Abstract
Background
Persistent air leak following pulmonary lobectomy can be very difficult to treat and results in prolonged hospitalization. We aimed to evaluate the efficacy of a new method of postoperative air leak management using intrapleurally infused fresh frozen plasma via the chest tube.
Material/Methods
Between June 2008 and June 2014, we retrospectively reviewed 98 consecutive patients who underwent lobectomy for lung cancer and postoperatively developed persistent air leak treated with intrapleural instillation of fresh frozen plasma.
Results
The study identified 89 men and 9 women, with a median age of 65.5 years (range 48–77 years), with persistent postoperative air leak. Intrapleural infusion of fresh frozen plasma was successful in stopping air leaks in 90 patients (92%) within 24 hours, and in 96 patients (98%) within 48 hours, following resumption of the procedure. In the remaining 2, air leak ceased at 14 and 19 days.
Conclusions
Intrapleural infusion of fresh frozen plasma is a safe, inexpensive, and remarkably effective method for treatment of persistent air leak following lobectomy for lung cancer.
MeSH Keywords: Lung, Plasma, Thoracic Surgical Procedures
Background
Persistent alveolar air leak (PAAL) following pulmonary resection remains a common complication despite the existing approaches of prevention and treatment. It is an unpleasant and frustrating problem for patients and thoracic surgeons because it prolongs hospital stay and cost, and increases the risk of other complications, such as pneumonia, empyema, and thrombosis [1–3].
Air leaks can be prevented intraoperatively by standard surgical techniques (e.g., electrocautery, stapler line buttresses, and suturing), or with the use of synthetic or biologic glues or other adhesives; postoperatively air leaks can be managed conservatively with chest tube drainage or by infusion of irritating substances (e.g., talc, tetracycline, and bleomycin), or autologous blood in the pleural cavity, via the chest tube, for pleurodesis [4–10]; surgical repair of persistent air leak is rarely necessary [11,12]. However, concerns regarding the effectiveness and adverse effects of pleurodesis have been reported: bleomycin, although rare, can cause life-threatening pneumonitis. Talc and tetracycline, in experimental studies, have been implicated in producing alveolar hemorrhage, cellular infiltration, and edema. Autologous blood, although non-toxic, produces insignificant pleurodesis, and despite a published high success rate with a low risk of complications, effectiveness and morbidity considerations have prevented its universal acceptance by thoracic surgeons [2,3,10,13–18].
The significance of plasma proteins in wound healing and the ability of human epithelial cells to produce coagulation cascade proteins in response to injury have been reported anecdotally, and there is only 1 case report regarding intrapleurally infused fresh frozen plasma (FFP) for PAAL management [19–21]. However, there are many reports in orthopedics, sports medicine, plastic and reconstructive surgery, and other surgical specialties regarding the use of autologous plasma enriched with platelets, which is currently considered a breakthrough in tissue healing and repairing processes [22–24].
During the recent fiscal crisis, and driven by a shortage of medical supplies, including intraoperatively used surgical adhesives, in our hospital, we studied an economical but safe and effective sealant to use, at least postoperatively, for the management of PAAL. Prompted by the reported similarities in the mechanism of autologous blood pleurodesis and biologic (fibrin) glue, we treated 98 consecutive patients who underwent lobectomy for lung cancer and postoperatively developed PAAL, using intrapleurally infused FFP during a 6-year period. In this study we present our experience with this.
Material and Methods
In our thoracic surgical department, which is the busiest in our country, each year we perform approximately 300 lobectomies, bilobectomies, sleeve lobectomies, wedge resections, and segmentectomies, for primary or metastatic lung cancer and benign diseases. We found that postoperative air leaks due to alveolar-pleural fistulae occur in about 18% of our lobectomies, with almost a third of them persisting after the 5th postoperative (PO) day, which are then considered as PAAL. In an attempt to have a uniform patient cohort, and having obtained institutional review board approval and informed patient consent, we retrospectively studied 98 consecutive patients (6%) out of 1609 patients subjected only to lobectomy or bilobectomy for primary non-small cell lung cancer, between June 2008 and June 2014, all who postoperatively developed PAAL treated with intrapleural instillation of FFP, provided that PAAL was due to an alveolar-pleural fistula, it was above moderate, expiratory, and remained unchanged until after the 5th PO day, as we favor discharging uncomplicated lobectomy patients on the 6th PO day.
