Abstract
Background
Catheter-directed therapy (CDT) for pulmonary embolism (PE) is considered as an alternative to systemic thrombolysis (ST) in patients with hemodynamically unstable acute PE who are considered at high bleeding risk for ST. We aimed to evaluate the efficacy and safety of CDT in the management of acute PE with right ventricular dysfunction (RVD). The primary outcomes were mortality, clinical success, and complications. Secondary outcomes were change in hemodynamic parameters in the first 24 hours following the procedure.
Material/Methods
Medical records of consecutive patients diagnosed as having acute massive or submassive PE with accompanying RVD treated by immediate CDT at our institution from January 2007 to January 2014 were reviewed. Patient characteristics, mortality, achievement of clinical success, and minor and major bleeding complications were analyzed in the overall study group, as well as massive vs. submassive PE subgroups. Change in hemodynamic parameters in the second, eighth, and 24th hours after the CDT procedure were also analyzed.
Results
The study included 15 consecutive patients (M/F=10/5) with a mean age of 54.2±16.6 years who underwent immediate CDT. Nine of the patients had submassive PE, and 6 had massive PE. In-hospital mortality rate was 13.3% (95% CI, 0.04–0.38). One major, but not life-threatening, bleeding episode was evident in the whole group. Hemodynamic parameters were stabilized and clinical success was achieved in 14/15 (93.3%; 95% CI, 70.2–98.8) of the patients in the first 24 hours. Notably, the hemodynamic recovery was significantly evident in the first 8 hours after the procedure.
Conclusions
CDT is a promising treatment option for patients with acute PE with RVD with no fatal bleeding complication. In experienced centers, CDT should be considered as a first-line treatment for patients with acute PE and RVD and contraindications for ST, with the advantage of providing early hemodynamic recovery.
MeSH Keywords: Mechanical Thrombolysis, Pulmonary Embolism, Thrombolytic Therapy
Background
Pulmonary embolism (PE) may cause acute life-threatening right ventricular dysfunction (RVD) [1]. The severity of PE is an estimate of PE-related mortality [2,3]. Systemic thrombolysis (ST) is the mainstay in the management of patients with massive and submassive PE with RVD [3–5]. However, a considerable proportion of patients with acute PE cannot receive ST because of contraindications [4]. Catheter-directed therapy (CDT), to recanalize occluded pulmonary arteries, is accepted as an alternative to ST when emergency surgical thrombectomy is unavailable [6,7]. Increasing evidence suggests CDT is a safe and effective treatment for acute PE [6–9]. However, current knowledge on efficacy and safety of CDT in management of submassive PE is limited [7,8]. Moreover, a close follow-up study exploring the acute changes in hemodynamic parameters after the procedure is not available in the current literature. In the present study, we aimed to evaluate the efficacy and safety of CDT in the management of acute PE with RVD in a group consisting of both patients with massive PE and patients with submassive PE. The primary outcomes were mortality, clinical success, and complications; the secondary outcomes were change in hemodynamic parameters in the first 24 hours after the procedure.
Material and Methods
Study population
We conducted a retrospective study enrolling consecutive patients diagnosed with acute massive or submassive PE with accompanying RVD and treated by immediate CDT at our institution from January 2007 to January 2014 [2,3]. Patients were eligible for CDT if they had massive or submassive PE and evidence of contraindication to ST. PE was initially diagnosed by pulmonary angiography, echocardiography, or ventilation-perfusion computed tomography (CT) scan. Patients’ medical records were reviewed for baseline characteristics including symptoms, vital signs, risk factors, electrocardiography, transthoracic echocardiography, follow-up data, laboratory tests including biomarkers of RVD and myocardial necrosis, procedures, and the outcome data (mortality, cause of death, minor and major complications, management of complications, hemodynamic parameters) at first admission and during the follow-up period. Patients were followed by hospital visit and/or phone contact after discharge. Excluded from the study were patients with nonmassive or chronic PE; patients receiving anticoagulant therapy; and patients who received fibrinolytics at first admission, required a treatment escalation because of hemodynamic and clinical instability, and underwent CDT. The local ethical committee at the Faculty of Medicine, Uludağ University, approved the protocol of the study.
Outcomes and definitions
Acute PE refers to a PE event with associated symptoms that lasts no longer than 14 days. Massive and submassive PEs were defined as described previously [3]. RVD was defined as the presence of 1 or both of the following criteria: (a) right ventricular (RV) dilatation or RV systolic dysfunction on echocardiography, (b) RV dilatation on CT [3]. None of the patients received fibrinolytics before the intervention.
