Abstract
Acute exposure to ambient fine particulate matter (PM2.5) is tied to cardiovascular morbidity and mortality, especially among those with prior cardiac injury. The mechanisms and pathophysiologic events precipitating these outcomes remain poorly understood but may involve inflammation, oxidative stress, arrhythmia, and autonomic nervous system imbalance. Cardiomyopathy results from cardiac injury, is the leading cause of heart failure, and can be induced in heart failure-prone rats through sub-chronic infusion of isoproterenol (ISO). To test whether cardiomyopathy confers susceptibility to inhaled PM2.5 and can elucidate potential mechanisms, we investigated the cardiophysiologic, ventilatory, inflammatory, and oxidative effects of a single nose-only inhalation of a metal-rich PM2.5 (580 μg/m3, 4h) in ISO-pretreated (35 days * 1.0 mg/kg/day sc) rats. During the 5 days post-treatment, ISO-treated rats had decreased HR and BP and increased pre-ejection period (PEP, an inverse correlate of contractility) relative to saline-treated rats. Before inhalation exposure, ISO-pretreated rats had increased PR and ventricular repolarization time (QT) and heterogeneity (Tp-Te). Relative to clean air, PM2.5 further prolonged PR-interval and decreased systolic BP during inhalation exposure; increased tidal volume, expiratory time, heart rate variability (HRV) parameters of parasympathetic tone, and atrioventricular block arrhythmias over the hours post-exposure; increased pulmonary neutrophils, macrophages, and total antioxidant status one day post-exposure; and decreased pulmonary glutathione peroxidase 8 weeks after exposure, with all effects occurring exclusively in ISO-pretreated rats but not saline-pretreated rats. Ultimately, our findings indicate that cardiomyopathy confers susceptibility to the oxidative, inflammatory, ventilatory, autonomic, and arrhythmogenic effects of acute PM2.5 inhalation.
Keywords: air pollution, particulate matter, cardiovascular, heart rate variability, arrhythmia, autonomic, electrocardiography, rats
Introduction
Epidemiological studies have shown that short-term exposure to particulate air pollution is associated with heart failure hospitalization and mortality (Burnett et al. 1995; Dominici et al. 2006; Goldberg et al. 2000; Shah et al. 2013; Wellenius et al. 2005). For instance, Shah and colleagues (2013) found that hospitalization or death related to heart failure increased by more than 2% for each 10 μg/m3 increase in short-term ambient PM2.5, with the strongest associations observed on the day of exposure. While the mechanisms underlying the increased physiological responses of individuals with heart failure to PM exposure remain largely unexplained, acute adverse cardiovascular outcomes are generally believed to result from physiologic disturbances involving modulation of the autonomic nervous system (ANS), cardiac ion channel, and vascular function; myocardial ischemia, oxidative stress and inflammation (Brook et al. 2010; Schulz et al. 2005). Given the high and growing prevalence of heart failure in the population, scientific investigation is merited to identify mechanisms that might explain why individuals with heart failure are at risk from short-term exposure to particulate pollution.
Animal models have proven valuable surrogates of human disease and provide a way to better understand fundamental biological mechanisms. The Spontaneously Hypertensive Heart Failure rat (SHHF, MccCrl-Leprcp) was established as a model of heart failure and has found utility in modeling heart failure in man. This model was developed from the Spontaneously Hypertensive Rat (SH) and has a phenotype that predisposes to hypertension, insulin resistance, and age-dependent cardiomyopathic remodeling of the left ventricle and heart failure (Carll et al. 2011b). The SHHF model has been used to demonstrate that inhalation of PM induces multiple biochemical and physiological responses (Carll et al. 2010; Carll et al. 2012; Carll et al. 2013a; Carll et al. 2013b) that can be summarized as changes in pulmonary and systemic inflammation, and modulation of autonomic input to the heart resulting an increased parasympathetic dominance. However, these investigations were limited to the study of diesel exhaust and high doses of residual oil fly ash (ROFA).
The purpose of this study is to examine the biological and physiological effects associated with a lower concentration of PM, more consistent with major urban areas throughout the world, in a rat model of cardiomyopathy. In the current study, we tested the hypothesis that rats with cardiomyopathy would be more susceptible to the cardiovascular effects of metal-rich PM2.5 than similarly exposed rats with minimal cardiomyopathy. To induce cardiomyopathy, young adult heart failure-prone rats were chronically infused with isoproterenol (ISO), a synthetic catecholamine. We assessed the effects of PM2.5 on cardiovascular and pulmonary physiology including electrocardiography (ECG), heart rate, heart rate variability (HRV, a measure of autonomic modulation of the heart), arrhythmia, BP, and ventilatory parameters, as well as on blood and pulmonary biomarkers of injury, inflammation, and oxidative stress.
Methods
Animals
Lean male Spontaneously Hypertensive Heart Failure (SHHF, MccCrl-Leprcp, n=48) rats were obtained from Charles River Laboratories (CRL, Kingston, NY). These rats acquire cardiac hypertrophy by 2 months of age and dilated cardiomyopathy and overt heart failure at 18 months (Carll et al. 2012). Intermediately, neurohormonal and sympathetic activation compensate for a loss of contractile function and promote myocardial remodeling. SHHF rats are more sensitive to cardiopulmonary effects of air pollutants than the normotensive and hypertensive strains from which they are derived (Carll et al. 2012), and this vulnerability is further accentuated by ISO-induced cardiomyopathy (Carll et al. 2010). Based on our previous experience with this model (Carll et al. 2010; Carll et al. 2011a), we modified our procedures in the present study to minimize premature mortality and incorporate PM exposures at levels more pertinent to environmental exposure.
Study Regimen
At 2-4 weeks after telemeter implantation, infusion pumps were implanted. Pumps were surgically removed after 5 weeks of continuous infusion, and all rats received nose-only acclimations at 3 d and 4 d post-pump removal. At 5 d post-pump removal, all rats were exposed for 4 h to sROFA or clean air. Necropsies occurred at 24 h post-exposure for non-telemetered rats and at 8 wks post-exposure for telemetered rats. Regimen summary: Implant telemeter → Implant infusion pump (ISO or saline) → Remove pump at 42 days → 5 d later, expose for 4 h to PM or filtered air.
Radiotelemeter Implantation
At 10-11 weeks of age, 20 rats were surgically implanted at CRL with radiotelemeters (model TL11M2-C50-PXT, Data Sciences International) capable of transmitting aortic BP, ECG, activity, and core body temperature. Surgeries involved catheterization of the descending abdominal aorta and subcutaneous ECG placement in an approximate Lead II configuration as previously described (Carll et al. 2010). After a 10-day recovery period, the 20 telemetered rats were shipped with 28 other SHHFs to our AAALAC International-approved animal facility at the U.S. EPA. At 12-13 weeks of age 9 more rats were implanted in-house with radiotelemeters measuring ECG, HR, and core body temperature (model TA11CTA-F40) in an approximate ECG Lead II configuration. All studies conformed to the guidelines of the U.S. EPA Institutional Animal Care and Use Committee.
Pharmacologic Model of Cardiomyopathy
Similar to our previous studies, continuous sub-chronic infusion with ISO (Carll et al. 2010; Carll et al. 2011a) was used to induce cardiomyopathy in heart failure-prone rats. Rats were surgically implanted with subcutaneous osmotic pumps (Alzet Model 2004) as detailed elsewhere (Carll et al. 2011a) at 14-15 weeks containing either dl-isoproterenol hydrochloride (ISO, 1.0 mg/kg/day; n=24) or saline (0.9% NaCl, n=24). Isoproterenol is a synthetic catecholamine that can cause myocardial infarct-like necrosis, cardiac hypertrophy and fibrosis while also potentially impairing cardiac performance. Accordingly, systolic failure has been observed after a 6 week infusion of ISO (1.2 mg/kg/day s.c.) in previously healthy Wistar rats (Takeshita et al. 2008). In the present study, pumps were surgically removed after 5 weeks of infusion.