With regard to postoperative air leak classification, we relied on the definition used by Cerfolio et al.: ‘…An air leak was defined as forced expiratory only if it was present with cough, expiratory only if it was present during expiration, inspiratory only if it was present during inspiration, or continuous if the leak was present throughout the respiratory cycle...’ [25]. Furthermore, as we never had digital or elaborate chest tube drainage devices in our hospital, so to quantify air leaks we had to differentiate between mild, moderate, and severe postoperative expiratory PAAL. We do not perform pleurodesis for mild to moderate expiratory PAAL, because the vast majority are self-limited, but only for above moderate to severe expiratory PAAL, as was the case in all 98 patients. In addition, none of our study patients had severe expiratory, inspiratory, or continuous PAAL. We always perform bronchoscopy when we encounter such postoperative air leaks to rule out bronchopleural fistula before consideration of any intervention.
Inclusion criteria for FFP pleurodesis in the 98 study patients besides an above moderate and expiratory only PAAL also comprised the following characteristics. First, it remained unchanged from 1st to 6th PO day, with chest tubes off suction and without pneumothorax or subcutaneous emphysema on daily obtained chest x-rays. In accordance with our chest tube management policy following lobectomy for lung cancer, we placed 2 chest tubes (apical and basal) and kept them on suction (15 cm of water) until air leak ceases (usually on the 2nd or 3rd PO day). We then place them to seal water, even if there is air leak, provided that there is no pneumothorax or subcutaneous emphysema on chest x-rays. Second, confounding factors, such as patient cooperation with physical therapy, mobilization, pain, cough, or chest tube malfunction or misplacement were excluded. Third, every effort was made intraoperatively to reduce postoperative air leaks.
All other aspects of surgical procedures (e.g., posterolateral thoracotomy and double-lumen endotracheal tube) and patient preoperative and postoperative management (e.g., daily chest X-rays, incentive spirometry, and physiotherapy) were also standardized.
Technique of FFP pleurodesis
A total of 98 patients presented with PAAL having the above-mentioned characteristics. On the 6th PO day we added an extension of the chest drain tubing between the apical chest tube and the underwater seal in the drainage device, so that it could be raised above the bed and be suspended over an IV drip stand (green or upward arrows), as shown in Figure 1. Using direct needle penetration of the apical chest tube with the regular IV infusion tubing (red or left arrows) of 1 FFP bag, matched in the ABO group, and hanged from the same IV drip stand, FFP was infused into the pleural cavity within minutes. In this manner, and having clamped the basal chest tube (black or right arrow), FFP was prevented from leaving the pleural space while air was allowed to be evacuated via the apical chest tube. The patient was then advised to remain in bed and to rollover and change positions every 15 minutes for 1 hour so that FFP is distributed evenly into the hemithorax. The following morning (7th PO day), the basal tube was unclamped, the extension tubing of the apical chest tube was taken out, and chest tubes were both connected again directly to their drainage system. When air leak has ceased the basal chest tube was removed in the afternoon so that on the next day (8th PO day) the apical chest tube could be pulled out and the patient could be discharged to home.
Figure 1.
