Abstract
Glomerular podocytes are pivotal in maintaining glomerular filtration barrier function. As severe podocyte injury results in proteinuria in patients with diabetic nephropathy, determining the pathogenesis of podocyte injury may contribute to the development of new treatments. We recently showed that autophagy is involved in the pathogenesis of diabetes-related podocyte injury. Insufficient podocyte autophagy and podocyte loss are observed in diabetic patients with massive proteinuria. Podocyte loss and massive proteinuria occur in high-fat diet-induced diabetic mice with podocyte-specific autophagy deficiency, with podocytes of these mice and of diabetic rats having huge damaged lysosomes. Sera from diabetic patients and from rodents with massive proteinuria cause autophagy insufficiency, resulting in lysosome dysfunction and apoptosis of cultured podocytes. These findings suggest the importance of autophagy in maintaining lysosome homeostasis in podocytes under diabetic conditions. Impaired autophagy may be involved in the pathogenesis of podocyte loss, leading to massive proteinuria in diabetic nephropathy.
Keywords: autophagy, diabetic nephropathy, lysosome, MTORC1, podocyte injury, proteinuria
Diabetic nephropathy is a leading cause of end-stage renal disease and is becoming a serious health problem worldwide. This condition is characterized initially by the appearance of microalbuminuria, followed by progression to overt proteinuria and a resultant decline in renal function over several years to decades. Recent clinical studies have shown that microalbuminuria and a degree of overt proteinuria can be halted and reversed by strict control of glycemia and blood pressure. However, some diabetic patients still develop massive and treatment-resistant proteinuria, resulting in a rapid decline of renal function. Thus, a better understanding of the pathogenesis of this refractory state and the "point of no return" in diabetic nephropathy may further improve renal and health outcomes in patients with diabetes.
Podocytes are a critical part of glomerular filtration barrier function, with injury to podocytes being a cause of massive proteinuria. Podocytes are terminally differentiated cells and generally do not replicate. Therefore, the intracellular degradation system may be important in maintaining podocyte homeostasis. However, the role of the autophagy-lysosome system in podocyte dysfunction under diabetic conditions remains unclear. We recently showed that impairment of the autophagy-lysosome system is associated with the pathogenesis of massive proteinuria due to diabetic nephropathy.
To determine how autophagy is activated in diabetic podocytes, we first investigated the relationships among levels of proteinuria, podocyte damage and autophagy activity in podocytes from human renal biopsy samples. Podocyte damage, defined as mislocalization of NPHS2/podocin, a key protein in the slit diaphragm of podocytes, is observed in patients with massive proteinuria, regardless of the underlying disease. A decrease in NPHS2-positive areas, indicating severe podocyte injury, and autophagy insufficiency, defined as the accumulation of the protein SQSTM1/p62, are observed in the glomeruli of diabetic patients with massive proteinuria. These histological results showed that insufficient podocyte autophagy is associated with severe podocyte injury and massive proteinuria in diabetic patients.
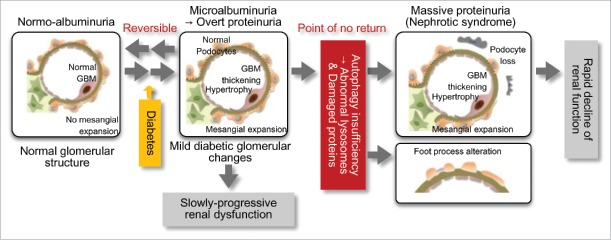
To assess this causal association, we investigated the effects of podocyte autophagy deficiency on minimal proteinuria induced by a high-fat diet (HFD). Over 32 wk of feeding with a HFD, control Atg5-floxed (Atg5f/f) and Nphs2.Cre-atg5-floxed (podo-ATG5−/−) mice showed similar development of obese diabetes. HFD-fed Atg5f/f mice develop minimal albuminuria, and podo-ATG5−/− mice fed a standard diet do not show increased urinary albumin excretion. However, HFD-fed podo-ATG5−/− mice develop massive albuminuria with severe podocyte injury, suggesting that autophagy insufficiency combined with diabetic conditions causes podocyte damage. Thus, autophagy activity in podocytes may be crucial in determining the progression of proteinuria to massive proteinuria, a "point of no return," in diabetic nephropathy (Fig. 1).
Figure 1.
Autophagy insufficiency is associated with the pathogenesis of podocyte injury and massive proteinuria in diabetic nephropathy. Diabetes alone results in the development of mild glomerular lesions, such as glomerular hypertrophy, basement membrane (GBM) thickening, mild mesangial expansion and minimal proteinuria, leading to slowly-progressive renal dysfunction. However, the combination of insufficient podocyte autophagy and diabetic conditions results in podocyte loss, foot process alterations and lysosome dysfunction. This results in massive refractory proteinuria and a rapid decline of renal function. Autophagy insufficiency-related podocyte loss may be involved in the "point of no return" in diabetic nephropathy.
To evaluate the mechanism by which autophagy insufficiency induces podocyte injury, we assessed morphological changes in podocytes. Electron microscopy analysis showed many vacuoles and lysosome-specific protein-positive structures in the podocytes of HFD-fed podo-ATG5−/− mice and diabetic rats with massive proteinuria. These results suggested that lysosomes are important targets of podocyte autophagy, and that maintenance of lysosome homeostasis by removing damaged lysosomes may be a crucial task of podocyte autophagy under diabetic conditions.
Several previous studies found that serum factors alter intracellular signaling pathways and autophagy activity under several physiological and pathological conditions. To investigate whether diabetic serum itself may alter autophagy activity in podocytes, we assessed sera from rodents with and without diabetes and with and without massive proteinuria. Although sera from nondiabetic rodents and diabetic rodents with minimal proteinuria have little effect on autophagy activity, sera from diabetic rodents and from diabetic patients with massive proteinuria induce autophagy insufficiency and apoptosis in cultured podocytes. These results suggested that several of the serum factors associated with massive diabetic proteinuria may inhibit autophagy and induce apoptosis in podocytes, and that autophagy insufficiency is particularly associated with the progression to massive proteinuria in patients with diabetic nephropathy (Fig. 1). Furthermore, bioassays, involving serum stimulation of cultured cells, may be useful in assessing in vivo autophagy activity in human subjects and experimental animals.
The mechanism underlying diabetes-related impairment of podocyte autophagy is still unclear. The mechanistic target of rapamycin complex 1 (MTORC1) is a nutrient-sensing signaling complex that inhibits autophagy. MTORC1 activity has been reported to be enhanced in podocytes of individuals with advanced diabetic nephropathy, suggesting that factors in diabetic sera may activate MTORC1, suppressing podocyte autophagy. Further studies are required to identify these serum factors and the intracellular molecular pathways regulating podocyte autophagy under diabetes.
Clinically, there are few effective treatments for diabetic patients with massive proteinuria. New therapeutic agents are needed to halt the stage progression of proteinuria, improving renal outcomes in patients with refractory diabetic nephropathy. Our findings suggest that deficient podocyte autophagy may be involved in the "point of no return" in diabetic nephropathy, and that autophagy activation may be a therapeutic target for diabetic patients with massive proteinuria and resultant rapid decline of renal function.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by Grants-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science (No. 25713033 to S. K., No. 26293217 to H. M, No. 15K19511 to M.Y.).