PM2.5 Inhalation Exposure
All rats were acclimated to nose-only restraint cones for 1 h on the third day after infusion ended and then again for 2 h on the next day. Five days after the infusion period, rats received a single 4-h exposure to either filtered air or 580 μg/m3 of PM2.5 (mass median aerodynamic diameter = 2.09 μm, geometric standard deviation = 3.37) in two separate 24-port nose-only flow-by inhalation chambers (Lab Products, Seaford, DE). All animals were allowed 20 min to stabilize in nose-only chambers and monitored by telemetry for the following 40 min while receiving filtered air for “Baseline” values. Subsequently, rats received either PM2.5 or clean air for 4 h, and filtered air for the 40-min immediately thereafter (“Recovery”). PM2.5 was suspended by a dry dust aerosol generator as previously described (Ledbetter et al. 1998). The PM2.5 used in this study was synthesized by combining metal compounds in molar ratios comparable to those in the historic ROFA sample collected as a post-control fugitive stack emission at a Florida Power and Light plant burning #6 grade residual oil containing 1% sulfur (Southern Research Institute, Birmingham, AL) (Hatch et al. 1985). ROFA-like dust was generated because of an insufficient supply of the original ROFA to perform inhalation studies. NiSO4, Fe2SO4, and NaVO3 (all from Sigma) were mixed in a porcelain mortar and placed on a rotary agitator for 24 h to generate a transition metal–rich PM2.5 suitable for inhalation exposure. During exposure, particle concentration was determined gravimetrically on Teflon filters (45-mm diameter with 1-μm pore size; VWR Scientific, West Chester, PA) using a Mercer cascade impactor (Intox Products, Albuquerque, NM), and real-time PM concentration was estimated with an aerosol monitor (Dust Track; TSI Inc., St Paul, MN) on the chamber exhaust. In a prior study, H2O- or HCl-extracts from the Teflon filters were analyzed quantitatively for 28 metals and sulfur (expressed as sulfate) (Hazari et al. 2009). Mean (± SD) of H2O-soluble content of metals and SO4 were as follows: Fe = 1118 ± 210, Ni = 10,144 ± 947, V = 21,849 ± 2342, and SO4 = 335,077 ± 28,529 μg/g. Mean (± SD) 1M HCl-soluble content of metals and SO4 in micrograms per gram were as follows: Fe = 135,586 ± 7904, Ni = 12,398 ± 713, V = 89,852 ± 5432, and SO4 = 421,845 ± 22,923 μg/g.
Cardiovascular Physiology
We continuously monitored activity, core body temperature, BP and ECG in conscious rats by radiotelemetry from the beginning of infusion to 8 weeks after inhalation exposure to PM2.5 (14 weeks after beginning of infusion) when all telemetered rats were euthanized. Arterial BP (mean, systolic, diastolic, and pulse pressures), HR, and aortic pre-ejection period (PEP) were automatically calculated by software (DataART 3.01; DSI) from pressure and ECG waveforms sampled at 1000 Hz for 2 of every 12 min within home cages (n=5/group) and 4 of every 10 min during nose-only inhalation exposure (n=5/group). ECG waveforms were analyzed with computer software (ECGauto 2.5.1.35; EMKA Technologies, Falls Church, VA) that enabled user identification and exclusion of arrhythmias and artifacts from automated analysis of ECG morphology and HRV parameters as previously detailed (Carll et al. 2012). ECG morphology parameters were based on P, QRS, and T waves and included the following: intervals of PR (beginning of P to R), QRS (Q to end of S), ST (S to T-peak), QT (Q to T-peak), QTc (HR-corrected QT using Fridericia correction), TpTe (T-peak to T-end, a measure of heterogeneity of repolarization) and amplitudes of Q, R, T-peak, and ST (mean amplitude between S and T-peak), relative to the isoelectric line (10 milliseconds [ms] before beginning of Q). ST negative area was calculated as total negative area underneath the isoelectric line from S-nadir to the beginning of the T. Q negative area was calculated as total area underneath the isoelectric line from beginning of Q to R. T-positive area was also calculated as the area above the isoelectric line from begin to end of the T.
HRV analysis generated HR and time-domain measures, including mean time between adjacent QRS-complex peaks (RR interval), standard deviation of the RR interval (SDNN), square root of the mean of squared differences of adjacent RR intervals (RMSSD), triangular index, and percent of adjacent normal RR intervals differing by ≥ 15 ms (pNN15). SDNN and triangular index represent overall HRV, whereas RMSSD and pNN15 represent parasympathetic influence over HR (Rowan et al. 2007). HRV analysis also provided frequency-domain parameters, including low-frequency (LF: 0.200–0.750 Hz) and high-frequency (HF: 0.75–2.00 Hz), and the ratio of these two frequency domains (LF/HF). For frequency-domain analysis, the signal was analyzed with a Hanning window for segment lengths of 512 samples with 50% overlapping. LF is generally believed to represent a combination of sympathetic and parasympathetic tone, whereas HF indicates cardiac parasympathetic (vagal) tone, and LF/HF serves as an index of sympathovagal balance (Rowan et al. 2007).
Arrhythmias were identified while blinded to treatment group according to previously described criteria (Carll et al. 2012). To facilitate statistical analysis and allow the data to converge under the Poisson distribution, zero values for each arrhythmia type within a sample interval were converted to 0.1. Arrhythmia frequencies were calculated over specific time-matched periods in home cages (pre-inhalation and post-inhalation, 7-h each) and in exposure chamber (baseline, mid-exposure, recovery), normalized to adjust for time differences between periods and gaps in data, and presented as number of events per hour of theoretically continuous ECG waveforms. Each premature beat was counted individually as a single event (e.g., 1 bigeminy = 2 ventricular premature beat [VPB] events), whereas atrioventricular (AV) or sinoatrial block arrhythmias were counted as one event regardless of duration or neighboring events. HRV and ECG morphologic analyses were conducted on ECG waveforms collected while rats resided in home cages at 1 day pre-exposure and immediately post-exposure during a 7-h time matched period to control for physiologic effects of circadian rhythm. ECG data collected within the exposure chamber (5 h 20 min total) were also analyzed according to the following periods: baseline (40 min), exposure (4 h), and recovery (40 min.). All ECG streams with less than 10 s of identifiable conduction cycles were excluded from ECG parameter calculation, and streams with less than 1 min of identifiable RR intervals were excluded from HRV analysis. Thorough visual inspection was conducted to identify and exclude arrhythmias and artifacts.
Respiratory Physiology
Data were collected on ventilatory parameters by a barometric whole-body plethysmography system (Buxco Electronics, Sharon, CT) permitting continuous monitoring of tidal volume, breathing frequency, minute volume, and related time parameters of breathing. At 1 d before inhalation exposure to PM2.5 or clean air, immediately following a 2-h nose-only acclimation, unrestrained rats (n=5 per group) were allowed to stabilize from handling in cylindrical plethysmographs for 1 min and then measured for 5 min. On the day of inhalation exposure, measurements were replicated immediately post-exposure as previously described (Carll et al. 2010). Ventilatory data were acquired again on the day following inhalation exposure immediately before necropsies. Daily calibration of plethysmography chambers (model PLY3213; Buxco Electronics) preceded every animal loading. A bias flow regulator delivered fresh air (1.8 L/min) to each cylindrical chamber, preventing CO2 buildup within the chamber.
