Abstract
Background & Aims
The MSH2 A636P mutation is a founder mutation in Ashkenazi Jews that causes Lynch syndrome with a prevalence of 0.4%–0.7%. Estimates of age-specific cumulative risk and lifetime risk for colorectal cancer (CRC) and endometrial cancer (EC) specific to carriers of this mutation are not available.
Methods
We studied 27 families with MSH2 A636P gene mutations identified in Israel; 13 were identified via a population-based case-control study and 14 from a clinical genetics service. Age-specific cumulative risks (penetrance) and hazard ratio (HR) estimates of CRC and EC risks were calculated and compared to the general Ashkenazi population using modified segregation analysis. An ascertainment-corrected likelihood that combined population-based and clinic-based sampling provided a powerful analysis for estimating penetrance. We analyzed 74 cases of CRC (40 in the clinic series and 34 in the population-based series), diagnosed at median ages of 50 years (men) and 49 years (women) in the combined sample.
Results
The cumulative risk of CRC at age 70 was 61.62 % (95% confidence interval [CI], 37.49%–76.45%) for men and 61.08% (95% CI, 39.39%–75.14%) for women, with overall HRs of 31.8 (19.9–51.0) and 41.8 (27.4–64.0), respectively. There were 28 cases of EC, diagnosed at median age of 53.0 years. Cumulative risk of EC was 55.64% (95% CI, 33.07%–70.58%) with overall HR of 66.7 (41.7–106.7).
Conclusions
Lifetime risks of CRC and EC in MSH2 A636P carriers are high even after adjusting for ascertainment. These estimates are valuable for patients and providers; specialized cancer screening is necessary for carriers of this mutation.
Keywords: Cancer risk, penetrance, ascertainment bias, Lynch Syndrome
Introduction
Lynch Syndrome (LS), formerly known as Hereditary Nonpolyposis Colorectal Cancer (HNPCC), is the most common hereditary colorectal cancer syndrome and accounts for 3–5% of CRC cases.1 Lynch syndrome tumors develop as a consequence of defective DNA mismatch repair caused by germline mutations in the mismatch repair (MMR) genes MLH1, MSH2, MSH6 and PMS2. Individuals with LS typically develop young-onset cancers, which usually demonstrate a phenotype of microsatellite instability. While there have been a variety of tumor types described in LS, adenocarcinomas of the colorectum and endometrium are the most common, and clinical management guidelines recommend aggressive surveillance and risk reducing surgery for these cancers.2 Previously, a rare founder mutation MSH2*1906C>G, also known as A636P was described in Ashkenazi Jews3 and was found in 8 of 1,345 individuals (0.6%) of Ashkenazi descent with colorectal cancer. A follow-up study sought to characterize the proportion of individuals of Ashkenazi heritage with very early-onset colon cancer (diagnosed at age 40 or younger) that could be attributed to MSH2*1906C>G.4 Although rare in the general population, the A636P mutation is detected in up to 7% of Ashkenazi Jewish patients with early age-of-onset colorectal cancer, and may account for up to one third of Lynch Syndrome in the Ashkenazi Jewish population.5
Prior to the discovery of MMR gene mutations, the diagnosis of LS was made on the basis of a family’s cancer history. The classic Amsterdam Criteria, originally developed for research purposes, required that a LS family contain 3 individuals with CRC in 2 generations with one case diagnosed at age less than 50 years.6 Once linkage analysis led to the positional cloning of the MMR gene mutations as the cause of Lynch Syndrome, it became possible to use molecular analysis to diagnose LS in clinical practice and offer predictive testing to at-risk relatives. As many MMR mutation carriers have family histories that do not meet Amsterdam Criteria,7 the more inclusive revised Bethesda guidelines 8 and risk prediction calculators 9–11 are now used to identify patients at risk for LS.
A number of studies have sought to quantify cancer risks in Lynch Syndrome. Lifetime risks for developing colorectal and endometrial cancer have previously been estimated at 70–80% and 40–60%, respectively, on the basis of data collected through European familial cancer registries.12–16 It has been suggested that estimates of cancer risk in LS may be artificially inflated, due to ascertainment bias resulting from overrepresentation of families with unusually striking cancer histories17 and failure to control for Amsterdam-defining tumors in calculations of cancer risks. Indeed, some studies that control for these potential biases have estimated risks for colorectal and endometrial cancer of 22–47% and 14–30%, respectively 17–19, while a more recent large study estimates risk for colorectal cancer as 66% for men, 43% for women, and 73% for either colorectal or endometrial cancer for women.20 Few papers quantify risk estimates specifically for carriers of any single founder mutation, although a study from Sweden21 found a cumulative risk of colorectal cancer of 60% in MSH6 founder mutation carriers, and an analysis of 2 founder mutations of MLH1 Spain described low-moderate penetrance for in the range of 7%–20% lifetime risk of colorectal cancer.22 In Israel, Lavie et al 23 concluded by basic descriptive analyses that 78.9% of MSH2 A636P carrier families had at least one family member diagnosed with gynecologic cancers, primarily endometrial cancers. The mean age at diagnosis was reported as 51.2 years. No such descriptive results are available for colorectal cancer.
We sought to quantify risk estimates for colorectal and endometrial adenocarcinoma in families with MSH2 A636P gene mutations associated with Lynch Syndrome. We used statistical methods that control for ascertainment bias and used information derived from observed genotype data and pedigree structure to probabilistically infer the genotypes of individuals who have not undergone genetic testing. An important analytical contribution is to combine two series of families, ascertained via different mechanisms under a combined likelihood framework.
