Abstract
Bone marrow macrophages (BMMs), in the presence of cyclooxygenase-2 (Cox2) produced PGE2, secrete an inhibitory factor in response to Rankl that blocks PTH-stimulated osteoblastic differentiation. This study was to determine if the inhibitory factor also blocks PTH-stimulated Wnt signaling. Primary calvarial osteoblasts (POBs) were co-cultured with conditioned medium (CM) from Rankl-treated wild type (WT) BMMs, which make the inhibitory factor, and Cox2 knockout (KO) BMMs, which do not. PTH induced cAMP production was blocked by WT CM but not by KO CM. In the presence of KO CM, PTH induced phosphorylation at β-catenin serine sites, ser552 and ser675, previously shown to be phosphorylated by protein kinase A (PKA). Phosphorylation was blocked by WT CM and by H89, a PKA inhibitor. PTH did not increase total β-catenin. PTH-stimulated transcription factor/lymphoid enhancer-binding factor response element activity in POBs was blocked by WT CM and by serum amyloid A (SAA), the human recombinant analog of murine Saa3, which has recently been shown to be the inhibitory factor. In POBs cultured with Cox2 KO CM, PTH increased expression of multiple genes associated with the anabolic actions of PTH and decreased expression of Wnt antagonists. This differential regulation of gene expression was not seen in POBs cultured with WT CM. These data highlight the ability of PTH to phosphorylate β-catenin directly via PKA and demonstrate the ability of a Cox2-dependent inhibitory factor, secreted by Rankl-stimulated BMMs, to abrogate PTH stimulated β-catenin signaling. Our results suggest that PTH can stimulate a novel negative feedback of its anabolic actions by stimulating Rankl and Cox2 expression.
Keywords: Wnt genes, serine 552/675, cyclooxygenase-2, serum amyloid A, protein kinase A
1. Introduction
Parathyroid hormone (PTH) is a potent regulator of bone homeostasis–able to stimulate both bone formation and resorption by stimulating the differentiation of both osteoblast and osteoclast populations [1, 2]. Intermittent therapy with human 1–34 PTH (teriparatide) was the first FDA approved anabolic therapy for osteoporosis [3]. In contrast, continuous PTH administration or elevation has been shown to cause bone loss [2]. PTH acts via its receptor PTH1R, a G-protein coupled receptor that is highly expressed by osteoblasts and that activates both Gαs and Gαq signaling pathways [4]. Gαs activates adenylyl cyclase resulting in cAMP production and protein kinase A (PKA) activation, while Gαq leads to activation of protein kinase C (PKC) and release of Ca2+. The anabolic effects of PTH are thought to be mediated via Gαs [5, 6].
PTH is also known to increase the expression of cyclooxygenase 2 (Cox2), an inducible enzyme responsible for acute production of prostaglandin E2 (PGE2), and PGE2 can also stimulate both bone formation and resorption [7–10]. We observed a Cox2-dependent inhibition of PTH’s osteogenic and anabolic actions both in vitro and in vivo [11–13]. In vitro, in bone marrow stromal cell (BMSC) cultures that have precursors for both osteoclast and osteoblast lineage, PTH can stimulate osteoblast differentiation in Cox2 KO BMSCs but not in WT BMSCs [11]. In these studies, PGE2, produced either by Cox2 in osteoblasts or Cox2 in bone marrow macrophages (BMMs), acted via the EP4 receptor to cause BMMs, committed to the osteoclastic lineage by Rankl, to secrete a factor that inhibited the PTH stimulation of osteoblastic differentiation [11]. In vivo studies showed that there was a greater anabolic effect of intermittent PTH in Cox2 KO mice compared to WT mice [12]. However, there was a more dramatic effect of Cox2 when PTH was administered continuously. In WT mice, PTH infusion was catabolic, while in Cox2 KO mice it was markedly anabolic [13]. Cox2 is a rapidly and transiently inducible gene, and PGE2 is rapidly metabolized in vivo [7]. Thus, with intermittently injected PTH, PGE2 is expected to be increased only transiently. With the continuous infusion of PTH, both Cox2 and PGE2 were expected to be persistently elevated and, indeed, Cox2 mRNA in bone and PGE2 in serum were found to be elevated after 12 days of infusion [13].
The inhibitory factor produced by the BMMs has recently been shown to be serum amyloid A3 (Saa3) [14]. Saa3 was found to be produced by the BMMs in a Cox2-dependent manner only when stimulated with Rankl, which drives these cells towards the osteoclast lineage. Saa3 was produced by RANKL-stimulated BMMs prior to the appearance of tartrate resistant acid phosphatase positive (TRAP+) multinucleated osteoclast-like cells, and Saa3 was produced only by the flow-sorted osteoclast precursor population, suggesting that Saa3 was produced by the preosteoclasts [14].
cAMP-dependent PKA has been shown to cause the phosphorylation of β-catenin at two distinct sites: serine 552 (Ser552) and serine 675 (Ser675) [15, 16]. These sites have been linked to increases in β-catenin mediated transcriptional activity. Traditionally, β-catenin has been linked with osteoblast differentiation via the canonical Wnt pathway, in which Wnt agonists act via Lrp5/6 and Frizzled receptors/co-receptors on the β-catenin-destruction-complex to prevent the ubiquitination and proteolysis of β-catenin [17]. The cAMP/PKA pathway provides for a novel Wnt-independent mechanism in which β-catenin proteins are acted upon directly to increase their signaling efficacy. PTH signaling has recently been shown to phosphorylate β-catenin at Ser552 in a cAMP-dependent manner and to increase the downstream transcriptional activity of β-catenin via this pathway [18].
The goal of the current study was to investigate the effects of the Cox2-dependent inhibitor on PTH-stimulated β-catenin signaling. We cultured POBs with conditioned medium (CM) from Rankl-treated WT BMMs, which produce the inhibitor, and Cox2 KO BMMs, which do not. Using this model we demonstrated that PTH stimulation of (1) phosphorylation of β-catenin at Ser552/675, (2) β-catenin transcriptional activity and (3) expression of genes thought to mediate osteoblast differentiation were blocked by the Cox2/PGE2-dependent inhibitor produced by Rankl-stimulated BMMs.
