Abstract
Microdialysis is often applied to understanding brain function. Because neurotransmission involves rapid events, increasing the temporal resolution of in vivo measurements is desirable. Here, we demonstrate microdialysis with on-line capillary liquid chromatography for the analysis of one-minute rat brain dialysate samples at one-minute intervals. Mobile phase optimization involved adjusting the pH, buffer composition, and surfactant concentration to eliminate interferences with the dopamine peak. By analyzing electrically evoked dopamine transients carefully synchronized with the switching of the on-line LC sample valve, we demonstrate that our system has both one-minute sampling capabilities and bona fide one-minute temporal resolution. Evoked DA transients were confined to single, one-minute brain dialysate samples. After uptake inhibition with nomifensine (20 mg/kg i.p.), responses to electrical stimuli of one-second duration were detected.
Graphical Abstract
Introduction
Dopamine (DA) is an important neurotransmitter in the central nervous system1 with relevance to human health stemming from its roles in disorders such as depression,2 schizophrenia,3,3 Parkinson’s disease4 and addiction5–8. Consequently, there is great value in techniques that provide insights into DA’s role in normal and abnormal brain function by measuring DA’s spatiotemporal dynamics in the extracellular space9,10.
Microdialysis combined with high-performance liquid chromatography is used widely for monitoring DA and other monoamine neurotransmitters in vivo11–20 (we will use “LC” for the abbreviations HPLC and UHPLC). Very often, microdialysis is performed with sampling times of several minutes or more, which limits the temporal resolution of the measurements. Notably, early papers by Justice described off-line dialysate DA analysis by microbore LC with one-minute temporal resolution21,22. In addition, there was considerable activity in the ‘90s and early 2000s on sub-one-minute measurements of amino acids by capillary electrophoresis (CE)23–29. Dopamine has been measured by CE with laser-induced fluorescence with 10–90s time resolution30–32. However, until recently there have been very few reports of sub-five minute time resolution measurements on dialysates. For instance, Andrews and coworkers report that none of the brain microdialysis papers published in 2012 used sampling times of 5 min or less19.
Nevertheless, interest in rapid microdialysis using LC appears to be resurging. Andrews and coworkers developed a 3-min LC method for serotonin based on readily available commercial equipment33. Reinhoud described rapid analyses for several neurochemicals34. Parrot and coworkers separated several related compounds including DA using UHPLC with a chromatographic run time for DA of less than three minutes35. The Kennedy group36 demonstrated and applied one-minute time resolution for DA with offline derivatization/LC/MS determinations. We developed an off-line system that uses sub-2-µm particles and high column temperatures to measure serotonin in one-minute brain dialysate samples37. We followed that up with an on-line capillary LC system and studied serotonin in awake rat striatum with one-minute temporal resolution38,39.
In practice, measuring DA in striatal dialysate should be easier than measuring serotonin because the DA concentration is ~10-fold higher. On the other hand, DA appears in a more crowded region of the chromatogram. In LC increasing speed generally decreases peak capacity, so we anticipate poorer resolution will accompany efforts to develop a one-minute on-line DA determination40,41. In addition, we found that divalent metal ions in the dialysates20 cause peaks near DA as well. We report here that optimizing the chromatographic conditions leads to a sub-one-minute analysis time for DA. We used these conditions to carry out microdialysis in vivo with on-line capillary LC and one-minute sampling times. Furthermore, by using this system to monitor electrically evoked DA transients, we document that this system provides bona fide sub-one-minute temporal resolution. Evoked transients appeared in single, one-minute dialysate samples.
Experimental
Chemicals
Disodium EDTA, sodium phosphate, o-phosphoric acid (85%, HPLC grade), acetonitrile (Optima LC/MS grade), acetone (HPLC grade), and 2-propanol (99.9 %, HPLC grade) were purchased from Fisher Scientific (Fair Lawn, NJ). Sodium acetate and glacial acetic acid (99.9 %) were from J.T. Baker (Phillipsburg, NJ). Sodium 1-octanesulfonate (SOS), L-ascorbic acid (AA), 3,4 dihydroxyphenylacetic acid (DOPAC), 5-hydroxyindole-3-acetic acid (5-HIAA), homovanillic acid (HVA), dopamine hydrochloride (DA), serotonin hydrochloride (5-HT), 3-methoxytyramine hydrochloride (3-MT) were from Sigma Aldrich (St. Louis, MO). Nomifensine maleate (Sigma) was dissolved in phosphate-buffered saline (PBS: 155 mM NaCl, 100 mM NaH2PO4, pH 7.40) and administered at 20 mg/kg (i.p.). Artificial cerebrospinal fluid (aCSF) was 144 mM NaCl, 2.7 mM KCl, 1.1.75 mM CaCl2, 1 mM MgCl2, 1.75 mM NaH2PO4 and 2.5 mM NaHCO3. All chemicals were used as received without further purification. Ultrapure water was obtained from a Millipore Milli-Q Synthesis A10 system (Bedford, MA).
Chromatography
Stock solutions of 1 mM DA, DOPAC, 5-HT, 5-HIAA, and HVA were prepared in 0.1 M acetic acid and stored frozen. Subsequent dilutions were made in 0.1 M acetic acid with final samples made in aCSF at the desired concentration. Mobile phase was prepared by mixing acetonitrile with appropriate aqueous buffer in a particular volume ratio. Acetate based buffers contained 75 mM sodium acetate, 0.15 mM disodium EDTA, and SOS (concentrations given below). Phosphate buffers contained 75 mM sodium phosphate, 0.15 mM sodium EDTA, and 1.75 mM SOS. The pH values of the aqueous buffers were adjusted with either acetic acid or phosphoric acid to the desired pH using a Fisher Acumet 10 pH meter. Prior to use mobile phase was passed through a 0.45 m nylon filter (Millipore).