Methods
Subjects
The National Familial Cancer Consultation Service in the Clalit Health Services (CHS) National Israeli Cancer Control Center in Haifa is responsible for counseling families with a multitude of cancer cases. The service evaluates and cares for families that are referred by treating physicians, by self-referral, or by families that are detected in population-based studies of cancer causes that are conducted at the National Cancer Control Center. Families of Ashkenazi descent with a family history that is suggestive of Lynch Syndrome were tested for the A636P mutation in the MSH2 gene. All participants of the population-based Molecular Epidemiology of Colorectal Cancer (MECC) case-control study were also studied for this mutation. Once a mutation had been diagnosed in a family, an effort was made to expand the testing to as many affected and non-affected family members as possible. DNA extraction from white blood cells was performed with the Puregene DNA isolation kit (Gentra Systems, Inc, Minneapolis, MN) according to the manufacturer’s recommendation. To genotype the samples for the hMSH2-A636P mutation, we used the 5’ nuclease TaqMan allelic discrimination assay with the ABI-Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) apparatus. Primers and probes were generated by the assay-by-design custom oligonucleotide reagent service (Applied Biosystems). All tested family members, from the MECC study or the clinical service sources, signed an informed consent form approved by the Carmel Medical Center. All cases of colorectal and endometrial cancers as reported by the proband or other family members were taken into account. An effort was made to ascertain the reported case through the study of medical records, when possible.
Statistical Analysis
We used the information on the occurrence of colorectal (CRC) and endometrial cancer (EC) in relatives of A636P mutation-positive index cases to estimate age and gender-specific incidences of CRC and EC (females) in A636P mutation carriers by maximum likelihood, using modified segregation analysis. The method was implemented in MENDEL (v3.3.5). 24, 25 Relatives were assumed to be followed from 20 years of age and to be censored at the age of death, at the age of last follow-up, or at age 70 years, whichever occurred earlier. Cancer events were measured at the age of diagnosis. We estimated the hazard function up to age 70, and we assumed that the hazard ratio stayed constant beyond the age of 70. Information on A636P mutation status in relatives was included whenever available. The segregation analysis implemented by MENDEL handles missing genotype information by including allele frequencies as parameters in the likelihood and marginalizes the observed joint likelihood of phenotype and genotype of the pedigree over the unobserved genotype matrix, summing over all possible genotype configurations for the unobserved genotype matrix of the pedigree. For individuals with missing age information the age was imputed based on the subject's relative generation with the proband as reference, deceased status (dead or alive at last follow up) and gender by the conditional mean of observed age in a given strata defined by these variables. As an example, if the proband’s unaffected father was missing age and he was reported as still alive, we imputed his age by using the average age among all fathers who were living. This led to a more conservative penetrance estimate than imputation conditional on disease status of the individual. In addition, for cases with missing age of onset, we used current age as the age of diagnosis, if current age was available. We also carried out a sensitivity analysis without imputing the age information to ensure that the age imputation did not artificially inflate estimates of penetrance and relative risk. In fact, the sensitivity analysis reveals that the penetrance estimates based on only observed age data are significantly higher. However, since a large fraction (83% ) of age data was missing, we present results with age imputation in the main text. The results of the sensitivity analysis with respect to different imputation strategies and with no imputed data are available at http://www.sph.umich.edu/bhramar/public_html/software/A636Psupplement.doc.
To correct for ascertainment in the service-based CHS series, we maximized the conditional likelihood of observing the phenotypes (CRC and/or EC) and genotype (mutations in A636P) of the entire pedigree given the phenotypic and genotypic information of the index case and phenotypic information of all CRC affected first degree relatives of the index case. This choice of conditioning was based on the belief that subjects were ascertained because they had a family history of CRC. Given the usual ascertainment and referral of probands to CHS based on diagnosis of CRC, this conditioning strategy seems reasonable. For the series ascertained through the identified case in the population-based case-control study (MECC), we implemented a less stringent ascertainment correction and we maximized the conditional likelihood of observing the phenotypes (CRC and/or EC) and genotypes (mutations in A636P) of the entire pedigree given the phenotypic and genotypic information of the index case. We present results stratified by each series first. We also consider the joint conditional likelihood of all 27 pedigrees and apply different ascertainment correction to families coming from each series to give us a maximum likelihood estimate based on the entire data.
Cancer incidence in carriers was assumed to follow a proportional hazards model, with λ(t)=λ0(t)exp[g(t)], where λ0(t) is the background incidence, which was assumed to follow the population incidence for Ashkenazi Jews from the National Israel Cancer Registry for the year 2005 (http://www.health.gov.il). Statistical tests of the assumptions of proportional hazards models were all consistent with proportionality. For CRC, the age and gender-specific relative risks in carriers as compared to the gender specific population rates are modeled through the function exp[g(t)]. For males and females we estimated the age specific log(RR) or log hazard ratio parameters for the two age intervals <50 and ≥50. The function g(t) takes the form , a piecewise constant RR in the kth age band k=1,2 and ith gender where i=Male, Female. When considering EC and either EC or CRC in females the age-specific relative risks in carriers as compared to the population rates were similarly modeled through the function exp[g(t)] . We estimated the age specific log(RR) or log hazard ratio parameters for the two age intervals <50 and ≥50, assuming that , a piecewise constant RR in the kth age band k=1,2. In all analysis, cancer incidences in non-carriers were assumed to follow the population cohort-specific rates as obtained through the Israel National Cancer Registry (2005 incidence rates).
To construct confidence intervals (CI) for the log(RR) estimates, we assumed that the maximum likelihood estimates of the parameters were asymptotically normally distributed with covariance matrix given by the inverse of the Fisher information matrix. Cumulative risk (e.g. penetrance) and 95% CI were calculated from the cumulative incidence A(t) given by where ik is the population incidence, tk is the length of the kth age interval and βk is the log(RR) in the kth age interval (and in the case of colorectal cancer this is gender specific). We only consider time intervals up to age t in this formula. The cumulative risk is given by F(t)=1-exp[-(t)] and a 95% confidence interval for F(t) is where .