The primary outcomes were mortality, clinical success, and complications. In-hospital mortality and 30-day mortality were recorded. Clinical success was defined as stabilization of hemodynamic parameters, resolution of hypoxia, and survival from PE [10]. Major and minor bleedings were defined as described previously [11].
Secondary outcomes were change in hemodynamic parameters: the change in systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), oxygen saturation (SaO2), and shock index (the ratio of HR to SBP), measured at first admission and compared with measurements the following second, eighth, and 24th hours of the intervention were analyzed [12,13]. Patients’ hemodynamic parameters were monitored hourly during hours 0 to 12, every 2 hours between 12th and 24th hours, and every 6 hours after the procedure. RVD was assessed by a cardiologist on echocardiography. Mean pulmonary artery pressure (PAP) was estimated by transthoracic echocardiography at first admission and 28±4 hours after catheter-directed intervention.
Catheterization procedure and treatment details
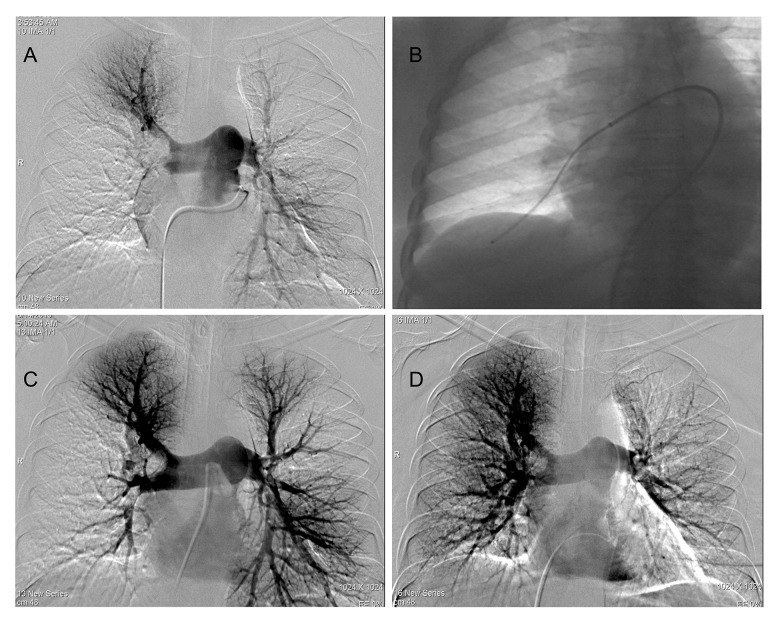
Under local anesthesia, a 5F sheath was inserted through the right common femoral vein with ultrasound guidance. 5F pigtail (Cook, Bloomington, USA) or angled (Picard) catheters guided with a 0.035-inch hydrophilic guide wire (Terumo, Tokyo, Japan) were used to catheterize the main pulmonary artery trunk. After measuring the PAP, pulmonary angiography was performed to identify the thrombus (Figure 1A). Then, the F sheath was exchanged via hydrophilic guide wire to an 8F/10F introducer sheath (Flexor Sheath, Cook Medical, Bloomington, USA). The introducer sheath positioned in the main or one of the right or left pulmonary artery. Sidearm of the sheath was connected to a heparinized saline flush (4000 IU heparin/1000 cc normal saline), infused at 15 cc/h. Before aspiration, 5 mg alteplase diluted in 20 cc saline injected slowly into the thrombus. The total alteplase dose varied depending on the interventionist’s discretion and the patient’s condition. Afterward, a guide catheter (100 cm-6F-Envoy, or 8F MPA-1Vista Brite Tip Cordis) was used for thrombus aspiration. A 20-mL syringe with a luer-lock connector was used to apply suction while moving the guide catheter slowly back and forth over several centimeters within the clot (Figure 1B). After several passes, control low-dose contrast injections were performed. If total or partial recanalization was achieved, we switched to another obstructed segment. The procedure was considered clinically successful when the subsegmental branches became visible behind recanalized pulmonary artery segments with an improvement in hemodynamic parameters without major complications (such as a perforation of the pulmonary vascular or cardiac structures, tamponade, or death) [9,14]. Pulmonary angiography was performed routinely before and 24 hours after the procedure (Figure 1C, 1D).
Figure 1.
(A) Pulmonary angiography showing filling defect of the right main and low left lobar pulmonary arteries. (B) Thrombus aspiration was performed with a guide catheter which was inserted through a long introducer sheath. (C, D) Final angiograms after catheter aspiration and local alteplase administration.