2. Materials and Methods
2.1 Materials
Bovine PTH (bPTH; 1–34) was obtained from Sigma-Aldrich (St. Louis, MO). Forskolin (cAMP agonist), H-89 (PKA inhibitor) and GF109203X (PKC inhibitor) were obtained from Enzo Life Sciences (Farmingdale, NY). Human recombinant SAA (Apo-SAA), which corresponds to human Apo-SAA1α, except for the presence of an N-terminal methionine and two substituted residues present in Apo-SAA2β, was purchased from PeproTech (Rocky Hill, NJ). Antibody for Actin C-11 (sc-1615) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for phospho-β-catenin Ser552 (#9566), phospho-β-catenin Ser675 (#9567), phospho-β-catenin Ser33/37/Thr41 (#9561), and amino terminal β-catenin (#9581) were obtained from Cell Signaling Technology (Danvers, MA).
2.2 Animals
Mice that produce non-functional Cox2 protein, due to disruption of Ptgs2 in a C57BL/6, 129SV background, which we call Cox2 KO mice, were the gift of Scott Morham [19]. Cox2 KO mice were backcrossed into the outbred CD-1 background [12]. Following 20 generations of backcrossing, the Cox2 KO mice no longer developed either renal failure or female infertility [12]. Maintenance colonies heterozygous for the Cox2 gene disruption were refreshed twice a year with WT mice from Jackson Laboratory (Bar Harbor, ME) to prevent genetic drift. Mice are genotyped as described previously, and experimental mice are bred by WT x WT or KO x KO mating [12]. Animal studies were performed in compliance with protocols approved by the Animal Care and Use Committee of UConn Health.
2.3 Cell Culture
All cell cultures were grown in humidified incubation conditions of 5% CO2 at 37°C. Basic medium was 10% heat inactivated fetal calf serum (HIFCS), 100 U/mL penicillin, and 50 μg/mL streptomycin in α-MEM (Invitrogen, Carlsbad, CA). Osteoblast differentiation medium was basic media supplemented with 50 μg/mL phosphoascorbate. Treatment vehicles were the following: 0.001 N hydrochloric acid-acidified 0.1% bovine serum albumin (BSA) in 1x phosphate buffered saline (PBS) for PTH; 0.1% BSA in 1x PBS for osteoprotegerin (OPG), macrophage colony stimulating factor (M-CSF), and receptor activator of nuclear factor κ-B ligand (Rankl); and dimethyl sulfoxide (DMSO) for isobutyl methyl xanthine (IBMX), H-89, GF109203X, and forskolin.
2.4 Primary Osteoblasts (POBs)
POBs were harvested from calvariae of neonatal mice. Sutures were removed and the calvariae were minced, washed multiple times with 1x PBS, and subsequently digested with 0.5 mg/mL collagenase P (Roche Diagnostics, Indianapolis, IN) solubilized in 1 mL trypsin/EDTA and 4 mL PBS at 37°C. Four 10 minute digests were performed followed by a fifth and final digest for 90 minutes. After each digest the reaction was halted by the addition of 10% HIFCS. Cells from digests 2–5 were collected, filtered through a Nitex membrane (Millipore, Bedford, MA), and plated at a density of 50,000 cells/well in 6-well cell culture plates in differentiation medium. Medium was changed every three days. We used only freshly plated cells for all experiments.
2.5 Culture of Conditioned Medium (CM) with POBs
CM was collected from BMM cultures, centrifuged for 5 minutes at 800 rpm at 4°C to remove debris and frozen for later use. BMMs were cultured following the Faccio protocol: http://www.orthoresearch.wustl.edu/content/Laboratories/2978/Roberta-Faccio/Faccio-Lab/Protocols.aspx. BMMs were obtained from 8 week old mice. In brief, nucleated bone marrow cells were plated in 150 mm petri dishes (Fisher Scientific, Pittsburgh, PA) at a density of 1 × 107 cells/dish in basic medium, supplemented with 100 ng/mL M-CSF. Cultures were expanded twice for three days each. Following expansion, BMMs were re-plated in 12-well cell culture plates at a density of 6 × 104 cells/well in basic medium and treated with M-CSF and Rankl (both at 30 ng/mL). CM was collected after 3 days of culture, a day before tartrate resistant acid phosphatase positive multinucleated cells formed in the BMM cultures. CM was added to Cox2 KO POBs 2 hours before agonist treatment at a concentration of 3 parts CM to 1 part differentiation medium. The only exception was for the study of differentiation, where treatments with agonists and CM were begun at the time of plating and continued for the entire 14 day period. Unless noted otherwise, all cultures were treated with 50 ng/mL OPG to prevent Rankl in the CM or PTH-stimulated Rankl in the POBs from inducing any remaining hematopoietic cells in the POB cultures from becoming osteoclasts and making more of the inhibitory factor [11].
2.6 Intracellular cAMP Measurement
On day five of culture, Cox2 KO POBs were treated with 3 parts WT or KO CM and 1 part differentiation medium for 2 hours, followed by 0.5 mM IBMX for 45 minutes, and PTH (10 nM) and FSK (10 μM) for 15 minutes. For extraction, 400 μL of ice-cold ethanol was added to each well and the cultures were detached from the plate by scraping and collected in 1.5 mL centrifuge tubes. Samples were then centrifuged at 1500 × g for 10 minutes at 4°C. The supernatant was collected and lyophilized and cAMP concentration was determined using an enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI).
2.7 Western Blotting
Proteins were extracted from culture by the addition of lysis buffer (2% SDS, 10 % glycerol, 62 mM Tris, pH: 6.85) and quantified using the BCA protein assay kit (Pierce, Rockford, IL). 10% SDS–PAGE was used to separate 15 μg of total protein, prior to transfer onto a nitrocellulose membrane. Membranes were washed with 1x Tris-buffered saline (TBS, pH: 7.6), blocked in blocking buffer (0.1% Tween-20, 5% (w/v) non-fat dry milk, 1x TBS), and incubated overnight at 4°C in blocking buffer supplemented with a primary antibody at the manufacturer’s suggested concentration. Membranes were subsequently washed in 1x TBS supplemented with 0.1% Tween-20 (TBST), incubated with HRP-conjugated secondary antibody, washed once more in 1x TBST, and developed using the LumiGLO chemiluminescence reagent (Cell Signaling, Danvers, MA). Densitometry was performed using ImageJ. Images of scanned films were converted into histogram form via the Analyze>Gels tool. Area under the curve was measured and normalized to the area under the corresponding β-actin curve.
