Abstract
Background
Accurate diagnosis of behavioral variant frontotemporal dementia (bvFTD) is important as patients’ behavioral symptoms have profound implications for their families and communities. Since the diagnosis of bvFTD derives from behavioral features, accurate identification of patients can be difficult for non-specialists. Concrete rates of diagnostic accuracy among non-specialists are unavailable.
Methods
To examine the accuracy of community clinicians’ diagnoses of bvFTD and to identify patient characteristics leading to misdiagnosis, we reviewed the charts and referral letters of 3,578 patients who were seen at our specialized center. Referral diagnosis and reasons, manifesting symptoms, demographic data, Mini-Mental State Examination score, Clinical Dementia Rating score and Neuropsychiatric Inventory score were extracted.
Results
60% of patients assigned a single diagnosis of bvFTD by community clinicians did not have bvFTD according to specialists. Compared to specialist-confirmed bvFTD patients, false bvFTD patients were more likely to be depressed and to be non-Caucasian, showed less euphoria, apathy, disinhibition and abnormal eating behaviors, had milder disease severity and better overall cognition. bvFTD was mentioned by referring clinicians in 86% of specialist-confirmed bvFTD cases, but missed cases were called Alzheimer’s, Parkinson’s or Huntington’s disease, or progressive aphasia.
Conclusion
These results revealed a widespread lack of familiarity with core diagnostic symptoms among non-specialists and suggest that community clinicians require specialized diagnostic support before providing a definitive diagnosis of bvFTD.
Keywords: Frontotemporal dementia, Misdiagnosis, Community clinicians, Diagnostic criteria
Introduction
Behavioral variant frontotemporal dementia (bvFTD) is a neurodegenerative disorder caused by focal degeneration of the frontal and anterior temporal lobes; it has an incidence and prevalence similar to Alzheimer’s disease (AD) among young-onset patients [1]. The diagnosis of bvFTD relies upon subjective behavioral features, including behavioral disinhibition, apathy or loss of interest, loss of sympathy or empathy, compulsive stereotypic behavior and dietary changes [2]. An accurate diagnosis is important because bvFTD affects patients’ lives and has profound implications for their families and communities [3]. Burden and stress are higher among bvFTD caregivers than those of patients with AD or other dementias [4–6]. When patients are given an incorrect diagnosis, they may receive inappropriate treatment causing increased distress. Previous reports suggest that problems leading to misdiagnosis of bvFTD include patients’ younger age at onset and failure of clinicians to obtain key diagnostic information [7].
Unfortunately, accurate identification of these patients can be difficult for clinicians, including primary care physicians, geriatricians, general psychiatrists and general neurologists, who do not specialize in the assessment of neurodegenerative syndromes. As a result, bvFTD is often mistaken for AD or other conditions including psychiatric disorders, such as late-onset schizophrenia, atypical psychosis and depression [8–11]. Our group has previously reported that patients with bvFTD were significantly more likely than patients with other neurodegenerative diseases to receive a psychiatric diagnosis from a non-specialist [11]. Alternatively, though non-specialist clinicians have become more aware of bvFTD as an entity, they may erroneously interpret their patients’ symptoms as indicating bvFTD when the patient has another neurologic or psychiatric disorder. Patients with AD presenting with agitation and aggression, which occur frequently in AD [12], can be diagnosed as bvFTD due to the difficulty of delineating the whole symptom profile necessary for differential diagnosis. The resulting confusion and social upheaval for the patient and their family can be highly distressing.
While this problem of under- and overdiagnosis of patients with bvFTD by non-specialist clinicians has been pointed out several times [13–17], concrete prevalence rates of misdiagnosis are largely unavailable. There is little opportunity to obtain secondary validation of diagnosis accuracy in the primary care setting. Thus, unbiased epidemiologic sampling to identify true rates of misdiagnosis of bvFTD is almost impossible. Instead, the best available data come from an analysis of referrals to tertiary care centers specializing in bvFTD diagnosis that perform rigorous and extensive diagnostic testing of patients in order to provide the most accurate diagnosis possible. While referrals to such ‘FTD centers’ are actually more likely to be biased, with providers more likely to send their patients suspected as having bvFTD, careful examination of such misdiagnoses can still provide important information about what is leading clinicians to misunderstand their patients’ symptoms. The University of California, San Francisco (UCSF) Memory and Aging Center is a behavioral neurology tertiary care center founded in 1998 that specializes in non-Alzheimer’s dementias such as bvFTD.