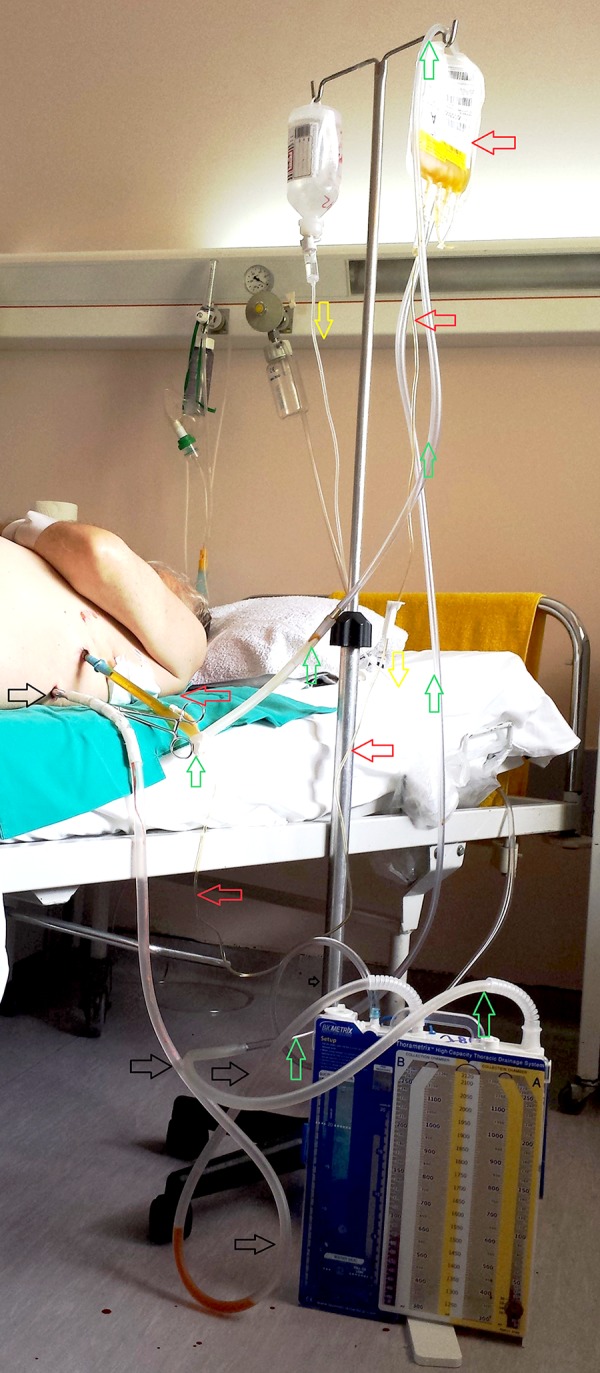
Fresh frozen plasma pleurodesis in a patient with persistent postoperative air leak; red (or left) arrows show the IV infusion tubing of the fresh frozen plasma bag and its point of needle penetration of the apical chest tube. B black (or right) arrows show the clamped basal chest tube tubing. Green (or upward) arrows show the extension of the apical chest tube tubing as it passes over the IV pole. Yellow or (downward) arrows show a normal saline solution bottle and its IV infusion tubing hanging over the same pole.
Results
Between June 2008 and June 2014, out of 1609 patients who underwent lobectomy or bilobectomy with systematic lymph node dissection for primary non-small cell lung cancer, we studied 98 consecutive patients (6%) – 89 men and 9 women, with a median age of 65.5 years (range 48–77 years), who postoperatively developed an above moderate, expiratory PAAL due to an alveolar-pleural fistula, which remained unchanged until after the 5th PO day.
Predisposing factors for PAAL, such as anatomical allocation of lobectomies (right upper lobectomy is associated with a higher incidence of PAAL), neoadjuvant treatment, smoking history, age, sex, and comorbidities (e.g., emphysema, diabetes, steroid use, and previous pulmonary infections) were all taken into account and are presented in Table 1. Besides the small percentage of women in our study patients, we did not observe incidence of any of the above-mentioned variables to be different from other cohorts of patients who underwent lobectomy for lung cancer in our institution or from published reports [4,5].
Table 1.