Tissue Collection and Analysis
At approximately 24 h after onset of the 4-h inhalation exposure, non-telemetered rats were deeply anesthetized with an ip injection of a sodium pentobarbital/phenytoin solution and euthanized by exsanguination. At 8 wk after inhalation exposure, telemetered rats were similarly anesthetized and euthanized. Blood, lung lavage fluid, and tissue samples (heart and lungs) were collected, processed, and analyzed as previously described (Carll et al. 2011a; Carll et al. 2012). Bronchoalveolar lavage fluid was processed for biochemical analyses, total cell counts, and cell differentials as previously described (Carll et al. 2011a). Lavage macrophages, neutrophils, lymphocytes, and eosinophils were enumerated using light microscopy (500 cells per sample). To examine for indications of cardiopulmonary inflammation, injury, oxidative stress, and risk, multiple biochemical markers were assayed. Lavage, serum, and plasma samples were analyzed with a Konelab 30 clinical chemistry analyzer (Thermo Clinical Labsystems, Espoo, Finland) as previously described (Carll et al. 2010; Carll et al. 2011a). Lavage supernatants were analyzed for albumin, lactate dehydrogenase activity, N-acetyl-b-d-glucosaminidase activity, total protein, and total antioxidant status. Lavage glutathione peroxidase along with serum glutathione peroxidase, reductase, and -S-transferase were analyzed as previously described (Jaskot et al. 1983). Serum was also analyzed for creatine kinase, C-reactive protein, α-hydroxybutyrate dehydrogenase, high- and low-density lipoprotein cholesterol, lactate dehydrogenase-1, total protein, myoglobin, sorbitol dehydrogenase, and triglycerides as previously described (Carll et al. 2010; Carll et al. 2012). Plasma was analyzed for angiotensin converting enzyme, creatinine, and fibrinogen with the Konelab 30 analyzer as well as B-type natriuretic peptide (BNP) by ELISA as detailed elsewhere (Carll et al. 2011a; Carll et al. 2012).
Statistics
Time-series telemetry parameter data (HR, BP, PEP, core body temperature) collected during and after drug infusion were analyzed using the Fishing License Method (FLM) software as previously described (Carll et al. 2010; Nadziejko et al. 2004). Data were analyzed for 4- to 24-h significant effects (P < 0.05) of ISO (n=8) relative to saline (n=9) from the beginning of infusion until immediately before inhalation exposure. Data collected in home cages from 32 h pre- until 82 h post-inhalation exposure were also analyzed by FLM for significant 4‐h effects of inhalation exposure. The statistical analyses for all remaining data in this study were performed using Prism version 4.03 (GraphPad Software Inc., San Diego, CA). Two-way ANOVA with Bonferroni post hoc test was used to detect significant differences between groups in biochemical and cytological endpoints, tissue weight, and arrhythmia frequency during inhalation exposure. Repeated measures two-way ANOVA with Bonferroni's post hoc test was performed on (1) arrhythmia frequency data over the 7-h pre- and post-exposure periods; (2) HRV and ECG morphology parameters during the exposure period, including baseline, and recovery periods to examine for between-group differences in 1-h means; (3) between-group differences in change in HRV at post-exposure relative to pre-exposure (7 h post-exposure means minus 7 h means from time-matched period 1 d prior); and (4) plethymosgraph data collected on the day before and immediately after inhalation exposure. A value of p < 0.05 was considered statistically significant.
Results
Heart Rate, Ventricular Function and Body Weight during and after ISO Infusion
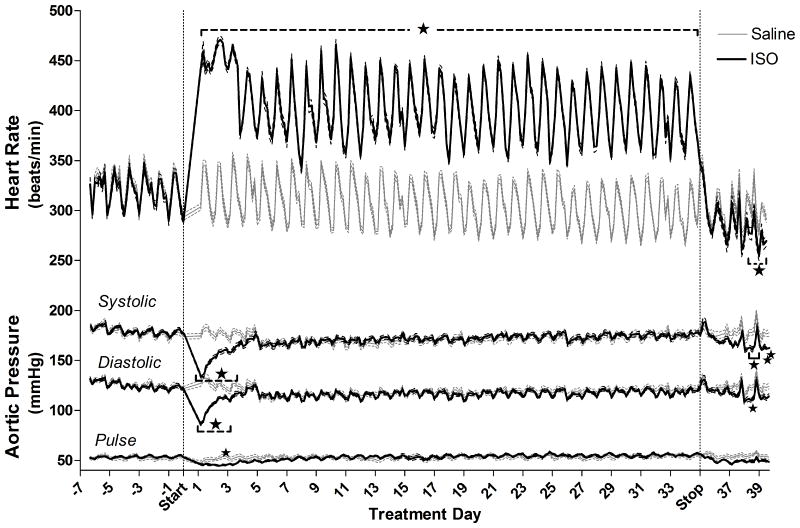
SO increased HR by 32% (effect estimate ± standard error: +97±5 BPM; Fig. 1), contractility by 13% as indicted by a decreased PEP (-5.0±0.2 ms; Fig. 2), and body weight by 15% (mean±standard error, ISO: 442±4, Saline: 384±6 g; P < 0.001) relative to saline over the entire 35-day infusion. ISO also significantly decreased systolic (effect estimate ± standard error: ‐25±3 mmHg) and diastolic (-23±3 mmHg) pressures for the first 3.5 d of infusion relative to saline, whereafter pressure normalized until osmotic pump removal (Figure 1). Although the groups had equal body mass at the beginning of infusion, ISO increased body weight by 15% relative to saline on the final infusion day (ISO: 442±4, Saline: 384±6 grams; P < 0.001).
Figure 1.
Effects of chronic ISO infusion on HR and aortic systolic, diastolic, and pulse pressures before exposure to PM. Solid lines indicate 4-h means of ISO- (black, n = 10) or saline-treated (gray, n = 11) rats, and are bordered by dashed lines indicating standard error. Stars and brackets mark periods of consistent significant differences from saline group, while individual stars indicate group differences for single 4-h means between saline and ISO-treated animals. For HR n = 13 for Saline and n = 11 for ISO, while for all pressure-derived parameters n = 9 for Saline and n=10 for ISO.
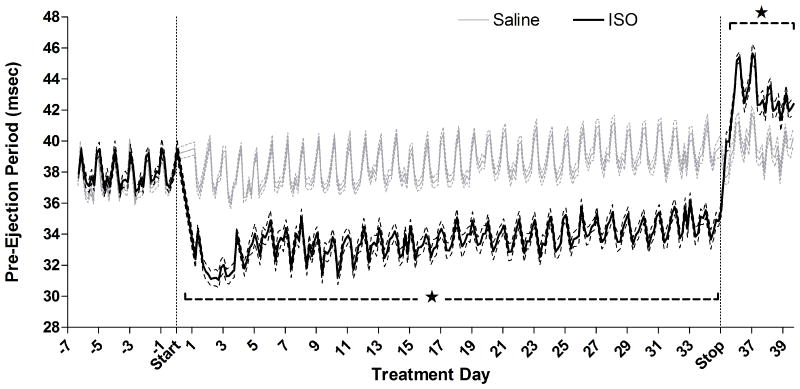
Figure 2.
Effects of chronic ISO infusion on pre-ejection period (an inverse index of cardiac contractility) before exposure to PM. Solid lines indicate 4-h means of ISO- (black, n = 10) or saline-treated (gray, n = 9) rats, and are bordered by dashed lines indicating standard error. Stars and brackets mark periods of consistent significant differences from saline group, while individual stars indicate group differences for single 4-h means between saline and ISO-treated animals.