2.8 TCF/LEF Luciferase Reporter Assay
Cox2 KO POBs were plated at 105 cells/well in differentiation medium in 12 well dishes. After 24 hours they were transduced with lentiviral particles using Cignal Lenti TCF/LEF luciferase reporter (Qiagen, Valencia, CA) and Cignal Lenti Renilla luciferase control kits. Twenty four hours after transduction, medium was changed to differentiation medium. Forty eight hours after transduction, cells were treated with recombinant human SAA (10 μg/mL) or WT CM or KO CM 1 hour prior to treatment with vehicle or PTH (10 nM) for 6 hours. Dual Luciferase assay was performed using Lumat LB 9507 (Berthold Technologies, Oak Ridge, TN). Promoter activity values are expressed as arbitrary units after normalization to the Renilla reporter activity. Experiments were done in triplicate for each group.
2.9 Real-time (quantitative) PCR Analysis
Total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol: 2–5 μg of total RNA was DNase treated (Ambion, Inc., Austin, TX) and subsequently converted to cDNA using the high capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Quantitative PCR was performed in 96-well plates using the Assays-on-Demand Gene Expression TaqMan primers (Applied Biosystems, Foster City, CA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the endogenous control gene. Samples were amplified in duplicate and primers were checked for equal efficiency amplification over a range of target gene concentrations. The Applied Biosystems ABI Prism 7300 Sequence detection instrument was used to amplify a PCR reaction mixture comprised of 2x TaqMan Universal PCR Master Mix, 20x Assays-on-Demand Gene Expression Assay Mix and 50 ng of cDNA in a total volume of 20 μL/well at universal thermal cycling parameters. Data analysis was performed using either comparative CT (ΔΔCT) or relative standard curve methods.
2.10 Statistics
All data are presented as means ± standard error of the mean (SEM). Statistical analysis was performed using GraphPad Prism for Microsoft Windows, Version 5.04. To compare results from experiments involving two independent variables, we used two-way ANOVA, followed by the Bonferroni pairwise multiple comparison post-test. If data were not normally distributed, a log10 transform was performed prior to ANOVA.
3. Results
3.1 WT CM abrogated PTH-stimulated cAMP production
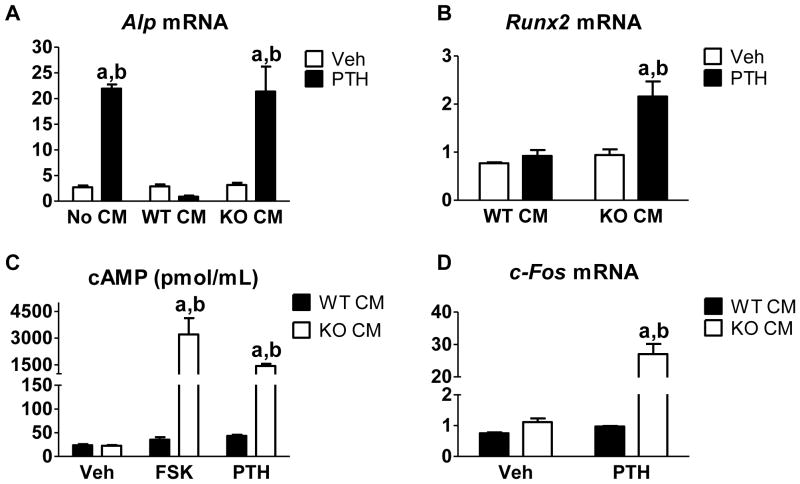
By co-culturing CM from Rankl-treated WT BMMs with POBs, we can model the inhibitory effect seen in PTH-treated BMSC cultures. We used POBs from Cox2 KO mice to eliminate the possibility of inducing additional Cox2/PGE2 in POBs with PTH or serum, which might affect osteoblastic differentiation. Even though POBs are washed multiple times during their collection, there can still be some hematopoietic cells remaining that can differentiate into osteoclast-like cells [11]. Hence, we treated the POBs with OPG to block Rankl in the CM or Rankl induced by PTH in the POBs from interacting with its receptor Rank on hematopoietic cells. We have previously shown that OPG does not affect PTH-stimulated osteoblast differentiation [11]. In Figure 1A we compare the effects of no CM, WT CM and Cox2 KO CM on PTH-stimulated differentiation as measured by alkaline phosphatase (Alp) mRNA expression at 14 days of culture. Consistent with previous results, there was no difference in Alp expression among groups of vehicle-treated POBs. PTH-stimulated Alp expression was inhibited by WT CM but not by normal differentiation medium or Cox2 KO CM. Because there was no difference in effects between normal differentiation medium and Cox2 KO CM, we only compared WT and KO CM in future experiments. PTH also stimulated expression of Runx2, an early marker of osteoblast differentiation, only in Cox2 KO CM (Fig. 1B). Because PTH has much of its downstream signaling via the cAMP/PKA pathway [6, 20–22], we examined the effect of WT CM on cAMP produced in response to PTH and to forskolin, a direct adenylyl cyclase (AC) agonist (Fig. 1C). Forskolin and PTH markedly stimulated cAMP production in the presence of Cox2 KO CM. WT CM blocked the stimulation of cAMP by both the PTH and forskolin. Similarly, PTH stimulated the expression of c-Fos, an early response gene mediated by the cAMP pathway [23], in POBs treated with KO, not WT, CM (Fig. 1D). Thus, WT CM blocked PTH-stimulated cAMP production and signaling.
Fig. 1. CM from WT BMMs, but not Cox2 KO BMMs, inhibited PTH-stimulated osteoblast differentiation and cAMP production.
For all experiments, CM was collected from either the WT BMMs (WT CM) or the Cox2 KO BMMs (KO CM) that were stimulated with Rankl for 3 days. (A) Cox2 KO POBs were cultured for 14 days in the presence of no CM (differentiation medium), WT CM, or Cox2 KO CM and either vehicle or PTH (10 nM). On day 14 of culture, Alp mRNA expression was measured by qRT-PCR. (B) On day 5 of culture, Cox2 KO POBs were treated with vehicle or PTH (10 nM) for 3 hours. Runx2 expression was measured by qRT-PCR. (C) On day 5 of culture, Cox2 KO POBs were treated with vehicle or PTH (10 nM) for 15 minutes. cAMP was measured by ELISA. (D) On day 5 of culture, Cox2 KO POBs were treated with vehicle or PTH (10 nM) for 3 hours. c-Fos expression was measured by qRT-PCR. Bars represent mean ± SEM, n=3. aSignificant effect of PTH, p<0.01. bSignificant difference compared to WT CM, p<0.01.