Demographics and characteristics of the 98 study patients underwent fresh frozen plasma pleurodesis for postoperative air leak management.
| Variable | Mean ±SD or No (%) |
|---|---|
| Age (years) | 65.32±6.929 |
| Females (%) | 9 (9.18%) |
| Smoking history | 97 (98.97%) |
| Neoadjuvant Tx | 15 (15.30%) |
| Coexisting diseases | |
| Emphysema | 40 (40.81%) |
| DM | 12 (12.24%) |
| Tbc or other lung infections | 18 (18.36%) |
| Steroid use | 2 (2.04%) |
| Anatomy of lobectomies | |
| RUL | 44 (44.89%) |
| RU/ML | 8 (8.16%) |
| RM/LL | 4 (4.08%) |
| RML | 2 (2.04%) |
| RLL | 12 (12.24%) |
| LUL | 19 (19.38%) |
| LLL | 9 (9.18%) |
SD – standard definition; Tx – treatment; DM – diabetes mellitus; Tbc – tuberculosis; RUL – right upper lobe; RU/ML – right upper/middle lobe; RM/LL – right middle/lower lobe; RML – right middle lobe; RLL – right lower lobe; LUL – left upper lobe; LLL – left lower lobe.
Infusion of fresh frozen plasma via the chest tube was successful in stopping air leaks in 90 patients (92%) within 24 hours and in 96 patients (98%) within 48 hours following resumption of the procedure. In the remaining 2, air leaks ceased 14 and 19 days later, respectively. There was no early or late morbidity or mortality associated with FFP pleurodesis. It is noteworthy that during this 6-year study period we never documented a single incidence of pain, fever, or dyspnea in our 98 patients.
After successful FFP pleurodesis, 92% of the study patients were discharged to home by the 8th PO day and 98% by the 9th PO day, without prolonging their hospital stay. In contrast, patients with PAAL, before the use of FFP pleurodesis, according to our records, remained in the hospital for at least 2 to 3 weeks, and some up to 4 weeks, because we avoid discharging patients on a Heimlich valve, and because of the poor results we had whenever we used talc or autologous blood for pleurodesis.
With regard to follow-up, all 96 patients underwent who had successful FFP pleurodesis were checked at 1 and 3 months postoperatively. They were all found to be asymptomatic without recurrence of air leak or any new collection of air or fluid on chest X-rays. However, we observed postoperative ‘space’ (small apical pneumothorax due to incomplete re-expansion of the remaining lobe or lobes) in 5 of them after discharge, which remained stable and without any sequels throughout follow-up.
Discussion
Postoperative air leaks following pulmonary lobectomy usually resolve within 2–3 days provided that the lung fully re-expands. When they persist for more than 5 days they are called persistent air leaks because most thoracic surgeons favor discharging uncomplicated lobectomy patients on the 6th postoperative day (the previous definition of PAAL involved postoperative air leaks persisting after the 7th postoperative day). PAAL due to alveolar-pleural fistulae are created by intraoperative injury of the visceral pleura somewhere in the periphery of the lung (raw lung surface) or during division of fused interlobar fissures, particularly in patients with history of pulmonary infections or neoadjuvant treatment because of the difficult dissection of adhesions. Therefore, etiology and management are completely different from those resulting from a broncho-pleural fistula. Despite the existing prevention and treatment approaches, postoperative PAAL remains the most common complication following pulmonary resection, and is reported to occur in 15–26% of patients. Although it usually is the only morbidity observed, PAAL increases the risk of other complications, prolongs hospital stay, and disconcerts patients and surgeons. Predisposing risk factors for PAAL (e.g., emphysema, steroid use, diabetes, and previous lung infections) may prolong hospital stay unpredictably by further hindering PAAL management [1–5].
Preventive intraoperative techniques include fissure-less technique, use of electrocautery or laser to coagulate air-leaking areas on the visceral pleura, buttressing of staplers, creation of a pleural tent, phrenic nerve-induced paresis, pneumoperitoneum, and use of various surgical sealants such as synthetic (polyethylene glycol or albumin based materials) or biologic (fibrin) glues, and collagen patches (coated with fibrinogen and thrombin) on the injured raw lung surface to achieve hemostasis and aerostasis [4–9]. In our hospital, due to the fiscal crisis, there was a temporary shortage of all medical supplies, particularly intraoperatively used surgical sealants. Regarding our surgical technique for fused interlobar fissures division (clamps with continuous suturing or staplers), it was generally similar, even in emphysematous lungs or following difficult dissection of adhesions. Due to supply shortages, stapler line buttresses or surgical adhesives were not used. After the lobe was resected, and following submersion in saline solution of the re-inflated remaining lobe(s), when we observed coalesced bubbles, we tried to reduce the air leak either by using electrocautery (to thermally shrink air leaking areas) or putting 3-0 silk sutures to seal air-leaking sites around stapler lines and on surgically injured areas on the visceral pleura. We also used fissure-less technique and phrenic nerve-induced paresis.