Results
A total of 27 families were identified, including 14 families from the CHS clinical series and 13 from the MECC series. A total of 1029 individuals were included in the analysis: 27 probands, 180 first-degree relatives (FDRs), and 849 other more distant relatives. Genotyping results were available on 146 subjects (27 probands + 119 relatives) with 88 subjects carrying the A636P mutation (24 proband, 64 relatives). The numbers of subjects genotyped (75 in CHS and 71 in MECC) were similar between the two series. The proportion of patients with a mutation was (49/75=65% and 39/71=55%) in CHS and MECC series respectively. Additional characteristics of study families are summarized in Table 1, stratified by series as well as combined.
Table 1.
Characteristics of study population by clinic (CHS) and population-based (MECC) ascertainment
| CHS | MECC | Total | |
|---|---|---|---|
| Number of probands/ pedigrees | 14 | 13 | 27 |
| Number of FDR | 93 | 87 | 180 |
| Total number of individuals | 518 | 511 | 1029 |
| Number of individuals with Known current/last available age(%) | 91 (18.6%) | 86 (16.8%) | 177(17.2%) |
| Number of females | 255 | 261 | 516 |
| (# CRC cases [*]) | (21[17]) | (19[14]) | (40[31]) |
| (# EC cases [*] | (12[12]) | (16[13]) | (28[25]) |
| Number of males | 263 | 250 | 513 |
| (# CRC cases[*]) | (19[13]) | (15[10]) | (34[23]) |
| CRC | |||
| female | |||
| range (median) | 28 − 79 (47) | 40 − 75 (52) | 28 − 79 (49) |
| -% of cancers diagnosed before age 50 |
58.9% | 42.8% | 51.6% |
| male | |||
| range (median) | 39 − 85 (52) | 23 − 63 (43.5) | 23 − 85 (50) |
| -% of cancers diagnosed before age 50 |
15.4% | 80.0% | 43.5% |
| EC (Range (median) | |||
| -% of cancers diagnosed | 13 − 76 (49.5) | 32 − 77 (55) | 13 − 77 (53) |
| before age 50 | 50% | 15.4% | 32% |
| Number of subjects genotyped | 75 | 71 | 146 |
| (Proband + FDR+SDR) | (14+44+17) | (13+41+7) | (27+85+24) |
| Number of mutation positive | 49 | 39 | 88 |
|
subjects (Proband + FDR+SDR) |
(11+32+6) | (13+26+0) | (24+58+6) |
MECC=Molecular Epidemiology of Colorectal Cancer, CHS= Clalit Health System
Indicate the number of age of onset that are observed, the remaining are imputed.
Risk of Colorectal Cancer
Seventy four cases of CRC were identified (CHS=40, MECC=34). Median age at diagnosis was 50 years for men (range 23–85) and 49 years for women (range 28–79). Among those affected 43.5 % of males and 51.6% of females had been diagnosed with CRC before the age of 50 years.
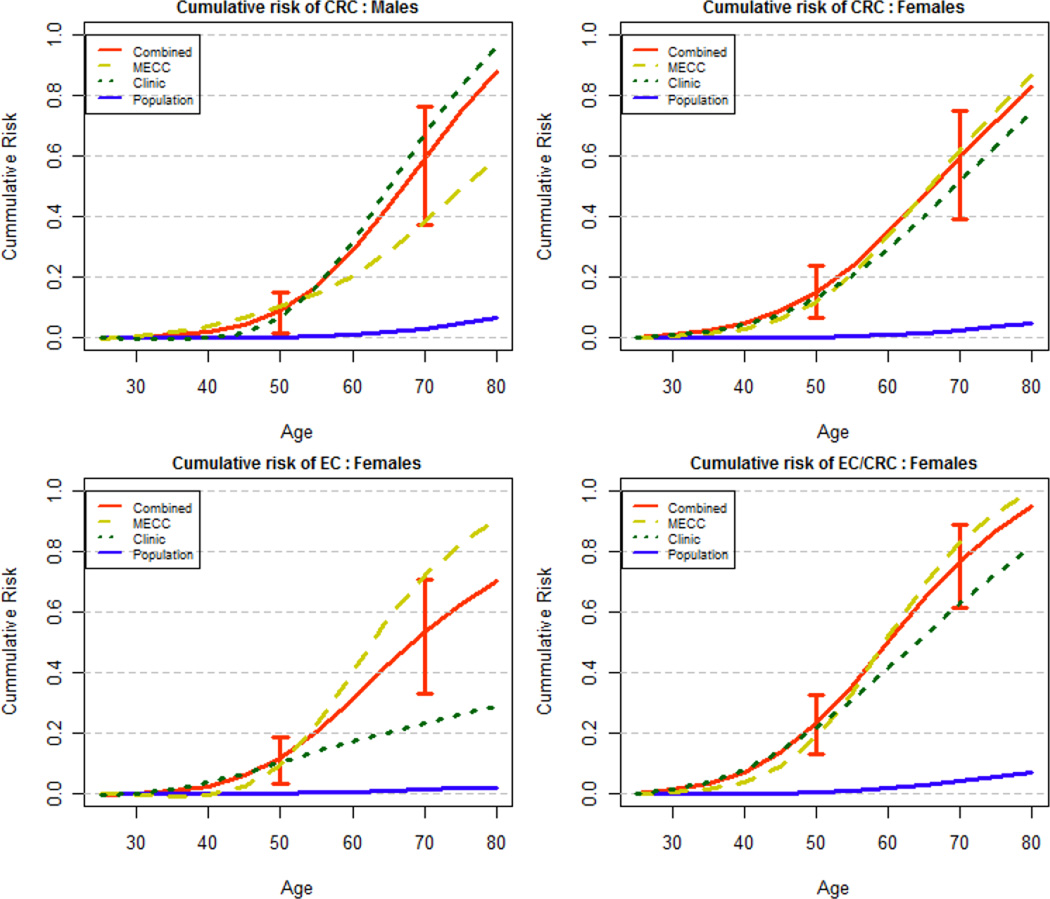
Age-specific cumulative risks of CRC by decade compared with the Ashkenazi population rates are shown in Table 2 and Figure 1. These results are based on all 27 families with different conditioning scheme applied to CHS and MECC series. For men with A636P mutations the risk of CRC significantly exceeds the risk in the general population by age 30 years and by age 50 is estimated at 8.52% (95% CI 1.46–15.21). Women who carry A636P mutations significantly exceed the population risk of CRC by 30 and by age 50 have a CRC risk of 15.54% (95% CI 6.78–23.94). Risk of CRC continues to increase and by age 70, cumulative risk for CRC in A636P gene mutation carriers is estimated at 61.62% (95% CI 37.49–76.45%) for men and 61.08% (95% CI 39.39–75.14%) for women. The