Abstract
Background
Animal studies have highlighted the role of vascular mineralocorticoid receptor during Cyclosporine A-induced nephrotoxicity. Mineralocorticoid receptor antagonists could improve kidney survival but are not commonly used during renal impairment and in association with several immunosuppressive drugs due to a supposed higher risk of adverse events. We tested the tolerance of eplerenone according to its expected adverse events: hyperkalemia, metabolic acidosis, hypotension, acute kidney failure, or any other adverse event.
Methods
We conducted a single-center, prospective, open-label study in 31 kidney-transplant recipients with impaired renal function (30 and 50 mL/min/1.73m2) and receiving cyclosporine A. All patients received eplerenone 25 mg/d for 8 weeks. Serum potassium, renal function and expected adverse events were closely monitored.
Results
Eight patients experienced mild hyperkalemia (>5 mmol/L), one moderate hyperkalemia (>5.5 mmol/L) and had to receive potassium-exchange resin. No severe hyperkalemia (>6 mmol/L) occurred. One acute kidney failure was observed, secondary to diarrhea. Basal serum potassium and bicarbonate were independently associated with a higher risk of developing mild hyperkalemia (>5 mmol/L) under treatment (OR 6.5, p = 0.003 and 0.7, p = 0.007, respectively). A cut-off value of 4.35 mmol/L for basal serum potassium was the best factor to predict the risk of developing mild hyperkalemia (>5 mmol/L).
Conclusions
Until eGFR falls to 30 mL/min/1.73m2, eplerenone could be safely given to kidney-transplant recipients receiving cyclosporine A, if kalemia is closely monitored. When renal function is impaired and if basal kalemia is >4.35 mmol/L, then clinicians should properly balance risk and benefit of eplerenone use and offer dietary advice. An adequately powered prospective randomized study is now needed to test its efficiency (and safety) in this population.
Trial Registration
ClinicalTrials.gov NCT01834768
Introduction
Calcineurin inhibitors (CNIs), such as Cyclosporine A (CsA) or tacrolimus, are the most commonly used maintenance immunosuppressive drugs after kidney transplantation [1] even if CNIs could lead to nephrotoxicity [2]. The mechanisms underlying CsA-induced nephrotoxicity (CIN) remain not fully elucidated [3]. Renal hemodynamic plays a central role during acute CIN: renal vasoconstriction has been reported as an initial event linked to CIN [3]. CsA is associated with renal afferent arteriolar vasoconstriction in rats and tubular injury during acute CsA nephrotoxicity [4].
The pharmacological antagonism of Mineralocorticoid Receptor (MR) reduces both cardiovascular and all-cause morbidity and/or mortality during chronic related (or not) heart failure [5, 6]. The MR expressed in endothelium and smooth muscle cells participates to the control of vascular tone: both endothelial and vascular smooth muscle MR modulate the responses to vasodilators and vasoconstrictors [7, 8]. Pharmacological antagonism of MR by both spironolactone [9, 10] and eplerenone [11, 12] is highly efficient to blunt CIN in experimental models. The vascular smooth muscle MR has been recently shown to play a key role during acute CIN in mice by preventing increased renal vascular resistance in acute CIN [13]: this could explain, at least partially, the beneficial effects of MR antagonism in CIN.
Chronic renal impairment could limit the use of MR antagonists (MRAs): even if hyperkalemia is feared, spironolactone and eplerenone could be safely used if a close monitoring of kalemia and renal function is ensured [14, 15]. However, the higher frequency of polypharmacy in chronic kidney disease patients could lead to drug-drug interactions and limit MRAs use, especially during kidney transplantation when immunosuppressive drugs metabolized by the P450 cytochrome (like CsA) are necessary. MRAs are not commonly used in this population despite the potential benefits to reduce cardiovascular risk and CIN after renal transplantation.
Gonzalez Monte et al. reported the benefits of adding spironolactone to a dual-blockade renin–angiotensin–aldosterone system (by both angiotensin-converting enzyme inhibitor [ACE-I] and type-2 angiotensin-receptor blockers [ARB]) in 11 kidney-transplant recipients with persistent proteinuria: after 6 months, proteinuria had decreased significantly with no adverse event [16]. Serum potassium remained stable (no severe hyperkalemia) and no serum bicarbonate was reported [16]. Since MRAs have never been tested in CsA-treated kidney-transplant recipients with impaired renal function, the present study was designed to test the tolerance of eplerenone in this population.
Subjects and Methods
We conducted a single-center, prospective, open-label study. The primary endpoint was the tolerance to eplerenone, assessed by the occurrence of the following expected adverse events: severe hyperkalemia (>6 mmol/L), metabolic acidosis (serum bicarbonate <15 mmol/L), hypotension (systolic blood pressure <100 mmHg), acute kidney failure (increase in serum creatinine >30% from baseline), or any adverse event that required discontinuation of eplerenone. Eplerenone was chosen due to its lower affinity for other steroid (progesterone, androgen and glucocorticoid) receptors and the absence of long-acting metabolites: this could lead to less frequent adverse events. We calculated the number of patients to include based upon the risk of severe hyperkalemia (>6 mmol/L), which was considered as the major adverse event.
Levels of kalemia were defined during the study as follow: normal (3.5 to ≤5 mmol/L), mild hyperkalemia (>5 to 5.4 mmol/L), moderate hyperkalemia (>5.5 to 5.9 mmol/L) and severe hyperkalemia (>6 mmol/L).
