Abstract
Known genetic causes of pediatric interstitial lung disease include disorders of surfactant metabolism, telomerase, and DNA repair. We report 4 children from 2 families with rapidly progressive and fatal pulmonary fibrosis. A novel DNA repair defect unrelated to the ataxia-telangiectasia mutated gene was found in 1 child from each family.
Recently discovered genetic disorders affecting surfactant metabolism,1 telomere length,2 and DNA repair3 now account for an increasing number of cases of interstitial lung disease (ILD) in children. Despite these discoveries, however, the etiology of many cases of familial ILD remains unknown.
We describe sibling pairs from 2 families who developed rapidly progressive ILD leading to terminal respiratory failure. A novel defect in DNA double-stranded break repair was found in 1 child tested from each family. This study was approved by Baylor College of Medicine’s Institutional Review Board.
Case Reports
Family A
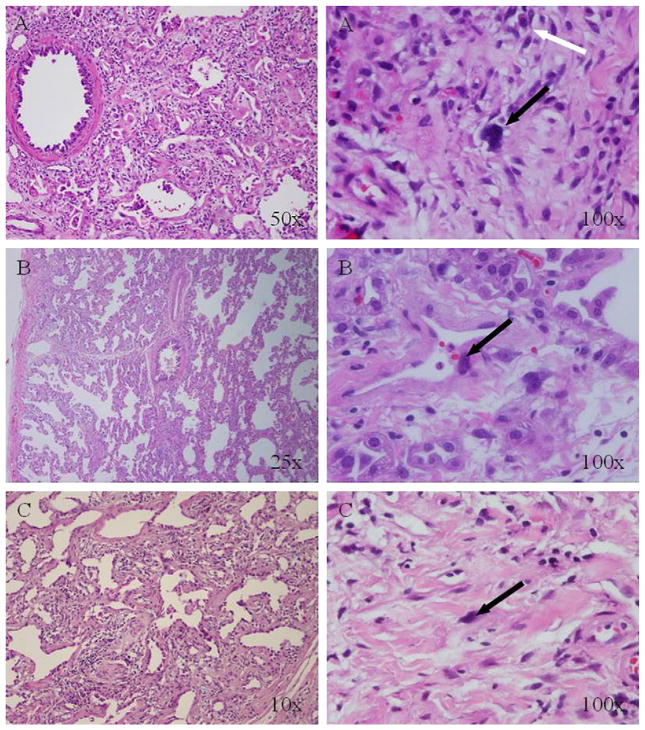
Patient A1 was a full-term male who exhibited failure to thrive (FTT) at age 6 months. At 14 months, he developed fever, diffuse pulmonary opacities, and progressive respiratory failure. Lung biopsy revealed proliferative diffuse alveolar damage with enlarged, bizarre pleiomorphic and hyperchromatic nuclei in pneumocytes and interstitial cells and eosinophils in the interstitium (Figure 1, A; available at www.jpeds.com). Despite treatment with intravenous methylprednisolone and intravenousimmunoglobulin, he died 52 days later.
Figure 1.
Histopathology of lung biopsy specimens from patients A1, B1, and B2 (hematoxylin and eosin staining). The findings were similar in all 3 patients and showed severe proliferative diffuse alveolar damage with large, bizarre, hyperchromatic nuclei (black arrows), suggestive of a DNA repair defect. Patient A1 also had multiple eosinophils (white arrows).
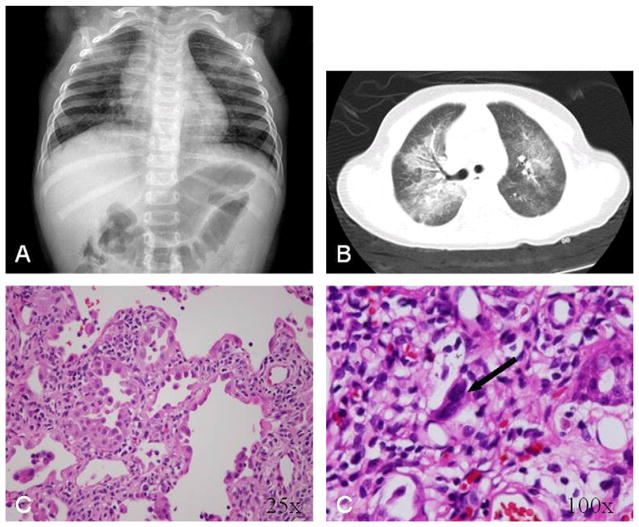
Patient A2, the younger sister of patient A1, was a full-term infant who had FTT at age 2 months. At 13 months, she developed progressive respiratory failure, likely related to viral illness. Imaging studies demonstrated bilateral diffuse infiltrates (Figure 2, A and B). Lung biopsy revealed proliferative diffuse alveolar damage with the same enlarged pleiomorphic and hyperchromatic nuclei in pneumocytes and interstitial cells seen in her brother’s biopsy specimen, but with an absence of eosinophilia (Figure 2, C). She underwent lung transplantation 51 days later, but died of progressive liver failure 1 year posttransplantation. Her liver demonstrated no pleomorphic cells on autopsy.