3.2 WT CM blocked PTH phosphorylation of β-catenin
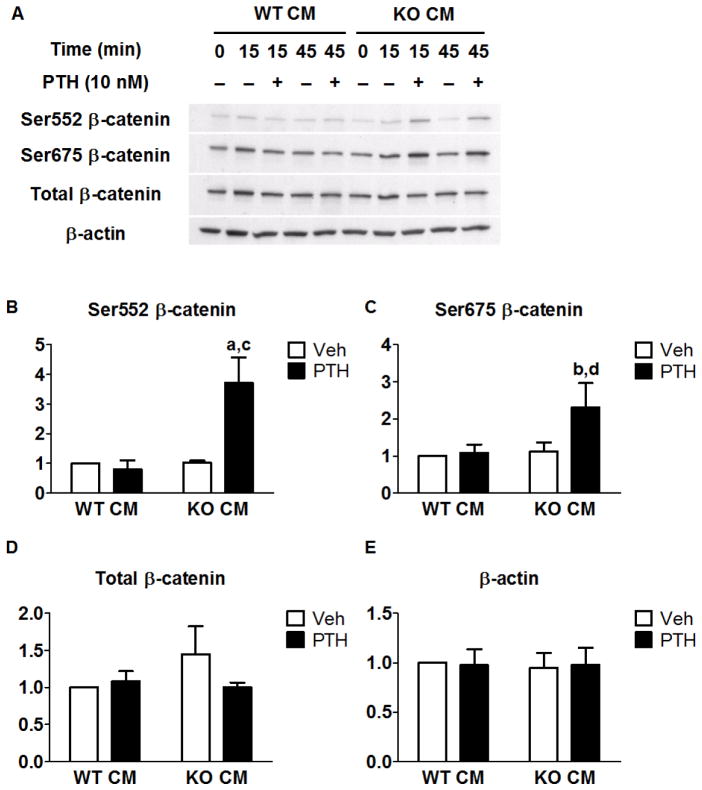
Previous studies identified a cAMP/PKA-dependent pathway that stimulates β-catenin signaling [15, 16, 18]. The hallmark of this pathway is the phosphorylation of β-catenin at Ser552 and/or Ser675 by PKA. We compared the effects of WT and Cox2 KO CM on PTH-stimulated phosphorylation at these two sites. PTH stimulated phosphorylation at both sites only in the presence of KO CM (Fig 2A). Phosphorylation was observed as early as 15 minutes of treatment and was sustained at 45 minutes of treatment (Fig. 2A). PTH caused a 3- and 2-fold increase of Ser552 and Ser675 phosphorylation, respectively, at 15 min (Fig. 2B–C), but did not increase total β-catenin levels (Fig. 2D).
Fig. 2. PTH increased phosphorylation of β-catenin at ser552 and ser675 in the presence of Cox2 KO CM but not WT CM.
CM was collected from either the WT BMMs (WT CM) or the Cox2 KO BMMs (KO CM) that were stimulated with Rankl for 3 days. Cox2 KO POBs were treated with vehicle or PTH (10 nM) on day 5 of culture and Western analysis was performed. (A) Time course examining effects of PTH on β-catenin phosphorylation. (B–E) Densitometry of bands was performed and normalized to β-actin. Vehicle-treated WT CM was set to 1. Bars represent means ± SEM, n=4. aSignificant effect of PTH, p<0.01; bp<0.05. cSignificant difference compared to WT CM, p<0.01; dp>0.05.
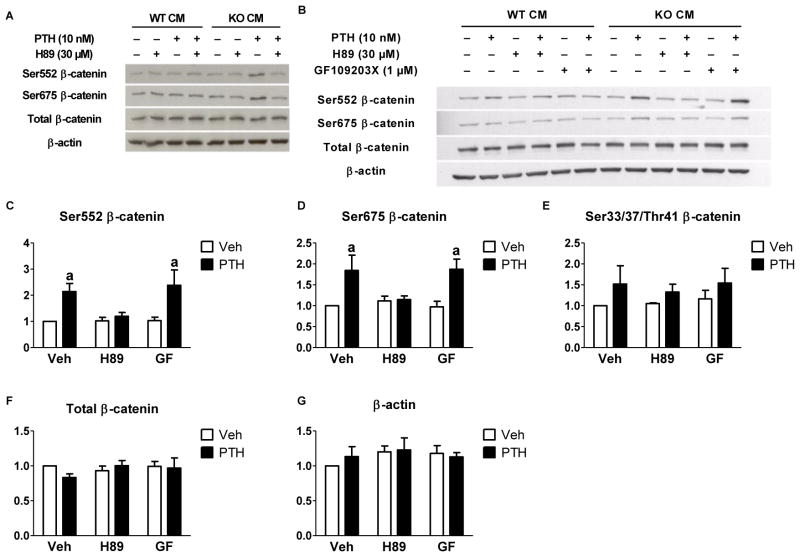
To determine if this effect was dependent on PKA, we treated with H89, a PKA inhibitor (Fig. 3). H89 had no effect on POBs treated with WT CM. In POBs co-cultured with KO CM, the PTH-stimulated increase in Ser552 and Ser675 phosphorylation was abrogated by the presence of H89 (Fig. 3A). Although H89 is a selective inhibitor of PKA, it has been reported to have off-target effects on PKC [24]. In order to rule out involvement of PKC, we treated with a specific PKC inhibitor, GF109203X, at a dose previously shown to inhibit the effects of phorbol myristate acetate [25]. GF109203X had no effect on PTH stimulation of β-catenin phosphorylation (Fig. 3B). To examine whether or not β-catenin destruction was being affected by PTH treatment we examined both total levels of β-catenin as well as the GSK3β-stimulated phosphorylation sites that mark β-catenin for ubiquitination and destruction (Ser33/37/Thr41). There was no effect of PTH on levels of total β-catenin or β-catenin marked for destruction in the presence of KO CM (Fig. 3E–F).
Fig. 3. PTH phosphorylated β-catenin at ser552 and ser675 in a PKA- dependent manner.
Cox2 KO POBs were treated with vehicle or 10 nM PTH on day 5 of culture and Western analysis was performed. CM was collected from either the WT BMMs (WT CM) or the Cox2 KO BMMs (KO CM) that were stimulated with Rankl for 3 days. (A) Cultures treated with 10 nM PTH for 15 minutes following WT/KO CM and ± H89 (30 μM) pretreatment. (B) Cultures treated as noted with the addition of ± GF109203X (1 μM). (C–G) Densitometry was performed on KO CM treated groups, values were normalized to β-actin and vehicle-treated groups were set to 1. Bars represent means ± SEM, n=3. aSignificant effect of PTH, p<0.05.