Study design
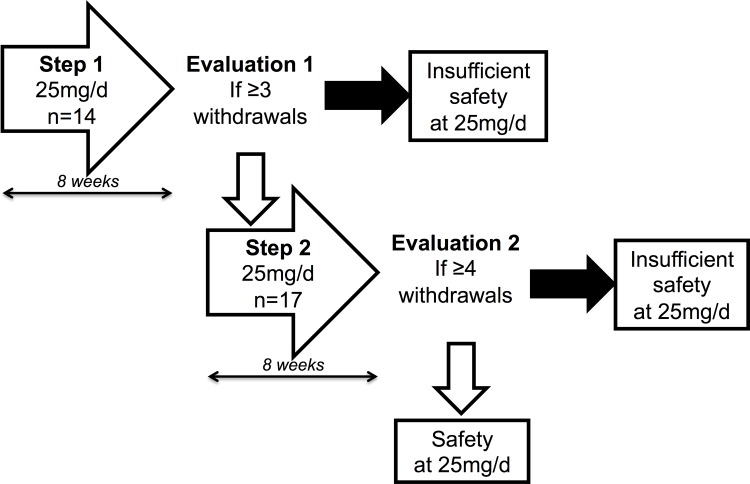
We performed the study by using a two-steps Simon’s plan (Fig 1) [17]. During the first step, 14 patients took eplerenone 25 mg/d for 8 weeks. This posology was chosen to be the minimum efficient. If three or more adverse events occurred, then study had to be discontinued. If not, 17 new patients were included within step 2 and also received the same treatment for 8 weeks. If four or more adverse events occurred in both steps (1 and 2), then study had to be discontinued, and the alternate hypothesis (a risk of adverse events >20%) could not be rejected: i.e., the safety of eplerenone could not be concluded. If not, we could conclude that eplerenone at 25 mg/d could be safety used in such a population.
Fig 1. Design of the EpleCsAT: Safety trial.
Sequential inclusion was performed: 14 patients during step 1; then 17 new patients during step 2.
All included patients were aged >18 years on the date of inclusion, belonged to a healthcare system, gave their informed written consent, had a functional kidney allograft for at least 1 year from the date of inclusion, was receiving CsA, and had impaired renal function, estimated by the MDRD formula [18], at between 30 and 50 mL/min/1.73 m2. Exclusion criteria were serum potassium of ≥5 mmol/L on the date of inclusion; one or more incidences of severe hyperkalemia (≥6 mmol/L), for whatever reason; currently receiving potassium-exchange resin treatment; on-going pregnancy or lack of effective contraception during the whole study period; uncontrolled high arterial blood pressure (systolic blood pressure >140 mmHg); orthostatic hypotension; systolic arterial blood pressure ≤110 mmHg; heart failure within the 3 months before the date of inclusion or chronic heart failure (NYHA III or IV); severe hepatic failure (Child-Pugh C score); allergy to one or more of the components of eplerenone (INSPRA®); on-going treatment, including spironolactone or eplerenone; on-going treatment that could not be withdrawn during the study period: e.g., potassium-sparing diuretics, potassium salts, CYP3A4 enzyme inhibitors other than CsA; malabsorption syndrome; abnormal galactose metabolism or a deficiency of galactase; on-going non-steroidal anti-inflammatory treatment, or lithium, or another nephrotoxic agent; or on-going treatment with a double-blockade of the renin–angiotensin–aldosterone system with ACE-I and ARB. The treatment could include ACE-I or ARB, but not in combination.
Clinical parameters (body weight, blood pressure, and adverse events) were monitored on days (D) 0, 14, 28, and 56. Serum potassium was closely monitored on days 0, 2, 7, 14, 21, 28, 35, 42, 49, and 56. Other biological parameters (such as serum creatinine and bicarbonate) were monitored on D 0, 14, 28, and 56.
At any time during the study period, adverse events that required discontinuation of eplerenone included serum potassium >6 mmol/L, serum potassium >5.5 mmol/L under potassium-exchange resin, metabolic acidosis assessed by a serum bicarbonate <15 mmol/L, and any other clinical outcome that required discontinuation of eplerenone.
Statistical methods and analyses
According to previously reported data, the probabilities to develop hyperkalemia (> 6 mmol/L) during 8 weeks of eplerenone treatment are <7% with the 25mg/d dose and <10% with the 50mg/d dose [5, 6, 16, 19]. Included patients exerted a better renal function than the population of the present study: herein, estimated glomerular filtration rate (eGFR) range was fixed between 50 and 70 mL/min/1.73m2. In our population, the expected risk (null hypothesis, H0) was supposed to be < 7% whereas a risk higher than 20% of developing major hyperkalemia (>6 mmol/L) was considered unacceptable (alternative hypothesis, Ha). Using a sample proportion test, the power to detect this adverse outcome was calculated at 95% (the β risk was 5%). Otherwise, in such conditions, the risk of not identifying an unacceptable risk of major hyperkalemia (>6 mmol/L) under eplerenone in these patients is 5%: the α risk was calculated at 22.5%. With these hypotheses, the inclusion of 31 patients was required: if 4 (/31) or more patients had to stop the treatment, the safety (< 20%) could not be assumed.
Quantitative data are described by their median and range and qualitative data as numbers and percentages. Variations of serum potassium at different times were evaluated using variance analysis for repeated measures. Comparisons between patients with mild hyperkalemia (>5 mmol/L) during the study protocol and those with normal kalemia (<5 mmol/L) at anytime of the study period were performed using univariate analyses (Wilcoxon's test or Fisher’s exact test, as appropriate) and multivariate analyses (stepwise logistic regression). The multivariate stepwise logistic regression included only significant factors at p ≤ 0.10 with entry and removal limits set at 0.10: basal cyclosporine A posology, creatininemia, serum potassium and bicarbonate. Sensitivities and specificities of basal serum potassium and bicarbonate were calculated, and a receiver-operating characteristic (ROC) curve was calculated to determine a cut-off value with optimal sensitivity and specificity: the statistical software (SAS) calculated automatically the coordinates of the ROC curve and calculated both the sensitivity and specificity (1—specificity for more precision) for all coordinates. Then, the cut-off value obtaining the best ratio between the sensitivity and the specificity was chosen.