Figure 2.
Diagnostic imaging studies and lung biopsy histopathology from patient A2. A, Chest radiograph at presentation. B, Chest computed tomography, performed 4 days after admission. C, Histopathological analysis showing severe proliferative diffuse alveolar damage with large, bizarre, hyperchromatic nuclei in interstitial cells (black arrow).
Family B
Patient B1 was a full-term male with FTT and chronic respiratory symptoms initially diagnosed as asthma. At age 4 years, he developed respiratory failure associated with influenza B and died 58 days later. Patient B2, the younger sibling of patient B1, was a full-term male who developed FTT at age 2 months. At 34 months, he developed respiratory failure associated with picornavirus and died 53 days later. Both brothers had lung biopsy findings similar to those seen in the siblings in family A (Figure 1, B and C).
Results of Genetic Testing and DNA Repair Studies
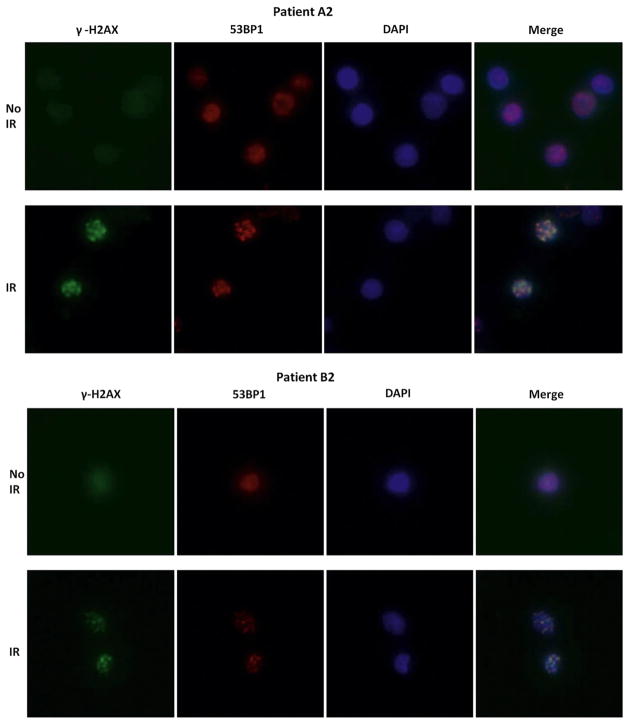
In all 4 cases, common causes of respiratory disease, including cystic fibrosis, immunodeficiency, and aspiration, were ruled out. Unfortunately, testing for genetic lung disorders was not performed on patients A1 and B1, because the familial pattern of disease was not appreciated until the second child in each family developed a similar disorder. The Table presents the results of genetic testing in patients A2 and B2. No mutations in surfactant and telomerase genes were found, and telomere length was normal. Lymphocytes from peripheral blood were immortalized using Epstein-Barr virus, and lymphoblastoid cell cultures were established. The cells were plated in 96-well trays and irradiated with 1 Gy. After 2 weeks, the wells with surviving colonies were scored and compared with daily controls from healthy cells and from patients with ataxia-telangiectasia (A-T). Both patients exhibited diminished colony survival, indicating a disorder of double-stranded DNA repair (Table). Tests performed for known causes of deficient double-stranded DNA repair mechanisms, including 53BP1 foci, were negative (Table and Figure 3; available at www.jpeds.com).
Table.
Evaluation of patients A2 and B2
| Studies | Patient A2 | Patient B2 |
|---|---|---|
| Surfactant | ||
| Mutations | SFTPC: none ABCA3: none |
SFTPB: none SFTBC: none ABCA3: none |
| Telomerase | ||
| Variants | TERT: 915G>A (A305A) TERC: none |
TERT: none TERC: r.58g>a |
| Telomere length (lung tissue) | Not short* | Not short* |
| DNA repair | ||
| Percent survival | 16% (normal-50% ± 13%)† | 7% (normal, 50% ± 13%)† |
| ATM protein level (intranuclear) | 48% Gene sequence: normal |
98% |
| Phosphorylation | Of SMC1: reduced Of Nibrin: absent |
Of SMC1: normal Of Nibrin: normal |
| Mre11 level | Not tested | Normal |
| 53BP1 foci | Normal | Normal |
ABCA3, ATP binding cassette protein A3; SFTPB, surfactant protein B; SFTPC, surfactant protein C; SMC1, structural maintenance of chromosome 1; TERC, telomerase RNA component; TERT, telomerase reverse transcriptase.