Capillary columns were prepared by packing Waters Acquity BEH C18, 1.7 µm particles (Millford, MA) into 150 µm i.d. fused-silica capillaries from Polymicro Technogies (Phoenix, AZ). Column frits were made using an electrical arc to sinter 2 µm solid borosilicate spheres (Thermo Scientific, Fremont, CA) into the end of the 15–20 cm long column blank. Frits were approximately 1 mm in length. Particles were slurried in isopropanol at a concentration of 65 mg/mL and sonicated for 20 minutes prior to packing using the downward slurry method. A Haskel pneumatic amplification pump (Model DSHF-302, Haskel, Burbank, CA) was used to pack column at 20,000 psi for 20 minutes. Acetone was used as the packing solvent. Following packing, column pressure was allowed to dissipate naturally. The packed column was flushed with acetonitrile and equilibrated with mobile phase overnight prior to use. Columns were then trimmed to a final length of 4.5 cm.
For chromatography, a pump (Model NanoLC-Ultra 1D, Eksigent, Dublin, CA) with a maximum pressure limit of 10,000 psi was used to deliver mobile phase. A Valco 8-port nano volume valve (100 µm port size) equipped with two 500 nL sample loops was connected directly to the capillary column. Mobile phase flow rate was 9 µL/min. Column and injection temperatures were maintained between 40 and 60 °C using two simple homemade heating assemblies previously described39.
A Bio-analytical Systems Inc. (BASi, West Lafayette, IN) thin-layer radial-style electrochemical detection flow cell with a glassy carbon (3-mm-diameter) electrode was used to detect analytes at 700 mV vs Ag/AgCl, 3M NaCl. The flow path and detector volume was defined by a 13 µM Teflon gasket. Potential control and data acquisition at 20 Hz were done by a BSAi Epsilon potentiostat and ChromGraph software (West Lafayette, IN). The LC system was calibrated by direct sample injection of DA standard solutions (100 nM) before and after in vitro and in vivo online experiments.
Microdialysis
Concentric microdialysis probes (300 µm diameter, 4 mm length) were constructed using 13 kDA MWCO hollow fiber membranes (Spectra/Por RC, Spectrum Laboratories Inc., Ranco Dominguez, CA). Inlet tubing (PE, Becton Dickinson, Franklin Lakes, NJ) was connected to a Pico-plus syringe pump (Harvard Apparatus, Holliston, MA) with 1.0 mL gastight syringe. The microdialysis perfusion rate was 0.61 µL/min: perfusion of the probes began before they were implanted into the brain and continued for the duration of the experiments. The probe outlet line (a 71-cm length of fused silica capillary, 75 µm I.D by 150 µm O.D., Polymicro Technologies, Phoenix, AZ) was plumbed directly to the LC injection valve for online dialysate analysis. For in vitro probe calibration, probes were immersed in a beaker of aCSF, transferred manually to a second beaker containing 1 µM DA in aCSF, held there for 30 s, and then returned to the original beaker of aCSF.
Surgical procedures
All procedures involving animals were approved by the Institutional Animal Care and Use of Committee of the University of Pittsburgh. Male Sprague-Dawley rats (250–350 g; Hilltop, Scottsdale, PA) were anesthetized with isoflurane (0.5 % by volume, Henry Schein Animal Health, Elizabethtown, PA). Rats were wrapped in a heating blanket (37 °C) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) set up for flat skull coordinates42. A small craniotomy was made over the striatum (1.6 mm anterior and 2.5 mm lateral from bregma). Probes were lowered into the striatum at 5 µm/s with an automated micropositioner (David Kopf Instruments, Tujunga, CA, Model 2660). The tip of the probes came to rest 7 mm beneath the brain surface. The probes were secured to the skull with bone screws and acrylic cement and the scalp incision was closed with sutures. The anesthesia was removed and the rats were placed in a Raturn Microdialysis Bowl Stand-Alone System (MD-1404, BASi) for 24 hr.
On the following day, 24-hr after the probe insertion, the rats were re-anesthetized and placed back into the stereotaxic frame. A carbon fiber microelectrode for fast-scan cyclic voltammetry (FSCV) of DA was lowered into the striatum to a position 1.5 mm away from the microdialysis probe as previously described43. A bipolar stimulating electrode was lowered towards the medial forebrain bundle (4.3 mm posterior and 1.2 mm lateral from bregma). The stimulating electrode was positioned 7.2 mm below the dura, then slowly lowered until robust electrically evoked DA responses were observed via the FSCV electrode. The stimulus was a biphasic constant current square wave delivered via a stimulus isolation unit (stimulus parameters: 300 µA, 4 ms pulse width for 1 s (60 Hz) or 25s (45Hz)).
In vivo experiments
With the rat still anesthetized and in the stereotaxic frame, online analysis of the dialysate was performed at one-minute intervals until a stable baseline DA signal was established: this typically required only a few minutes. Then, the medial forebrain bundle was electrically stimulated for 25 s with an approximately 20 minute interval between stimulations. The rats then received a dose of nomifensine (20 mg/kg i.p.), a dopamine uptake inhibitor and sampling continued until a new DA baseline was attained (20 minutes). After nomifensine administration, two more stimuli were delivered, one with a duration of 1 s and one with a duration of 25 s.
Quantitation
Optimization of the LC conditions was performed by the analysis of microdialysate samples that had been mixed 1:1 with 0.1 M acetic acid, frozen, and stored. Concentrations were determined by external calibration without correction for probe recovery. Peak area was used for calibration and determined by integration in MATLAB. Data (20 points per second) were output from the instrument as a text file and input into the MATLAB workspace as a single, continuous stream of data containing the series of individual, one-minute chromatograms. The data were first separated into individual runs for analysis. Each run was analyzed for a peak in the 50 to 55 second window using the “findpeaks” command from the MATLAB Signal Processing Toolbox. The “findpeaks” command finds local maxima within each data set by specifying the minimum peak height and minimum distance between peaks. From the maximum of the identified peak, the algorithm moves down the left and right sides of the peak until reaching the baseline. The baseline was identified by measuring the slope between two adjacent points (typically 0.5 to 1 second apart) until the slope reaches or passes zero. This window can be adjusted to prevent the algorithm from being stopped at noise. Once the baseline was determined, the peak area was calculated using trapezoidal integration.