Whatever the test used, a p-value <0.05 was considered statistically significant. All statistical analyses were performed using SAS software, release 9.3 (SAS INC, Cary, California).
Ethical considerations
This trial (S1 and S2 Figs) received specific agreements from an appropriate independent ethics committee, was registered in the European registry (EudraCT 2011-003759-20) and in clinicaltrials.gov (NCT01834768) and has therefore been performed in accordance with the ethical standards laid down in an appropriate version of the Helsinki Declaration of 1975, as revised in 2000, as well as the Declaration of Istanbul 2008. All persons gave their informed written consent prior to their inclusion to the study. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism”.
Results
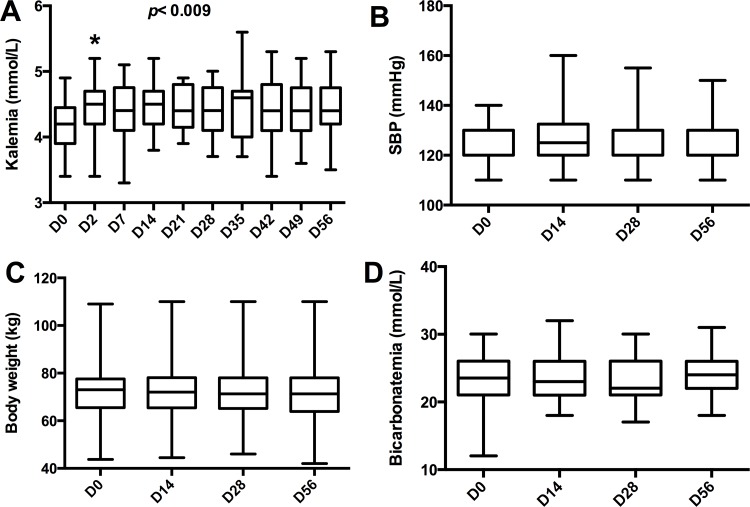
A total of 31 patients were included (Table 1) and all completed the study period (8 weeks), except one (last follow-up on D35 due to an unplanned move). Serum potassium increased slightly from baseline (4.2±0.4 mmol/L): on d2, serum potassium became increased and then remained in a steady state (Fig 2A). Nine patients experienced at least one episode of mild hyperkalemia (>5 mmol/L) but there was only one episode of moderate hyperkalemia (>5.5 mmol/L). This patient received a specific intervention (potassium-exchange resin) on D35. Half the incidences of mild hyperkalemia (>5 mmol/L) occurred within 7 days after beginning eplerenone treatment.
Table 1. Characteristics of included patients.
| Demography | n = 31 |
| Age (years) | 56 [32–70] |
| Gender ratio (M/F) | 18/13 |
| Time since transplantation (months) | 126 [18–326] |
| Body-mass index at inclusion (kg/m2) | 23.8 [18.2–36.8] |
| Diabetes, n (%) | 3 (10) |
| Biology | |
| Creatininemia (μmol/L) | 145 [87–239] |
| eGFR (mL/min/1.73 m2) | 41 [26–59] |
| Serum potassium at inclusion (mmol/L) | 4.2 [3.4–4.9] |
| Serum bicarbonate at inclusion (mmol/L) | 24 [12–30] |
| Natriuresis at inclusion (mmol/d) | 136 [29–360] |
| Kaliuresis at inclusion (mmol/d) | 60 [0–176] |
| Proteinuria at inclusion (mg/d) | 123 [0–648] |
| Drug therapies | |
| Cyclosporine posology at inclusion (mg/kg/d) | 2.1 [1.4–4.0] |
| Cyclosporinemia at inclusion (ng/mL) | 94 [38–152] |
| MMF/azathioprine, n (%) / n (%) | 24 (77) / 4 (1) |
| ACE-i/ARB, n (%) / n (%) | 13 (42) / 6 (2) |
| Diuretics, n (%) | 12 (39) |
| β-blockers, n (%) | 14 (45) |
| Oral bicarbonate, n (%) | 7 (23) |
| Steroids, n (%) | 5 (16) |
M: male; F: female; eGFR: estimated glomerular-filtration rate; MMF: mycophenolate mofetil. Data are expressed by their median [range].
Fig 2. Eplerenone induced mild hyperkalemia.
(A) Kalemia increased from day 2 (D2) and became stable during the treatment period. (B) Systolic blood pressure (SBP), (C) body weight, and (D) serum bicarbonate did not change during the treatment period. Data are represented as their median and range (whiskers). * p <0.05 vs. D0.
Three patients presented with other adverse events: two unspecific outcomes (diarrhea and sweats) and one acute kidney injury (>30% increased creatininemia from baseline) on D56, secondary to acute diarrhea. None of these adverse events needed specific management. We observed no modifications to systolic blood pressure (Fig 2B), body weight (Fig 2C), or serum bicarbonate (Fig 2D). Other biological or clinical parameters remained stable.
The risk of at least one episode of mild hyperkalemia (>5 mmol/L) under eplerenone was studied using baseline data: demographic and biological parameters were analyzed as well as treatments. Two groups were individualized (patients with at least one episode of mild hyperkalemia (>5 mmol/L) versus others). After stepwise multivariate analyses (including CsA posology, creatininemia, serum potassium and bicarbonate), only serum potassium and bicarbonate at baseline were independently associated with a higher risk of developing at least one episode of mild hyperkalemia (>5 mmol/L) under eplerenone treatment (Table 2). Higher serum potassium at baseline was associated with a higher risk (OR 6.5 [1.4;30.5]) of developing mild hyperkalemia and lower serum bicarbonate was also associated with a higher risk (OR 0.7 [0.5;0.9]) of developing mild hyperkalemia.