There is no statistical difference between these telomere lengths and those measures from genomic DNA isolated from lung tissue of 3 patients aged 20–60. There are no reference norms for telomere length of lung tissue.
Normal ranges for colony survival have been published by Sun et al.6
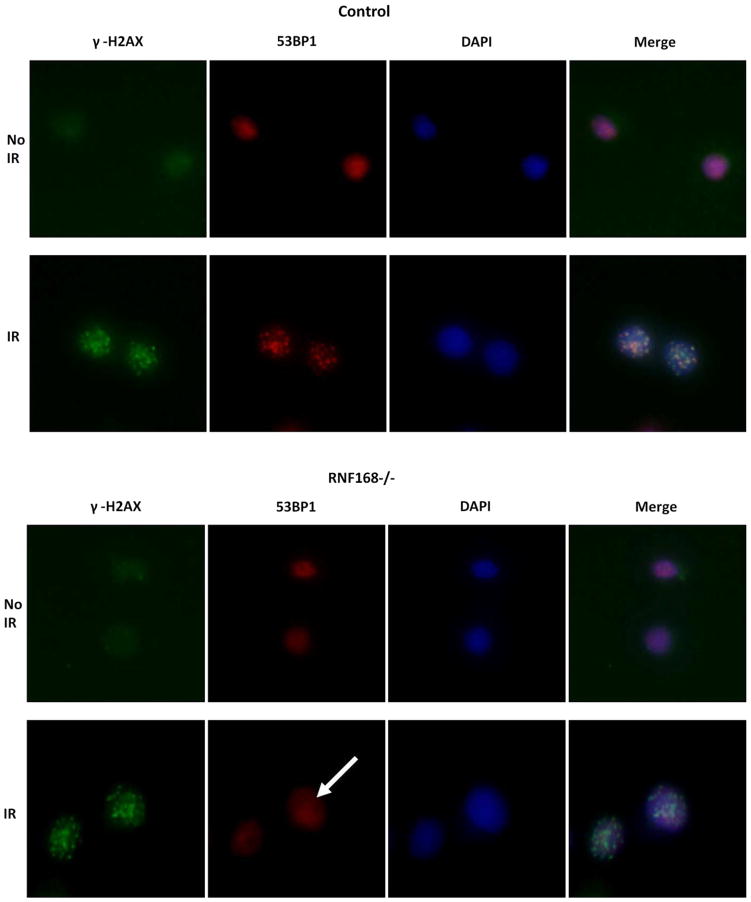
Figure 3.
53BP1 nuclear foci formation was normal in patients A2 and B2 compared with the wild-type cell control and RNF168−/− cell control, the latter failing to show 53BP1 foci at 1 hour after 3-Gy DNA damage (arrow). Gamma-H2AX foci were evaluated at 1 hour after 10 Gy to document ionizing radiation damage. DAPI (Vector Laboratories, Burlingame, California), which binds the minor groove of double-stranded DNA, was used to identify the nuclei of cells. Merging 53BP1 and DAPI demonstrated that the 53BP1 foci were within the nuclei. Cells were stained with a mouse antibody to γ-H2AX (1:300 dilution; Upstate, Billerica, Massachusetts) or with rabbit antibody to 53BP1 (1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, California).
Discussion
These cases reported here represent a novel form of familial, rapidly progressive respiratory failure associated with FTT in young children. No surfactant or telomerase mutations were found in the child tested from each family, and telomere length was normal. However, an unknown defect in DNA repair was found.