For this study we (1) quantified the accuracy of bvFTD diagnoses of patients referred to our center over the last decade and (2) examined the clinical characteristics leading to misdiagnosis by referring clinicians. We also analyzed referral information to reveal any major factors that could be used to encourage non-specialists to refer when the patient’s symptom profile is sufficiently unclear or has features commonly leading to misdiagnosis.
Methods
During their clinical visit at our center, patients underwent diagnostic evaluation by a multidisciplinary team who performed behavioral, neurological and neuropsychological assessments; in addition, caregiver interviews were done to ensure comprehensive diagnostic histories. After a multistage selection process (see online supplementary detailed methods, www.karger.com/doi/10.1159/000438454) we reviewed the charts of 3,578 patients to extract referring symptoms, diagnostic differential, referring clinician’s level of certainty and any previous psychiatric diagnosis. Additionally, information pertaining to the patients’ clinical evaluation at UCSF was also reviewed, including demographic factors such as sex, age, race, education, UCSF final diagnosis, Mini-Mental State Examination (MMSE) score [18], Neuropsychiatric Inventory (NPI) score [19] and Clinical Dementia Rating (CDR) score [20].
All patients included in the study were assigned a diagnosis during their UCSF evaluation consistent with the research criteria available at the time of evaluation. Careful attention was paid to issues of FTD terminology, and cases where the intended diagnosis was not identifiable due to unclear language were designated as such. We determined referring clinicians’ diagnostic sensitivity, specificity, positive predictive value and false-positive rates over every year of the study, and examined clinical features of patients in various referral categories (see online supplementary detailed methods).
Results
General Results
Analysis of referring clinicians’ specialty showed that the majority of referrals came from primary care or internal medicine physicians (32.0%), followed by neurologists (22.1%), mental health professionals (psychiatrists or psychologists) (18.6%) and other specialist physicians (8.7%), with 18.6% coming from sources where the referring clinician’s specialty could not be determined. 38.8% of all subjects came from the San Francisco Bay Area, 61.4% from California and 94.8% from the United States. The referral rate from other departments within UCSF was 18.5%.
Of the 3,578 patients included in the study, 682 (19.1%) were suspected to have an FTD syndrome by referring clinicians (i.e. bvFTD, semantic variant primary progressive aphasia or nonfluent variant primary progressive aphasia). The mean age of these FTD syndrome patients was 61.2 years (standard deviation 12.7), 53% were male, mean education was 15.9 years (standard deviation 5.8), and 85% of them were Caucasians.
These patients’ referral diagnoses were categorized as (1) just bvFTD without any differential (n = 247, 36.2%), (2) mentioning bvFTD but not definitively (n = 188, 27.6%), (3) bvFTD and/or AD (n = 90, 13.2%), (4) bvFTD and/or motor neuron disease (n = 50, 7.3%), (5) bvFTD or other types of dementia (n = 11, 1.6%), (6) bvFTD or Pick’s disease (n = 10, 1.5%), (7) bvFTD or psychosis (n = 12, 1.8%) and (8) primary progressive aphasia (PPA) syndrome (n = 74, 10.9%). The cross-tabulation of referral diagnosis and specialist diagnosis is summarized in table 1.
Table 1.
Cross-tabulation of referral diagnosis versus expert diagnosis (bold figures represent incorrectly diagnosed bvFTD patients, i.e. false-positive and false-negative diagnoses)
| Expert diagnosis | Total | ||||||
|---|---|---|---|---|---|---|---|
| bvFTD | PPA | not FTD | |||||
| Referral diagnosis | FTD mentioned | bvFTD mentioned |
only bvFTD | 100 | 18 | 129 | 247 |
| bvFTD + other | 66 | 50 | 245 | 361 | |||
| ‘PPA’ mentioned | 6 | 32 | 36 | 74 | |||
| no mention of FTD | just ‘dementia’ | 2 | 0 | 106 | 108 | ||
| other diagnosis | 18 | 15 | 2,359 | 2,392 | |||
| no clear diagnosis mentioned | 2 | 4 | 390 | 396 | |||
| Total | 194 | 119 | 3,265 | 3,578 | |||
Accuracy Identifying FTD Syndrome
The ability of the referring clinicians to predict that their patient had one of the three FTD syndromes (i.e. their positive predictive value as diagnosticians) was 0.40 ([172 + 100]/682), while their ability to correctly include FTD syndromes in their diagnostic differential had a sensitivity of 0.87 ([172 + 100]/313) and a specificity of 0.87 (2,855/3,265). This describes their likelihood of mentioning an FTD syndrome diagnosis as one possibility in their referral, not their ability to definitively isolate the FTD syndrome. Among patients later identified as correctly having an FTD syndrome, their referring clinician included other diagnoses on the differential in 52.9% of cases.