Results and Discussion
Optimizing the mobile phase
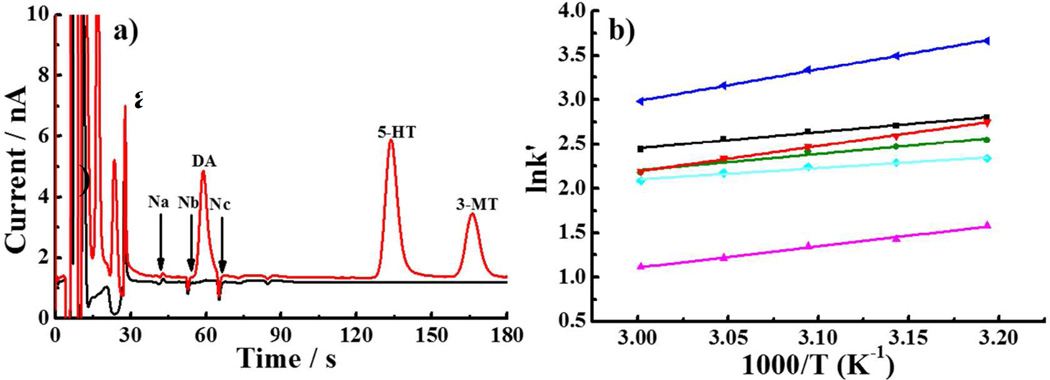
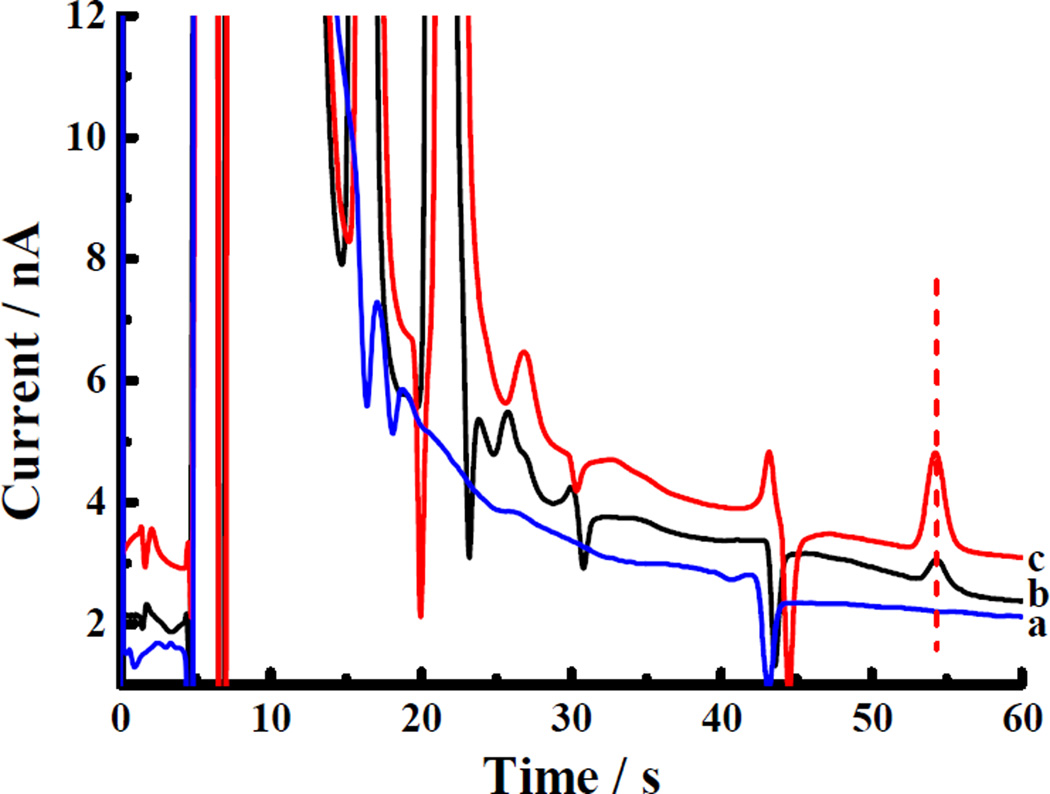
Figure 1a shows a chromatogram of standards (100 nM DOPAC, 5-HIAA, HVA, DA, 5-HT, 3-MT) using mobile phase of Zhang and coworkers39 The column temperature was lowered to increase the retention of DA. Three small negative peaks (labeled Na, Nb, Nc) appear near the DA peak and interfere with the determination of dialysate DA levels (~1–10 nM). van ‘t Hoff plots (Fig. 1b) of the negative peaks44 suggest that temperature control alone cannot move them sufficiently away from the DA peak. Two of the negative peaks, Na, and Nb, are caused by divalent metal ions, Mg2+ and Ca2+ in the aCSF (Figure S1), which are retained on the column due to the presence of SOS in the mobile phase and create a signal by adsorbing/desorbing to the carbon electrode45.
Figure 1.
(a) Chromatogram of standard mixture (red) and blank (black). Temperature: 45 °C. (b) Van ‘t Hoff plots for analytes. Top to bottom (near the center of the plot): 5-HT (dark blue), Na (black), DA (red), Nb (green), Nc (light blue), HVA (fuchsia). Mobile phase: 75mM Sodium acetate, 0.15 mM disodium EDTA, 10 mM SOS (pH 4); column: 150 µm×45 mm, 9.0 µL/min, injection volume: 500 nL.