In a systematic review of surgical sealants, a significant reduction in postoperative air leak duration was reported in 2 of the 3 studies using polyethylene-glycol-based sealants, compared to a single trial in which no difference was found. In another study, using a glutaraldehyde-based sealant, a significant reduction in postoperative air leak duration was noted in the treatment group as opposed to its controls. In 7 studies using fibrin-based sealants, postoperative air leak duration was significantly reduced in 3 but made no difference in the remaining 4. The conclusion of the review was that routine use of surgical adhesives for all operations cannot yet be recommended, although surgical sealants may be useful in selected situations [6]. In another prospective randomized trial, the use of a collagen patch coated with human fibrinogen and thrombin resulted in significant reduction of postoperative air leaks and hospital stay [7].
We were encouraged by reports on intraoperative use of fibrin glue, which can be administered by means of various delivery systems (topical adhesive or aerosolized), or as a mixture of 2 components: a protein concentrate (fibrinogen, plasma fibronectin, factor XIII, plasminogen) and thrombin reconstituted in calcium chloride solution. It is considered a natural adhesive, provided by cryoprecipitate or fresh frozen plasma, and generated by the interaction between fibrinogen and thrombin, in the presence of calcium ions, to produce fibrin, simulating the final step of the coagulation cascade [8,9]. Furthermore, we focused on the plasma mechanism of action, which entails protein systems (complement, coagulation, and kinin) that contain inactive enzymes (proenzymes), being activated sequentially. The first proenzyme is converted to an active enzyme, which utilizes the next component in the series as a substrate, and so forth. Particularly as regards the activation of the coagulation system, it forms a fibrinous meshwork at an injured or inflamed site, which prevents the spread of infection, keeps microorganisms and foreign bodies at the site of greatest inflammatory activity, and forms a clot that stops bleeding and provides a framework for repair and healing, the main substance of which is the insoluble protein fibrin. Moreover, we researched the scarce literature on the contribution of plasma proteins in the natural healing process, supporting tissue repair by metabolic and functional activity, and regarding the ability of human epithelial cells to produce coagulation cascade proteins independently of plasma proteins, in response to injury. We also found 1 case report of PAAL treatment using intrapleural instillation of FFP in a 95-year-old patient with pneumothorax, which was also encouraging [19–21]. Finally, our concept was supported by the plethora of reports on application of platelet-rich plasma, which is an autologous product, derived from whole blood through the process of gradient density centrifugation, and promotes natural wound healing, and bone and soft tissue reconstruction, in a broad range of clinical practice in orthopedics, sports medicine, plastic surgery, oral and maxillofacial surgery, dentistry, and other medicine fields. Its efficacy in comparison with platelet poor plasma, which is FFP, lies in the local delivery of many growth factors (e.g., epidermal growth factor, insulin-like growth factor, platelet derived growth factor, and vascular endothelial growth factor), and proteins derived from the high concentration of platelets, and the normal concentration of fibrinogen it contains [22–24]. Therefore, we consider the mechanism of FFP pleurodesis to be the following: using 1 unit of fresh frozen plasma (about 250 ml), a significant quantity of clotting factors (larger in infused volume as compared to autologous blood instillation – usually 50–100 cc) can be injected intrapleurally. With patient movements, FFP can be distributed evenly so as to reach the site of the surgically traumatized surface of the visceral pleura and initiate the coagulation cascade, similar to the IV injection, resulting in fibrin scaffold formation over the air-leaking lung area within a few hours. This, in conjunction with local bioactive factors of the alveolar epithelium, seals the air leak and protects also from infection. We call it “FFP pleurodesis”; it is similar to the reported autologous blood pleurodesis, although the mechanism of action for both is the formation of a fibrin ‘patch’ (clot), which seals the air leak and, subsequently, a mild pleural inflammation that causes pleurodesis.