Table 2. Candidate parameters for predicting the risk of mild hyperkalemia.
| Parameter | Kalemia >5 mmol/L (n = 9) | No kalemia > 5 mmol/L (n = 22) | Univariate analysis# | Multivariate analysis* | ||
|---|---|---|---|---|---|---|
| p | p | OR | 95%CI | |||
| Demography | ||||||
| Age (years) | 50.7 [32.7–70.1] | 57.4 [35.8–66.5] | 0.31 | |||
| Gender (M/F) | 7/2 | 11/11 | 0.12 | |||
| Body weight (kg) | 75.0 [66–90] | 70.1 [43.8–109] | 0.17 | |||
| Body-mass index (kg/m2) | 23.8 [23.0–28.2] | 24.4 [18.2–36.8] | 0.84 | |||
| Diabetes at inclusion (n) | 0 | 3 | 0.34 | |||
| Time since transplantation (months) | 152.0 [24–326] | 119.5 [18–264] | 0.29 | |||
| Systolic blood pressure (mmHg) | 120 [110–140] | 130 [110–140] | 0.42 | |||
| Biology | ||||||
| Creatininemia on day 0 (μmol/L) | 170.0 [121.0–232.0] | 138.0 [87.0–239.0] | 0.06 | |||
| eGFR (mL/min/1.73 m2) | 36.0 [26.0–53.0] | 44.5 [26.0–59.0] | 0.17 | |||
| Serum potassium at baseline (mmol/L) | 4.7 [4.0–4.9] | 4.1 [3.4–4.7] | <0.01 | 0.003 | 6.5 | [1.4;30.5] |
| Kaliuresis (mmol/d) | 62.0 [33.0–92.0] | 57.0 [0–176] | 0.33 | |||
| Kaliuresis/creatininuria (mmol/mmol) | 4.8 [0.3–7.0] | 4.7 [0–21.5] | 0.57 | |||
| Natriuresis (mmol/d) | 143 [71–300] | 135.5 [29–360] | 0.84 | |||
| Natriuresis/creatininuria (mmol/mmol) | 10 [1.2–14.8] | 12.7 [1.8–64.6] | 0.25 | |||
| Serum bicarbonate at baseline (mmol/L) | 21.0 [12.0–25.0] | 24.0 [19.0–30.0] | 0.02 | 0.007 | 0.7 | [0.5;0.9] |
| Drug therapy | ||||||
| Cyclosporine A posology at inclusion (mg/d) | 180 [120–220] | 140 [100–280] | 0.08 | |||
| Cyclosporine A posology at inclusion (mg/kg/d) | 2.1 [1.6–2.8] | 2.0 [1.4–4.0] | 0.37 | |||
| Cyclosporinemia at inclusion (ng/mL) | 98 [38–145] | 92.5 [40–152] | 0.81 | |||
| ACE-I at inclusion (n) | 3 | 10 | 0.26 | |||
| ARB at inclusion (n) | 2 | 4 | 0.36 | |||
| Diuretics at inclusion (n) | 4 | 8 | 0.29 | |||
| β-blockers at inclusion (n) | 4 | 10 | 0.31 | |||
| Oral bicarbonate at inclusion (n) | 2 | 4 | 0.36 | |||
| Steroids at inclusion (n) | 1 | 4 | 0.39 | |||
M: male; F: female; eGFR: estimated glomerular-filtration rate; OR: odds ratio; CI: confidence interval. Data are expressed as their median [range]. All urine tests were performed on 24-h urine collections.
# Univariate analyses using Wilcoxon tests for quantitative variables and Fisher exact test for qualitative variables.
* Multivariate analysis by stepwise logistic regression was performed including creatininemia, serum potassium, serum bicarbonate and cyclosporine A posology on day 0.
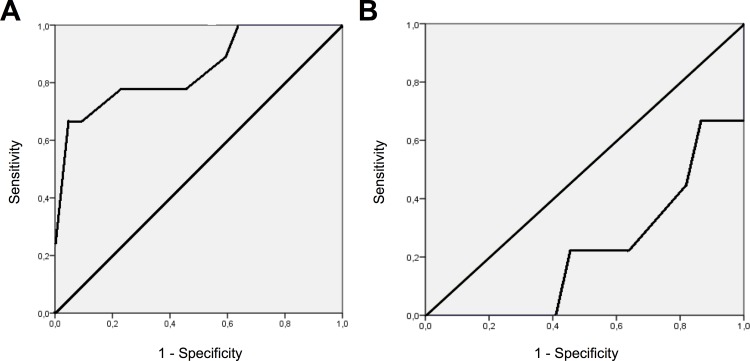
ROC analyses were performed to test if a cut-off value for serum potassium and/or bicarbonate at baseline could distinguish which patients had a higher risk of developing mild hyperkalemia (>5 mmol/L) under eplerenone treatment. Only serum potassium at baseline (Fig 3A) showed this ability (AUC = 0.846 [0.681–1.0]), whereas serum bicarbonate at baseline (Fig 3B) did not (AUC = 0.222 [0.048–0.397]). Serum potassium of >4.35 mmol/L at baseline was a marker for a higher risk of developing mild hyperkalemia (>5 mmol/L) during the treatment period, with a sensitivity of 78% and a specificity of 77%.
Fig 3. Risk factors for developing mild hyperkalemia under treatment.
Receiver-operating characteristic (ROC) curves for (A) serum potassium and (B) serum bicarbonate at baseline.
Discussion
During this study, we found that eplerenone could be safely given to kidney-transplant recipients treated with CsA and impaired renal function. The only acute renal failure observed during eplerenone treatment was not considered to be associated to this treatment due to the diarrhea. This gastro-intestinal adverse outcome was no longer related to eplerenone, regarding to the context of known contact. Moreover, other ongoing drugs could have facilitated this event.