Accuracy Identifying bvFTD Syndrome
The ability of referring clinicians to accurately predict that their patient had bvFTD was much lower, at only 0.27 (166/608), while their diagnostic sensitivity was 0.86 (166/194) and specificity was 0.87 ([32 + 36 + 19 + 2,855]/[119 + 3,265]).
False-Positive Diagnosis of bvFTD
There were 247 patients whose clinician referred them with a sole diagnosis of bvFTD. Of these, 60.0% (147/247) did not have bvFTD according to specialists.
Specialist Diagnoses of Patients Mistaken for bvFTD
Among the 147 subjects incorrectly declared to have bvFTD by referring clinicians, the most common correct diagnoses were AD (n = 27, 18.4%), followed by PPA (n = 18, 12.2%), clinically normal (n = 12, 8.2%), corticobasal syndrome or progressive supranuclear palsy (n = 11, 7.5%) and dementia with Lewy bodies (n = 3, 2.0%) according to specialists. The other diagnoses included psychiatric conditions such as depression, bipolar spectrum disorder and psychosis.
Symptoms Triggering a bvFTD Diagnosis
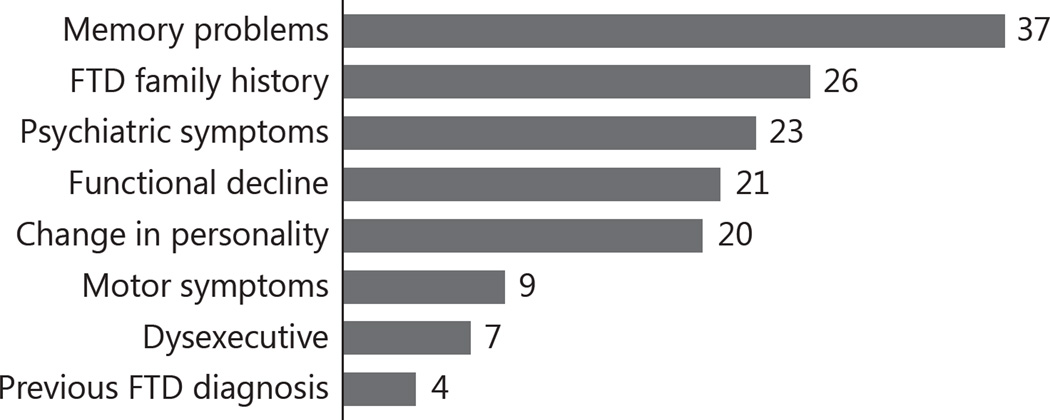
The referral documentation was examined to identify the primary symptoms on which referring clinicians focused to make their diagnosis of bvFTD (fig. 1). Among them, the most frequent symptom was unspecified memory problems (n = 37, 25.2%), followed by family history without any specific symptom (n = 26, 17.7%), psychiatric symptoms including depression, bipolar spectrum symptom and psychosis (n = 23, 15.6%), general functional decline (n = 21, 14.3%) and behavior and personality change (n = 20, 13.6%).
Fig. 1.
Main symptoms described by referring physicians in their referral documentation, among 147 subjects mistakenly diagnosed with bvFTD.
