC, et al. Rab5a overexpression promoting ovarian cancer cell proliferation may be associated with appl1-related epidermal growth factor signaling pathway. Cancer Sci. 2010;101:1454–62. doi: 10.1111/j.1349-7006.2010.01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan Y, You H, Wu C, Altomare DA, Testa JR. Appl1 is dispensable for mouse development, and loss of Appl1 has growth factor-selective effects on Akt signaling in murine embryonic fibroblasts. J Biol Chem. 2010;285:6377–89. doi: 10.1074/jbc.M109.068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bidkhori G, Narimani Z, Hosseini Ashtiani S, Moeini A, Nowzari-Dalini A, Masoudi-Nejad A. Reconstruction of an integrated genome-scale co-expression network reveals key modules involved in lung adenocarcinoma. PLoS One. 2013;8:e67552. doi: 10.1371/journal.pone.0067552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauro L, Pellegrino M, De Amicis F, et al. Evidences that estrogen receptor α interferes with adiponectin effects on breast cancer cell growth. Cell Cycle. 2014;13:553–64. doi: 10.4161/cc.27455. [DOI] [PubMed] [Google Scholar]
- 13.Bai YQ, Zhang JY, Bai CY, et al. Low EphA7 expression correlated with lymph node metastasis and poor prognosis of patients with esophageal squamous cell carcinoma. Acta Histochem Cytochem. 2015;48:75–81. doi: 10.1267/ahc.14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer L, Takacs A, Slotta-Huspenina J, et al. Clinical significance of NOTCH1 and NOTCH2 expression in gastric carcinomas: an immunohistochemical study. Front Oncol. 2015;5:94. doi: 10.3389/fonc.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao XH, Yang XQ, Wang BC, Liu SP, Wang FB. Overexpression of Twist and matrix metalloproteinase-9 with metastasis and prognosis in gastric cancer. Asian Pac J Cancer Prev. 2013;14:5055–60. doi: 10.7314/APJCP.2013.14.9.5055. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Sun K, Ding J, et al. Harmine induces apoptosis and inhibits tumor cell proliferation, migration and invasion through down-regulation of cyclooxygenase-2 expression in gastric cancer. Phytomedicine. 2014;21:348–55. doi: 10.1016/j.phymed.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Zhang EB, Han L, Yin DD, Kong R, De W, Chen J. c-Myc–induced, long, noncoding H19 affects cell proliferation and predicts a poor prognosis in patients with gastric cancer. Med Oncol. 2014;31:914. doi: 10.1007/s12032-014-0914-7. [DOI] [PubMed] [Google Scholar]
- 18.Alevizos L, Gomatos IP, Smparounis S, Konstadoulakis MM, Zografos G. Review of the molecular profile and modern prognostic markers for gastric lymphoma: how do they affect clinical practice? Can J Surg. 2012;55:117–24. doi: 10.1503/cjs.002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oki E, Hisamatsu Y, Ando K, Saeki H, Kakeji Y, Maehara Y. Clinical aspect and molecular mechanism of dna aneuploidy in gastric cancers. J Gastroenterol. 2012;47:351–8. doi: 10.1007/s00535-012-0565-4. [DOI] [PubMed] [Google Scholar]
- 20.Johnson IR, Parkinson-Lawrence EJ, Shandala T, Weigert R, Butler LM, Brooks DA. Altered endosome biogenesis in prostate cancer has biomarker potential. Mol Cancer Res. 2014;12:1851–62. doi: 10.1158/1541-7786.MCR-14-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki T, Kuniyasu H. Significance of Akt in gastric cancer. Int J Oncol. 2014;45:2187–92. doi: 10.3892/ijo.2014.2678. [DOI] [PubMed] [Google Scholar]