As regards postoperative approaches for PAAL management, the usual approach is first watchful waiting, occasionally with new chest tube placement or repositioning of the existing one, and often patients are discharged to home with a Heimlich valve. We avoid discharging patients on a Heimlich valve. We consider that its pressure threshold is higher than a water seal, and sometimes air can be trapped in the hemithorax, especially if there is an accidental blockade, the valve becomes clogged or dislodged, resulting in serious problems for the patients, as we experienced in the past. Other postoperative approaches for PAAL management include pleurodesis by irritating the pleura substances (e.g., talc, bleomycin, and tetracycline), or autologous blood infused via the chest tube, and, rarely, reoperation with surgical repair of the air-leaking lung area [1–5,11,12]. Nevertheless, effectiveness and morbidity concerns regarding sclerosants or autologous blood pleurodesis have been raised. Talc is the most effective sclerosant available for pleurodesis, and tetracycline, although fairly effective, has fewer adverse effects, but both have been reported to produce local (e.g., alveolar collapse with hemorrhage, inflammation, and edema), and systematic adverse effects in animal studies. Bleomycin is an alternative sclerosant, with modest efficacy, but it is expensive, and, rarely, causes fatal pulmonary toxicity and anaphylactic reactions, even at low systematic doses, as 45% of the intrapleurally infused bleomycin enters the bloodstream. Autologous blood, although non-toxic, seems to produce insignificant pleurodesis and despite a reported high success rate with a low risk of complications, there remains skepticism about its use in the management of PAAL because of efficiency and morbidity concerns in clinical and experimental studies [13–18]. Chambers et al., in a review of several small prospective studies, including 2 randomized control trials, reported that autologous blood pleurodesis has superior outcomes when compared with conservative treatment of PAAL. They noted an overall success rate of 92.7% for patients subjected to pulmonary surgery and 91.7% for patients with pneumothorax and PAAL, with an overall 0–18% risk of complications (e.g., empyema, fever, and pneumonia). However, in 1 of the reviewed studies empyema was observed in 9% of the patients and minor complications in 28%. Two other studies involving autologous blood pleurodesis reported success rates of 64.3% and 72.7%, respectively [10]. Karangelis et al. further questioned the efficacy of autologous blood pleurodesis, as they observed only a 27% success rate in 15 patients with spontaneous pneumothorax and PAAL [17]. Additionally, the optimal volume of the injected blood needed, since it is an ideal medium for bacterial growth, to patch the air leak and avoid complications has not been specified yet. It is usually suggested to be 50 to 100 cc, preferably instilled in 1 course, and the chest tube must be flushed with normal saline afterwards in order to avoid clotting. Life-threatening tension pneumothorax caused by a clotted chest tube following autologous blood injection for pleurodesis was reported in a 19-year-old patient with cystic fibrosis who presented with PAAL [18]. In our experience, we had encountered only failures in attempted pleurodesis with both talc and autologous blood, when we had used them for PAAL management following lobectomy for lung cancer, so we have discontinued their use. On the contrary, we have noted only advantages associated with the use of FFP pleurodesis. First, it is safe because it was not accompanied by any adverse effects in our 98 patients, probably because plasma proteins protect from infection. Second, it was simple to perform and easily tolerated by patients. Third, it was inexpensive and cost effective for our hospital administrator because on the one hand, 98% of the treated patients were discharged between the 8th and 9th PO days, which was a creditable achievement considering that in our past experience patients with PAAL remained in the hospital at for 2–3 weeks, and on the other hand regarding cost of FFP preparation in our country apply the following: blood donation is only voluntary and in state hospitals all patients, in order to get their planned operations, have to find 2–3 blood donors in advance; surgeons are also obligated to ask for 2–3 units of cross-matching packed red blood cells for any planned lobectomy on their operative list; therefore, as the hospital Blood Bank prepares the packed red blood cells, they can also provide with 2–3 units of FFP without extra charges for the patient, if asked.