HR returned to pre-infusion rates when ISO infusion ceased, yet, from 3 d post-infusion until inhalation exposures began, ISO pretreatment significantly decreased HR by 33 BPM (‐10%) relative to saline-infused rats. By contrast, PEP rapidly increased relative to saline-infused rats, peaking at 28 h after pump removal (+4.4 msec, ISO: 45.6±0.3 vs. saline: 41.2±0.6, P < 0.05) suggesting decreased contractility (Fig. 2). Until inhalation exposures at 4.5 d post-infusion, ISO pretreatment significantly increased PEP by 9% (+3.7±0.6 msec, P < 0.05). ISO pretreatment also significantly decreased systolic BP for 16 h at 3 d after pump removal (ISO: 165±3 vs. saline: 183±3 mmHg, P < 0.05) and for 8 h at 4 d post-infusion (ISO: 162±2 vs. saline: 177±3 mmHg, P < 0.05) just before inhalation exposure to PM. In addition, diastolic pressure was significantly decreased for 4 h at 3.5 d post-ISO (ISO: 109±2 vs. saline: 122±2 mmHg, P < 0.05).
Immediately prior to inhalation exposures, ISO-treated rats had significantly increased PR interval relative to saline-treated rats (48.4±0.8 vs. 44.9 ± 0.9 ms, P = 0.01), as well as increased ST (30.3±1.3 vs. 21.9±1.0 ms), QT (49.5±1.3 vs. 42.4±1.5 ms) and TpTe (31.6±2.6 vs. 18.1±1.7 ms) intervals (P < 0.01 for all). Accordingly, ISO-treated rats also had increased T-area relative to saline-treated rats (1.3±0.3 vs. 0.4±0.2 ms2, P = 0.04). As well, ISO-treated rats retained an 11% greater body mass than saline-treated rats (ISO: 413±4, Saline: 372±6 grams; P < 0.001) just before inhalation exposure. ISO infusion did not affect any parameters of ventilatory function at 4 d after infusion cessation, corresponding with immediately after the second acclimation to nose-only exposure (Fig. 4; P > 0.05).
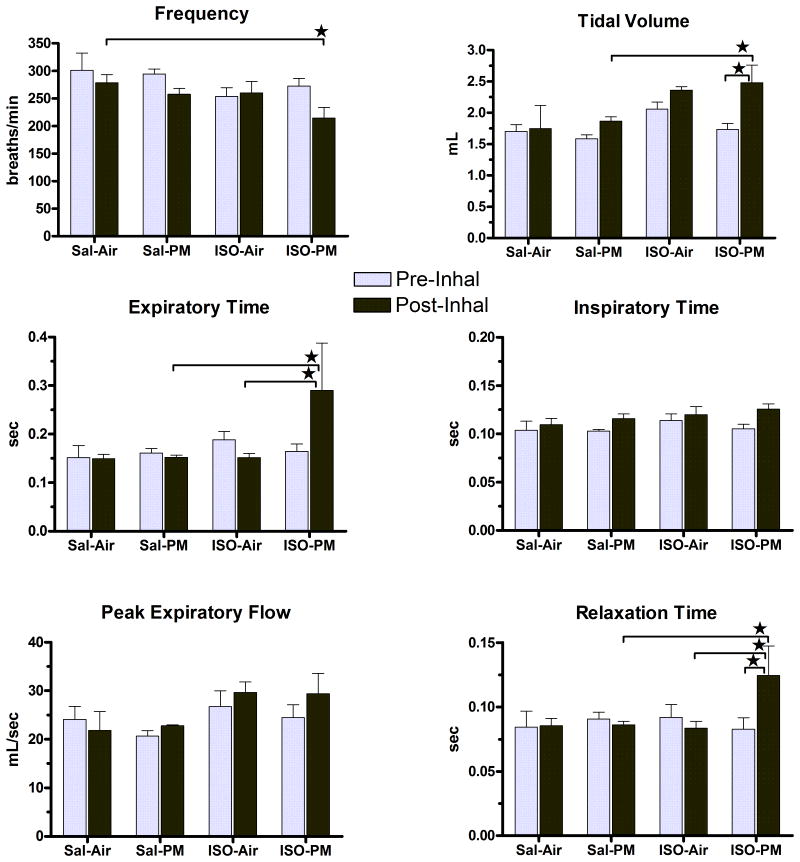
Figure 4.
PM inhalation increased tidal volume, expiratory time, and relaxation time only in ISO-pretreated animals. Values represent group means (± standard error) immediately after nose-only inhalation exposure (“Post-Inhal”) or the time-matched period on the preceding day (“Pre-Inhal”) after a 2-h acclimation to nose-only restraint. Star indicates significant difference between values indicated by bracket (P < 0.05). N = 5 per group.
Cardiac electrophysiology, Contractility, and Hemodynamics during Particulate Inhalation
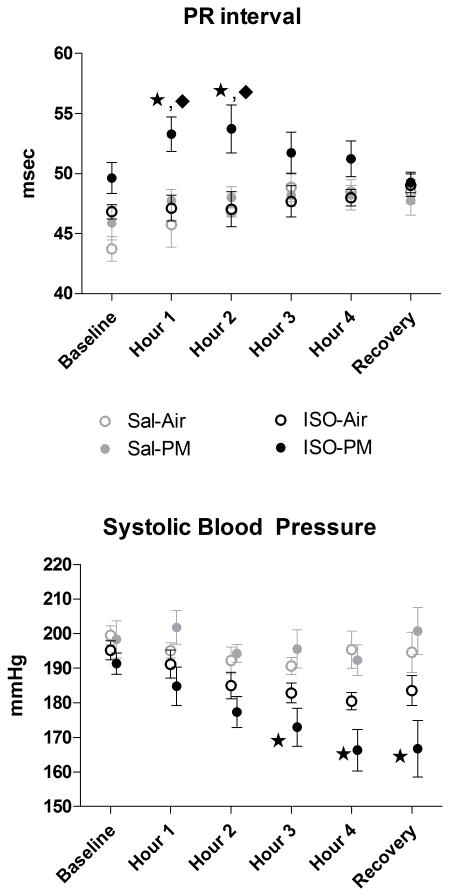
At baseline immediately before particulate exposure, but while animals resided in nose-only cones, both the ISO-PM and ISO-Air groups had significantly lower HR than saline pretreated rats assigned to receive the same inhalant (ISO-PM: 337±8 vs. Sal-PM: 370±10 BPM; ISO-Air: 327±6 vs. Sal-Air: 379±9 BPM, P < 0.05). There were no significant differences between ISO- and saline- pretreated rats in BP at this baseline period. At 1 h and 2 h of inhalation exposure, PM increased PR interval in ISO-pretreated rats relative to the ISO-air (P<0.05) and Sal-PM groups (p<0.05, Fig. 3). PM did not affect the remaining ECG intervals (ST, QT, QRS, TpTe), amplitudes (ST segment, T, Q), or areas (negative Q, negative ST, positive T-wave) in either the saline- or ISO-pretreated groups (all P > 0.05, data not shown). PM inhalation in ISO-pretreated rats significantly decreased systolic BP relative to Sal-PM rats at 3 h (173±5 vs. 196±6 mmHg), 4 h (166±6 vs. 192±4 mmHg), and the recovery period of inhalation exposure (167±8 vs. 200±7 mmHg; all P < 0.05). No similar effects occurred in the ISO-Air group. Additionally, ISO-PM rats had decreased diastolic BP at the recovery period of inhalation exposure relative to Sal-PM rats (122±9 vs. 144±6 mmHg; P < 0.05). At the recovery period, the ISO-PM group appeared to have an accentuated decline in HR relative to the ISO-Air group (254±11 vs. 266±18 BPM), but these values did not significantly differ (P>0.05).