After the RALES study [19], MRAs were considered to be at risk of major hyperkalemia [20], especially in patients with chronic kidney disease [21]. Most of the cases of severe hyperkalemia were due to the lack of serum potassium monitoring after initiating the treatment [22]. All CNIs increase the risk of hyperkalemia [23], especially after adding a renin–angiotensin–aldosterone-system blocker, such as ACE-I or ARB [24]: the underlying mechanisms may rely on the decreased efficacy of loop diuretics (like furosemide) [25], the activation of the sodium-chloride co-transporter [26, 27], and decreased ROMK channel activity [28].
CNIs are also associated with mild renal tubular acidosis in about one-third of patients [29]. The acidosis observed during CsA-treatment [30] can worsen potassium shift from the intracellular to the extracellular compartment: distal renal tubular acidosis [31] appears to be related to Na+/K+ ATPase pump impairment [32] under CsA-treatment. MRAs could worsen metabolic acidosis, especially when it pre-exists [33]. In our study, serum bicarbonate was closely monitored: if it was associated with a higher risk of developing mild hyperkalemia (>5 mmol/L) during the treatment period, a basal cut-off value could not be proposed. The use of oral bicarbonate was allowed and was monitored due to its possible effects on preserving renal function decline [34] and counteracting the acidotic effect of MRAs. Even if patients that had at least one episode of mild hyperkalemia (>5 mmol/L) had lower serum bicarbonate at baseline, they were not more frequently treated with oral bicarbonate (2/9 patients) than other patients (4/22, p = 0.36). Other treatments that could interact with the metabolism of potassium were screened: the frequencies of ACE-I, ARB, diuretics and/or β-blockers were not different between both groups.
Renal impairment is a risk factor of hyperkalemia: during chronic kidney disease, renal potassium handling increases as glomerular-filtration rate decreases [35], leading to hyperkalemia because of the loss in nephron mass. In the present study (where all patients had a renal impairment), renal function assessed by the MDRD formula [18] was not associated with a higher risk of developing mild hyperkalemia (>5 mmol/L) during the treatment period. Because of the creatininemia assay used in our study (modified Jaffe’s method), the use of the CKD-EPI formula–which necessitates an enzymatic assay–was not appropriate [36]. For ranges of eGFR between 30 to 59 mL/min/1.73m2, the MDRD formula misclassifies 5% of patients that should be mostly in the upper eGFR group (60 to 89 mL/min/1.73m2) [36]: as the included patients in the present study had eGFR ranging from 30 to 50 mL/min/1.73m2, misclassifications may had been rare. Even if creatininemia at baseline tended to be different between patients that experienced at least one episode of mild hyperkalemia (>5 mmol/L) during the study, eGFR was not different. Moreover, multivariate analysis included creatininemia at inclusion: it did not explain the higher frequency of mild hyperkalemia (>5 mmol/L) observed in these patients.
The risk of developing moderate to severe hyperkalemia during treatment with eplerenone is less than 10% in both hypertension and heart-failure indications, depending on the definition of hyperkalemia (>5.5 mmol/L or 6 mmol/L) and drug dosage [37]: in our study, only one (1/31, about 3%) moderate hyperkalemia (>5.5 mmol/L) was observed. Laboratory monitoring (serum potassium and renal function), after initiating MRA treatment, is the best way to prevent hyperkalemia and hospitalization [38]. Even though our cohort was relatively small (n = 31), higher serum potassium at baseline was associated with a higher risk of developing mild hyperkalemia (>5 mmol/L) during the treatment period. In our study, mild hyperkalemia was not associated with a higher rate of 24-h kaliuresis, neither at baseline nor during the follow-up. At a steady state, kaliuresis reflects potassium intake and is not related to a higher risk of developing hyperkalemia: this is consistent with a previous study [39].
To evaluate the risk of developing mild hyperkalemia during treatment with eplerenone, a cut-off value of 4.35 mmol/L at baseline was determined to have both the best sensitivity and specificity. A previous study also reported that, during hypertension therapy, predictive factors for developing moderate hyperkalemia (>5.5 mmol/L) under MRA treatment were eGFR <45 mL/min/1.73 m2 and baseline serum potassium >4.5 mmol/L [40]: this is consistent with our findings. Such data are easy to use in clinical practice, especially when hyperkalemia is feared: in our study, among patients who experienced at least one episode of hyperkalemia (>5 mmol/L), only two had serum potassium levels at baseline that were lower than this cut-off value, defining a negative predictive value of close to 90%.
The beneficial effects of MRAs have been well established during heart failure, with strong data obtained during randomized controlled trials, especially when cardiac ejection fraction is reduced [41]: both eplerenone and spironolactone have demonstrated improved survival benefits. During chronic kidney disease, the use of MRAs is associated with reducing proteinuria [42]. As proteinuria is one of the most common predictive factors for the progression of kidney disease [43], a beneficial effect of MRAs on kidney survival could be expected but has not been demonstrated previously, due to a lack of randomized controlled trials with kidney survival as the primary endpoint. MRAs could also be useful during CIN [44]: both drugs (MRAs and CsA) act on vascular function. MRAs could limit CsA-induced vascular toxicity. Several animal studies suggest a beneficial effect of MRA use under CsA treatment [9, 10, 13]. It could be related to vascular MR-induced remodeling [45]. To date, no study was published using MRAs and including tacrolimus-treated patients.
The beneficial effects of MRAs could be related to their diuretic effects or their pleiotropic actions (tissue remodeling), as occurs during heart failure [41]. In our study, no effect was observed on systolic, diastolic or mean blood pressure nor on body weight. This is consistent with previous studies: during-end stage renal disease in anuric hemodialyzed patients, MRAs use was effective in reducing mortality without causing a diuretic effect [46], and post-hoc analysis of the EPHESUS trial showed that the beneficial effects of eplerenone were independent of diuretic effects [47].