With regard to the drawbacks of our study, although it was observational and retrospective, there were no selection biases since our 98 consecutive patients were only selected on account of the kind of operation they underwent and the kind of air leak they developed, without presenting as a cohort increased incidence of any specific risk factor for PAAL, as shown in Table 1. Regarding the reduced percentage of women in our study, there are reports considering either male or female sex as a significant risk factor for PAAL [4,5]. In addition, we did not have a chance to perform a prospective cohort study due to the better the outcome of FFP pleurodesis. We attempted to use it, trying to check and record its notable results. We have so far used it in more than 300 patients with postoperative PAAL following not only lobectomy for lung cancer, but also wedge resection, segmentectomy, lung biopsies, bullectomies, and even decortication for empyema, with similar results and without a single complication. Nevertheless, we are confident that comparable results can be reproduced in any thoracic surgical unit and our peers will take the opportunity to perform prospective randomized trials so as to strengthen our findings before generalized evaluation and adoption of this new treatment modality.
Conclusions
Intrapleural infusion of FFP was proved to be a safe, simple, inexpensive, and remarkably successful method for the management of postoperative PAAL following lobectomy for lung cancer, with a 98% overall success rate and without recurrences or complications.
Footnotes
Source of support: Departmental sources
Acknowledgements and disclosures
None declared.
Conflict of interest statement
None declared.
References
- 1.Venuta F, Rendina EA, De Giacomo T, Coloni GF. Postoperative strategies to treat permanent air leaks. Thorac Surg Clin. 2010;20(3):391–97. doi: 10.1016/j.thorsurg.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Lang-Lazdunski L, Coonar AS. A prospective study of autologous ‘blood patch’ pleurodesis for persistent air leak after pulmonary resection. Eur J Cardiothorac Surg. 2004;26:897–900. doi: 10.1016/j.ejcts.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 3.Shackcloth MJ, Poulis M, Jackson M, et al. Intrapleural instillation of autologous blood in the treatment of prolonged air leak after lobectomy: a prospective randomized controlled trial. Ann Thorac Surg. 2006;82:1052–56. doi: 10.1016/j.athoracsur.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Venuta F, Rendina EA, De Giacomo T, et al. Technique to reduce air leaks after pulmonary lobectomy. Eur J Cardiothorac Surg. 1998;13:361–64. doi: 10.1016/s1010-7940(98)00038-4. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman M, Muzikanski A, Wright CD, et al. Incidence and risk factors of persistent air leak after major pulmonary resection and use of chemical pleurodesis. Ann Thorac Surg. 2010;89:891–89. doi: 10.1016/j.athoracsur.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Tambiah J, Rawlins R, Robb D, Treasure T. Can tissue adhesives and glues significantly reduce the incidence and length of postoperative air leaks in patients having lung resections? Interact Cardiovasc Thorac Surg. 2007;6:529–33. doi: 10.1510/icvts.2007.153080. [DOI] [PubMed] [Google Scholar]
- 7.Anegg U, Lindenmann J, Matzi V, et al. Efficiency of fleece-bound sealing (TachoSil) of air leaks in lung surgery: a prospective randomised trial. Eur J Cardiothorac Surg. 2007;31:198–202. doi: 10.1016/j.ejcts.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 8.Wong K, Goldstraw P. Effect of fibrin glue in the reduction of post-thoracotomy alveolar air leak. Ann Thorac Surg. 1997;64:979–81. doi: 10.1016/s0003-4975(97)00820-5. [DOI] [PubMed] [Google Scholar]