Statistical analyses
Statistical analyses were performed using the IBM SPSS Statistics for Windows 22.0 software program (Armonk, NY, IBM Corp, USA). The variables were investigated using visual (histograms, probability plots) and analytical methods (Shapiro-Wilk test) to determine distribution. Descriptive analyses were presented using medians and interquartile range (IQR, 25–75) and mean plus or minus standard deviation (±SD) for the nonnormally and normally distributed ordinal variables, respectively. Categorical variables were reported as proportions and 95% confidence intervals (CIs). For group comparisons, Pearson’s chi-square test was used for categorical variables, and the Mann Whitney-U test was used for continuous variables. Hemodynamic parameters between baseline and postprocedure were compared using the Wilcoxon rank test. Two-tailed statistical significance level was considered as 5%, and unadjusted P values were reported.
Results
Study population
During the study period, 15 acute PE patients with RVD (6 with massive PE and 9 with submassive PE) having a mean age of 54±17 years who had consecutively undergone immediate CDT were enrolled in the study. Clinical characteristics of enrolled patients are presented in Table 1. PE was diagnosed by spiral CT angiography in 93.3% (n=14) patients; high-probability perfusion scans in 6.7% (n=1) patients. PE was also verified by pulmonary angiograms in all patients before CDT. Preexisting comorbid diseases in the study group were cardiovascular disease (40%), malignancy (20%), diabetes mellitus (6.7%), hypertension (6.7%), chronic renal failure (6.7%), chronic obstructive pulmonary disease (6.7%), and ulcerative colitis (6.7%). Median length of stay was 11 [IQR, 25–75; 9–17] days.
Table 1.
Patient characteristics, clinical presentation, risk factors and comorbidities.
| Catheter directed therapy (n=15) | |
|---|---|
| Age | 54.2±16.6 |
|
| |
| Sex (M/F) | 5/10 |
|
| |
| Symptom duration (day) | 3 [1–5.5] |
|
| |
| Risk factors for PE | |
| Active malignancy | 4 (26.7) |
| Obesity | 1 (6.7) |
| Immobilization (<3 months)* | 8 (53.3) |
| Recent trauma (<4 weeks) | 3 (20) |
| Recent surgery (<4 weeks) | 7 (46.7) |
| Previous venous thromboembolism | 2 (13.3) |
| Hereditary predisposition | 3 (20) |
| Hormone therapy# | 1 (6.7) |
|
| |
| Massive PE/Submassive PE (n) | 6/9 |
|
| |
| Risk stratification marker | |
| Markers of RV dysfunction## | 14 (93.3) |
| Markers of myocardial necrosis& | 11 (73.3) |
| Clinical markers, syncope and hypotension&& | 2 (13.3) |
|
| |
| sPES index | |
| 0 (n,%) | 4 (26.7) |
| 1 (n,%) | 11 (73.3) |
|
| |
| Location of PE | |
| Central bilateral | 10 (66.7) |
| Central single side | 5 (33.3) |
| Paracentral | 10 (66.7) |
|
| |
| Systolic blood pressure (mmHg) | 97 [83–112] |
|
| |
| Diastolic blood pressure (mmHg) | 60 [50–62] |
|
| |
| PAP (mmHg) | 59.9±12.5 |
|
| |
| Shock index | 1.02±0.3 |
|
| |
| SpO2 (%) | 92.4±5.7 |
|
| |
| D-Dimer (mg/L) | 5.4 [2.6–9.2] |
|
| |
| Troponin-I (ng/mL) | 0.14 [0.03–1.2] |
Data are mean ± standard deviation or median IQR 25–75]; number of cases (percentage ratios within the grup) as appropriate.
Immobilization: bed ridden >72 hours, or long distance travel >6 hours;
Hormone therapy: oral contraceptive pills, hormone replacement therapy or Tamoxifen use;
Markers of RV dysfunction defined as RV dilatation, hypokinesis on echocardiography;
Myocardial injury was based on cardiac troponin T positivity;
Hypotension was defined as systolic blood pressure <90 mmHg not caused by arrhtmia, hypovolemia or sepsis.
M – male; F – female; RV – right ventricul; sPES index – Simplified Pulmonary Embolism Severity Index; PAP – pulmonary artery pressure; SpO2 – oxygen saturation measured at room air.
Treatment details and technical complications regarding CDT
Mean rt-PA dose per patient was 30±10 mg, and total dose applied was higher for patients with massive PE than for patients with submassive PE (37±7 mg vs. 24±10 mg; P=0.034, respectively). Technical complications related with the procedure were recorded in 2 patients, both as hematoma in the femoral puncture site, and neither patient required blood transfusions. Clinical and procedural characteristics, as well as safety and efficacy outcomes of the patients, are summarized in Table 2. Of those 15 patients with relative or absolute contraindications to ST, 4 (26.7%; 95% CI, 12.2–51.9) received either mechanical thrombectomy or thrombus aspiration, and 11 (73.3%; 95% CI, 48.0–89.1) received CDT in addition to mechanical thrombectomy or thrombus aspiration (Table 2).