cumulative lifetime risk to age 80 is estimated with less precision, but the point estimate risk of CRC for men is 86%, and for women is 82%. The overall hazard ratio (HR) for CRC is numerically lower for men at 31.8 (95% CI 19.9–51.0), compared with 41.8 (95% CI 27.4–64.0) for women (Table 3), though the difference between men and women is not statistically significant. Table 2 and Table 3 also indicate that an analyses stratified by the two series may lead to wider CI for cumulative risk and HR estimates, as there are only 13 and 14 families in each series and relatively few events, but a combined analyses integrates the two datasets and provides more plausible inference.
Table 2.
Age-specific cumulative risk of CRC and EC for Male and Female A636P mutation carriers compared with Israeli population incidence rates for Ashkenazi Jews to age 70.|
| Colorectal Cancer (Males) | Colorectal Cancer (Females) | |||||||
|---|---|---|---|---|---|---|---|---|
| Age | Cumulative Risk Population % |
Cumulative Risk A636P Carriers % Combined |
Cumulative Risk A636P Carriers % MECC |
Cumulative Risk A636P Carriers % CHS |
Cumulative Risk Population % |
Cumulative Risk A636P Carriers % Combined |
Cumulative Risk A636P Carriers % MECC |
Cumulative Risk A636P Carriers % CHS |
| 30 | 0.01 | 0.45 | 0.63 | 0.23 | 0.02 | 1.19 | 0.81 | 1.05 |
| 40 | 0.06 | 2.32 | 3.22 | 1.17 | 0.08 | 4.59 | 3.15 | 4.03 |
| 50 | 0.23 | 8.52 (1.46–15.21) |
11.66 (0–22.52) |
4.35 (0–10.27) |
0.26 | 15.54 (6.78–23.94) |
10.86 (1.15–19.92) |
13.75 (2.37–24.20) |
| 60 | 1.06 | 28.74 | 20.34 | 32.14 | 1.11 | 35.61 | 35.09 | 30.12 |
| 70 | 3.10 | 61.62 (37.49–76.45) |
38.35 (7.12–59.16) |
71.00 (37.37–86.57) |
2.66 | 61.08 (39.39–75.14) |
63.96 (24.71–82.83) |
52.71 (21.71–71.60) |
| 80 | 6.51 | 85.89 | 59.29 | 92.65 | 5.04 | 81.60 | 84.98 | 73.59 |
| Endometrial Cancer (Females) | Colorectal or Endometrial Cancer (Females) | |||||||
|---|---|---|---|---|---|---|---|---|
| Age | Cumulative Risk Population % |
Cumulative Risk A636P Carriers % Combined |
Cumulative Risk A636P Carriers % MECC |
Cumulative Risk A636P Carriers % CHS |
Cumulative Risk Population % |
Cumulative Risk A636P Carriers % Combined |
Cumulative Risk A636P Carriers % MECC |
Cumulative Risk A636P Carriers % CHS |
| 30 | 0.00 | 0.23 | 0.09 | 0.25 | 0.03 | 1.38 | 0.84 | 1.41 |
| 40 | 0.03 | 2.88 | 1.09 | 3.15 | 0.11 | 6.77 | 4.17 | 6.94 |
| 50 | 0.13 | 11.22 (3.22–18.58) |
4.37 (0–10.66) |
12.23 (1.13–22.08) |
0.39 | 23.14 (12.81–32.65) |
14.77 (3.65–24.87) |
23.65 (9.28–36.13) |
| 60 | 0.60 | 32.45 | 44.26 | 16.89 | 1.70 | 52.12 | 55.99 | 41.90 |
| 70 | 1.32 | 55.64 (33.07–70.58) |
75.72 (37.78–90.53) |
23.60 (2.19–40.33) |
3.95 | 79.01 (61.32–88.69) |
86.09 (59.66–95.21) |
63.91 (34.25–80.31) |
| 80 | 1.91 | 68.43 | 87.63 | 28.63 | 6.95 | 92.68 | 96.79 | 80.36 |
Incidence reference site: Ministry of Health (http://www.health.gov.il/seker/default.asp)
Figure 1.
Cumulative risk of CRC and EC by gender in A636P mutation carriers compared to Israeli Ashkenazi population. (males (top left), females (top right), endometrial cancer in females (bottom left) and either colorectal or endometrial cancer in females (bottom right) in MSH2 Carriers and the population. 95% Confidence intervals at reported at age 50 and at age 70.
Table 3.