- 9.Fabian T, Federic JA, Ponn RB. Fibrin glue in pulmonary resection: a prospective randomized blinded study. Ann Thorac Surg. 2003;75:1587–92. doi: 10.1016/s0003-4975(02)04994-9. [DOI] [PubMed] [Google Scholar]
- 10.Chambers A, Routledge T, Bille A, Scarci M. Is blood pleurodesis effective for determining the cessation of persistent air leak? Interact Cardiovasc Thorac Surg. 2010;11:468–72. doi: 10.1510/icvts.2010.234559. [DOI] [PubMed] [Google Scholar]
- 11.Suter M, Bettschart V, Vandoni RE, Cuttat JF. Thoracoscopic pleurodesis for prolonged (or intractable) air leak after lung resection. Eur J Cardiothorac Surg. 1997;12:160–61. doi: 10.1016/s1010-7940(97)00118-8. [DOI] [PubMed] [Google Scholar]
- 12.Thiestlethwaite PA, Luketich JD, Ferson PF, Jamieson SW. Ablation of persistent air leaks after thoracic procedures with fibrin sealants. Ann Thorac Surg. 1999;67:575–77. doi: 10.1016/s0003-4975(98)01292-2. [DOI] [PubMed] [Google Scholar]
- 13.Antunes G, Nevile E, Duffy J, Ali N on behalf of the BTS Pleural Disease Group, a subgroup of the BTS Standards of Care Committee. BTS guidelines for the management of malignant pleural effusions. Thorax. 2003;58(Suppl 2):ii29–35. doi: 10.1136/thorax.58.suppl_2.ii29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sleijfer S. Bleomycin-induced pneumonitis. Chest. 2001;120(2):617–24. doi: 10.1378/chest.120.2.617. [DOI] [PubMed] [Google Scholar]
- 15.Gözübüyük A, Özpolat B, Cicek AF, et al. Comparison of side effects of oxytetracycline and talc pleurodesis: an experimental study. J Cardiothorac Surg. 2012;5:128–33. doi: 10.1186/1749-8090-5-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchem RE, Herndon BL, Fiorella RM, et al. Pleurodesis by autologous blood, doxycline, and talc in the rabbit model. Ann Thorac Surg. 1999;67:917–21. doi: 10.1016/s0003-4975(99)00160-5. [DOI] [PubMed] [Google Scholar]
- 17.Karangelis D, Tagarakis GI, Daskalopoulos M, et al. Intrapleural instillation of autologous blood for persistent air leak in spontaneous pneumothorax – is it as effective as it is safe? J Cardiothorac Surg. 2010;5:61–64. doi: 10.1186/1749-8090-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams P, Laing R. Tension pneumothorax complicating autologous ‘blood patch’ pleurodesis. Thorax. 2005;60:1066–67. doi: 10.1136/thx.2004.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrio MJ, Ewen D, Trevethick MA, et al. Fibrin formation by wounded bronchial epithelial cell layers in vitro is essential for normal epithelial repair and independent of plasma proteins. Clin Exp Allergy. 2007;37(11):1688–700. doi: 10.1111/j.1365-2222.2007.02829.x. [DOI] [PubMed] [Google Scholar]
- 20.Powanda MC, Moyer ED. Plasma proteins and wound healing. Surg Gynecol Obstet. 1981;153(5):749–55. [PubMed] [Google Scholar]
- 21.Tan P, Lee C, Beckert L. Biological glue for persistent air leak: a case report. N Z Med J. 2012;125:67–68. [PubMed] [Google Scholar]
- 22.Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21:739–48. doi: 10.5435/JAAOS-21-12-739. [DOI] [PubMed] [Google Scholar]
- 23.Marx RE. Platelet rich-plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–96. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (Platelet Gel) and autologous platelet-poor plasma (Fibrin Glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107:229–37. doi: 10.1097/00006534-200101000-00037. [DOI] [PubMed] [Google Scholar]
- 25.Cerfolio RJ, Tummala RP, Holman WL, et al. A Prospective algorithm for the management of air leaks after pulmonary resection. Ann Thorac Surg. 1998;66:1726–31. doi: 10.1016/s0003-4975(98)00958-8. [DOI] [PubMed] [Google Scholar]