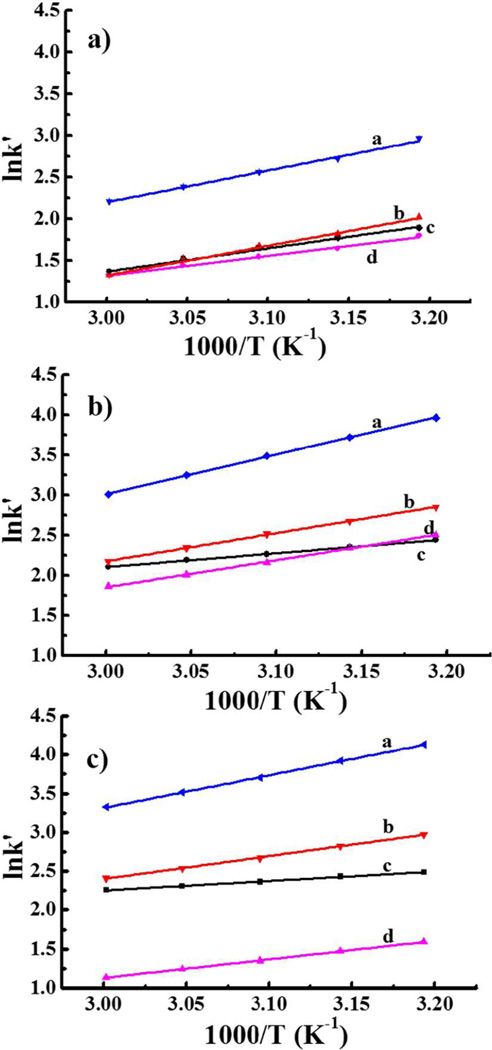
The aCSF must contain Mg2+ and Ca2+ so as not to disturb the ionic composition of the brain tissue near the probe. So, we attempted to manipulate the negative peaks by adjusting SOS concentration. Two of the negative peaks disappeared at 1.75 mM SOS. However, such a low SOS concentration compromised the resolution of DA and HVA (Figure 2a). Somewhat better performance (Fig. 2b) was obtained by switching from acetate to phosphate buffer. The phosphate buffer solved the problem of the negative peak but DA is not sufficiently resolved from HVA, which is a problem because brain dialysates contain large amounts of this anion. Because the pKa of HVA is 4.39, increasing the pH to 5.00 reduced its retention with little impact on DA retention (Fig. 2c and Fig. S2). Consideration of the van ‘t Hoff plot in Fig. 2c shows that as temperature increases, the DA and Na peaks approach each other. At a temperature of 48 °C (1000/T = 3.12, Fig. 2c) the DA peak elutes in less than one minute and the separation from the interfering peak Na is adequate. In summary, the optimized mobile phase contains 75 mM phosphate buffer with 1.75 mM SOS at pH 5.00 and the column is operated at 48 °C. In addition, based on the retention times of standards, late-eluting peaks from earlier chromatograms do not interfere with the DA peak from a given sample. At the operating temperature of 48 °C the serotonin peak from an injection at time zero elutes at an apparent retention time of 20 s in the chromatogram from the injection made at 120 s. Thus there is no interference with the DA peak which elutes much later in the chromatogram.
Figure 2.
Van ‘t Hoff plots for detection of DA. Mobile phase: (a) 75 mM Sodium acetate, pH 4.00; (b) 75 mM Sodium phosphate, pH 4.00; (c) 75 mM Sodium phosphate, pH 5.00 with 0.15 mM disodium EDTA, 1.75 mM SOS. (column: 150 µm×4.5 mm, 9.0 µL/min, injection volume: 500 nL. a: 5-HT; b: DA; c: Na; d: HVA.
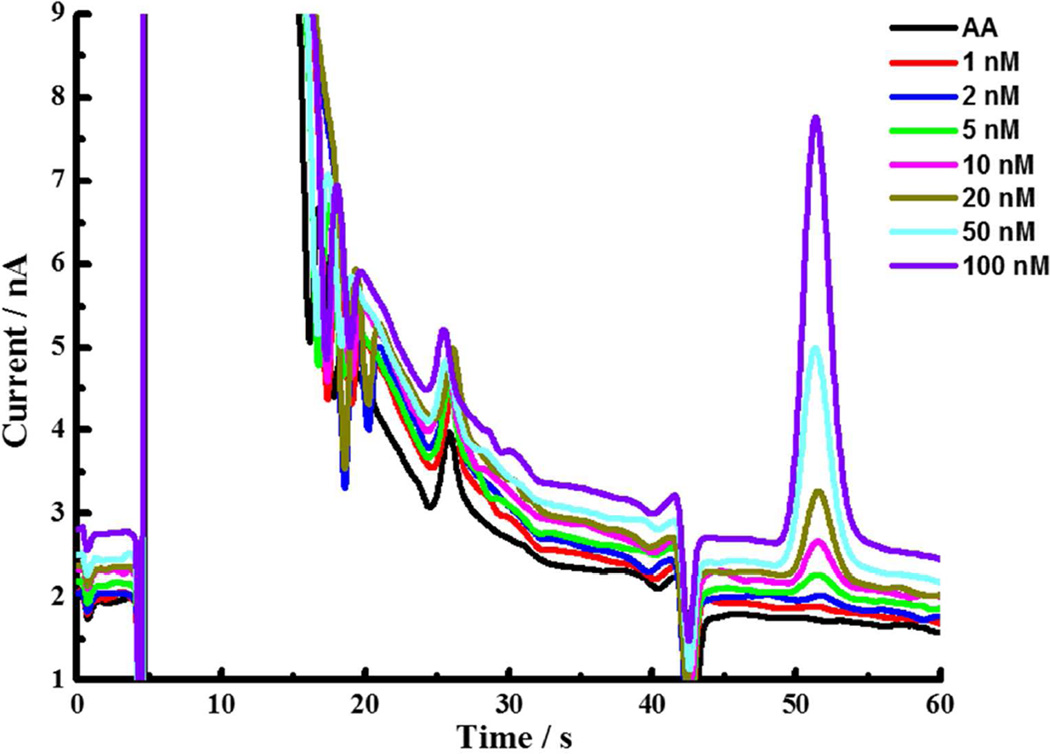
Figure 3 shows chromatograms of DA standards containing 50 µM ascorbic acid (AA), which is approximately the AA concentration of brain dialysates. The area of the DA peaks is linearly dependent on its concentration. Calibration curves were linear over a wide range (Fig. S3). The detection limit, based on the signal-to-noise ratio of three, is 0.15 nM.
Figure 3.