Age specific and overall hazard ratio for Colorectal and Endometrial Cancers for A636P mutation carriers.
| Colorectal Cancer (Males) | Colorectal Cancer (Females) | |||||
|---|---|---|---|---|---|---|
| Age |
Hazard Ratio A636P Carriers (95% CI) Combined |
Hazard Ratio A636P Carriers (95% CI) MECC |
Hazard Ratio A636P Carriers (95% CI) CHS |
Hazard RatioA636P Carriers (95% CI) Combined |
Hazard Ratio A636P Carriers (95% CI) MECC |
Hazard Ratio A636P Carriers (95% CI) CHS |
| 20–49 | 39.8 (17.3–91.9) |
55.4 (19.6–156.7) |
19.9 (4.8–81.9) |
65.9 (36.5–119.0) |
44.8 (18.2–110.3) |
57.7 (24.9–133.7) |
| 50–79 | 29.7 (17.1–51.6) |
12.3 (4.2–36.0) |
40.8 (21.5–77.4) |
31.8 (18.3–55.2) |
37.2 (16.7–82.9) |
24.7 (11.0–55.2) |
| Overall HR |
31.8 (19.9–51.0) |
20.3 (9.4–44.1) |
34.8 (19.3–62.8) |
41.8 (27.4–64.0) |
40.2 (21.6–74.7) |
34.2 (18.5–63.2) |
| Endometrial Cancer (Females) | Colorectal and Endometrial Cancer (Females) | |||||
|---|---|---|---|---|---|---|
| Age |
Hazard Ratio A636P Carriers (95% CI) Combined |
Hazard Ratio A636P Carriers (95% CI) MECC |
Hazard Ratio A636P Carriers (95% CI) CHS |
Hazard Ratio A636P Carriers (95% CI) Combined |
Hazard Ratio A636P Carriers (95% CI) MECC |
Hazard Ratio A636P Carriers (95% CI) CHS |
| 20–49 | 88.8 (43.0–183.5) |
33.3 (7.3–152.8) |
97.3 (39.1–242.3) |
67.4 (41.5–109.5) |
40.9 (19.0–88.4) |
69.2 (36.4–131.5) |
| 50–79 | 58.1 (32.9–102.7) |
114.8 (58.0–227.4) |
11.6 (2.5–53.9) |
35.8 (22.7–56.4) |
50.0 (28.0–89.2) |
20.7 (9.7–43.9) |
| Overall HR |
66.7 (41.7–106.7) |
83.3 (45.4–153.1) |
33.0 (14.7–74.1) |
46.0 (32.4–65.4) |
46.4 (28.8–74.8) |
35.6 (20.9–60.9) |
Risk of Endometrial Cancer and Cumulative Cancer Risk in Women
Twenty-eight cases of endometrial cancer were identified (CHS=12, MECC=16). Median age at diagnosis was 53 years (range 13–77 years) and 32% of cases were diagnosed before age 50 years. Age-specific cumulative risks of endometrial cancer by decade compared with the population incidence rates are shown in Table 2 and Figure 1. The risk of EC in A636P gene mutation carriers is significantly higher than the general population by age 30 and by age 50 is 11.22% (95% CI 3.22–18.58%). By age 70 the risk of EC is estimated at 55.64% (95% CI 33.07–70.58%). Cumulative lifetime risk to age 80, again estimated with slightly less precision, is 68%. The overall HR for endometrial cancer was 66.7 (95% CI 41.7–106.7) (Table 3).
In addition to the risk estimates derived from modified segregation analysis, we also calculated risks using an alternative, but less powerful statistical method called kin-cohort analysis26–28. Rates of cancer in the relatives of mutation carriers were compared to rates of cancer in the relatives of non-carriers, all within the population-based MECC study. The results of this kin-cohort analysis showed high risk of any Lynch-associated cancer (52.6% at age 70 with 95% confidence interval, 21.5% - 80.1%). However, our estimates for colorectal+endometrial cancer by using modified segregation analysis in females at age 70 in Table 2 is 79.01% (95% CI 61.32%–88.69%) are considerably higher with narrower CI than the estimates provided by the kin-cohort approach. The kin-cohort method ignores information about genetic testing status of the relatives themselves, and therefore it is not surprising that this cumulative risk estimate is lower than the estimates derived from modified segregation analysis with adjustment for ascertainment.
Discussion
Our estimates of cancer risk using data from families with Lynch Syndrome ascertained through one population-based and one clinically ascertained series in Israel reveal a cumulative risk of colorectal cancer in male and female A636P gene mutation carriers of approximately 60% by age 70. We calculated an overall hazard ratio for CRC of 31.8 for men and 41.8 for women. For women, cumulative risk of endometrial cancer approaches 55% by age 70 with a hazard ratio of 66.7. Our data demonstrate that the cumulative risk for colon and endometrial cancer continue to increase with age, with the most dramatic elevation in age-specific hazard ratios most often seen in individuals age 20 to 49 years.