Table 2.
Clinical and procedural characteristics, primary and secondary outcomes of the total group.
| Pt | Age/sex | Clinic risk | Comorbidity | Treatment | Contraindication for ST | Precedural complication | Major bleeding | Minor bleeding | Clinical success | Mortality |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53/F | Massive | CAD | PMT | Recent major surgery | +, hematoma in the puncture site | – | +, hematoma | + * | Alive |
| 2 | 38/M | Massive | None | PMT | Trauma, recent major surgery | – | – | – | + * | Alive |
| 3 | 33/F | Massive | None | PMT | Recent major surgery | – | – | – | + * | Alive |
| 4 | 51/F | Massive | CAD | PMT | Recent major surgery | – | – | – | – | Dead, in-hospital mortality due to advanced malignancy |
| 5 | 61/M | Massive | Malignancy | PMT | Recent major surgery | – | – | – | + * | Dead, 1 month, after discharge, PE-related |
| 6 | 73/F | Massive | CAD, DM, malignancy | PMT | CNS neoplasm | – | – | – | + * | Alive |
| 7 | 59/F | Submassive | ITP | PMT | Recent major surgery | – | – | +, hematoma | + * | Alive |
| 8 | 25/M | Submassive | None | PMT | Recent major surgery | – | – | – | + * | Alive |
| 9 | 73/F | Submassive | UC | PMT | Recent GİS bleeding | – | – | – | + * | Alive |
| 10 | 53/M | Submassive | Malignancy | MT | CNS neoplasm | – | – | +, ecchimosis | + * | Alive |
| 11 | 58/F | Submassive | HT | MT | Recent ischaemic stroke | – | – | – | + * | Alive |
| 12 | 52/F | Submassive | CRF | PMT | Recent major surgery | – | – | – | + * | Alive |
| 13 | 34/F | Submassive | None | PMT | Recent major surgery | – | +, hematoma, 3 units ES transfusion | – | + * | Alive |
| 14 | 65/F | Submassive | Malignancy | MT | Central nervus system neoplasm | +, hematoma in the puncture site | – | +, hematoma | + * | Dead, in-hospital PE-related |
| 15 | 85/F | Submassive | HT, aritmi | MT | Refractory hypertension | – | – | – | + * | Alive |
Pt – patient number; F – female; M – male; N – no; Y – yes.
Hemodynamic stabilization achieved in the first 24 hours.
ST – systemic thrombolysis; CAD – coronary artey disease; DM – diabetes mellitus; ITP – immun thrombocytopenic purpura; UC – ulcerative colitis; CRF – chronic renal failure; HT – hypertension; PMT – pharmacomechanical thrombolysis; T – thrombolytic therapy, alteplase; MT – mechanical thrombectomy, thrombus aspiration; ES – erythrocyte suspension; CNS – central nervous system.
Outcomes
In-hospital mortality and 30-day mortality rates were 13.3% (95% CI, 3.7–37.9) and 20% (95% CI, 7.0–45.2), respectively (Table 3). Major bleeding occurred in 1 patient (6.7%; 95% CI, 1.2–29.8) from a rectus hematoma that required 3 units of erythrocyte transfusion. This single major bleeding event was observed in a submassive PE patient who received pharmacomechanical thrombolysis. Recorded minor bleeding events (4/15) were hematomas (n=3) and ecchymosis (n=1); 26.7% (95% CI, 10.9–51.9; Table 3).
Table 3.
Primary and secondary outcome measures of the study groups.
| CDT (n=15) | P | |||
|---|---|---|---|---|
| Total (N=15) | Massive PE (N=6) | Submassive PE (N=9) | ||
| In-hospital mortality | 2 (13.3) | 1 (6.7) | 1 (6.7) | 1.00 |
| 30-day mortality | 3 (20) | 2 (13.3) | 1 (6.7) | 0.52 |
| Hemodynamic stabilization achieved | 14 (93.3) | 5 (33.3) | 9 (60) | 0.40 |
| Δ Pulmonary artery pressure, median [IQR] | 25 [9–30] | 26 [10–37] | 20 [9–30] | 0.64 |
| Major bleeding | 1 (6.7) | 0 | 1 (6.7) | 1.00 |
| Minor bleeding | 4 (26.7) | 1 (6.7) | 3 (20) | 0.60 |
Data presented as number of cases (% of the total study population) unless otherwise stated. Statistical significance was determined by the Pearson’s chi-square goodness-of-fit test for group comparisons, the Mann-Whitney U test for comparison of continuous non-normal data between massive PE vs. submassive PE groups. CDT – catheter-directed therapy; PE – pulmonary emboli; IQR – interquartile range 25 to 75.