Chromatograms of standard solutions of DA containing 50 µM AA, from bottom to top: 0 nM, 2 nM, 5 nM, 10 nM, 20 nM, 50 nM, 100 nM DA. Mobile phase: 75 mM sodium phosphate, 0.15 mM disodium EDTA, 1.75 mM SOS, (pH 5.00); column: 150 µm × 4.5 mm; T=48 °C, 9.0 µL/min; injection volume: 500 nL.
System performance: in vitro determinations
The chromatogram of a 50 µM AA standard shows no peaks near DA’s retention time (53 s), while a clear peak is observed in the chromatogram of a frozen, stored, and thawed dialysate sample (Fig. 4). Spiking the dialysate sample with 25 nM DA increased the peak area, which confirms the identity of the dialysate component as DA. The DA peak was very stable upon repeated injection of 60 samples (Fig. S4). There was no significant change in the DA peak area: the average concentration of DA in these 60 samples was 11.5±1.0 nM.
Figure 4.
Chromatograms of microdialysis samples. (a) 50 µM AA; (b) microdialysis sample; (c) microdialysis sample with 25 nM DA added. Red dotted line indicates the typical DA peak position. Conditions as in Fig. 3.
Synchronizing microdialysis with on-line LC
Rapid on-line microdialysis should allow for more accuracy in knowing when a transient occurs in the brain. It is necessary to account for the time it takes the sample to travel from the probe to the sample loop. To accomplish this we created 30-s DA boluses in the outlet line of the probe (see methods). If the bolus is properly synchronized with the actuation of the sampling valve, it should appear in a single one-minute dialysate sample. Preliminary experiments showed that when the bolus was created 30 s, but not 15 s or 45 s, after a valve actuation, then DA appeared entirely in the 7th following sample. We then confined our attention to time intervals of 25, 30, and 35 s. For the 30 s time interval, as Table 1 demonstrates, only 1.2% of the DA was found in the chromatogram at the 6th minute, while the majority of the signal was found in the 7th minute. The 30-s synchronization time was then used in the following in vivo experiments.
Table 1.
Establishment of time delay between event and chromatogram
Time interval between injection valve actuation and probe insertion in DA-containing solution.
Percent of total signal in a particular chromatogram.
Number of replicates
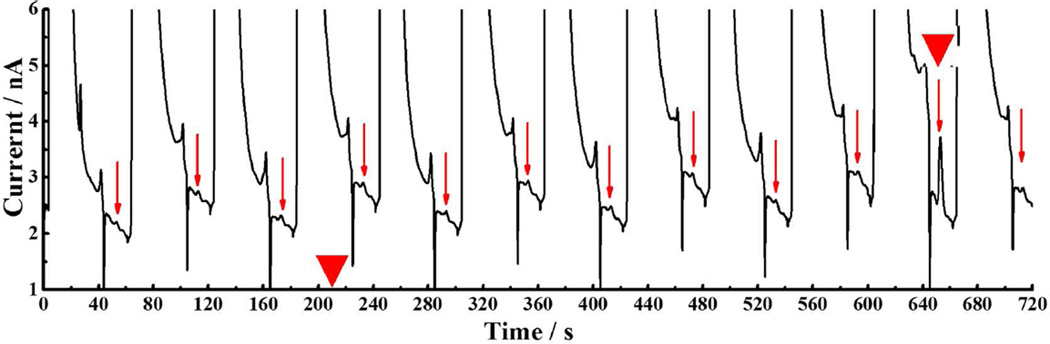
In vivo monitoring of basal and electrically evoked DA
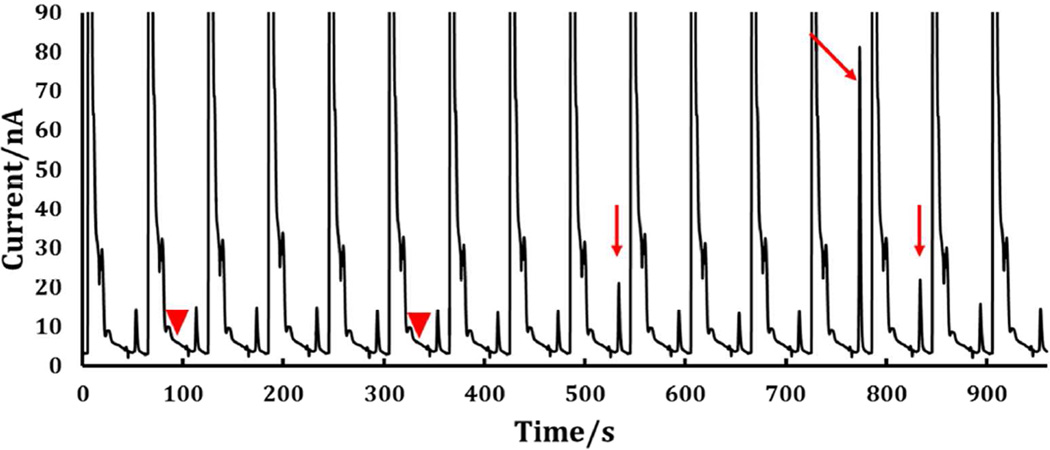
Figure 5 shows a series of chromatograms of brain dialysate samples obtained by means of the optimized on-line capillary LC system. The basal DA concentration is 10.7±1.5 nM (mean ± standard deviation of n=68 one-minute chromatograms), which is comparable to reports in the literature using similar microdialysis flow rates46,47. A 25-s electrical stimulus was delivered to the medial forebrain bundle at t = 210s (red arrowhead, Fig. 5), which resulted in a transient DA increase in a single chromatogram 7 samples later. Figure S6 shows series of chromatograms showing the effect on DA of nomifensine administration. Figure 6 shows chromatograms of post-nomifensine dialysate samples. A one-s stimulus was performed at t = 90 s and a 25-s stimulus was performed at t = 330s (red arrowheads, Fig. 6): transient increases in DA were again observed 7 samples later (red arrows, Fig. 6), After the 25-s stimulus, some DA was observed in the 8th following sample. This is expected as nomifensine is known to increase the duration of evoked transients. Experiments in three animals resulted in the following observations. The two 25s stimulations before nomifensine resulted in concentrations of 37 ± 16 and 38 ± 18 nM (mean ± standard error), which were not significantly different from one another. In the absence of uptake inhibition, we did not see a response from a one-second stimulation (data not shown). Following the administration of nomifensine a one-second stimulus elicited a response of 220 ± 17 nM (only attempted in 2 of the 3 trials). The 25s stimulation after nomifensine produced an average concentration of 975± 316 nM. The administration of nomifensine increased the signal obtained from 25s stimulations approximately twenty-five-fold.