Our risk estimates for CRC and EC in A636P mutation carriers are the first rigorous estimates specific to this relatively frequent single founder mutation in Ashkenazi population. Using “ascertainment-corrected maximum likelihood estimation”, Quehenberger et. al. analyzed cancer histories of 84 families with mutations in MLH1 or MSH2 from the Dutch HNPCC registry and found a cumulative risk of CRC by age 70 of 26% for men and 22% for women, which led them to conclude that risk of CRC in LS is considerably lower than suggested by the original reports.17 In examining cancer histories of 17 families with different MMR mutations, Jenkins et al. calculated cumulative risks for CRC of 45% for men and 38% for women.18 In addition to concluding that risks of CRC were lower than expected, they reported that while CRC risk increases to age 50, the incidence decreases to general population levels at older ages 18. However, there are no such penetrance estimates specific to the MSH2 A636P mutation, and this is the first study to directly address mutation-specific risks in Lynch Syndrome using modified segregation analysis. From other gene penetrance studies it is clear that specific mutations at specific locations can have different clinical implications as has been shown in breast cancer29 and familial adenomatous polyposis.30, 31 Our data suggest, that whenever possible in a founder population, risk estimates should be calculated for particular mutations to enhance clinical decision making. This approach would also be helpful for newly identified, less common founder mutations as more carriers are identified over time. For example, two newly described Ashkenazi Jewish founder mutations in MSH6 are too rare to calculate mutation specific risks, although the relative risks ranging from 10- to 20-fold for these two new specific mutations suggest that mutational heterogeneity by gene and mutation is relevant and important to quantify. 32
Our study, which used similar statistical methodology to control for ascertainment bias, yielded strikingly different risk estimates than those of Quehenberger and Jenkins. Our data demonstrate that the risk of CRC and EC for A636P gene mutation carriers is significantly higher than the population risk by age 40 and continues to increase with age. This is more in line with the recent study findings from Stoffel et al.20 Our results provide evidence in support of current cancer screening recommendations for LS which include CRC screening every 1–2 years in all A636P gene mutation carriers beginning at age 20–25 and annual uterine cancer screening for women beginning at age 30–35 2 with the options of continued surveillance over the patients lifetime or prophylactic surgery. In addition, our results provide new and important data that can be easily incorporated into computational software routinely used to estimate the lifetime risk of cancer among gene carriers, such as MMRpro.9
Our study has several strengths. The use of segregation analysis affords many advantages to estimating cancer risk, as it calculates the probability of being a mutation carrier for all relatives whose mutation status is unknown. By conditioning the analysis on the available phenotypic information from the pedigree, we were able to minimize ascertainment bias by excluding diagnoses of CRC in probands and first-degree relatives in the CHS series and diagnoses of the index case in the MECC series. Finally, our findings confirm the substantially elevated risk estimates for CRC and EC presented in the original descriptions of Lynch Syndrome. We compared the population-based (MECC) and clinically-based (CHS) series to assess whether carriers of MSH2 A636P gene mutations evaluated through clinical genetics services may be phenotypically different from those that do not seek genetic evaluation. The stratified and the combined analyses and ascertainments schemes are both helpful in that sense. Indeed, it is worthwhile to note that the risk estimates derived from our population-based study and our clinically ascertained families are similar in most cases. This could possibly be attributed to the ease and wide application of genetic testing for the MSH2-A636P founder mutation in our clinical series of families suspicious of Lynch syndrome, even for those families not fulfilling the Amsterdam criteria. Careful consideration of the point estimates of the absolute risks and cumulative hazard ratios shows that the population-based risks are actually higher than the clinically-based estimates. This may reflect over-adjustment for ascertainment in the clinical series, since the population-based (MECC) series was conditioned on the genotype and the phenotype of the proband alone, whereas the clinical series (CHS) was conditioned on the genotype and the phenotype of the proband, as well as the phenotype of all first degree relatives affected with colorectal cancer. Despite the possibility of attenuation of risks in the clinical series, the risks from both series are very high and clinically meaningful.
Our study has certain limitations. Only half of the families with Lynch Syndrome were population-based. Unconfirmed cancer diagnoses were another limitation. The cancer histories included in each family’s pedigree were obtained mostly through proband reports and only a minority of cancer diagnoses had corresponding medical record confirmation. Although a number of studies have demonstrated that patient reports of family cancer history are largely accurate,33, 34 reports of gynecologic cancers may be less accurate 35 and it is likely that some cancers may have been misclassified. Nevertheless, non-differential misclassification would be expected to attenuate our findings, and would be unlikely to substantially alter our conclusions. Missing information was a limitation, especially since phenotypic information was often truncated in older generations who perished in the holocaust. However, we imputed subject ages in a conservative manner and checked these in sensitivity analyses which demonstrated no significant inflation of risk estimates. When we considered both colorectal cancer and endometrial cancer as the endpoint for females, we noted that 10 women had both types of cancer among 40 women with colorectal cancer and 28 with endometrial cancer. This type of data can be better modeled within the context of a competing risks framework with cause-specific hazard functions, or via bivariate failure time models, but this approach was not feasible due to small sample size. Without directly accounting for these competing risks, Figure 1 showing the cumulative risk of EC/CRC for females may be slightly inflated. It is also worthwhile to note that mutation carriers in the clinical series were referred for enhanced surveillance with colonoscopy and endometrial cancer screening, consistent with consensus practice guidelines. Therefore it is possible that screen-detected cancers might increase the cumulative lifetime risk (or decrease the average age at diagnosis) within the clinical series. However, the comparison of population-based and clinical series analyses suggests that cumulative lifetime risks are quite comparable through both ascertainment mechanisms when appropriate statistical analyses are performed.
As genetic testing becomes more broadly available for common conditions like Lynch Syndrome,36 it is clear that mutation-specific penetrance analyses will be possible for many different conditions. Ascertainment-adjusted statistical analyses will enhance the precision of risk estimation, and should facilitate better, personalized risk assessment and management for patients and families with inherited susceptibility to cancer.
Acknowledgement
The authors wish to thank Antonis Antoniou for his technical advice in implementing the modified segregation analysis and penetrance models.
Grant Support: This work was supported in part by the National Cancer Institute RO1 CA81488 (Dr. Gruber), the University of Michigan's Cancer Center Support Grant (5 P30 CA46592), R03 CA130045 (Dr. Mukherjee).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors report no disclosures or conflicts.