Clinical success was achieved in 93.3% (95% CI, 70.2–98.8) of the total study population. Clinical success rate was 83.3% and 100% in patients with massive or submassive PE, respectively. Two of 3 deaths were recorded in massive PE patients and 1 in a submassive PE patient. Two deaths occurred during hospitalization. One occurred in a patient with massive PE because of advanced malignancy, although clinical success and hemodynamic stabilization were achieved in the first 24 hours after the CDT procedure. The other patient had a submassive presentation and an accompanying malignant disease. Clinical success was not achieved after the CDT procedure. Death was caused by a recurrent PE event on the 20th day of hospitalization.
Hemodynamic parameters
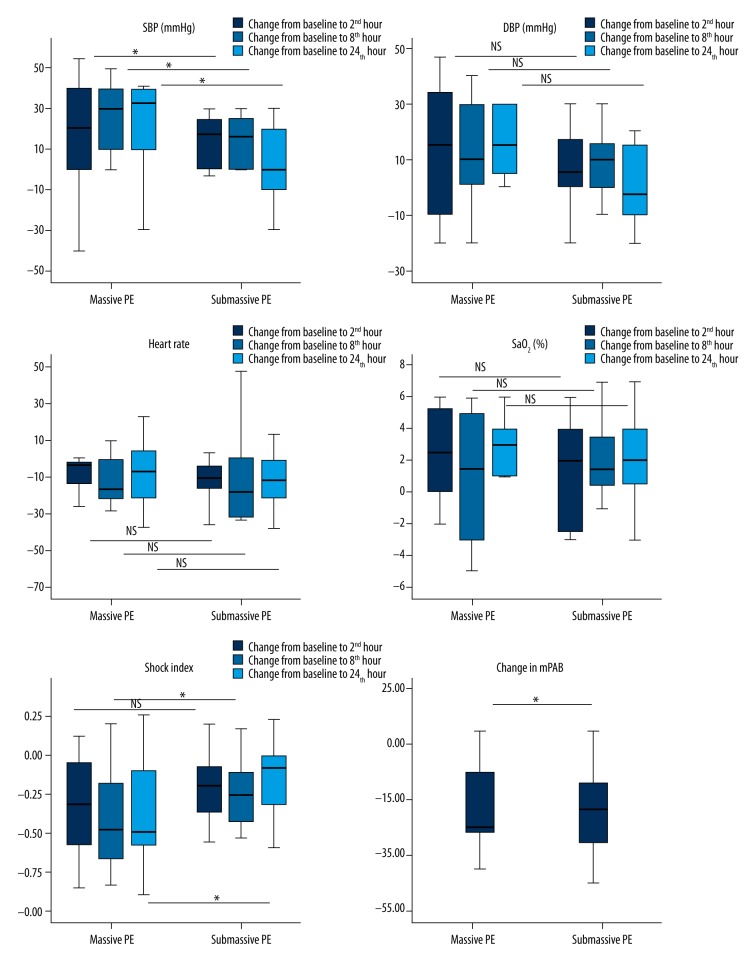
SBP, DBP, HR, shock index (SI), and SaO2 significantly improved within 24 hours after the CDT procedure (Figure 2). Notably, recovery started in the first 2 hours and was significantly evident in all hemodynamic parameters in the first 8 hours after intervention and hemodynamic improvement continued to occur over a 24-hour period. PAP improved from 59.9±12.5 mm Hg to 38.9±8.5 mm Hg (P<0.0001). Stabilization of hemodynamic parameters was achieved in the first 24 hours in 93.3% (95% CI, 70.2–98.8) of patients. The 1 unstabilized patient had massive PE; this patient died of advanced cancer during hospitalization.
Figure 2.
Comparison of change in hemodynamic parameters in massive (n=6) vs. submassive (n=9) pulmonary embolism (PE) groups. Dark grey bars represent change from baseline to second hour, grey bars represent change from baseline to the eighth hour, dark grey bars represent change from baseline to 24th hour, and the error bars 95% CIs for the medians. Statistical significance was determined by the the Mann-Whitney U test for comparison of continuous nonnormal data between massive PE vs. submassive PE groups. SpO2=oxygen saturation measured at room air.
Subgroup analyses revealed significantly higher improvement in SBP and SI in patients with massive PE vs. submassive PE but no evidence of significant difference in DBP, HR, and peripheral capillary oxygen saturation (SpO2; Figure 2).