Figure 3.
Effects of PM inhalation on 1-h group means of ECG PR interval and Systolic BP during a 4 h nose-only exposure. Baseline and Recovery represent 40-min periods before initiation and after cessation of particle or filtered air exposure. Star indicates significant difference from Sal-PM. Diamond represents significant difference from ISO-Air (P < 0.05). N = 4-5 per group.
Autonomic Balance and Arrhythmia during Particulate Inhalation
ISO did not alter measures of HRV or arrhythmia during nose-only baseline or the subsequent hours of inhalation exposure relative to saline-pretreated rats of the corresponding inhalation exposure. PM did not significantly alter HRV or arrhythmia during inhalation exposure regardless of pretreatment (P > 0.05 relative to corresponding pretreatment, data not shown).
Ventilatory Function after Particulate Inhalation
Immediately after exposure, PM exposure significantly increased several measures of ventilatory function in ISO-pretreated rats (Figure 4). In these rats PM increased tidal volume by 43% relative to pre-inhalation and by 33% relative to saline-pretreated rats exposed to PM. Exposure to PM in ISO-pretreated rats also increased expiratory time by 91% from both the air-exposed ISO-pretreated rats and the PM-exposed saline-pretreated rats. PM also increased relaxation time to 50% greater than pre-inhalation, to 44% greater than the Sal-PM group, and to 49% greater than the ISO-Air group (all P < 0.05). There were no significant differences in ventilatory function on the day following exposure.
Heart Rate, Contractility, and Hemodynamics after Particulate Inhalation
In ISO-pretreated rats, PM decreased HR at 5.5–6 h post-exposure relative to air (Fig. 5; P < 0.05). Other than this isolated effect, PM did not alter HR, PEP, BP, or pulse pressure over the 64 h after nose-only exposure in either saline- or ISO-pretreated rats relative to their corresponding air-exposed counterparts (all P > 0.05).
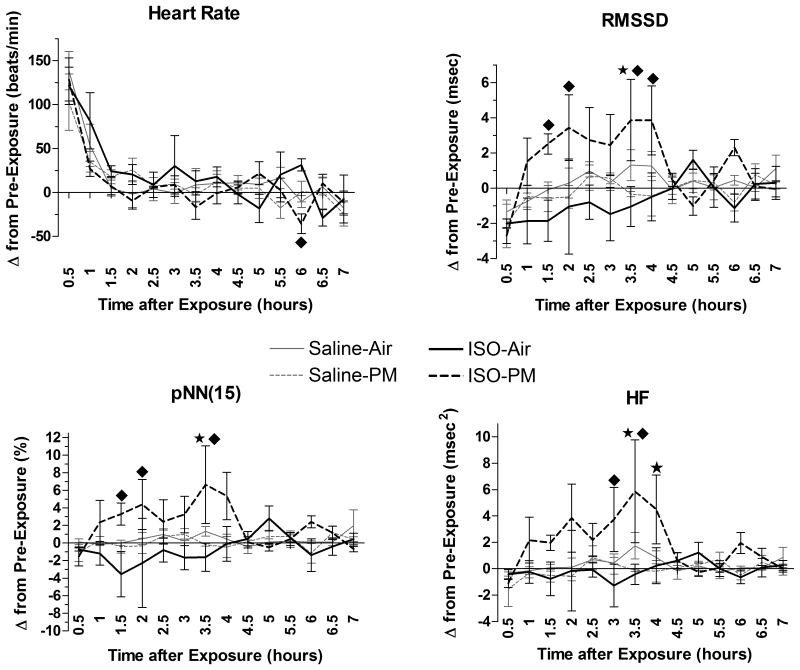
Figure 5.
ISO alters the HR and HRV effects of a 4 h nose-only PM inhalation. Data represents each group's mean change (± standard error) at the 7 h immediately after exposure relative to its own values on the preceding day at the same time. During both monitoring periods, animals resided in home cages. Star indicates significant difference from Sal-PM (P < 0.05). Diamond represents significant difference from ISO-Air (P < 0.05). N = 5 per group.
Autonomic Balance and Arrhythmia after Particulate Inhalation
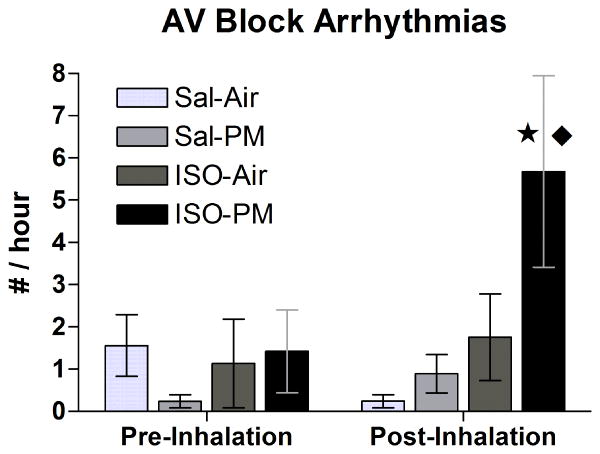
PM inhalation significantly increased the frequency of AV block (non-conducted P-waves) in ISO-pretreated rats to 5.7±2.3 events / h over the seven-hour post-exposure period relative to both the ISO-Air (1.8±1.0 events/h) and Sal-PM (0.9±0.5 events/h) groups (Fig. 6). Most of these AV block arrhythmias (29 of 33) occurred within the first 4 h of rats returning to their home cages, and approximately half (17) resembled Second Degree Mobitz type I AV block. PM increased RMSSD significantly in ISO pretreated rats at 1–2 h and 3–4 h post-exposure relative to the ISO-Air group and at 3–3.5 h post-exposure relative to the Sal-PM group (Fig. 5). Likewise, pNN15 significantly increased for the ISO-PM group in several instances (at 1–2 h and 3–3.5 h post-exposure vs. ISO-Air and at 3–3.5 h post-exposure vs. Sal-PM). HF also significantly increased for the ISO-PM group at similar time-points (at 2.5–3.5 h post-exposure vs. ISO-Air, and at 3–4 h post-exposure vs. Sal-PM; all P < 0.05). Additionally, ISO-PM rats had significantly increased LF from 3–4 h post-exposure relative to the Sal-PM group and from 3–3.5 h relative to ISO-Air (P < 0.05; data not shown).
Figure 6.
PM inhalation increased AV block arrhythmias only in ISO-pretreated animals. Values represent group means (± standard error) over the 7 h after nose-only inhalation exposure or the time-matched 7-h period on the preceding day (“Pre-Inhalation”). Star indicates significant difference from Sal-PM (P < 0.05). Diamond represents significant difference from ISO-Air (P < 0.05). N = 5 per group.