In our study, MRA dosage was low but was efficient enough at increasing serum potassium. Dose-efficiency has been demonstrated for both spironolactone [48] and eplerenone [49] in reducing morbi-mortality. Moreover, electrolyte disturbances (hyperkalemia) appear to be also dose-dependent [41]. Survival benefits in heart failure have been shown with low posologies: the means were 26 mg/d for spironolactone during the RALES trial [19] and 42 mg/d and 39 mg/d for eplerenone during the EPHESUS [6] and EMPHASIS-HF [5] trials, respectively. Such a low dose has been shown to be efficient during end-stage renal disease in reducing mortality in hemodialyzed patients [46] and morbidity in peritoneal dialysis patients [50]. All these data are consistent with the dosage we chose in the present study: it appeared to be the best compromise between achieving higher efficacy and lower toxicity in our population. Further studies should test the safety and efficiency of higher doses (50 mg/d) that should be facilitated by the use of potassium binders [51].
Taken together, our data show the safe use of eplerenone in CsA-treated transplant recipients, despite renal impairment. This is consistent with a previous study in another population of chronic kidney-disease patients [15]. Of note, our study is the first performed on kidney-transplant recipients.
Further studies are needed to analyze the potential benefits of MRAs in kidney-allograft transplantation: an adequately powered prospective randomized controlled trial should test the efficiency (and safety) of eplerenone in reducing chronic renal-allograft dysfunction, and the potential benefits to survival.
Supporting Information
To improve the quality of nonrandomized trials, this checklist helped verifying all items.
(PDF)
All the extended methods used in this trial are available here in English and was approved by legal authorities.
(PDF)
All the extended methods used in this trial are available here in French and was approved by legal authorities.
(PDF)
Acknowledgments
Reims University Hospital and the Institut National de la Santé et de la Recherche Médicale funded the present study. The authors thank Drs Aldjia Hocine and Antoine Braconnier for the follow-up of participants and Mr Eymeric Lagonotte and Dr Vincent Vuiblet for blood and urine management and storage.
Data Availability
To ensure the best quality of data report, raw data used in this trial are available upon request (jean-philippe.bertocchio@aphp.fr).
Funding Statement
Reims University Hospital and the Institut National de la Santé et de la Recherche Médicale funded the present study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Menon MC, Murphy B. Maintenance immunosuppression in renal transplantation. Current opinion in pharmacology. 2013;13(4):662–71. Epub 2013/06/05. 10.1016/j.coph.2013.05.004 . [DOI] [PubMed] [Google Scholar]
- 2.Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349(24):2326–33. Epub 2003/12/12. 10.1056/NEJMoa020009349/24/2326 [pii]. . [DOI] [PubMed] [Google Scholar]
- 3.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4(2):481–508. Epub 2009/02/17. doi: 4/2/481 [pii] 10.2215/CJN.04800908 . [DOI] [PubMed] [Google Scholar]
- 4.English J, Evan A, Houghton DC, Bennett WM. Cyclosporine-induced acute renal dysfunction in the rat. Evidence of arteriolar vasoconstriction with preservation of tubular function. Transplantation. 1987;44(1):135–41. Epub 1987/07/01. . [DOI] [PubMed] [Google Scholar]
- 5.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21. Epub 2010/11/16. 10.1056/NEJMoa1009492 . [DOI] [PubMed] [Google Scholar]
- 6.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–21. Epub 2003/04/02. 10.1056/NEJMoa030207 NEJMoa030207 [pii]. . [DOI] [PubMed] [Google Scholar]
- 7.Nguyen Dinh Cat A, Griol-Charhbili V, Loufrani L, Labat C, Benjamin L, Farman N, et al. The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB J. 2010;24(7):2454–63. Epub 2010/03/20. doi: fj.09-147926 [pii] 10.1096/fj.09-147926 . [DOI] [PubMed] [Google Scholar]
- 8.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nature medicine. 2012;18(9):1429–33. Epub 2012/08/28. 10.1038/nm.2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feria I, Pichardo I, Juarez P, Ramirez V, Gonzalez MA, Uribe N, et al. Therapeutic benefit of spironolactone in experimental chronic cyclosporine A nephrotoxicity. Kidney Int. 2003;63(1):43–52. Epub 2002/12/11. doi: kid707 [pii] 10.1046/j.1523-1755.2003.00707.x . [DOI] [PubMed] [Google Scholar]
- 10.Perez-Rojas JM, Derive S, Blanco JA, Cruz C, Martinez de la Maza L, Gamba G, et al. Renocortical mRNA expression of vasoactive factors during spironolactone protective effect in chronic cyclosporine nephrotoxicity. Am J Physiol Renal Physiol. 2005;289(5):F1020–30. Epub 2005/07/07. doi: 00166.2005 [pii] 10.1152/ajprenal.00166.2005 . [DOI] [PubMed] [Google Scholar]
- 11.Nielsen FT, Jensen BL, Marcussen N, Skott O, Bie P. Inhibition of mineralocorticoid receptors with eplerenone alleviates short-term cyclosporin A nephrotoxicity in conscious rats. Nephrol Dial Transplant. 2008;23(9):2777–83. Epub 2008/04/22. doi: gfn204 [pii] 10.1093/ndt/gfn204 . [DOI] [PubMed] [Google Scholar]