A-T, caused by mutations in the ataxia-telangiectasia mutated (ATM) gene, is the most common inherited form of defective repair of DNA double-stranded breaks.4 Schroeder et al5 identified ILD in 25 of 97 patients with A-T and chronic lung disease. The mean age of onset was 17.5 years (range, 9–28 years), and 19 of 25 patients died within 2 years of onset. In 13 patients, lung histology showed chronic inflammation and fibrosis with atypical epithelial and interstitial cells containing large hyperchromatic and pleiomorphic nuclei. This histological pattern is likely caused by accumulation of DNA resulting in failure of the cells to divide secondary to the deficient DNA repair. Our patients demonstrated a histological pattern on lung biopsy similar to that described in patients with A-T, but they were much younger at presentation, had a more rapidly progressive course, and did not have any other systemic manifestations of A-T, such as progressive ataxia or abnormal cells in other tissues, such as the liver. Similar to patients with A-T, our patients demonstrated decreased lymphoblastoid colony survival after DNA damage with 1 Gy of ionizing radiation.6 However, both patients had an ATM protein level inconsistent with A-T. Because patient A2 had an ATM protein level of 48%, his ATM genes were sequenced; no ATM mutations were found.
A new DNA repair defect consequent to mutations in the RNF168 gene has been described recently. RNF168 is a ubiquitin ligase protein in the chromatin ubiquitin ligase cascade.7–9 In 1 of the 2 patients reported to date, a rapidly progressive and fatal respiratory failure in association with growth retardation was described.10 Absence of RNF168 protein at sites of double-stranded breaks also prevents formation and retention of 53BP1 nuclear foci at these sites. Postirradiation 53BP1 foci formation was intact in both patients A2 and B2, arguing against RNF168 deficiency as a cause of respiratory failure.
In summary, we report 4 children from 2 families who presented with FTT and rapidly progressive, fatal ILD who most likely had a novel double-stranded DNA break repair defect of unknown etiology. Other causes of double-stranded DNA break repair-associated disorders were not found. We recommend that patients with diffuse lung disease of unknown origin undergo open lung biopsy for diagnostic purposes. If a pattern of diffuse alveolar damage that includes lung cells containing bizarre, hyperchromatic and pleiomorphic nuclei is found, an evaluation for DNA repair is appropriate, including testing for lymphoblastoid colony survival.6
Acknowledgments
We thank Okan Elidemir, MD, Jennifer Rama, MD, and George Mallory, MD, for their participation in the care of these children.
Glossary
- ATM
Ataxia-telangiectasia mutated
- A-T
Ataxia-telangiectasia
- FTT
Failure to thrive
- ILD
Interstitial lung disease
Footnotes
The authors declare no conflicts of interest.
References
- 1.Nogee LM. Genetic basis of children’s interstitial lung disease. Pediatr Allergy Immunol Pulmonol. 2010;23:15–24. doi: 10.1089/ped.2009.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–65. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nahas SA, Gatti RA. DNA double-strand break repair defects, primary immunodeficiency disorders, and “radiosensitivity”. Curr Opin Allergy Clin Immunol. 2009;9:510–6. doi: 10.1097/ACI.0b013e328332be17. [DOI] [PubMed] [Google Scholar]
- 4.McGrath-Morrow SA, Gower WA, Rothblum-Oviatt C, Brody AS, Langston C, Fan LL, et al. Evaluation and management of pulmonary disease in ataxia-telangiectasia. Pediatr Pulmonol. 2010;45:847–59. doi: 10.1002/ppul.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroeder SA, Swift M, Sandoval C, Langston C. Interstitial lung disease in patients with ataxia-telangiectasia. Pediatr Pulmonol. 2005;39:537–43. doi: 10.1002/ppul.20209. [DOI] [PubMed] [Google Scholar]
- 6.Sun X, Becker-Catania S, Chun HH, Hwang MJ, Huo Y, Wang Z, et al. Early diagnosis of ataxia-telangiectasia using radiosensitivity testing. J Pediatr. 2002;40:724–31. doi: 10.1067/mpd.2002.123879. [DOI] [PubMed] [Google Scholar]
- 7.Stewart GS, Panier S, Townsend K, Al-Hakim AK, Kolas NK, Miller ES, et al. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell. 2009;136:420–34. doi: 10.1016/j.cell.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 8.Stewart GS. Solving the RIDDLE of 53BP1 recruitment to sites of damage. Cell Cycle. 2009;8:1532–8. doi: 10.4161/cc.8.10.8351. [DOI] [PubMed] [Google Scholar]
- 9.Doil C, Mailand N, Bekker-Jensen S, Menard P, Larsen DH, Pepperkok R, et al. RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell. 2009;136:435–46. doi: 10.1016/j.cell.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 10.Devgan S, Sanal O, Doil C, Nakamura K, Nahas SA, Pettijohn K, et al. Homozygous deficiency of ubiquitin-ligase ring-finger protein RNF168 mimics the radiosensitivity syndrome of ataxia-telangiectasia. Cell Death Differ. 2011;18:1500–6. doi: 10.1038/cdd.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]