Demographics and Neuropsychiatric Symptoms of Patients Mistaken for bvFTD
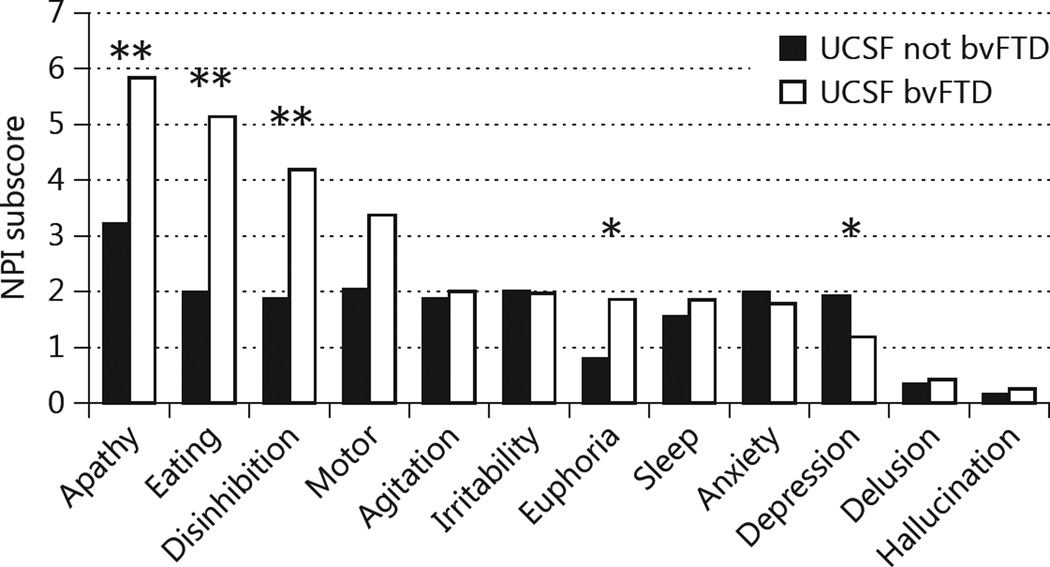
We examined data from the UCSF clinical evaluation for the 247 patients referred as bvFTD and compared the demographics and neuropsychiatric symptoms between those who were correctly and incorrectly diagnosed by community clinicians (table 2). Non-Caucasians were significantly more likely be incorrectly diagnosed as bvFTD by non-specialists; 6 were Asian Indian (vs. 0 with specialist-confirmed bvFTD) and 13 were East Asian (vs. 3 with specialist-confirmed bvFTD). Patients incorrectly diagnosed as bvFTD had significantly better general cognitive function (MMSE) and functional capacity (CDR Sum of Boxes) than specialist-confirmed bvFTD patients. They were significantly more depressed, but less likely to show euphoria, apathy, disinhibition or abnormal eating behaviors than patients diagnosed with bvFTD by experts (fig. 2).
Table 2.
Comparison of the demographics and result at the initial assessment between those who were correctly diagnosed and those who were mistaken for bvFTD
| Referred as bvFTD |
Signifi- cance |
Test | ||
|---|---|---|---|---|
| UCSF not bvFTD (n = 147) |
UCSF bvFTD (n = 100) |
|||
| Age, years | 57.32 ± 15.70 | 59.93 ± 8.79 | 0.133 | t test |
| Sex, m:f | 61:86 | 39:61 | 0.695 | χ2 test |
| Education, years | 15.62 ± 3.43 | 16.06 ± 3.27 | 0.336 | t test |
| Race, Caucasian:others | 103:44 | 82:18 | 0.034 | χ2 test |
| MMSE | 25.01 ± 6.20 | 20.99 ± 8.01 | 0.000 | t test |
| CDR-SOB | 4.02 ± 3.55 | 7.80 ± 3.70 | 0.000 | Mann-Whitney test |
| NPI-total | 30.49 ± 27.46 | 30.49 ± 27.46 | 0.028 | Mann-Whitney test |
Figures are mean ± SD or n. CDR-SOB = CDR Sum of Boxes.
Fig. 2.
Comparison of neuropsychiatric features between patients correctly and incorrectly diagnosed with bvFTD. * p < 0.05; ** p < 0.01.
Missed Diagnosis of bvFTD (False-Negative Errors)
Among 194 subjects UCSF-diagnosed as bvFTD, 28 (14.4%) did not have bvFTD mentioned in their referring clinician’s differential diagnosis, i.e. these bvFTD cases were apparently missed by their doctors.
False Referral Diagnoses of Actual bvFTD Patients
Among the 28 false-negative subjects, the referral diagnosis was AD (n = 12, 42.9%), PPA (n = 6, 21.4%), Parkinson’s disease-related dementia (n = 5, 17.9%), unspecified dementia (n = 2, 7.1%), Huntington’s disease (n = 1, 3.6%) and no clear diagnosis mentioned (n = 2, 7.1%). Among these 28 patients, 3 (10.7%) had previously been diagnosed at some point in their history with a psychiatric disorder, including depression and bipolar disorder.