- 22.Cheng KK, Lam KS, Wu D, et al. appl1 potentiates insulin secretion in pancreatic β cells by enhancing protein kinase Akt-dependent expression of snare proteins in mice. Proc Natl Acad Sci U S A. 2012;109:8919–24. doi: 10.1073/pnas.1202435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schenck A, Goto-Silva L, Collinet C, et al. The endosomal protein appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–97. doi: 10.1016/j.cell.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 24.Katoh M. Functional proteomics, human genetics and cancer biology of GIPC family members. Exp Mol Med. 2013;45:e26. doi: 10.1038/emm.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu SH, Huang JZ, Xu ML, et al. ack1 promotes gastric cancer epithelial–mesenchymal transition and metastasis through Akt-pou2f1-ecd signalling. J Pathol. 2015;236:175–85. doi: 10.1002/path.4515. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Zhang Y, Xu R, et al. pi3k/Akt–dependent phosphorylation of gsk3β and activation of RhoA regulate Wnt5a-induced gastric cancer cell migration. Cell Signal. 2013;25:447–56. doi: 10.1016/j.cellsig.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Tabata S, Ikeda R, Yamamoto M, et al. Thymidine phosphorylase activates nfκb and stimulates the expression of angiogenic and metastatic factors in human cancer cells. Oncotarget. 2014;5:10473–85. doi: 10.18632/oncotarget.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hupalowska A, Pyrzynska B, Miaczynska M. appl1 regulates basal nf-κb activity by stabilizing nik. J Cell Sci. 2012;125:4090–102. doi: 10.1242/jcs.105171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian C, Jin X, Ye X, et al. Long term intake of 0.1% ethanol decreases serum adiponectin by suppressing pparγ expression via p38 mapk pathway. Food Chem Toxicol. 2014;65:329–34. doi: 10.1016/j.fct.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Haagenson KK, Zhang JW, Xu Z, Shekhar MP, Wu GS. Functional analysis of mkp-1 and mkp-2 in breast cancer tamoxifen sensitivity. Oncotarget. 2014;5:1101–10. doi: 10.18632/oncotarget.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu YF, Sheu JR, Lin CH, et al. mapk phosphatase-1 contributes to trichostatin A inhibition of cyclooxygenase-2 expression in human umbilical vascular endothelial cells exposed to lipopolysaccharide. Biochim Biophys Acta. 2011;1810:1160–9. doi: 10.1016/j.bbagen.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Song J, Su H, Zhou YY, Guo LL. Cyclooxygenase-2 expression is associated with poor overall survival of patients with gastric cancer: a meta-analysis. Dig Dis Sci. 2014;59:436–45. doi: 10.1007/s10620-013-2917-1. [DOI] [PubMed] [Google Scholar]
- 33.Khajeniazi S, Allameh A, Soleimani M, Mortaz E. Changes in cox-2 and oxidative damage factors during differentiation of human mesenchymal stem cells to hepatocyte-like cells is associated with downregulation of P53 gene. Biol Chem. 2013;394:1213–22. doi: 10.1515/hsz-2012-0355. [DOI] [PubMed] [Google Scholar]
- 34.Kim S, Sato Y, Mohan PS, et al. Evidence that the Rab5 effector appl1 mediates app-βctf-induced dysfunction of endosomes in Down syndrome and Alzheimer’s disease. Mol Psychiatry. 2015. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 35.Torres VA. Rab’ing tumor cell migration and invasion: focal adhesion disassembly driven by Rab5. Cell Adh Migr. 2014;8:84–7. doi: 10.4161/cam.28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen C, Yang JM, Hu TT, et al. Prognostic role of human epidermal growth factor receptor in gastric cancer: a systematic review and meta-analysis. Arch Med Res. 2013;44:380–9. doi: 10.1016/j.arcmed.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Lee JR, Hahn HS, Kim YH, et al. Adaptor protein containing ph domain, ptb domain and leucine zipper (appl1) regulates the protein level of egfr by modulating its trafficking. Biochem Biophys Res Commun. 2011;415:206–11. doi: 10.1016/j.bbrc.2011.10.064. [DOI] [PubMed] [Google Scholar]
- 38.Hennig J, McShane MP, Cordes N, Eke I. appl proteins modulate dna repair and radiation survival of pancreatic carcinoma cells by regulating atm. Cell Death Dis. 2014;5:e1199. doi: 10.1038/cddis.2014.167. [DOI] [PMC free article] [PubMed] [Google Scholar]