Measures of Pulmonary Inflammation, Endogenous Antioxidants, and Injury
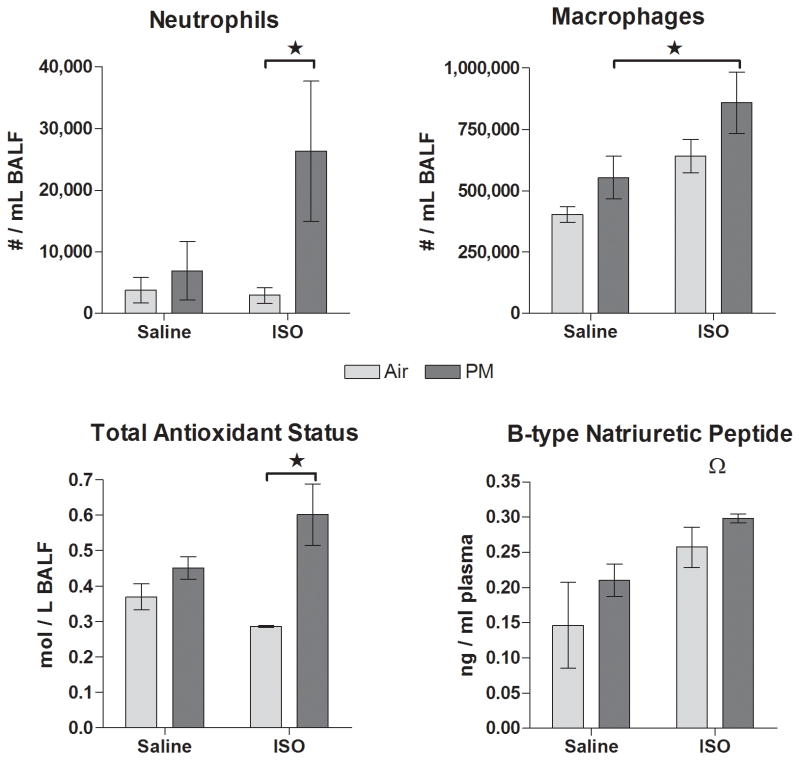
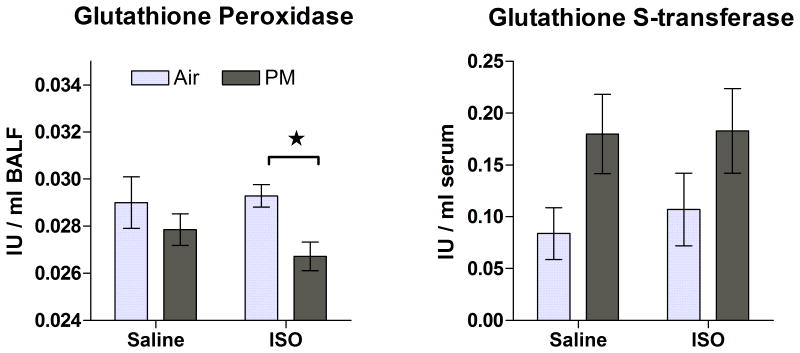
At 1 d post-exposure, PM significantly increased pulmonary neutrophils and total antioxidant status only in ISO-pretreated rats relative to their corresponding air control (Fig. 7). The ISO-PM group also significantly exceeded the Sal-PM group in pulmonary macrophages. ISO overall increased plasma BNP (a marker of myocardial stretch) at 6 days after infusion had ended (P=0.01) without affecting total lavage protein (data not shown, P>0.05). Nevertheless, PM with or without ISO pre-treatment did not significantly alter plasma BNP or lavage protein (a marker of pulmonary edema) when compared to control groups (P>0.05). At 8 wk after nose-only exposure, only the ISO-PM group had significantly decreased glutathione peroxidase in lung lining fluid relative to its air-exposed counterpart (Fig. 8). At this same time point, PM overall increased circulating glutathione S-transferase relative to air exposure (P = 0.03), but there were no differences between individual treatment groups when controlling for drug pretreatment (Figure 8). There were no other significant effects of ISO or PM on biochemical or cytological endpoints examined in lavage or blood (P > 0.05).
Figure 7.
Markers of cardiopulmonary inflammation (neutrophils and macrophages), antioxidant response (total antioxidant status), and myocardial stretch (BNP) at 1 d post-exposure to PM or filtered air in saline- or ISO-pretreated rats. Means ± standard error, n = 4-5 / group. Stars and brackets represent significant differences between groups (P < 0.05). Omega indicates a significant overall effect of ISO on BNP as detected by two-way ANOVA (P=0.01).
Figure 8.
Endogenous antioxidants (pulmonary glutathione peroxidase and serum glutathione S-transferase) at 8 weeks after a single 4-h exposure to PM or filtered air in saline- or ISO-pretreated rats. Means ± standard error, n = 6-7 per group. Star and bracket represents significant difference between groups (P < 0.05).
Gross Pathology
Neither ISO nor PM altered heart-to-body weight ratio at 1 day post-inhalation exposure (6 days post-infusion). At 8 wk post-inhalation exposure (9 wk post-infusion), overall, ISO treatment increased heart-to-body weight ratio by 3.6% (3.47±0.02 mg/g vs. 3.35±0.05 mg/g in saline-treated rats; P < 0.05) despite that the ISO-PM group did not significantly differ from the Sal-PM group in this measure.
Discussion
We demonstrate that catecholamine-induced cardiomyopathy confers susceptibility to inhaled PM. Subchronic infusion of isoproterenol (ISO) in heart failure-prone SHHF rats caused post-treatment decrements in cardiac mechanical function and ventricular repolarization as measured by an increase in the pre-ejection period and QT and TpTe intervals, respectively. These effects were consistent with cardiomyopathic changes in mechanical function and electrical properties that accompany hypertrophic cardiomyopathy and which are commonly associated with increased risk of arrhythmia and sudden cardiac death (Savelieva et al. 1998). Thereafter, PM exposure caused significant cardiopulmonary changes only in ISO-pretreated rats, including pulmonary inflammation, AV block arrhythmias, an acute surge in pulmonary antioxidants, and a chronic depletion of pulmonary glutathione peroxidase, whereas PM had no significant effects in saline-pretreated rats. PM inhalation also caused parasympathetic dominance in ISO-pretreated rats but not saline-pretreated rats, as evidenced by prolonged AV conduction and decreased systolic BP during PM exposure as well as increased HRV and decreased HR after PM exposure.
The current study used lower concentrations of PM than our prior research to reveal that inhalation of metal-rich ROFA can cause arrhythmia in heart failure-prone rats with preexisting cardiac injury. In an earlier study, exposure of Spontaneously Hypertensive rats to ROFA at 26-fold the present concentrations and with similar metal content also caused AV block arrhythmias (Farraj et al. 2009). Likewise, two 5-hour exposures to ROFA at 20-fold higher concentrations than our current study, but with lower Vn, Ni, and Fe and higher Zn content also increased AV block in ISO-pretreated heart failure-prone rats (Carll et al. 2010). We have also found that exposing hypertensive rats to 20% lower concentrations of ROFA than the current study does not affect spontaneous arrhythmias but does decrease the dose at which aconitine, an arrhythmogenic drug, induces arrhythmia (Hazari et al. 2009). More importantly, in a past study with hypertensive rats exposed to higher concentrations of sROFA PM, AV block arrhythmias occurred at 6-fold but not at 1.7-fold the current study's concentration (Farraj et al. 2011), further suggesting heightened susceptibility in our current model. It is also noteworthy that the PM2.5 concentrations in our present study fell well below others used to investigate the cardiovascular effects of a similarly metal-rich PM (Kodavanti et al. 2002; Muggenburg et al. 2000; Wellenius et al. 2002) and even below some recent extremes in urban environments (Johnson 2013; NASA 2013). Together, these findings indicate that the inhalation of PM at more environmentally relevant levels has greater arrhythmogenicity in the setting of cardiac remodeling.