Symptoms Noted by Referring Clinicians
Among these 28 subjects, the main symptoms that the referring clinicians focused on in their referral reason included language symptoms such as poor language production (n = 16, 57.1%), memory symptoms (n = 6, 21.4%) and behavior changes, which included apathy, irritability or mood change and stereotypic behaviors.
Demographics and Neuropsychiatric Symptoms of bvFTD Patients Misdiagnosed by Their Referring Clinicians
We examined the demographics and clinical features at initial UCSF assessment for all true bvFTD patients, including both the 166 patients for whom bvFTD was correctly mentioned by their referring clinicians and the 28 patients in whom bvFTD was missed entirely. There were no significant differences between the two groups in age, race, education, MMSE score, CDR score, NPI total or any NPI subscore. There was a significant difference between the two groups only in sex ratio (p = 0.028); male patients with true bvFTD were more likely to have their diagnosis missed by their referring doctor.
Sensitivity/Specificity Rates over the Last Decade
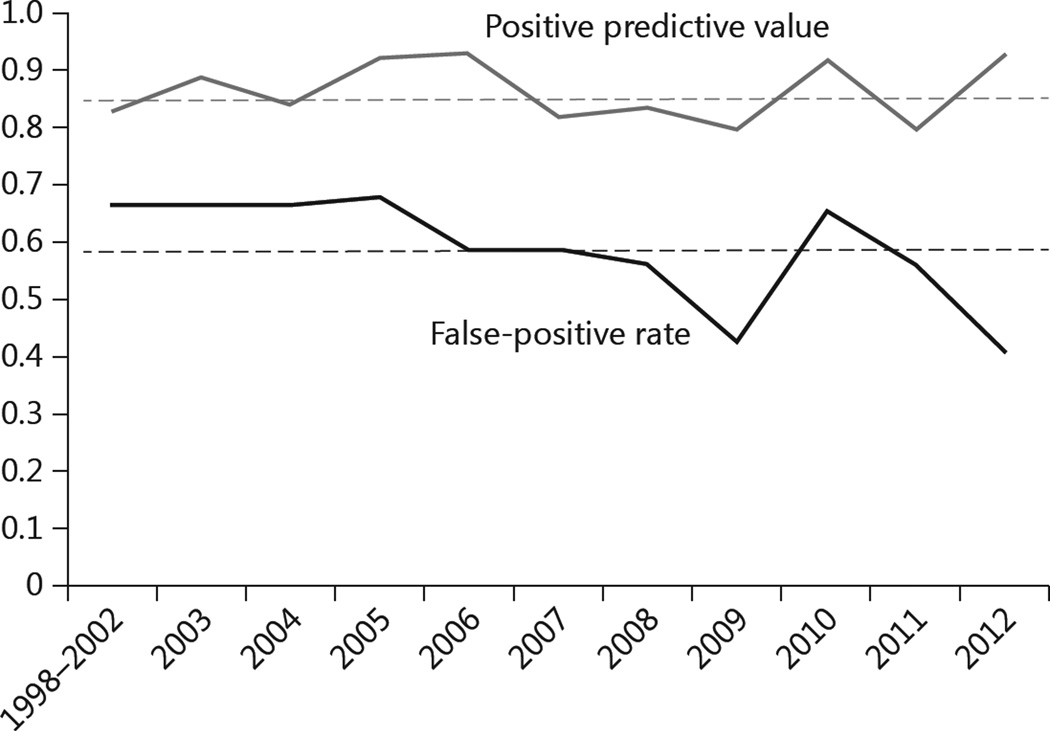
We analyzed diagnostic specificity and sensitivity rates for community referrals across a 14-year time period. The positive predictive value and the false-positive rate of referring doctors for each year are summarized in figure 3. The positive predictive value, i.e. the ratio that the referring doctors put bvFTD on their differential among UCSF-diagnosed bvFTD cases, tended to remain stable around a mean of 0.86 throughout the research period. On the other hand, false-positive rates, i.e. the tendency for referring clinicians to definitively diagnose bvFTD in cases where the patient did not have bvFTD, fluctuated significantly around a mean of 0.59, but appear to have decreased overall through the research period.
Fig. 3.
Changes of positive predictive value and false-positive rate by year. The positive predictive value includes patients whose referral mentioned bvFTD alone or with other diagnoses. The falsepositive rate includes subjects referred as bvFTD only.