Author Contributions:
Study Concept and Design: Bhramar Mukherjee, Gad Rennert, Stephen B. Gruber. Acquisition of Data: Gad Rennert, Hedy S. Rennert, Sara Dishon, Flavio Lejbkowicz, Stacey Shiovitz. Drafting of Manuscript: Bhramar Mukherjee, Jaeil Ahn, Gad Rennert, Stephen B.Gruber. Revision of Manuscript for Intellectual Content: All Authors. Statistical Analysis: Bhramar Mukherjee, Jaeil Ahn, Victor Moreno, Stephen B. Gruber
References
- 1.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 2.Lindor NM, Petersen GM, Hadley DW, Kinney AY, Miesfeldt S, Lu KH, Lynch P, Burke W, Press N. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. Jama. 2006;296:1507–1517. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- 3.Foulkes WD, Thiffault I, Gruber SB, Horwitz M, Hamel N, Lee C, Shia J, Markowitz A, Figer A, Friedman E, Farber D, Greenwood CM, Bonner JD, Nafa K, Walsh T, Marcus V, Tomsho L, Gebert J, Macrae FA, Gaff CL, Paillerets BB, Gregersen PK, Weitzel JN, Gordon PH, MacNamara E, King MC, Hampel H, De La Chapelle A, Boyd J, Offit K, Rennert G, Chong G, Ellis NA. The founder mutation MSH2*1906G-->C is an important cause of hereditary nonpolyposis colorectal cancer in the Ashkenazi Jewish population. Am J Hum Genet. 2002;71:1395–1412. doi: 10.1086/345075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guillem JG, Rapaport BS, Kirchhoff T, Kolachana P, Nafa K, Glogowski E, Finch R, Huang H, Foulkes WD, Markowitz A, Ellis NA, Offit K. A636P is associated with earlyonset colon cancer in Ashkenazi Jews. J Am Coll Surg. 2003;196:222–225. doi: 10.1016/S1072-7515(02)01808-2. [DOI] [PubMed] [Google Scholar]
- 5.Guillem JG, Moore HG, Palmer C, Glogowski E, Finch R, Nafa K, Markowitz AJ, Offit K, Ellis NA. A636P testing in Ashkenazi Jews. Fam Cancer. 2004;3:223–227. doi: 10.1007/s10689-004-0899-z. [DOI] [PubMed] [Google Scholar]
- 6.Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34:424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 7.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J, Panescu J, Fix D, Lockman J, Comeras I, de la Chapelle A. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 8.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, Hamilton SR, Hiatt RA, Jass J, Lindblom A, Lynch HT, Peltomaki P, Ramsey SD, Rodriguez-Bigas MA, Vasen HF, Hawk ET, Barrett JC, Freedman AN, Srivastava S. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Wang W, Lee S, Nafa K, Lee J, Romans K, Watson P, Gruber SB, Euhus D, Kinzler KW, Jass J, Gallinger S, Lindor NM, Casey G, Ellis N, Giardiello FM, Offit K, Parmigiani G. Prediction of germline mutations and cancer risk in the Lynch syndrome. Jama. 2006;296:1479–1487. doi: 10.1001/jama.296.12.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balmana J, Stockwell DH, Steyerberg EW, Stoffel EM, Deffenbaugh AM, Reid JE, Ward B, Scholl T, Hendrickson B, Tazelaar J, Burbidge LA, Syngal S. Prediction of MLH1 and MSH2 mutations in Lynch syndrome. JAMA. 2006;296:1469–1478. doi: 10.1001/jama.296.12.1469. [DOI] [PubMed] [Google Scholar]
- 11.Kastrinos F, Steyerberg EW, Mercado R, Balmana J, Holter S, Gallinger S, Siegmund KD, Church JM, Jenkins MA, Lindor NM, Thibodeau SN, Burbidge LA, Wenstrup RJ, Syngal S. Gastroenterology. The PREMM(1,2,6) Model Predicts Risk of MLH1, MSH2, and MSH6 Germline Mutations Based on Cancer History. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aarnio M, Mecklin JP, Aaltonen LA, Nystrom-Lahti M, Jarvinen HJ. Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. Int J Cancer. 1995;64:430–433. doi: 10.1002/ijc.2910640613. [DOI] [PubMed] [Google Scholar]
- 13.Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, Peltomaki P, Mecklin JP, Jarvinen HJ. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214–218. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Dunlop MG, Farrington SM, Carothers AD, Wyllie AH, Sharp L, Burn J, Liu B, Kinzler KW, Vogelstein B. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105–110. doi: 10.1093/hmg/6.1.105. [DOI] [PubMed] [Google Scholar]
- 15.Watson P, Lynch HT. Cancer risk in mismatch repair gene mutation carriers. Fam Cancer. 2001;1:57–60. doi: 10.1023/a:1011590617833. [DOI] [PubMed] [Google Scholar]
- 16.Vasen HF, Wijnen JT, Menko FH, Kleibeuker JH, Taal BG, Griffioen G, Nagengast FM, Meijers-Heijboer EH, Bertario L, Varesco L, Bisgaard ML, Mohr J, Fodde R, Khan PM. Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology. 1996;110:1020–1027. doi: 10.1053/gast.1996.v110.pm8612988. [DOI] [PubMed] [Google Scholar]
- 17.Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet. 2005;42:491–496. doi: 10.1136/jmg.2004.024299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins MA, Baglietto L, Dowty JG, Van Vliet CM, Smith L, Mead LJ, Macrae FA, St John DJ, Jass JR, Giles GG, Hopper JL, Southey MC. Cancer risks for mismatch repair gene mutation carriers: a population-based early onset case-family study. Clin Gastroenterol Hepatol. 2006;4:489–498. doi: 10.1016/j.cgh.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Alarcon F, Lasset C, Carayol J, Bonadona V, Perdry H, Desseigne F, Wang Q, Bonaiti-Pellie C. Estimating cancer risk in HNPCC by the GRL method. Eur J Hum Genet. 2007;15:831–836. doi: 10.1038/sj.ejhg.5201843. [DOI] [PubMed] [Google Scholar]