It is evident from this and our past research that acute inhalation of a variety of air pollutants, including diesel exhaust (DE) in its whole or particle-free form, ozone, and ROFA, can modulate autonomic neural input to the heart in the rat and shift autonomic balance to parasympathetic predominance. These studies collectively suggest that CVD and/or cardiac injury predispose animals to pollutant-induced responses consistent with parasympathetic dominance, including AV block arrhythmias, decreased BP and HR, prolonged PR-interval, increased HRV—which all occurred in at least 3 of our past studies (Carll et al. 2012; Carll et al. 2013a; Farraj et al. 2009; Hazari et al. 2009; Lamb et al. 2012)—and, presently, changes in pulmonary physiology. While PR is known to increase not only with parasympathetic dominance but also with age and arterial stiffness in humans (Gosse et al. 2011), we and others (Campen et al. 2003) have noted PR prolongation with increasing age, cardiovascular disease, and/or air pollutant exposure in awake rats, with SH rats < DE-exposed SH < SHHF < DE-exposed SHHF ≈ PM-exposed SHHF < aged SHHF rats (Carll et al. 2012; Carll et al. 2013b; Lamb et al. 2012). Interestingly, when PM concentrations are below 1 mg/m3, only SHHF rats appear to manifest AV block arrhythmias, which may partly derive from their pronounced AV conduction slowing. PM exposure increased tidal volume and elicited parasympathetic cardiovascular responses only in ISO-pretreated rats—effects consistent with pulmonary irritant reflexes (Coleridge and Coleridge 2011; Widdicombe and Lee 2001). The increases in tidal volume may have prolonged expiration through Hering-Breuer parasympathetic reflexes to pulmonary stretch (Hayashi et al. 1996; Marek et al. 2008) that can in some cases induce AV block (James et al. 1980). Although arrhythmias and parasympathetic influence over HRV did not change during exposure, we cannot rule out the possibility that PM enhanced deposition in ISO-pretreated rats by altering pulmonary function during exposure. Still, these findings point to enhanced cardiopulmonary susceptibility to PM in this model of cardiomyopathy. Others have found that carbon black PM increases parasympathetic HRV indices in a mouse model of terminal senescence, but not in younger adult mice (Tankersley et al. 2004). Air pollutant susceptibility and parasympathetic responses may also be associated in humans. Exposure of coronary heart disease patients—but not healthy—patients to DE caused near-significant increases in a HRV parameter (pNN50, P= 0.07) (Mills et al. 2011). Likewise, ambient PM2.5 correlated with increased RMSSD in elderly patients with cardiopulmonary diseases—effects the authors noted could promote bradyarrhythmias and hypotension (Pope et al. 1999). These observations in humans and animals appear to conflict with the prevailing view that PM exposure compromises cardiovascular function through sympathetic dominance (Brook et al. 2010). However, we recently found evidence that air pollutants may cause time-dependent oscillations between sympathetic and parasympathetic dominance. Specifically, a brief sympathetic dominance during DE exposure was followed shortly after exposure by parasympathetic dominance and one day later by sympathetic dominance (Carll et al. 2013a). This prior study used time-matched sham exposures and exercise stress tests that likely enhanced the sensitivity of our analyses. In contrast, our current study did not include a full 5-h sham exposure to normalize for each rat's stress responses to nose-only restraint, nor did it use exercise challenges to evoke periods of uniform physiological conditions before and after exposure. Accordingly, we saw no significant effects on BP and HR over the 60 hours following PM exposure, and HRV effects were restricted to the first 4 hours after exposure. Although it is plausible that the various air pollutants we have examined differ in their physiological effects, it is also likely that certain procedures, including stress tests and sham exposures, can unmask latent changes in autonomic and cardiovascular physiology. Thus, the parasympathetic effects of PM that we observed herein may only represent a fraction of the autonomic imbalance incurred by PM exposure.
The parasympathetic dominance and bradyarrhythmias that we observed may result from the stimulation of receptors and sensory nerve fiber pathways that propagate autonomic imbalance. Two candidate mechanisms include the triggering of acute pulmonary irritant reflexes and the activation of myocardial stretch receptors (Carll et al. 2013b; Hazari et al. 2012). The activation of the transient-receptor potential ankyrin-1 (TRPA1) channel appears partly responsible for pulmonary-derived autonomic imbalance (Hazari et al. 2012). Additionally, left ventricular dilation can trigger parasympathetic mechanoreceptor reflexes and has been observed with inhalation exposures to carbon black or DE that also both increased HRV and AV block (Carll et al. 2013b; Tankersley et al. 2004; Tankersley et al. 2008). Although the mechanism of PM-induced bradyarrhythmias remains unclear, we have seen positive correlations between HRV and AV block events in DE-exposed rats that may result from parasympathetic activation of acetylcholine-sensitive K+ channels (IKach) (Carll et al. 2013b; Carll et al. 2012). Moreover this phenomenon may involve transient inhibition of sinoatrial node-specific vagal neurons, as demonstrated in mice exposed to iron-rich PM (Pham et al. 2009), which could cause heart block during parasympathetic reflexes (James et al. 1980). The potential for IKach blockade or broadcast vagal inhibition to mitigate these effects deserves further investigation.
Similar to our prior studies, cardiomyopathic remodeling was induced in heart failure-prone rats via continuous sub-chronic infusion of the synthetic catecholamine isoproterenol (Carll et al. 2010; Carll et al. 2011a). Previously, a 4-wk infusion of ISO at 2.5 mg/kg/d caused cardiomyopathic changes (degenerating myofibers, mixed inflammatory cells, proliferating fibroblasts, and fibrosis) and attendant functional changes including decreases in HR and contractility assessed by PEP (Carll et al. 2010; Carll et al. 2011a). Because of premature mortality in the previous study (Carll et al. 2011a), we reduced the daily dose to 1 mg/kg and prolonged infusion to 5 wk in our current study. During infusion, this new regimen increased HR (32% increase) and contractility (13% decrease in PEP) comparable to our past regimen, suggesting a similar extent of pharmacologic activation of β1 adrenergic receptors. We also observed a 3-day hypotension during infusion that was consistent with prior findings that ISO causes a transient vasodilation through β2 adrenergic activation that dissipates with β2 downregulation (Brouri et al. 2002; Brouri et al. 2004; Nanoff et al. 1989). The pathologic implications of these effects are unclear; nevertheless, gross cardiac lesions and telemetry data indicate that ISO infusion caused post-treatment cardiomyopathy, including decreased contractility, increased heterogeneity of transmural repolarization (TpTe) and delayed atrioventricular conduction (PR). TpTe has been shown to correlate with post-infarct LV remodeling (Szydlo et al. 2010) and hypertrophic cardiomyopathy (Savelieva et al. 1998) while also predicting ventricular tachycardia and sudden cardiac death in patients with hypertrophic cardiomyopathy (Shimizu et al. 2002). Furthermore, we have found that PR interval increases between related rat strains with increasing propensity for cardiomyopathy, including young adult WKY, SH, and SHHF rats as well as the aged SHHF (Carll et al. 2012; Carll et al. 2013b). Although histopathology was not performed in the current study, we previously demonstrated that infused ISO elicits significant cardiomyopathy (Carll et al. 2010; Carll et al. 2011a). Similarly, a 2-wk ISO infusion in rats at a 28% lower dose than our current regimen caused a virtually identical distribution and magnitude of fibrosis as with our prior regimen (Brouri et al. 2004). Like our prior regimen, the current one caused gross lesions on the cardiac apices of all ISO-treated rats, which we previously confirmed to be dense areas of myocardial fibrosis and thus pathognomonic of ISO-induced cardiomyopathy (Carll et al. 2010; Carll et al. 2011a).