Discussion
In the present study, CDT for patients with acute PE with RVD rapidly improved hemodynamic parameters and was free of major fatal bleeding. Because current data for efficacy and safety of CDT in patients with submassive PE is limited, we observed that mortality, clinical success, complication rates, and hemodynamic improvement profile did not differ markedly between patients with massive or submassive PE.
CDT options include thrombus fragmentation and various thrombectomy methods that can be performed with no or low-dose local thrombolytic [15]. Currently, there is no agreement on the indications and the optimum technique for CDT [6,16]. CDT is recommended as an alternative to ST in the presence of contraindications, ST failure, or an alternative if surgery is unavailable in high-risk PE [2]. The American Heart Association extends these recommendations to patients with submassive PE with adverse prognostic clinical evidence [3]. Acute PE is related with a poor prognosis for short- and long-term survival [17]. Our in-hospital mortality rate was 13.3%, slightly less than the 15.7% reported by Chechi [7]. We detected that PE was responsible for 50% (1/2) of the in-hospital deaths (95% CI, 9.4–90.5), whereas PE was responsible for the vast majority (2/3, 66.7%; 95% CI, 20.8–93.8) of the 30-day mortality. One of the patients who died of PE had had a massive PE and had received a pharmacomechanical thrombectomy. The second patient who died of PE had had a submassive PE and had not received thrombolytics. Although we achieved clinical stabilization in the first 24 hours after CDT, this patient died of a recurrent PE during hospitalization. Of note, both of these patients also had cancer. Even though these patients underwent immediate CDT, this finding emphasizes the suggestion that PE-related events seem to be responsible for the majority of early mortality [1]. We think one possible explanation for high PE-related mortality observed may be the high coexistence of malignant and cardiovascular disease (20% and 40%, respectively) in our study population. Cancer and congestive heart failure have been identified as prognostic factors affecting mortality in PE [1,18,19].
Overall clinical success achieved with CDT was quite high (93.3%) in our study, consistent with the pooled clinical success rate reported as 86.5% [10]. Similar to PERFECT trial results, clinical success rates achieved in our study were 83.3% for massive PE and 100% for submassive PE [8]. On the other hand, Skaf and colleagues demonstrated that administration of local thrombolytics, in addition to mechanical thrombectomy and aspiration, improved clinical success rate from 81% to 95% in another systematic review [20]. There were no technical complications such as perforation of a cardiac/vascular structure, tamponade, or procedure-related death in our study. Interventionalist experience is known to inflence the technical success [21]. The high success rate in our study may be a result of all procedures being performed by the same experienced interventionalist. Another explanation may be that more than half of the catheter intervention group (60%) included submassive PE patients, among whom clinical success expectancy is high. Furthermore, 73% of the CDT group received local thrombolytic infusion with mechanical thrombectomy, which may have a role in the increased success rate of the procedure.
Thrombolytic therapy carries the risk of major bleeding including intracranial hemorrhage [22,23]. The rate of major hemorrhage with ST administration was 20% to 22% [1,22,24]. Because of high bleeding risk, alternative revascularization strategies safer than ST are required. The rate of major complications due to CDT was 6.7%, and there was no recorded fatal bleeding. Eid-Lidt and colleagues observed a major bleeding rate of 11.1% following percutaneous mechanical thrombectomy in massive PE, and Chechi reported a rate of 7.8% [7,14]. The minor and major complication rate of CDT were reported as 7.9% and 2.4%, in a recent meta-analysis [10]. Although, major bleeding rate observed in our study is consistent with previous reports, minor bleeding rate is higher than reported (26.7%). We think that bleeding rates in our study should be interpreted with caution. In this study the only reason for a patient to undergo the CDT procedure was the presence of an absolute/relative contraindication for ST. Despite present relative contraindication for ST, almost three-quarters (73.3%) of our study group recieved local thrombolytic (mean dose of 30±10.5 mg) and did not develop major fatal bleeding.
As the presence of RVD is an important determinant of the adverse clinical outcome, these patients are considered to be candidates for early reperfusion therapy [25,26]. However, the role of thrombolytic therapy in patients with submassive PE is controversial. Fibrinolytic therapy improves clinical outcome and prevents hemodynamic decompensation and clinical deterioration in submassive PE patients [27,28]. In addition to ST, the efficacy of the CDT techniques in the management of patients with submassive PE is also a subject of interest [7,8,29]. Mortality rates, major and minor bleeding complications, and clinical success rate did not differ between massive and submassive PE patients in our study. The only major but nonfatal bleeding event was observed in a patient with submassive PE treated by pharmacomechanical thrombectomy, but it should be kept in mind that interpretations of our study are limited because of the small number of patients.