- 20.Stoffel E, Mukherjee B, Raymond VM, Tayob N, Kastrinos F, Sparr J, Wang F, Bandipalliam P, Syngal S, Gruber SB. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621–1627. doi: 10.1053/j.gastro.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cederquist K, Emanuelsson M, Wiklund F, Golovleva I, Palmqvist R, Gronberg H. Two Swedish founder MSH6 mutations, one nonsense and one missense, conferring high cumulative risk of Lynch syndrome. Clin Genet. 2005;68:533–541. doi: 10.1111/j.1399-0004.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- 22.Borras E, Pineda M, Blanco I, Jewett EM, Wang F, Teule A, Caldes T, Urioste M, Martinez-Bouzas C, Brunet J, Balmana J, Torres A, Ramon y, Cajal T, Sanz J, Perez-Cabornero L, Castellvi-Bel S, Alonso A, Lanas A, Gonzalez S, Moreno V, Gruber SB, Rosenberg NA, Mukherjee B, Lazaro C, Capella G. MLH1 founder mutations with moderate penetrance in Spanish Lynch syndrome families. Cancer Res. 2010;70:7379–7391. doi: 10.1158/0008-5472.CAN-10-0570. [DOI] [PubMed] [Google Scholar]
- 23.Lavie O, Gruber SB, Lejbkowicz F, Dishon S, Rennert G. Gynecologic malignancies in Ashkenazi families with the MSH2 A636P founder mutation. Am J Obstet Gynecol. 2008;199(148):e1–e3. doi: 10.1016/j.ajog.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Lange K, Weeks D, Boehnke M. Programs for Pedigree Analysis: MENDEL, FISHER, and dGENE. Genet Epidemiol. 1988;5:471–472. doi: 10.1002/gepi.1370050611. [DOI] [PubMed] [Google Scholar]
- 25.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjakoski K, Kallioniemi OP, Thompson D, Evans C, Peto J, Lalloo F, Evans DG, Easton DF. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wacholder S, Hartge P, Struewing JP, Pee D, McAdams M, Brody L, Tucker M. The kincohort study for estimating penetrance. Am.J.Epidemiol. 1998;148:623–630. doi: 10.1093/aje/148.7.623. [DOI] [PubMed] [Google Scholar]
- 27.Begg CB, Orlow I, Hummer AJ, Armstrong BK, Kricker A, Marrett LD, Millikan RC, Gruber SB, Anton-Culver H, Zanetti R, Gallagher RP, Dwyer T, Rebbeck TR, Mitra N, Busam K, From L, Berwick M. Lifetime risk of melanoma in CDKN2A mutation carriers in a population-based sample. J Natl Cancer Inst. 2005;97:1507–1515. doi: 10.1093/jnci/dji312. [DOI] [PubMed] [Google Scholar]
- 28.Chatterjee N, Wacholder S. A marginal likelihood approach for estimating penetrance from kin-cohort designs. Biometrics. 2001;57:245–252. doi: 10.1111/j.0006-341x.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- 29.Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Fan I, Tang J, Li S, Zhang S, Shaw PA, Narod SA. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694–1706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 30.Giardiello FM, Petersen GM, Piantadosi S, Gruber SB, Traboulsi EI, Offerhaus GJ, Muro K, Krush AJ, Booker SV, Luce MC, Laken SJ, Kinzler KW, Vogelstein B, Hamilton SR. APC gene mutations and extraintestinal phenotype of familial adenomatous polyposis. Gut. 1997;40:521–525. doi: 10.1136/gut.40.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giardiello FM, Brensinger JD, Luce MC, Petersen GM, Cayouette MC, Krush AJ, Bacon JA, Booker SV, Bufill JA, Hamilton SR. Phenotypic expression of disease in families that have mutations in the 5' region of the adenomatous polyposis coli gene. Annals of Internal Medicine. 1997;126:514–519. doi: 10.7326/0003-4819-126-7-199704010-00003. [DOI] [PubMed] [Google Scholar]
- 32.Raskin L, Schwenter F, Freytsis M, Tischkowitz M, Wong N, Chong G, Narod S, Levine D, Bogomolniy F, Aronson M, Thibodeau S, Hunt K, Rennert G, Gallinger S, Gruber S, Foulkes W. Characterization of two Ashkenazi Jewish founder mutations in MSH6 gene causing Lynch syndrome. Clin Genet. 2010 doi: 10.1111/j.1399-0004.2010.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murff HJ, Spigel DR, Syngal S. Does this patient have a family history of cancer? An evidence-based analysis of the accuracy of family cancer history. Jama. 2004;292:1480–1489. doi: 10.1001/jama.292.12.1480. [DOI] [PubMed] [Google Scholar]
- 34.Douglas FS, O'Dair LC, Robinson M, Evans DG, Lynch SA. The accuracy of diagnoses as reported in families with cancer: a retrospective study. J Med Genet. 1999;36:309–312. [PMC free article] [PubMed] [Google Scholar]
- 35.Sijmons RH, Boonstra AE, Reefhuis J, Hordijk-Hos JM, de Walle HE, Oosterwijk JC, Cornel MC. Accuracy of family history of cancer: clinical genetic implications. Eur J Hum Genet. 2000;8:181–186. doi: 10.1038/sj.ejhg.5200441. [DOI] [PubMed] [Google Scholar]
- 36.Dinh TA, Rosner BI, Atwood JC, Boland CR, Syngal S, Vasen HF, Gruber SB, Burt RW. Health Benefits and Cost-Effectiveness of Primary Genetic Screening for Lynch Syndrome in the General Population. Cancer Prev Res (Phila) 2010 doi: 10.1158/1940-6207.CAPR-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]