3.3 WT CM or SAA blocked PTH stimulated β-catenin transcriptional activity
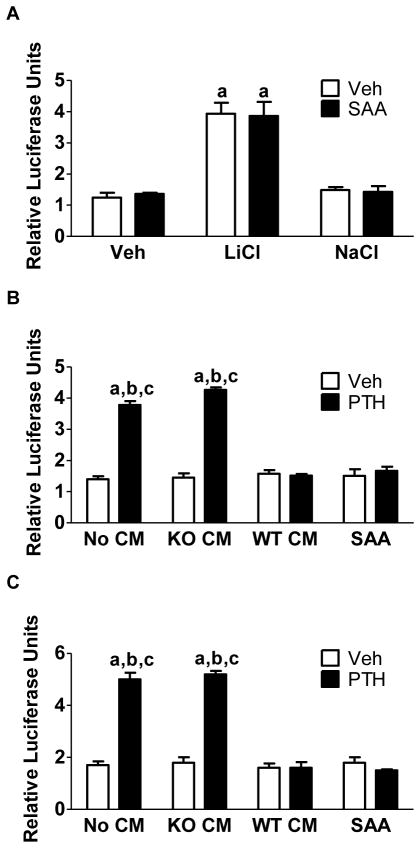
β-catenin signals by binding to TCF/LEF transcription factors in the nucleus, which leads to the transcription and expression of Wnt-responsive genes [26]. Although previous reports have differed on whether or not phosphorylation of β-catenin at Ser552 and Ser675 results in an increase in stability and nuclear translocation, they have generally reported increased transcriptional activity of β-catenin [15, 16, 18, 27]. To examine effects on transcriptional activity in POBs, we measured activity of a TCF/LEF-inducible firefly luciferase reporter normalized to activity of a constitutively active renilla luciferase construct. We compared the effects on PTH-stimulated TCF/LEF activity of WT CM and SAA, a human analog of Saa3, which we have shown to be the specific factor in the WT CM that inhibits the effects of PTH [14].
LiCl, which can activate Wnt signaling by inhibiting GSK3β, stimulated luciferase activity that was not inhibited by SAA (Fig. 4A). In the experiments shown in Fig. 4B–C, 6 hours of treatment with PTH induced a 4- to 5-fold increase in TCF/LEF activity in POBs treated with no CM or with Cox2 KO CM. Both WT CM and SAA abrogated the PTH stimulation of luciferase activity. Hence, WT CM and SAA have similar inhibitory effects. These data suggest that SAA does not inhibit a Wnt signaling pathway acting via GSK3β but does inhibit the PTH stimulation of β-catenin activity mediated by phosphorylation of β-catenin at Ser552 and Ser675.
Fig. 4. PTH-stimulated TCF/LEF reporter activity is inhibited by WT CM and SAA.
(A) Cultures were treated for 6 hours with LiCl (20 mM) or NaCl (20 mM). CM was collected from either the WT BMMs (WT CM) or the Cox2 KO BMMs (KO CM) that were stimulated with Rankl for 3 days. (B, C) Cultures were treated for 2 hours with no CM, WT CM, Cox2 KO CM, or SAA (10 μg/mL) prior to 6 hours of treatment with vehicle or PTH (10 nM). Panels (B) and (C) represent independent transductions. Promoter activity values are expressed as relative firefly luciferase units normalized to Renilla reporter activity. Bars are means ± SEM, n=3. aSignificant difference compared to vehicle, p<0.01. bSignificant difference compared to WT CM, p<0.01. cSignificant effect compared to SAA, p<0.01.
3.4 WT CM blocked the PTH regulation of genes involved in Wnt signaling
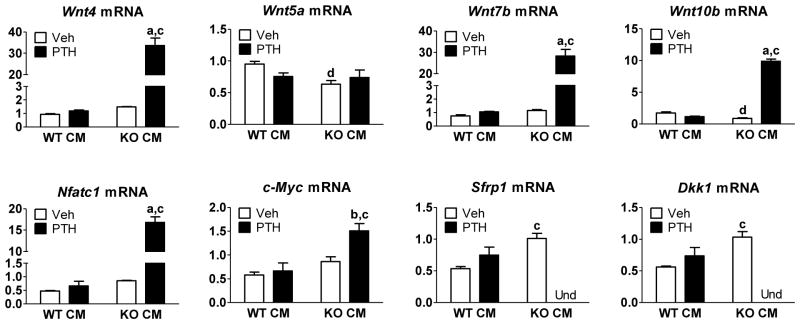
To examine effects of the inhibitory CM on the PTH regulation of specific genes known to be involved in Wnt signaling, we treated POBs for 3 hours with PTH and compared responses in WT or Cox2 KO CM. Several Wnts--Wnt4, Wnt7b, and Wnt10b--were increased in expression only in KO CM treated groups (Fig. 5). Wnt4 has been associated with the anabolic effects of PTH and found to be stimulatory for osteoblastogenesis and bone formation [5, 28]. Expression of Wnt7b can be induced by PTH, has been shown to increase with osteoblastic differentiation and to increase bone mass as well as osteoblast number in vivo [29]. Wnt10b is an important factor in coupling the actions of osteoblasts and osteoclasts, while also being able to enhance commitment to the osteoblastic lineage [30, 31]. In contrast, the expression of Wnt5a, reported to antagonize canonical Wnt signaling by stimulating destruction of β-catenin via GSK3β [32], was unaffected by PTH treatment in both WT and KO CM treated groups (Fig. 5). We were unable to detect Wnt3a mRNA (data not shown). Expression of Nfatc1, shown to be important for non-canonical Wnt signaling [33] and for the PTH induction of Cox2 [34], was markedly induced by PTH only in KO CM. Expression of c-Myc, a Wnt target gene in colorectal cancer [35], was also increased by PTH only in KO CM.
Fig. 5. CM from WT BMMs attenuates PTH-regulated expression of genes associated with Wnt signaling.
CM was collected from either the WT BMMs (WT CM) or the Cox2 KO BMMs (KO CM) that were stimulated with Rankl for 3 days. Cox2 KO POBs were treated for 2 hours with WT CM or Cox2 KO CM prior to treatment with vehicle or 10 nM PTH for 3 hours on day 5 of culture. mRNA was measured by qRT-PCR. Genes whose expression was undetectable are marked Und. Bars are means ± SEM, n=3. aSignificant effect of PTH, p<0.01; bp<0.05. cSignificant effect compared to WT CM, p<0.01; dp<0.05.