One of the most striking results of our study was a dramatic early hemodynamic improvement starting from the second hour after CDT. Significant improvement was evident in all hemodynamic parameters in the first 8 hours, followed by a continued recovery in 24 hours after the procedure (Figure 2). Interestingly, hemodynamic response was similar between patients with massive or submassive PE, except a marked significant improvement in SBP and SI in massive PE cases (Figure 2). Percutaneous CDT frequently combining mechanical thrombus reduction modalities with local thrombolysis offers a rapid relieving in hemodynamic burden of PE [16,30]. This early improvement with CDT may be explained by successful trombus debulking, rapid recanalization of a central occlusion, and relieving RVD [2,31]. Mohan and colleagues reported a significant hemodynamic improvement after the first 24 hours after CDT [9]. Early improvement seems to have a prognostic importance; Zeni and colleagues found that 15 of 17 patients treated with rheolytic thrombectomy who were able to survive in the first 24 hours after the procedure were all alive at a 19-month follow-up visit [32].
Few limitations should be noted regarding our study. This study reflects our real-life practice in the management of massive/submassive PE patients with clinical evidence of adverse prognosis. Despite expert recommendation on integration of CDT into the PE treatment algorithm, there is lack of controlled randomized prospective studies and official treatment indication [10,33]. Although data collection was retrospective, our medical records including hemodynamic data were strict and reliable. In order to prevent selection bias, all CDT performed patients were enrolled consecutively. Essentially, clinical success rate of CDT was reported to be similar in prospective and retrospective studies [10]. All procedures were performed by the same experienced interventional radiologist. Unfortunately, full-spectrum echocardiography parameters were not available for echocardiographic assessments in some of the patients, so the improvement in RVD was assessed after the procedure. Even though pulmonary angiograms were performed, Miller scores were not recorded, and we were unable to report Miller scores of the patients.
Conclusions
Acute PE is associated with a poor prognosis. In experienced centers, CDT can be performed safely and effectively in most patients with massive or submassive PE. CDT is useful when ST is contraindicated. Our results suggest that CDT improves hemodynamic parameters rapidly in patients with massive or submassive PE. In experienced centers, CDT may be considered as a first-line treatment for patients with acute PE with accompanying RVD who have contraindications to ST.
Footnotes
Source of support: Departmental sources
Conflicts of interest
Nothing to declare.
References
- 1.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: Clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–89. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 2.Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2008;29:2276–315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 3.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation. 2011;123:1788–830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 4.Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: Results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165–71. doi: 10.1016/s0735-1097(97)00319-7. [DOI] [PubMed] [Google Scholar]
- 5.Condliffe R, Elliot CA, Hughes RJ, et al. Management dilemmas in acute pulmonary embolism. Thorax. 2014;69:174–80. doi: 10.1136/thoraxjnl-2013-204667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelberger RP, Kucher N. Catheter-based reperfusion treatment of pulmonary embolism. Circulation. 2011;124:2139–44. doi: 10.1161/CIRCULATIONAHA.111.023689. [DOI] [PubMed] [Google Scholar]
- 7.Chechi T, Vecchio S, Spaziani G, et al. Rheolytic thrombectomy in patients with massive and submassive acute pulmonary embolism. Catheter Cardiovasc Interv. 2009;73:506–13. doi: 10.1002/ccd.21858. [DOI] [PubMed] [Google Scholar]
- 8.Kuo WT, Banerjee A, Kim PS, et al. Pulmonary embolism response to fragmentation, embolectomy, and catheter thrombolysis (PERFECT): Initial results from a prospective multicenter registry. Chest. 2015;148:667–73. doi: 10.1378/chest.15-0119. [DOI] [PubMed] [Google Scholar]