- 12.Sun QL, Li M, Rui HL, Chen YP. Inhibition of local aldosterone by eplerenone reduces renal structural damage in a novel model of chronic cyclosporine A nephrotoxicity. Journal of the renin-angiotensin-aldosterone system: JRAAS. 2015;16(2):301–10. Epub 2014/12/17. 10.1177/1470320314561248 . [DOI] [PubMed] [Google Scholar]
- 13.Amador CA, Bertocchio JP, Andre-Gregoire G, Placier S, Duong Van Huyen JP, El Moghrabi S, et al. Deletion of mineralocorticoid receptors in smooth muscle cells blunts renal vascular resistance following acute cyclosporine administration. Kidney Int. 2015. Epub 2015/10/01. 10.1038/ki.2015.312 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossignol P, Dobre D, McMurray JJ, Swedberg K, Krum H, van Veldhuisen DJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circulation Heart failure. 2014;7(1):51–8. Epub 2013/12/04. 10.1161/CIRCHEARTFAILURE.113.000792 . [DOI] [PubMed] [Google Scholar]
- 15.Edwards NC, Steeds RP, Chue CD, Stewart PM, Ferro CJ, Townend JN. The safety and tolerability of spironolactone in patients with mild to moderate chronic kidney disease. British journal of clinical pharmacology. 2012;73(3):447–54. Epub 2011/09/29. 10.1111/j.1365-2125.2011.04102.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez Monte E, Andres A, Polanco N, Toribio MJ, Santana R, Gutierrez Martinez E, et al. Addition of spironolactone to dual blockade of renin angiotensin system dramatically reduces severe proteinuria in renal transplant patients: an uncontrolled pilot study at 6 months. Transplant Proc. 2010;42(8):2899–901. Epub 2010/10/26. doi: S0041-1345(10)01224-8 [pii] 10.1016/j.transproceed.2010.08.024 . [DOI] [PubMed] [Google Scholar]
- 17.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. . [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70. . [DOI] [PubMed] [Google Scholar]
- 19.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–17. Epub 1999/09/02. . [DOI] [PubMed] [Google Scholar]
- 20.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351(6):543–51. Epub 2004/08/06. 10.1056/NEJMoa040135351/6/543 [pii]. . [DOI] [PubMed] [Google Scholar]
- 21.Hernandez AF, Mi X, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA, et al. Associations between aldosterone antagonist therapy and risks of mortality and readmission among patients with heart failure and reduced ejection fraction. Jama. 2012;308(20):2097–107. Epub 2012/11/29. 10.1001/jama.2012.14795 . [DOI] [PubMed] [Google Scholar]
- 22.Vardeny O, Claggett B, Anand I, Rossignol P, Desai AS, Zannad F, et al. Incidence, predictors, and outcomes related to hypo- and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circulation Heart failure. 2014;7(4):573–9. Epub 2014/05/09. 10.1161/CIRCHEARTFAILURE.114.001104 . [DOI] [PubMed] [Google Scholar]
- 23.Kaplan B, Wang Z, Abecassis MM, Fryer JP, Stuart FP, Kaufman DB. Frequency of hyperkalemia in recipients of simultaneous pancreas and kidney transplants with bladder drainage. Transplantation. 1996;62(8):1174–5. Epub 1996/10/27. . [DOI] [PubMed] [Google Scholar]
- 24.Mitterbauer C, Heinze G, Kainz A, Kramar R, Horl WH, Oberbauer R. ACE-inhibitor or AT2-antagonist therapy of renal transplant recipients is associated with an increase in serum potassium concentrations. Nephrol Dial Transplant. 2008;23(5):1742–6. Epub 2008/02/01. 10.1093/ndt/gfm864 . [DOI] [PubMed] [Google Scholar]
- 25.Hocherl K, Kees F, Kramer BK, Kurtz A. Cyclosporine A attenuates the natriuretic action of loop diuretics by inhibition of renal COX-2 expression. Kidney Int. 2004;65(6):2071–80. Epub 2004/05/20. 10.1111/j.1523-1755.2004.00627.x . [DOI] [PubMed] [Google Scholar]
- 26.Melnikov S, Mayan H, Uchida S, Holtzman EJ, Farfel Z. Cyclosporine metabolic side effects: association with the WNK4 system. European journal of clinical investigation. 2011;41(10):1113–20. Epub 2011/03/26. 10.1111/j.1365-2362.2011.02517.x . [DOI] [PubMed] [Google Scholar]
- 27.Hoorn EJ, Walsh SB, McCormick JA, Furstenberg A, Yang CL, Roeschel T, et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nature medicine. 2011;17(10):1304–9. Epub 2011/10/04. 10.1038/nm.2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Lin DH, Wang ZJ, Jin Y, Yang B, Wang WH. K restriction inhibits protein phosphatase 2B (PP2B) and suppression of PP2B decreases ROMK channel activity in the CCD. American journal of physiology Cell physiology. 2008;294(3):C765–73. Epub 2008/01/11. 10.1152/ajpcell.00528.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keven K, Ozturk R, Sengul S, Kutlay S, Ergun I, Erturk S, et al. Renal tubular acidosis after kidney transplantation—incidence, risk factors and clinical implications. Nephrol Dial Transplant. 2007;22(3):906–10. Epub 2007/01/11. 10.1093/ndt/gfl714 . [DOI] [PubMed] [Google Scholar]
- 30.Stahl RA, Kanz L, Maier B, Schollmeyer P. Hyperchloremic metabolic acidosis with high serum potassium in renal transplant recipients: a cyclosporine A associated side effect. Clinical nephrology. 1986;25(5):245–8. Epub 1986/05/01. . [PubMed] [Google Scholar]
- 31.Heering P, Grabensee B. Influence of ciclosporin A on renal tubular function after kidney transplantation. Nephron. 1991;59(1):66–70. Epub 1991/01/01. . [DOI] [PubMed] [Google Scholar]
- 32.Tumlin JA, Sands JM. Nephron segment-specific inhibition of Na+/K(+)-ATPase activity by cyclosporin A. Kidney Int. 1993;43(1):246–51. Epub 1993/01/01. . [DOI] [PubMed] [Google Scholar]