Discussion
This study found that clinicians referring to our specialized center made both falsepositive and false-negative errors in diagnosing bvFTD. Only 40% of the 247 subjects referred with a definitive diagnosis of bvFTD were found to have bvFTD by specialists. False bvFTD subjects were more likely to be depressed and non-Caucasian. Compared to true bvFTD patients, they showed less euphoria, apathy, disinhibition and abnormal eating behaviors, had milder disease severity and had better overall cognition. In contrast, 86% of true bvFTD patients were mentioned as bvFTD at least on the referring doctor’s differential diagnosis. Males with true bvFTD were significantly more likely to be missed by referring clinicians than females.
Reasons for False-Positive Diagnosis of bvFTD
The patients most likely to be erroneously diagnosed with bvFTD by referring doctors were likely to have AD or psychiatric disorders. AD has a wide symptomatic diversity and can produce behavioral changes resembling bvFTD [21, 22], particularly with comorbid pathology such as cardiovascular disease [23]. Our results suggest that non-specialists are more likely to use memory problems, psychiatric symptoms and general functional decline as reasons for a diagnosis of bvFTD. This suggests that one reason for incorrect bvFTD diagnosis by non-specialists is their lack of familiarity with the consensus bvFTD diagnostic criteria [2], which require at least three specific behavioral symptoms (i.e. disinhibition, apathy, loss of empathy, stereotypic behavior or dietary changes). Additionally, these criteria indicate that there should be comparative sparing of memory, thus the fact that memory was the primary deficit in 25% of falsely diagnosed bvFTD patients again suggests that non-specialists are not correctly using standard diagnostic criteria.
On behavioral assessment, false bvFTD patients showed significantly less euphoria, apathy, disinhibition and abnormal eating behaviors, which are typical bvFTD behavioral symptoms, than specialist-confirmed bvFTD patients [2]. On the other hand, falsely diagnosed bvFTD patients were found to have significantly higher levels of depression. While depression and apathy are neurologically distinct symptoms important for differential diagnosis, they are clinically related and can easily be confused [24–26]. Non-Caucasians, especially individuals of Indian or East Asian descent, were more likely to be erroneously diagnosed with bvFTD. This suggests that some ethnic or cultural bias might increase clinicians’ confusion about the diagnosis of bvFTD. These issues could be valuable targets for further continuing education of clinicians.
In the past decade, efforts have been made to educate the community about bvFTD and related disorders, which was almost unrecognized until 20 years ago [27]. Although awareness of the disorder has likely increased among community doctors, our data suggest that clinicians remain undereducated about the disease-specific symptoms and clinical characteristics, resulting in a high likelihood that they will give a definitive bvFTD diagnosis incorrectly. One encouraging finding is that a large subset of referring clinicians raised several differential diagnoses including bvFTD. Given the high likelihood of diagnostic inaccuracy suggested in this study, non-specialists should not be confident in their ability to definitively diagnose bvFTD, and in the absence of specialized diagnostic support, should simply refer patients to a specialist when bvFTD is suspected. General clinicians treating middle-aged and older adults should maintain active connections with neurodegenerative syndrome specialists.
Reasons Clinicians Miss the Diagnosis of bvFTD
Fourteen percent of patients diagnosed at UCSF as having bvFTD had no mention of bvFTD in their referring doctor’s differential diagnosis. The referral diagnoses of these false-negative bvFTD patients were diverse, but AD was most common. Reasons for referral included language and memory symptoms, but behavior changes more consistent with bvFTD were also noted, including apathy, irritability, mood change and stereotypic behaviors. Referring doctors may be more aware that language symptoms are common in AD, which may in turn have led them to rule out bvFTD; however, this error was mitigated by the fact that clinicians chose to refer these patients for evaluation at our center, where more careful assessment of these language symptoms could be performed for an accurate differential diagnosis. Males were significantly more likely to be missed by referring clinicians, which may be due to the influence of sociocultural factors on these clinicians’ interpretation of bvFTD-specific behaviors, which they may have been more likely to recognize as pathological when they appeared in females than in males.
Diagnostic Accuracy over the Last Decade
The likelihood that the referring clinicians would put bvFTD on their differential in cases where UCSF specialists confirmed bvFTD appears have remained stable at our center over the past 14 years. The tendency for referring clinicians to diagnose bvFTD in cases where the patient did not have bvFTD has fluctuated widely during this time. The 60% overall rate of false-positive diagnosis of bvFTD by community non-specialists referring to UCSF incorporates past years, i.e. 1998–2005, when this problem was more obvious, though rates have decreased below 60% in the past 2 years. The factors leading to this recent decrease are unclear, however rates were still high as recently as 2010, and it is uncertain whether this trend will continue as data are collected in future years.