In contrast to genes that positively regulate Wnt signaling, the expression of genes thought to inhibit Wnt signaling was inhibited by PTH in Cox2 KO CM (Fig. 5). Genes coding for the canonical Wnt signaling inhibitors Sfrp1 and Dkk1, which inhibit Wnt agonist interaction with frizzled and Lrp5/6, were measured. Sfrp1 and Dkk1 mRNA expression were undetectable in cultures treated with PTH in the presence of KO CM (Fig. 5). Sost mRNA expression was undetectable in these cultures (data not shown). Hence, the inhibitory CM blocked the PTH stimulation of multiple genes that may enhance osteogenic or anabolic responses and the PTH inhibition of several genes that inhibit these Wnt signaling.
4. Discussion
Our results suggest a prominent role for the β-catenin sites Ser552 and Ser675 in mediating PTH-stimulated β-catenin transcriptional activity and Wnt-related gene expression. We demonstrated that a novel inhibitor, produced by Rankl-stimulated Cox2-expressing BMMs and shown to block PTH-stimulated osteoblast differentiation in vitro [11], blocked PTH-stimulated cAMP production and PKA phosphorylation at these sites, along with subsequent β-catenin transcriptional activity. We have recently identified this inhibitory factor as Saa3 [14]. BMMs require both Rankl and Cox2/PGE2 to produce Saa3 [14]. PTH induces Cox2/PGE2 and Rankl in the osteoblast lineage. Rankl drives the commitment of BMMs towards the osteoclast lineage and induces more Cox2/PGE2 in these cells. The combination of Rankl and PGE2 cause the BMMs to secrete Saa3, which in turn acts on osteoblasts to block PTH-stimulated cAMP and β-catenin signaling (Fig. 6). Although PTH can activate both PKA and PKC signaling pathways, our observations strongly suggest that the direct effects of PTH on β-catenin signaling and osteoblast differentiation occur via Gαs/cAMP-initiated pathways as previously reported [5, 6].
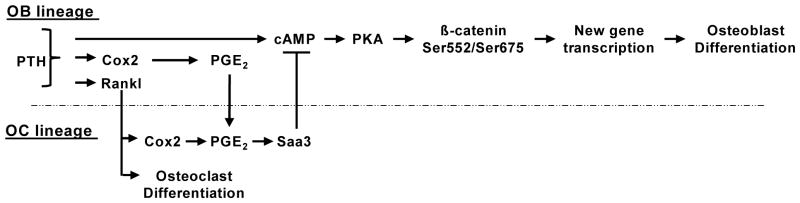
Fig. 6. Proposed means by which PTH inhibits its own osteogenic/anabolic actions.
PTH stimulates Rankl and Cox2/PGE2 in osteoblast (OB) lineage cells. Rankl acts on osteoclast (OC) lineage cells to commit them to become osteoclasts and to stimulate more Cox2/PGE2. The combination of Rankl and PGE2 cause cells committed to become osteoclasts to secrete Saa3. In the presence of Saa3, PTH-stimulated cAMP production is abrogated, preventing subsequent PKA activation and β-catenin signaling. When PTH is unable to initiate this negative feedback pathway, either by absence of osteoclasts or Cox2, β-catenin is phosphorylated at Ser552 and Ser675 and can stimulate gene transcription associated with osteoblastic differentiation.
A number of studies have reported that PTH promotes increased β-catenin transcriptional activity but the specific steps by which this occurs are still not clear. PTH has been shown to be a potent inhibitor of canonical Wnt antagonists, including Dkk1, Sost, and Sfrp, which can lead to enhanced Wnt signaling and stabilization of β-catenin [36–39], and to increase the expression of Wnt agonists [40]. An early study in rat osteosarcoma UMR-106 cells reported that PTH acted via the canonical Wnt signaling pathway to increase Lrp6 and Fzd-1 expression, decrease Dkk1 expression, and increase β-catenin levels and activity and that these effects were dependent on cAMP signaling [41]. However, PTH was also reported to activate β-catenin signaling by directly recruiting dishevelled independently of Wnt or Lrp5/6 in UMR cells [42] and by directly inactivating GSK-3β in human osteosarcoma Saos-2 cells [43]. Another study in UMR cells and in vivo showed that PTH stabilized β-catenin in osteoblasts by activating binding of its receptor PTH1R to Lrp6 and by activating PKA to phosphorylate Lrp6 [16, 27, 44]. One other group has linked PTH to phosphorylation of Ser675 on β-catenin [18]. They used bone marrow stromal cells and MC3T3-E1 cells and found that PTH stimulation increased β-catenin activity but did not increase total β-catenin levels. In our murine POB model, we did not find any evidence of GSK-3β mediated phosphorylation of β-catenin at Ser33/37/Thr41 nor did we observe any increases in total β-catenin, suggesting that PTH acted to increase the signaling efficiency of the current population of β-catenin rather than preventing its destruction. Our results agree with Taurin et al who reported that PKA phosphorylation of β-catenin at Ser552 and Ser675 increased β-catenin transcriptional activity, while leaving the destruction markers and complex unaffected [16, 36].
Our in vitro results in POBs treated continuously with PTH are similar to our in vivo effects in WT and Cox2 KO mice continuously infused with PTH for 12 or 21 days[13]. Infused PTH was anabolic for bone only in Cox2 KO mice, not in WT mice. PTH infusion increased expression in tibiae of Wnt4 and Wnt10b and decreased expression of Sfrp1, Dkk1 and Sost only in Cox2 KO mice. Expression of Wnt3a was undetectable in our in vitro studies and was not regulated by PTH in either WT or KO mice in the infusion studies[13]. Wnt3a is frequently used as an exogenous agent to induce canonical Wnt signaling. Our data suggest that other Wnts (Wnt4, Wnt7b, and Wnt10b) are more likely to be involved in the direct osteogenic/anabolic effects of PTH. One interesting possibility is that the PTH-induced phosphorylation of β-catenin at Ser552 and Ser675 could be priming osteoblasts for subsequent Wnt-signaling to take place by increasing expression of these Wnts and decreasing the expression of Wnt inhibitors.