- 9.Mohan B, Chhabra ST, Aslam N, et al. Mechanical breakdown and thrombolysis in subacute massive pulmonary embolism: A prospective trial. World J Cardiol. 2013;5:141–47. doi: 10.4330/wjc.v5.i5.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo WT, Gould MK, Louie JD, et al. Catheter-directed therapy for the treatment of massive pulmonary embolism: Systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol. 2009;20:1431–40. doi: 10.1016/j.jvir.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non surgical patients. J Thromb Haemost. 2005;3:692–94. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 12.Rady MY, Smithline HA, Blake H, et al. A comparison of the shock index and conventional vital signs to identify acute, critical illness in the emergency department. Ann Emerg Med. 1994;24:685–90. doi: 10.1016/s0196-0644(94)70279-9. [DOI] [PubMed] [Google Scholar]
- 13.Kucher N, Luder C, Dörnhöfer T, et al. Novel management strategy for patients with suspected pulmonary embolism. Eur Heart J. 2003;24:366–76. doi: 10.1016/s0195-668x(02)00476-1. [DOI] [PubMed] [Google Scholar]
- 14.Eid-Lidt G, Gaspar J, Sandoval J, et al. Combined clot fragmentation and aspiration in patients with acute pulmonary embolism. Chest. 2008;134:54–60. doi: 10.1378/chest.07-2656. [DOI] [PubMed] [Google Scholar]
- 15.Kuo WT. Endovascular therapy for acute pulmonary embolism. J Vasc Interv Radiol. 2012;23:167–179.e4. doi: 10.1016/j.jvir.2011.10.012. quiz 179. [DOI] [PubMed] [Google Scholar]
- 16.Saad N. Aggressive management of pulmonary embolism. Semin Interv Radiol. 2012;29(1):52–56. doi: 10.1055/s-0032-1302452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klok FA, Zondag W, van Kralingen KW, et al. Patient outcomes after acute pulmonary embolism: A pooled survival analysis of different adverse events. Am J Respir Crit Care Med. 2010;181:501. doi: 10.1164/rccm.200907-1141OC. [DOI] [PubMed] [Google Scholar]
- 18.Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326:1240–45. doi: 10.1056/NEJM199205073261902. [DOI] [PubMed] [Google Scholar]
- 19.Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877–905. doi: 10.1378/chest.121.3.877. [DOI] [PubMed] [Google Scholar]
- 20.Skaf E, Beemath A, Siddiqui T, et al. Catheter-tip embolectomy in the management of acute massive pulmonary embolism. Am J Cardiol. 2007;99:415–20. doi: 10.1016/j.amjcard.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 21.Goldhaber SZ. Percutaneous mechanical thrombectomy for acute pulmonary embolism: A double-edged sword. Chest. 2007;132:363–65. doi: 10.1378/chest.07-0591. [DOI] [PubMed] [Google Scholar]
- 22.Fiumara K, Kucher N, Fanikos J, Goldhaber SZ. Predictors of major hemorrhage following fibrinolysis for acute pulmonary embolism. Am J Cardiol. 2006;97:127–29. doi: 10.1016/j.amjcard.2005.07.117. [DOI] [PubMed] [Google Scholar]
- 23.Ramadan MR, Khan IS, Mahdi O. Spontaneous hemarthrosis following fibrinolytic therapy for acute myocardial infarction: A case report and literature review. Am J Case Rep. 2014;15:514–17. doi: 10.12659/AJCR.892138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konstantinides S, Geibel A, Olschewski M, et al. Association between thrombolytic treatment and the prognosis of hemodynamically stable patients with major pulmonary embolism: Eesults of a multicenter registry. Circulation. 1997;96:882–88. doi: 10.1161/01.cir.96.3.882. [DOI] [PubMed] [Google Scholar]
- 25.Konstantinides S. Acute pulmonary embolism. N Engl J Med. 2008;359:2804–13. doi: 10.1056/NEJMcp0804570. [DOI] [PubMed] [Google Scholar]
- 26.Konstantinides S, Goldhaber SZ. Pulmonary embolism: Risk assessment and management. Eur Heart J. 2012;33(24):3014–22. doi: 10.1093/eurheartj/ehs258. [DOI] [PubMed] [Google Scholar]
- 27.Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402–11. doi: 10.1056/NEJMoa1302097. [DOI] [PubMed] [Google Scholar]
- 28.Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347:1143–50. doi: 10.1056/NEJMoa021274. [DOI] [PubMed] [Google Scholar]
- 29.Görek A, Akçay Ş, İbiş OA, et al. Herpes simplex virus infection, massive pulmonary thromboembolism, and right atrial thrombi in a single patient: Case report. Heart Lung. 2007;36:148–53. doi: 10.1016/j.hrtlng.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Todoran TM, Sobieszczyk P. Catheter-based therapies for massive pulmonary embolism. Prog Cardiovasc Dis. 2010;52:429–37. doi: 10.1016/j.pcad.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Kucher N, Goldhaber SZ. Management of massive pulmonary embolism. Circulation. 2005;112:e28–e32. doi: 10.1161/CIRCULATIONAHA.105.551374. [DOI] [PubMed] [Google Scholar]
- 32.Zeni PT, Blank BG, Peeler DW. Use of rheolytic thrombectomy in treatment of acute massive pulmonary embolism. J Vasc Interv Radiol. 2003;14:1511–15. doi: 10.1097/01.rvi.0000099526.29957.ef. [DOI] [PubMed] [Google Scholar]
- 33.Goldhaber SZ. Integration of catheter thrombectomy into our armamentarium to treat acute pulmonary embolism. Chest. 1998;114:1237–38. doi: 10.1378/chest.114.5.1237. [DOI] [PubMed] [Google Scholar]