Limitations
Referrals to ‘FTD centers’ are more likely to be biased, with general clinicians more likely to send their patients who they suspect to have bvFTD symptoms. Because of this, our base rates for diagnosis of bvFTD among referring clinicians are likely to be inflated. Second, this was a retrospective study based on examination of referral clinical documentation, thus there was necessarily some uncertainty around pinpointing the referral clinicians’ thought process. Another source of ambiguity in this study came from the shifting clinical feature definitions and terminology used for the FTD syndromes. Importantly, in the chart review process, all efforts were made to ascertain the syndrome to which clinicians were actually referring by using all data provided, and cases where there was ambiguity about the intentions of the referring clinician were excluded from our analysis. The result is that our study represents a conservative estimate of the problem of bvFTD overdiagnosis in the community, because it is likely that an even larger number of patients were believed to have bvFTD by referring clinicians, but because this diagnosis did not come through clearly in their report, it was not considered to be a false-positive diagnosis in our study. Also, because of our study design, multiple referrals might have come from the same clinician, which may have distorted the referral bias rates.
Conclusion
Reports by referring physicians, as well as the symptoms found in falsely diagnosed bvFTD patients, suggest a lack of familiarity among non-specialists with the diagnostic criteria of bvFTD. More than a decade after the community has become aware of bvFTD, more widespread and accurate training about the clinical features of bvFTD and the standards for diagnosing this syndrome is still needed to better educate non-specialists. These results suggest that, in the absence of specialized diagnostic support, non-specialist clinicians should be encouraged not to provide a definitive diagnosis of bvFTD themselves, but to refer patients for a specialized diagnostic consultation in cases where they suspect bvFTD.
Supplementary Material
Acknowledgments
Financial support for this research and any potential conflicts are as follows: Dr. Rankin received funding from NIH (5-R01 AG029577) and UCSF (REAC). Dr. Miller receives institutional support from NIH (P50AG023501, P01AG019724) and the Larry L. Hillblom Foundation (2007/2I).
Footnotes
Author Contributions
Dr. Shinagawa: design and conceptualization of the study, acquisition of data, analysis and interpretation of data, drafting of the manuscript. Dr. Catindig: design and conceptualization of the study, acquisition of data, analysis and interpretation of data, drafting of the manuscript. Dr. Block: acquisition of data, drafting of the manuscript. Dr. Rankin: design and conceptualization of the study, analysis and interpretation of the data, drafting of the manuscript. Dr. Miller: design and conceptualization of the study, drafting of the manuscript.
Disclosures Statement
The other authors have nothing to declare.
References
- 1.Shinagawa S, Ikeda M, Toyota Y, Matsumoto T, Matsumoto N, Mori T, Ishikawa T, Fukuhara R, Komori K, Hokoishi K, Tanabe H. Frequency and clinical characteristics of early-onset dementia in consecutive patients in a memory clinic. Dement Geriatr Cogn Disord. 2007;24:42–47. doi: 10.1159/000102596. [DOI] [PubMed] [Google Scholar]
- 2.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diehl-Schmid J, Schmidt EM, Nunnemann S, Riedl L, Kurz A, Förstl H, Wagenpfeil S, Cramer B. Caregiver burden and needs in frontotemporal dementia. J Geriatr Psychiatry Neurol. 2013;26:221–229. doi: 10.1177/0891988713498467. [DOI] [PubMed] [Google Scholar]
- 4.Riedijk SR, De Vugt ME, Duivenvoorden HJ, Niermeijer MF, Van Swieten JC, Verhey FR, Tibben A. Caregiver burden, health-related quality of life and coping in dementia caregivers: a comparison of frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22:405–412. doi: 10.1159/000095750. [DOI] [PubMed] [Google Scholar]
- 5.Boutoleau-Bretonnière C, Lebouvier T, Volteau C, Jaulin P, Lacomblez L, Damier P, Thomas-Anterion C, Vercelletto M. Prospective evaluation of behavioral scales in the behavioral variant of frontotemporal dementia. Dement Geriatr Cogn Disord. 2012;34:75–82. doi: 10.1159/000341784. [DOI] [PubMed] [Google Scholar]