These studies highlight the potential role of the Cox2-dependent inhibitory factor, Saa3, for modulating the osteogenic/anabolic actions of PTH. It seems likely that Saa3 suppresses the anabolic responses to continuously elevated PTH [13] and may also inhibit the anabolic responses to intermittently elevated PTH in vivo [12, 13]. Saa3 is an acute phase protein, generally considered to be associated with acute and chronic inflammation, and its levels can be increased many fold under inflammatory circumstances [45]. Many pro-inflammatory agents and cytokines can increase both Cox2 and Rankl and are expected, therefore, to increase Saa3 expression [7,10]. Under these circumstances, Saa3 may inhibit PTH-stimulated cAMP signaling, Wnt signaling and bone formation. Hence, Saa3 might be an important means by which inflammatory agents restrain PTH-stimulated anabolic actions and cause bone loss and, therefore, Saa3 might be implicated in the bone loss associated with aging and chronic inflammation.
Our results suggest that the suppression of the osteogenic/anabolic responses to PTH, associated with Cox2 expression, is due to the inhibition of PTH/cAMP/PKA stimulated β-catenin signaling by Rankl-stimulated BMMs. Thus, the osteogenic/anabolic effects of PTH might be enhanced by interrupting the production of the Cox2-mediated induction of Saa3.
Supplementary Material
Highlights.
PTH stimulated cAMP/PKA dependent phosphorylation of β-catenin at serines 552 and 675
Rankl-treated bone marrow macrophages (BMMs) inhibited PTH stimulated β-catenin transcriptional activity
In the absence of cyclooxygenase 2 (Cox2), BMMs were not inhibitory
Serum amyloid A also inhibited PTH-stimulated β-catenin transcriptional activity
The anabolic effects of PTH may be enhanced by inhibition of Cox2 or serum amyloid A
Acknowledgments
Funding was provided by the National Institute of Arthritis and Musculoskeletal and Skin Disease award number NIH-AR060286 to CP and by the National Institute of Dental and Craniofacial Research Training Grant 1T90DE021989 to TE.
The authors thank Drs. Anne Delany and Mina Mina for critical review of the manuscript.
Footnotes
Disclosures: The authors of this manuscript state that they have no conflicts of interest to disclose.
Authors’ roles: Study design: TE, SC, CP. Study conduct: TE, SC. Data collection: TE, SC. Data analysis: TE. Data interpretation: TE, SC, CP. Drafting manuscript: TE, SC, CP. Revising manuscript content: TE, CP. Approving final version of manuscript: TE, SC, CP. TE takes responsibility for the integrity of the data analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26:688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- 2.Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol. 2015;22:41–50. doi: 10.1016/j.coph.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane NE, Silverman SL. Anabolic therapies. Curr Osteoporos Rep. 2010;8:23–7. doi: 10.1007/s11914-010-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vilardaga JP, Romero G, Friedman PA, Gardella TJ. Molecular basis of parathyroid hormone receptor signaling and trafficking: a family B GPCR paradigm. Cell Mol Life Sci. 2011;68:1–13. doi: 10.1007/s00018-010-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Liu H, Qin L, Tamasi J, Bergenstock M, Shapses S, Feyen JH, Notterman DA, Partridge NC. Determination of dual effects of parathyroid hormone on skeletal gene expression in vivo by microarray and network analysis. J Biol Chem. 2007;282:33086–97. doi: 10.1074/jbc.M705194200. [DOI] [PubMed] [Google Scholar]
- 6.Yang D, Singh R, Divieti P, Guo J, Bouxsein ML, Bringhurst FR. Contributions of parathyroid hormone (PTH)/PTH-related peptide receptor signaling pathways to the anabolic effect of PTH on bone. Bone. 2007;40:1453–61. doi: 10.1016/j.bone.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pilbeam C, Choudhary S, Blackwell K, Raisz L. Prostaglandins and bone metabolism. In: Bilezikian JP, Raisz L, Martin TJ, editors. Principles of Bone Biology. Vol. 2. San Diego, CA, USA: Elsevier; 2008. pp. 1235–71. [Google Scholar]
- 8.Tetradis S, Pilbeam CC, Liu Y, Herschman HR, Kream BE. Parathyroid hormone increases prostaglandin G/H synthase-2 transcription by a cyclic adenosine 3′,5′-monophosphate-mediated pathway in murine osteoblastic MC3T3-E1 cells. Endocrinology. 1997;138:3594–600. doi: 10.1210/endo.138.9.5391. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi H, Raisz LG, Voznesensky OS, Alander CB, Hakeda Y, Pilbeam CC. Regulation of the two prostaglandin G/H synthases by parathyroid hormone, interleukin-1, cortisol, and prostaglandin E2 in cultured neonatal mouse calvariae. Endocrinology. 1994;135:1157–64. doi: 10.1210/endo.135.3.8070358. [DOI] [PubMed] [Google Scholar]
- 10.Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab. 2010;21:294–301. doi: 10.1016/j.tem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choudhary S, Blackwell K, Voznesensky O, Deb Roy A, Pilbeam C. Prostaglandin E2 acts via bone marrow macrophages to block PTH-stimulated osteoblast differentiation in vitro. Bone. 2013;56:31–41. doi: 10.1016/j.bone.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M, Choudhary S, Voznesensky O, Gao Q, Adams D, Diaz-Doran V, Wu Q, Goltzman D, Raisz LG, Pilbeam CC. Basal bone phenotype and increased anabolic responses to intermittent parathyroid hormone in healthy male COX-2 knockout mice. Bone. 2010;47:341–52. doi: 10.1016/j.bone.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhary S, Canalis E, Estus T, Adams D, Pilbeam C. Cyclooxygenase-2 suppresses the anabolic response to PTH infusion in mice. PLoS One. 2015;10:e0120164. doi: 10.1371/journal.pone.0120164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choudhary S, Goetjen A, Estus T, Jacome-Galarza CE, Aguila HL, Lorenzo J, Pilbeam C. Serum Amyloid A3 Secreted by Preosteoclasts Inhibits Parathyroid Hormone-Stimulated cAMP Signaling in Murine Osteoblasts. J Biol Chem. 2015 Dec 23; doi: 10.1074/jbc.M115.686576. pii: jbc.M115.686576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taurin S, Sandbo N, Yau DM, Sethakorn N, Dulin NO. Phosphorylation of beta-catenin by PKA promotes ATP-induced proliferation of vascular smooth muscle cells. Am J Physiol Cell Physiol. 2008;294:C1169–74. doi: 10.1152/ajpcell.00096.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006;281:9971–6. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- 17.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 18.Revollo L, Kading J, Jeong SY, Li J, Salazar V, Mbalaviele G, Civitelli R. N-cadherin restrains PTH activation of Lrp6/beta-catenin signaling and osteoanabolic action. J Bone Miner Res. 2015;30:274–85. doi: 10.1002/jbmr.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, Smithies O. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–82. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 20.Lee SK, Lorenzo JA. Regulation of receptor activator of nuclear factor-kappa B ligand and osteoprotegerin mRNA expression by parathyroid hormone is predominantly mediated by the protein kinase a pathway in murine bone marrow cultures. Bone. 2002;31:252–9. doi: 10.1016/s8756-3282(02)00804-9. [DOI] [PubMed] [Google Scholar]
- 21.Dolson GM, Hise MK, Weinman EJ. Relationship among parathyroid hormone, cAMP, and calcium on proximal tubule sodium transport. Am J Physiol. 1985;249:F409–16. doi: 10.1152/ajprenal.1985.249.3.F409. [DOI] [PubMed] [Google Scholar]
- 22.Goldring SR, Dayer JM, Krane SM. Regulation of hormone-induced cyclic AMP response to parathyroid hormone and prostaglandin E2 in cells cultured from human giant cell tumors of bone. Calcif Tissue Int. 1979;29:193–200. doi: 10.1007/BF02408080. [DOI] [PubMed] [Google Scholar]
- 23.Pearman AT, Chou WY, Bergman KD, Pulumati MR, Partridge NC. Parathyroid hormone induces c-fos promoter activity in osteoblastic cells through phosphorylated cAMP response element (CRE)-binding protein binding to the major CRE. J Biol Chem. 1996;271:25715–21. doi: 10.1074/jbc.271.41.25715. [DOI] [PubMed] [Google Scholar]
- 24.Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–72. [PubMed] [Google Scholar]
- 25.Mehrotra M, Saegusa M, Wadhwa S, Voznesensky O, Peterson D, Pilbeam C. Fluid flow induces Rankl expression in primary murine calvarial osteoblasts. J Cell Biochem. 2006;98:1271–83. doi: 10.1002/jcb.20864. [DOI] [PubMed] [Google Scholar]
- 26.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol. 2005;25:9063–72. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergenstock MK, Partridge NC. Parathyroid hormone stimulation of noncanonical Wnt signaling in bone. Ann N Y Acad Sci. 2007;1116:354–9. doi: 10.1196/annals.1402.047. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Tu X, Esen E, Joeng KS, Lin C, Arbeit JM, Ruegg MA, Hall MN, Ma L, Long F. WNT7B promotes bone formation in part through mTORC1. PLoS Genet. 2014;10:e1004145. doi: 10.1371/journal.pgen.1004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ota K, Quint P, Ruan M, Pederson L, Westendorf JJ, Khosla S, Oursler MJ. TGF-beta induces Wnt10b in osteoclasts from female mice to enhance coupling to osteoblasts. Endocrinology. 2013;154:3745–52. doi: 10.1210/en.2013-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati N, Martinez-Santibanez G, MacDougald OA. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a beta-catenin-dependent mechanism. Bone. 2012;50:477–89. doi: 10.1016/j.bone.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugimura R, He XC, Venkatraman A, Arai F, Box A, Semerad C, Haug JS, Peng L, Zhong XB, Suda T, Li L. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell. 2012;150:351–65. doi: 10.1016/j.cell.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang H, Chikazu D, Voznesensky OS, Herschman HR, Kream BE, Drissi H, Pilbeam CC. Parathyroid hormone induction of cyclooxygenase-2 in murine osteoblasts: role of the calcium-calcineurin-NFAT pathway. J Bone Miner Res. 2010;25:819–29. doi: 10.1359/jbmr.091019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR, Vass JK, Athineos D, Clevers H, Clarke AR. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–9. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 36.Guo J, Liu M, Yang D, Bouxsein ML, Saito H, Galvin RJ, Kuhstoss SA, Thomas CC, Schipani E, Baron R, Bringhurst FR, Kronenberg HM. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab. 2010;11:161–71. doi: 10.1016/j.cmet.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keller H, Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37:148–58. doi: 10.1016/j.bone.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Qin L, Qiu P, Wang L, Li X, Swarthout JT, Soteropoulos P, Tolias P, Partridge NC. Gene expression profiles and transcription factors involved in parathyroid hormone signaling in osteoblasts revealed by microarray and bioinformatics. J Biol Chem. 2003;278:19723–31. doi: 10.1074/jbc.M212226200. [DOI] [PubMed] [Google Scholar]
- 39.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146:4577–83. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 40.Kakar S, Einhorn TA, Vora S, Miara LJ, Hon G, Wigner NA, Toben D, Jacobsen KA, Al-Sebaei MO, Song M, Trackman PC, Morgan EF, Gerstenfeld LC, Barnes GL. Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. J Bone Miner Res. 2007;22:1903–12. doi: 10.1359/jbmr.070724. [DOI] [PubMed] [Google Scholar]
- 41.Kulkarni NH, Halladay DL, Miles RR, Gilbert LM, Frolik CA, Galvin RJ, Martin TJ, Gillespie MT, Onyia JE. Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem. 2005;95:1178–90. doi: 10.1002/jcb.20506. [DOI] [PubMed] [Google Scholar]
- 42.Romero G, Sneddon WB, Yang Y, Wheeler D, Blair HC, Friedman PA. Parathyroid hormone receptor directly interacts with dishevelled to regulate beta-Catenin signaling and osteoclastogenesis. J Biol Chem. 2010;285:14756–63. doi: 10.1074/jbc.M110.102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki A, Ozono K, Kubota T, Kondou H, Tachikawa K, Michigami T. PTH/cAMP/PKA signaling facilitates canonical Wnt signaling via inactivation of glycogen synthase kinase-3beta in osteoblastic Saos-2 cells. J Cell Biochem. 2008;104:304–17. doi: 10.1002/jcb.21626. [DOI] [PubMed] [Google Scholar]
- 44.Wan M, Yang C, Li J, Wu X, Yuan H, Ma H, He X, Nie S, Chang C, Cao X. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 2008;22:2968–79. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eklund KK, Niemi K, Kovanen PT. Immune functions of serum amyloid A. Crit Rev Immunol. 2012;32:335–48. doi: 10.1615/critrevimmunol.v32.i4.40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.