Figure 5.
Typical chromatograms during continuous DA measurements. Arrows indicate the DA peaks. A 25 s electrical stimulus was initiated at the time marked by the first triangle. The DA response is indicated by a second triangle. Conditions as in Fig. 3.
Figure 6.
Typical chromatograms during continuous DA measurements following administration of nomifensine. An electrical stimulus excited the median forebrain bundle for 1 s at time = 90 s (left triangle) and at time = 330 s for 25 s (right triangle). Arrows indicate the corresponding DA release events. Conditions as in Fig. 3.
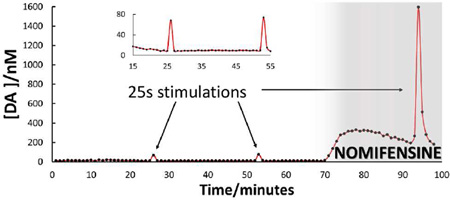
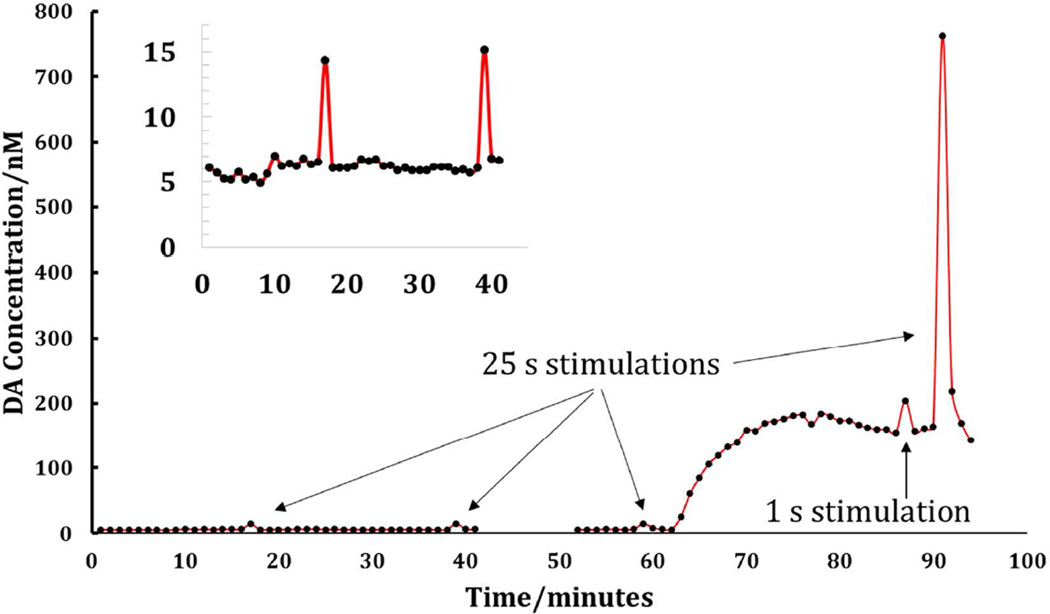
Figure 7 summarizes a 2-h in vivo DA monitoring experiment by microdialysis with optimized on-line capillary LC analysis with a one-minute sampling time. The figure clearly shows five features. The one-minute transients at t = 17, 39 and 59 min are responses to 25-s electrical stimuli delivered prior to nomifensine administration. The prolonged increase in DA that begins at t = 62 min is the response to the nomifensine injection. The transients at t = 87 min and at t = 91 min are responses to one-s and 25-s stimuli, respectively, delivered after nomifensine. The one-s stimulus response is confined to a single sample. The 25-s response spans two samples, consistent with the known tendency of nomifensine to prolong the duration of evoked responses.
Figure 7.
On-line in vivo DA measurements. Three electrical stimulations were carried out prior to administration of nomifensine. Twenty minutes after i.p. injection of nomifensine, 1 second and 25 second stimulations were carried out. The arrows indicate the responses. The inset provides an expanded view of the pre-nomifensine stimulations at 17 and 39 min. Note that a technical malfunction prevented accurate integrations of DA peaks from about 42 to 51 minutes.
The DA concentrations reported in Fig. 7 were determined by external calibration without correction for the unknown in vivo recovery of microdialysis probes. The response seen earlier by in vivo FSCV to the stimulus parameters used during this work is an evoked DA transient with amplitudes in the micromolar range43,48,49. The nanomolar concentrations observed in the dialysate samples confirm the view that the in vivo recovery of DA by microdialysis probes, especially during transients that do not attain steady state, is very low. Moreover, the increased evoked amplitude observed after nomifensine administration likely has two origins: uptake inhibition increases evoked DA overflow50–52 but also increases the microdialysis recovery of DA53–55. While these quantitative issues need to be considered when relating microdialysis data to in vivo events, they do not diminish the value of monitoring DA on time scales more closely relevant to those of DA neurotransmission.
Separately, we compared three injections of a 100 nM standard DA solution before and after more than two hours of continuous online in vivo experiments (Fig. S5). There was no significant change in the sensitivity or retention time of DA peak (Table 2). However, we did observe a decrease in theoretical plate count, the origin of which is under investigation. It is worth mentioning, however, that the column exhibited ~5000 theoretical plates, which is more than 110,000 plates per meter, prior to the in vivo work. Plate counts for DA peaks were calculated under conditions identical to those used for in vivo experiments and were not corrected for extra column contributions to band spreading. This column performance makes a significant contribution to system performance (rapid analysis and low detection limits) because it is the source of the narrow, tall, well-resolved, and easily integrated peaks.
Table 2.