- 6.Mioshi E, Bristow M, Cook R, Hodges JR. Factors underlying caregiver stress in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2009;27:76–81. doi: 10.1159/000193626. [DOI] [PubMed] [Google Scholar]
- 7.Nunnemann S, Kurz A, Leucht S, Diehl-Schmid J. Caregivers of patients with frontotemporal lobar degeneration: a review of burden, problems, needs, and interventions. Int Psychogeriatr. 2012;24:1368–1386. doi: 10.1017/S104161021200035X. [DOI] [PubMed] [Google Scholar]
- 8.Mendez MF. Functional neuroimaging and presenting psychiatric features in frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2006;77:4–7. doi: 10.1136/jnnp.2005.072496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendez MF, Shapira J, Woods RJ, Licht EA, Saul RE. Psychotic symptoms in frontotemporal dementia: prevalence and review. Dement Geriatr Cogn Disord. 2008;25:206–211. doi: 10.1159/000113418. [DOI] [PubMed] [Google Scholar]
- 10.Passant U, Elfgren C, Englund E, Gustafson L. Psychiatric symptoms and their psychosocial consequences in frontotemporal dementia. Alzheimer Dis Assoc Disord. 2005;19(suppl 1):S15–S18. doi: 10.1097/01.wad.0000183084.22562.5a. [DOI] [PubMed] [Google Scholar]
- 11.Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease. J Clin Psychiatry. 2011;72:126–133. doi: 10.4088/JCP.10m06382oli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballard CG, Gauthier S, Cummings JL, Brodaty H, Grossberg GT, Robert P, Lyketsos CG. Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol. 2009;5:245–255. doi: 10.1038/nrneurol.2009.39. [DOI] [PubMed] [Google Scholar]
- 13.Cardarelli R, Kertesz A, Knebl JA. Frontotemporal dementia: a review for primary care physicians. Am Fam Physician. 2010;82:1372–1377. [PubMed] [Google Scholar]
- 14.Seltman RE, Matthews BR. Frontotemporal lobar degeneration: epidemiology, pathology, diagnosis and management. CNS Drugs. 2012;26:841–870. doi: 10.2165/11640070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Cerami C, Scarpini E, Cappa SF, Galimberti D. Frontotemporal lobar degeneration: current knowledge and future challenges. J Neurol. 2012;259:2278–2286. doi: 10.1007/s00415-012-6507-5. [DOI] [PubMed] [Google Scholar]
- 16.Warren JD, Rohrer JD, Rossor MN. Clinical review. Frontotemporal dementia. BMJ. 2013;347:f4827. doi: 10.1136/bmj.f4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pressman PS, Miller BL. Diagnosis and management of behavioral variant frontotemporal dementia. Biol Psychiatry. 2014;75:574–581. doi: 10.1016/j.biopsych.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5 suppl 6):S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 20.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 21.Warren JD, Fletcher PD, Golden HL. The paradox of syndromic diversity in Alzheimer disease. Nat Clin Pract Neurol. 2012;8:451–464. doi: 10.1038/nrneurol.2012.135. [DOI] [PubMed] [Google Scholar]
- 22.Lam B, Masellis M, Freedman M, Stuss DT, Black SE. Clinical, imaging, and pathological heterogeneity of the Alzheimer’s disease syndrome. Alzheimers Res Ther. 2013;5:1. doi: 10.1186/alzrt155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rankin KP, Santos-Modesitt W, Kramer JH, Pavlic D, Beckman V, Miller BL. Spontaneous social behaviors discriminate behavioral dementias from psychiatric disorders and other dementias. J Clin Psychiatry. 2008;69:60–73. doi: 10.4088/jcp.v69n0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tagariello P, Girardi P, Amore M. Depression and apathy in dementia: same syndrome or different constructs? A critical review. Arch Gerontol Geriatr. 2008;49:246–249. doi: 10.1016/j.archger.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Landes AM, Sperry SD, Strauss ME. Prevalence of apathy, dysphoria, and depression in relation to dementia severity in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2005;17:342–349. doi: 10.1176/jnp.17.3.342. [DOI] [PubMed] [Google Scholar]
- 26.Starkstein SE, Ingram L, Garau ML, Mizrahi R. On the overlap between apathy and depression in dementia. J Neurol Neurosurg Psychiatry. 2005;76:1070–1074. doi: 10.1136/jnnp.2004.052795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J Neurol Neurosurg Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.