- 33.Henger A, Tutt P, Riesen WF, Hulter HN, Krapf R. Acid-base and endocrine effects of aldosterone and angiotensin II inhibition in metabolic acidosis in human patients. J Lab Clin Med. 2000;136(5):379–89. Epub 2000/11/18. 10.1067/mlc.2000.110371 . [DOI] [PubMed] [Google Scholar]
- 34.Dobre M, Rahman M, Hostetter TH. Current status of bicarbonate in CKD. J Am Soc Nephrol. 2015;26(3):515–23. Epub 2014/08/26. 10.1681/ASN.2014020205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moranne O, Froissart M, Rossert J, Gauci C, Boffa JJ, Haymann JP, et al. Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol. 2009;20(1):164–71. Epub 2008/11/14. 10.1681/ASN.2008020159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. Epub 2009/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danjuma MI, Mukherjee I, Makaronidis J, Osula S. Converging indications of aldosterone antagonists (spironolactone and eplerenone): a narrative review of safety profiles. Current hypertension reports. 2014;16(2):414 Epub 2014/01/11. 10.1007/s11906-013-0414-8 . [DOI] [PubMed] [Google Scholar]
- 38.Allen LA, Shetterly SM, Peterson PN, Gurwitz JH, Smith DH, Brand DW, et al. Guideline concordance of testing for hyperkalemia and kidney dysfunction during initiation of mineralocorticoid receptor antagonist therapy in patients with heart failure. Circulation Heart failure. 2014;7(1):43–50. Epub 2013/11/28. 10.1161/CIRCHEARTFAILURE.113.000709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smyth A, Dunkler D, Gao P, Teo KK, Yusuf S, O'Donnell MJ, et al. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int. 2014;86(6):1205–12. Epub 2014/06/12. 10.1038/ki.2014.214 . [DOI] [PubMed] [Google Scholar]
- 40.Khosla N, Kalaitzidis R, Bakris GL. Predictors of hyperkalemia risk following hypertension control with aldosterone blockade. American journal of nephrology. 2009;30(5):418–24. Epub 2009/09/10. 10.1159/000237742 . [DOI] [PubMed] [Google Scholar]
- 41.Zannad F, Gattis Stough W, Rossignol P, Bauersachs J, McMurray JJ, Swedberg K, et al. Mineralocorticoid receptor antagonists for heart failure with reduced ejection fraction: integrating evidence into clinical practice. European heart journal. 2012;33(22):2782–95. Epub 2012/09/04. 10.1093/eurheartj/ehs257 . [DOI] [PubMed] [Google Scholar]
- 42.Bertocchio JP, Warnock DG, Jaisser F. Mineralocorticoid receptor activation and blockade: an emerging paradigm in chronic kidney disease. Kidney Int. 2011;79(10):1051–60. Epub 2011/03/18. 10.1038/ki.2011.48 . [DOI] [PubMed] [Google Scholar]
- 43.Ruggenenti P, Perna A, Mosconi L, Pisoni R, Remuzzi G. Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. "Gruppo Italiano di Studi Epidemiologici in Nefrologia" (GISEN). Kidney Int. 1998;53(5):1209–16. Epub 1998/05/09. 10.1046/j.1523-1755.1998.00874.x . [DOI] [PubMed] [Google Scholar]
- 44.Bobadilla NA, Gamba G. New insights into the pathophysiology of cyclosporine nephrotoxicity: a role of aldosterone. Am J Physiol Renal Physiol. 2007;293(1):F2–9. Epub 2007/04/13. doi: 00072.2007 [pii] 10.1152/ajprenal.00072.2007 . [DOI] [PubMed] [Google Scholar]
- 45.Galmiche G, Pizard A, Gueret A, El Moghrabi S, Ouvrard-Pascaud A, Berger S, et al. Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension. 2014;63(3):520–6. Epub 2013/12/04. 10.1161/HYPERTENSIONAHA.113.01967 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumoto Y, Mori Y, Kageyama S, Arihara K, Sugiyama T, Ohmura H, et al. Spironolactone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. Journal of the American College of Cardiology. 2014;63(6):528–36. Epub 2013/11/05. 10.1016/j.jacc.2013.09.056 . [DOI] [PubMed] [Google Scholar]
- 47.Rossignol P, Menard J, Fay R, Gustafsson F, Pitt B, Zannad F. Eplerenone survival benefits in heart failure patients post-myocardial infarction are independent from its diuretic and potassium-sparing effects. Insights from an EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) substudy. Journal of the American College of Cardiology. 2011;58(19):1958–66. Epub 2011/10/29. 10.1016/j.jacc.2011.04.049 . [DOI] [PubMed] [Google Scholar]
- 48.RALES. Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). The American journal of cardiology. 1996;78(8):902–7. Epub 1996/10/15. . [DOI] [PubMed] [Google Scholar]
- 49.Weinberger MH, Roniker B, Krause SL, Weiss RJ. Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens. 2002;15(8):709–16. Epub 2002/08/06. . [DOI] [PubMed] [Google Scholar]
- 50.Ito Y, Mizuno M, Suzuki Y, Tamai H, Hiramatsu T, Ohashi H, et al. Long-term effects of spironolactone in peritoneal dialysis patients. J Am Soc Nephrol. 2014;25(5):1094–102. Epub 2013/12/18. 10.1681/ASN.2013030273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–21. Epub 2014/11/22. 10.1056/NEJMoa1410853 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
To improve the quality of nonrandomized trials, this checklist helped verifying all items.
(PDF)
All the extended methods used in this trial are available here in English and was approved by legal authorities.
(PDF)
All the extended methods used in this trial are available here in French and was approved by legal authorities.
(PDF)
Data Availability Statement
To ensure the best quality of data report, raw data used in this trial are available upon request (jean-philippe.bertocchio@aphp.fr).