As with our past study (Carll et al. 2010), the period after ISO infusion corresponded with substantial decreases in HR and contractility (HR: ‐10% vs. -9% previously; PEP: +9% vs. +7% previously) which, incidentally, was accompanied by increased body mass potentially due to renin-angiotensin-aldosterone system-induced hypervolemia. Collectively, these findings indicate that prolonging ISO infusion and decreasing the daily dose induces similar cardiac dysfunction and injury while avoiding premature mortality. Likewise, prolonging infusion of ISO from 4wk to 6wk at a comparable dose has been shown to further exacerbate systolic failure in Wistar rats (Takeshita et al. 2008). The decrease in HR and PEP after ISO may have been due to down-regulation of β1-adrenoceptors, which are mediators of sympathetic augmentation of contractility and HR. β1 down-regulation, decreased contractility, and cardiomyopathy are all common pathologic traits of heart failure, which often results from myocardial injury causing a compensatory increase in endogenous catecholamines that promote myocardial remodeling and thereby eventually decrease ventricular ejection and filling. Given that ISO increased plasma BNP overall while decreasing PEP and HR, this model of ISO-induced cardiomyopathy likely recapitulates several key facets of heart failure. Thus, the effects of PM exposure that we observed in ISO-pretreated rats might have clinical relevance to the epidemiological associations between ambient PM exposure and exacerbation of heart failure.
Likewise, we have previously demonstrated that either ISO-induced cardiomyopathy or advanced age in heart failure-prone rats enhances the pulmonary inflammation and cardiopulmonary responses elicited by PM or DE exposure (Carll et al. 2010; Carll et al. 2013b). Research suggests this propagates autonomic imbalance, especially when transition metals are involved. For instance, exposure of healthy men to traffic-derived PM correlated with increased HRV (SDNN and pNN50), supraventricular ectopic beats, and pulmonary neutrophils (Riediker et al. 2004a; Riediker et al. 2004b; Riediker 2007), which the authors found were primarily mediated by copper, sulfur, and aldehydes. Accordingly, transition metal content, not total mass, has been found to mediate PM-induced pulmonary oxidative stress and inflammation (Costa and Dreher 1997). Recent research has demonstrated that nickel-sulfate inhalation increases HRV and beat-to-beat interval dependent on oxidative stress and inflammation (Chuang et al. 2013). In the present study, PM exposure caused greater cardiopulmonary inflammation in rats with ISO-induced cardiomyopathy than in saline-treated rats. Nevertheless, it remains unclear whether the pulmonary inflammatory effects of PM in ISO-pretreated rats mediated the overt parasympathetic responses. Notably, because parasympathetic activation is anti-inflammatory (Pavlov and Tracey 2005; Thayer 2009), it is possible that the increases in HRV reflect innate neural defense mechanisms against pro-inflammatory agents. This may partly explain our past observations of air pollutant-induced autonomic imbalance that were unaccompanied by detectable cardiopulmonary inflammation or oxidative stress (Carll et al. 2010; Carll et al. 2013a). Our findings merit deeper exploration into the anti- and pro-inflammatory effects as well as the mechanistic origins of pollutant-induced autonomic imbalance.
The short- and long-term effects that acute PM exposure had on pulmonary antioxidants offer novel insight to potential mechanisms of air pollutant toxicity. Exposure to PM in ISO-pretreated rats acutely increased total antioxidants within the lung lining fluid. This effect was consistent with an acute antioxidant defense response that others have noted with pulmonary inflammation from hyperoxia or mineral dust exposure (Quinlan et al. 1994). Interestingly, two months after this single acute exposure, PM persistently decreased pulmonary glutathione peroxidase, a major antioxidant enzyme, only in rats with ISO-induced cardiomyopathy. Thus, preexisting cardiomyopathy conferred susceptibility to PM-induced chronic depletion of antioxidants within the lung lining fluid. Moreover, the data indicate that acute exposure to PM can impair pulmonary defenses long after exposure. Given the prolonged effect of this single exposure on glutathione peroxidase, it seems plausible that repeat exposure would further enhance cardiopulmonary susceptibility in our model of cardiomyopathy by cumulatively depleting antioxidants. Whether the transition metal content of sROFA PM mediated this effect remains unknown. These findings merit further investigation into whether and how acute PM exposure may chronically enhance susceptibility to pulmonary injury.
Taken together, a single 4-hour inhalation exposure to a ROFA-like PM caused more cardiopulmonary responses in a rat model of drug-induced cardiomyopathy than in rats without cardiomyopathy. These responses included immediate decreases in BP and prolonged PR-interval, subsequent vagal dominance and—concomitantly—bradyarrhythmias, and later pulmonary inflammation and oxidative stress. The possibility that such changes can lead to adverse health effects and whether these responses in rats translate to human responses is unclear. Nevertheless, exaggerated sensitivity to PM in rats with cardiomyopathy is consistent with the pattern observed in heart failure patients. Ultimately, this study demonstrates that preexisting cardiac remodeling confers susceptibility to the cardiopulmonary effects of air pollution and may help elucidate the mechanisms by which PM exposure exacerbates heart failure in man.
Acknowledgments
The authors thank Justin B. Callaway of UNC-Chapel Hill for help with animal handling and surgical procedures, and Dr. Barbara Buckley, Dr. William Polk and Dr. Ian Gilmour of the U.S. EPA for their expert reviews of this manuscript.
Footnotes
Publisher's Disclaimer: Disclaimer: This paper has been reviewed and approved for release by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency. Approval does not signify that the contents necessarily reflect the views and policies of the U.S. EPA, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Declaration of Interest. The authors have no conflicts of interest to disclose.
Contributor Information
Alex P. Carll, Email: carll.alex@epa.gov.
Najwa Haykal-Coates, Email: coates.najwa@epa.gov.
Darrell W. Winsett, Email: winsett.darrell@epa.gov.
Mehdi S. Hazari, Email: hazari.mehdi@epa.gov.
Allen D. Ledbetter, Email: ledbetter.allen@epa.gov.
Judy H. Richards, Email: richards.judy@epa.gov.
Wayne E. Cascio, Email: cascio.wayne@epa.gov.
Daniel L. Costa, Email: costa.dan@epa.gov.
Aimen K. Farraj, Email: farraj.aimen@epa.gov.
References
- Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Brouri F, Findji L, Mediani O, Mougenot N, Hanoun N, Naour GL, et al. Toxic cardiac effects of catecholamines: role of beta-adrenoreceptor downregulation. Eur J Pharmacol. 2002;456:69. doi: 10.1016/s0014-2999(02)02643-2. [DOI] [PubMed] [Google Scholar]
- Brouri F, Hanoun N, Mediani O, Saurini F, Hamon M, Vanhoutte PM, et al. Blockade of beta 1- and desensitization of beta 2-adrenoceptors reduce isoprenaline-induced cardiac fibrosis. Eur J Pharmacol. 2004;485:227. doi: 10.1016/j.ejphar.2003.11.063. [DOI] [PubMed] [Google Scholar]
- Burnett RT, Dales R, Krewski D, Vincent R, Dann T, Brook JR. Associations between ambient particulate sulfate and admissions to Ontario hospitals for cardiac and respiratory diseases. Am J Epidemiol. 1995;142:15–22. doi: 10.1093/oxfordjournals.aje.a117540. [DOI] [PubMed] [Google Scholar]
- Campen MJ, McDonald JD, Gigliotti AP, Seilkop SK, Reed MD, Benson JM. Cardiovascular effects of inhaled diesel exhaust in spontaneously hypertensive rats. Cardiovasc Toxicol. 2003;3:353–361. doi: 10.1385/ct:3:4:353. [DOI] [PubMed] [Google Scholar]
- Carll AP, Haykal-Coates N, Winsett DW, Rowan WH, 3rd, Hazari MS, Ledbetter AD, et al. Particulate matter inhalation exacerbates cardiopulmonary injury in a rat model of isoproterenol-induced cardiomyopathy. Inhal Toxicol. 2010;22:355–368. doi: 10.3109/08958370903365692. [DOI] [PubMed] [Google Scholar]
- Carll AP, Haykal-Coates N, Winsett DW, Hazari MS, