Abstract
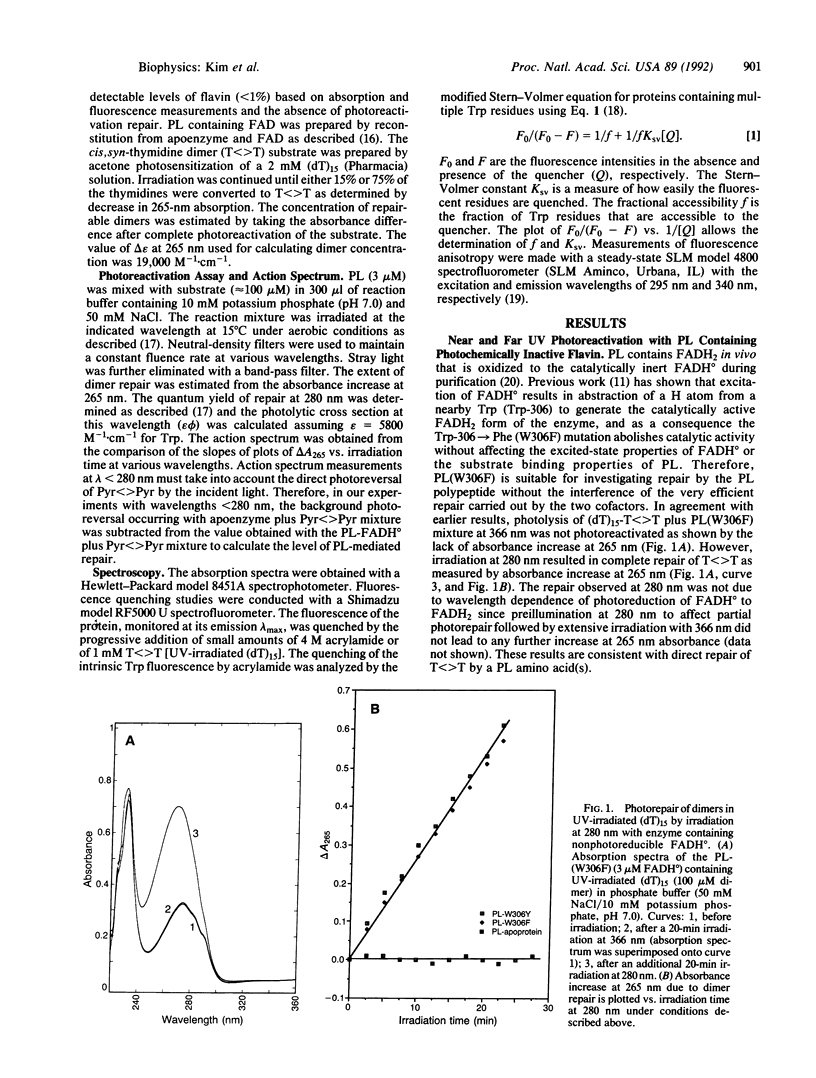
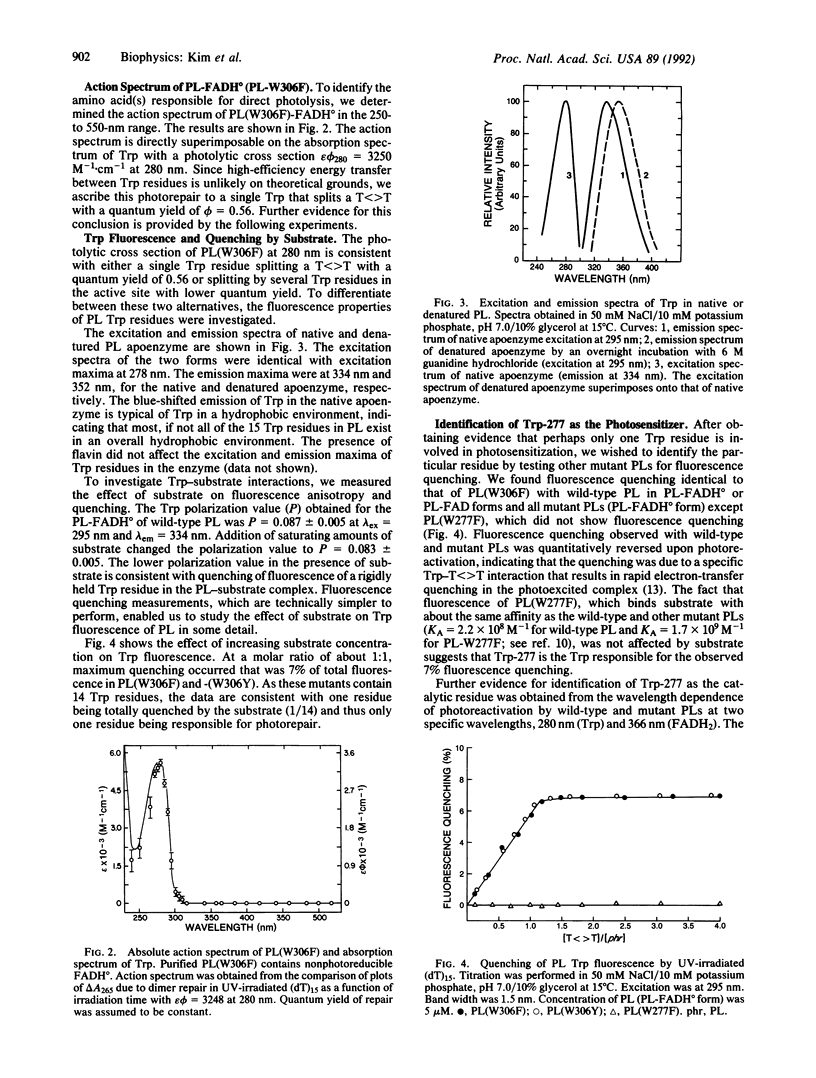
Photolyases repair pyrimidine dimers in DNA by converting the light energy of 300- to 500-nm photons into chemical energy. Enzymes from various organisms contain two chromophore cofactors (FADH2 and either methenyltetrahydrofolate or 8-hydroxy-5-deazaflavin) that absorb the low-energy photons and initiate splitting of the cyclobutane ring by a radical mechanism. Here, we show that, in addition to these two chromophores, in the far UV range, direct excitation of one specific tryptophan residue (out of 15 total) in the polypeptide chain of Escherichia coli photolyase leads to splitting of the cyclobutane ring with high quantum yield (phi = 0.56), independent of the other chromophores. The specific tryptophan residue responsible for photosensitized repair was identified as Trp-277 by site-specific mutagenesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock G. T., Barry B. A., Debus R. J., Hoganson C. W., Atamian M., McIntosh L., Sithole I., Yocum C. F. Water oxidation in photosystem II: from radical chemistry to multielectron chemistry. Biochemistry. 1989 Dec 12;28(25):9557–9565. doi: 10.1021/bi00451a001. [DOI] [PubMed] [Google Scholar]
- Baer M., Sancar G. B. Photolyases from Saccharomyces cerevisiae and Escherichia coli recognize common binding determinants in DNA containing pyrimidine dimers. Mol Cell Biol. 1989 Nov;9(11):4777–4788. doi: 10.1128/mcb.9.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow D. A., Lentz B. R. The use of isochronal reference standards in phase and modulation fluorescence lifetime measurements. J Biochem Biophys Methods. 1983 May;7(3):217–234. doi: 10.1016/0165-022x(83)90031-3. [DOI] [PubMed] [Google Scholar]
- Eker A. P., Kooiman P., Hessels J. K., Yasui A. DNA photoreactivating enzyme from the cyanobacterium Anacystis nidulans. J Biol Chem. 1990 May 15;265(14):8009–8015. [PubMed] [Google Scholar]
- Hamm-Alvarez S., Sancar A., Rajagopalan K. V. Role of enzyme-bound 5,10-methenyltetrahydropteroylpolyglutamate in catalysis by Escherichia coli DNA photolyase. J Biol Chem. 1989 Jun 5;264(16):9649–9656. [PubMed] [Google Scholar]
- Heelis P. F., Okamura T., Sancar A. Excited-state properties of Escherichia coli DNA photolyase in the picosecond to millisecond time scale. Biochemistry. 1990 Jun 19;29(24):5694–5698. doi: 10.1021/bi00476a008. [DOI] [PubMed] [Google Scholar]