Column performance evaluation before and after in vivo measurementsa.
| Peak Area | Retention Time / s | Plate Number | |
|---|---|---|---|
| Before | 9.12 ±0.22 × 104 b | 53.0 ± 0.3 | 4976 ± 56 |
| After | 9.21 ± 0.20 × 104 | 53.0 ± 0.2 | 3733 ± 39 |
Evaluation based on 100 nM DA standard.
Errors are standard errors of the mean.
As far as we are aware, the fastest on-line microdialysis/dopamine measurements are those by Shou et al56. They used capillary electrophoresis/derivatization/laser-induced fluorescence to achieve 90-s time resolution with a detection limit of 2 nM. As described in the introduction, there are several prior reports of sub-minute measurements of samples obtained by microdialysis and online measurements of compounds at higher concentrations (amino acids). Here we demonstrate that we can quantify DA over an extended period with one-minute time resolution, online analysis, and a detection limit of 0.15 nM. By appropriately synchronizing a stimulation with the LC injection cycle, we are able to see the response to a 25-s stimulation in a single chromatogram. Shifts of that phasing by 15 s give obvious peaks in adjacent chromatograms. Thus, in situations where the DA response to an event is known, when that event occurred can be determined with a time resolution of fifteen seconds. Remarkably, in the presence of nomifensine, we see a robust response to a one-second stimulation. We believe that this level of performance in a microdialysis experiment will lead to wider use of microdialysis in behavior experiments and other experiments in which the goal is to measure the time at which a transient of DA occurs. Microdialysis is becoming increasingly capable of measuring neurotransmitter on the fast time scales relevant to neurotransmission.
Conclusion
On-line analysis of DA in brain dialysate with one-minute resolution was achieved through detailed attention to the capillary LC separation conditions including column temperature, buffer composition, and surfactant concentration. The major interferences were HVA and the divalent metal ions in the samples. Optimizing the mobile phase pH, buffer composition (acetate to phosphate), surfactant content and temperature solved these interference problems. By analysis of electrically evoked DA transients, we confirmed that the system has bona fide sub-one-minute temporal resolution, temporal resolution being distinct from the sampling time.
Supplementary Material
Acknowledgments
This projected was supported by funding from the National Institutes of Health (R21NS081744 (ACM) and R01MH104386 (SGW)). ELV and SGR acknowledge support from the NSF for Graduate Research Fellowships. We are grateful to Dr. Ed Bouvier of Waters for the gift of packing materials
Footnotes
Associated content
Supporting information
Supporting figures Scheme 1 and S1 through S6
The authors declare no competing financial interest.
Literature cited
- 1.Grace AA. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai H-C, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizuno Y, Bies RR, Remington G, Mamo DC, Suzuki T, Pollock BG, Tsuboi T, Watanabe K, Mimura M, Uchida H. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:182–187. doi: 10.1016/j.pnpbp.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Lotharius J, Brundin P. Nat Rev Neurosci. 2002;3:932–942. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- 5.Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, Schwandt ML, Sommer WH, George DT, Parsons LH, Herscovitch P, Hommer D, Heilig M. Mol. Psychiatry. 2011;16:809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willuhn I, Wanat MJ, Clark JJ, Phillips PEM. Curr Top Behav Neurosci. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DCS, Jones SR. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 10.Schultz W. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 11.Yoshitake T, Yoshitake S, Fujino K, Nohta H, Yamaguchi M, Kehr J. J. Neurosci. Methods. 2004;140:163–168. doi: 10.1016/j.jneumeth.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 12.Watson CJ, Venton BJ, Kennedy RT. Anal. Chem. (Washington, DC, U. S.) 2006;78:1391–1399. doi: 10.1021/ac0693722. [DOI] [PubMed] [Google Scholar]
- 13.Yoshitake T, Kehr J, Todoroki K, Nohta H, Yamaguchi M. Biomed. Chromatogr. 2006;20:267–281. doi: 10.1002/bmc.560. [DOI] [PubMed] [Google Scholar]
- 14.Ji C, Li W, Ren X-d, El-Kattan AF, Kozak R, Fountain S, Lepsy C. Anal. Chem. (Washington, DC, U. S.) 2008;80:9195–9203. doi: 10.1021/ac801339z. [DOI] [PubMed] [Google Scholar]
- 15.Greco S, Danysz W, Zivkovic A, Gross R, Stark H. Anal. Chim. Acta. 2013;771:65–72. doi: 10.1016/j.aca.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Hershey ND, Kennedy RT. ACS Chem. Neurosci. 2013;4:729–736. doi: 10.1021/cn300199m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos-Fandila A, Zafra-Gomez A, Barranco A, Navalon A, Rueda R, Ramirez M. Talanta. 2013;114:79–89. doi: 10.1016/j.talanta.2013.03.082. [DOI] [PubMed] [Google Scholar]
- 18.Suominen T, Uutela P, Ketola RA, Bergquist J, Hillered L, Finel M, Zhang H, Laakso A, Kostiainen R. Plos One. 2013;8:e68007. doi: 10.1371/journal.pone.0068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang H, Thompson AB, McIntosh BJ, Altieri SC, Andrews AM. ACS Chem. Neurosci. 2013;4:790–798. doi: 10.1021/cn400072f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferry B, Gifu E-P, Sandu I, Denoroy L, Parrot S. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2014;951–952:52–57. doi: 10.1016/j.jchromb.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Newton AP, Justice JB. Anal. Chem. (Washington, DC, U. S.) 1994;66:1468–1472. doi: 10.1021/ac00081a018. [DOI] [PubMed] [Google Scholar]