- Husain I., Sancar G. B., Holbrook S. R., Sancar A. Mechanism of damage recognition by Escherichia coli DNA photolyase. J Biol Chem. 1987 Sep 25;262(27):13188–13197. [PubMed] [Google Scholar]
- Johnson J. L., Hamm-Alvarez S., Payne G., Sancar G. B., Rajagopalan K. V., Sancar A. Identification of the second chromophore of Escherichia coli and yeast DNA photolyases as 5,10-methenyltetrahydrofolate. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2046–2050. doi: 10.1073/pnas.85.7.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorns M. S., Sancar G. B., Sancar A. Identification of a neutral flavin radical and characterization of a second chromophore in Escherichia coli DNA photolyase. Biochemistry. 1984 Jun 5;23(12):2673–2679. doi: 10.1021/bi00307a021. [DOI] [PubMed] [Google Scholar]
- Jorns M. S., Wang B. Y., Jordan S. P., Chanderkar L. P. Chromophore function and interaction in Escherichia coli DNA photolyase: reconstitution of the apoenzyme with pterin and/or flavin derivatives. Biochemistry. 1990 Jan 16;29(2):552–561. doi: 10.1021/bi00454a032. [DOI] [PubMed] [Google Scholar]
- Kiener A., Husain I., Sancar A., Walsh C. Purification and properties of Methanobacterium thermoautotrophicum DNA photolyase. J Biol Chem. 1989 Aug 15;264(23):13880–13887. [PubMed] [Google Scholar]
- Kim S. T., Hartman R. F., Rose S. D. Solvent dependence of pyrimidine dimer splitting in a covalently linked dimer-indole system. Photochem Photobiol. 1990 Oct;52(4):789–794. doi: 10.1111/j.1751-1097.1990.tb08683.x. [DOI] [PubMed] [Google Scholar]
- Kim S. T., Heelis P. F., Okamura T., Hirata Y., Mataga N., Sancar A. Determination of rates and yields of interchromophore (folate----flavin) energy transfer and intermolecular (flavin----DNA) electron transfer in Escherichia coli photolyase by time-resolved fluorescence and absorption spectroscopy. Biochemistry. 1991 Nov 26;30(47):11262–11270. doi: 10.1021/bi00111a011. [DOI] [PubMed] [Google Scholar]
- Kim S. T., Sancar A. Effect of base, pentose, and phosphodiester backbone structures on binding and repair of pyrimidine dimers by Escherichia coli DNA photolyase. Biochemistry. 1991 Sep 3;30(35):8623–8630. doi: 10.1021/bi00099a019. [DOI] [PubMed] [Google Scholar]
- Li Y. F., Heelis P. F., Sancar A. Active site of DNA photolyase: tryptophan-306 is the intrinsic hydrogen atom donor essential for flavin radical photoreduction and DNA repair in vitro. Biochemistry. 1991 Jun 25;30(25):6322–6329. doi: 10.1021/bi00239a034. [DOI] [PubMed] [Google Scholar]
- Li Y. F., Sancar A. Active site of Escherichia coli DNA photolyase: mutations at Trp277 alter the selectivity of the enzyme without affecting the quantum yield of photorepair. Biochemistry. 1990 Jun 19;29(24):5698–5706. doi: 10.1021/bi00476a009. [DOI] [PubMed] [Google Scholar]
- Payne G., Heelis P. F., Rohrs B. R., Sancar A. The active form of Escherichia coli DNA photolyase contains a fully reduced flavin and not a flavin radical, both in vivo and in vitro. Biochemistry. 1987 Nov 3;26(22):7121–7127. doi: 10.1021/bi00396a038. [DOI] [PubMed] [Google Scholar]
- Payne G., Sancar A. Absolute action spectrum of E-FADH2 and E-FADH2-MTHF forms of Escherichia coli DNA photolyase. Biochemistry. 1990 Aug 21;29(33):7715–7727. doi: 10.1021/bi00485a021. [DOI] [PubMed] [Google Scholar]
- Payne G., Wills M., Walsh C., Sancar A. Reconstitution of Escherichia coli photolyase with flavins and flavin analogues. Biochemistry. 1990 Jun 19;29(24):5706–5711. doi: 10.1021/bi00476a010. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. Escherichia coli DNA photolyase is a flavoprotein. J Mol Biol. 1984 Jan 15;172(2):223–227. doi: 10.1016/s0022-2836(84)80040-6. [DOI] [PubMed] [Google Scholar]
- Sancar A., Smith F. W., Sancar G. B. Purification of Escherichia coli DNA photolyase. J Biol Chem. 1984 May 10;259(9):6028–6032. [PubMed] [Google Scholar]
- Stubbe J. Radicals in biological catalysis. Biochemistry. 1988 May 31;27(11):3893–3900. doi: 10.1021/bi00411a001. [DOI] [PubMed] [Google Scholar]
- Van Camp J. R., Young T., Hartman R. F., Rose S. D. Photosensitization of pyrimidine dimer splitting by a covalently bound indole. Photochem Photobiol. 1987 Mar;45(3):365–370. doi: 10.1111/j.1751-1097.1987.tb05388.x. [DOI] [PubMed] [Google Scholar]
- Wurtman R. J. The effects of light on the human body. Sci Am. 1975 Jul;233(1):69–77. [PubMed] [Google Scholar]
- Young T., Kim S. T., Van Camp J. R., Hartman R. F., Rose S. D. Transient intermediates in intramolecularly photosensitized pyrimidine dimer splitting by indole derivatives. Photochem Photobiol. 1988 Nov;48(5):635–641. doi: 10.1111/j.1751-1097.1988.tb02874.x. [DOI] [PubMed] [Google Scholar]