- 22.Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Psychopharmacology (Berl) 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- 23.Bert L, Robert F, Denoroy L, Stoppini L, Renaud B. J. Chromatogr. A. 1996;755:99–111. doi: 10.1016/s0021-9673(96)00595-x. [DOI] [PubMed] [Google Scholar]
- 24.Bowser MT, Kennedy RT. Electrophoresis. 2001;22:3668–3676. doi: 10.1002/1522-2683(200109)22:17<3668::AID-ELPS3668>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Lada MW, Kennedy RT. Anal. Chem. (Washington, DC, U. S.) 1996;68:2790–2797. doi: 10.1021/ac960178x. [DOI] [PubMed] [Google Scholar]
- 26.Lada MW, Vickroy TW, Kennedy RT. Anal. Chem. (Washington, DC, U. S.) 1997;69:4560–4565. doi: 10.1021/ac970518u. [DOI] [PubMed] [Google Scholar]
- 27.Robert F, Parisi L, Bert L, Renaud B, Stoppini L. J. Neurosci. Meth. 1997;74:65–76. doi: 10.1016/s0165-0270(97)02261-9. [DOI] [PubMed] [Google Scholar]
- 28.Rossell S, Gonzalez LE, Hernandez L. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003;784:385–393. doi: 10.1016/s1570-0232(02)00826-7. [DOI] [PubMed] [Google Scholar]
- 29.Tucci S, Rada P, Sepulveda MJ, Hernandez L. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1997;694:343–349. doi: 10.1016/s0378-4347(96)00488-4. [DOI] [PubMed] [Google Scholar]
- 30.Bert L, Parrot S, Robert F, Desvignes C, Denoroy L, Suaud-Chagny MF, Renaud B. Neuropharmacology. 2002;43:825–835. doi: 10.1016/s0028-3908(02)00170-3. [DOI] [PubMed] [Google Scholar]
- 31.Parrot S, Sauvinet V, Riban V, Depaulis A, Renaud B, Denoroy L. J. Neurosci. Methods. 2004;140:29–38. doi: 10.1016/j.jneumeth.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 32.Shou MS, Ferrario CR, Schultz KN, Robinson TE, Kennedy RT. Anal. Chem. (Washington, DC, U. S.) 2006;78:6717–6725. doi: 10.1021/ac0608218. [DOI] [PubMed] [Google Scholar]
- 33.Yang H, Thompson AB, McIntosh BJ, Altieri SC, Andrews AM. ACS Chem. Neurosci. 2013;4:790–798. doi: 10.1021/cn400072f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinhoud NJ, Brouwer HJ, van Heerwaarden LM, Korte-Bouws GAH. ACS Chem. Neurosci. 2013;4:888–894. doi: 10.1021/cn400044s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parrot S, Neuzeret P-C, Denoroy L. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2011;879:3871–3878. doi: 10.1016/j.jchromb.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 36.Vander Weele CM, Porter-Stransky KA, Mabrouk OS, Lovic V, Singer BF, Kennedy RT, Aragona BJ. Eur. J. Neurosci. 2014;40:3041–3054. doi: 10.1111/ejn.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu YS, Zhang J, Xu XM, Zhao MK, Andrews AM, Weber SG. Anal. Chem. (Washington, DC, U. S.) 82:9611–9616. doi: 10.1021/ac102200q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Liu Y, Jaquins-Gerstl A, Shu Z, Michael AC, Weber SG. J. Chromatogr., A. 2012;1251:54–62. doi: 10.1016/j.chroma.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Jaquins-Gerstl A, Nesbitt KM, Rutan SC, Michael AC, Weber SG. Anal. Chem. (Washington, DC, U. S.) 2013 doi: 10.1021/ac4023605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muzzi C, Bertocci E, Terzuoli L, Porcelli B, Ciari I, Pagani R, Guerranti R. Biomed Pharmacother. 2008;62:253–258. doi: 10.1016/j.biopha.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Suominen T, Uutela P, Ketola RA, Bergquist J, Hillered L, Finel M, Zhang HB, Laakso A, Kostiainen R. Plos One. 2013;8 doi: 10.1371/journal.pone.0068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press; 2005. [Google Scholar]
- 43.Nesbitt KM, Varner EL, Jaquins-Gerstl A, Michael AC. ACS Chem. Neurosci. 2015;6:163–173. doi: 10.1021/cn500257x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levin S, Grushka E. Anal. Chem. (Washington, DC, U. S.) 1986;58:1602–1607. [Google Scholar]
- 45.Chen J-G, Vinski E, Colizza K, Weber SG. J. Chromatogr. A. 1995;705:171–184. doi: 10.1016/0021-9673(95)00286-v. [DOI] [PubMed] [Google Scholar]
- 46.Tang A, Bungay PM, Gonzales RA. J Neurosci Meth. 2003;126:1–11. doi: 10.1016/s0165-0270(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 47.Jaquins-Gerstl A, Shu Z, Zhang J, Liu Y, Weber SG, Michael AC. Anal. Chem. (Washington, DC, U. S.) 2011;83:7662–7667. doi: 10.1021/ac200782h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borland LM, Shi G, Yang H, Michael AC. J Neurosci Methods. 2005;146:149–158. doi: 10.1016/j.jneumeth.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Nesbitt KM, Jaquins-Gerstl A, Skoda EM, Wipf P, Michael AC. Anal. Chem. (Washington, DC, U. S.) 2013;85:8173–8179. doi: 10.1021/ac401201x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garris PA, Ciolkowski EL, Pastore P, Wightman RM. J Neurosci. 1994;14:6084–6093. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones SR, Garris PA, Wightman RM. J Pharmacol Exp Ther. 1995;274:396–403. [PubMed] [Google Scholar]
- 52.Wu Q, Reith MEA, Kuhar MJ, Carroll FI, Garris PA. J. Neurosci. 2001;21:6338–6347. doi: 10.1523/JNEUROSCI.21-16-06338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu Y, Peters JL, Michael AC. J. Neurochem. 1998;70:584–593. doi: 10.1046/j.1471-4159.1998.70020584.x. [DOI] [PubMed] [Google Scholar]
- 54.Floresco SB, West AR, Ash B, Moore H, Grace AA. Nat. Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 55.Bungay PM, Newton-Vinson P, Isele W, Garris PA, Justice JB. J. Neurochem. 2003;86:932–946. doi: 10.1046/j.1471-4159.2003.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shou M, Ferrario CR, Schultz KN, Robinson TE, Kennedy RT. Anal. Chem. (Washington, DC, U. S.) 2006;78:6717–6725. doi: 10.1021/ac0608218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.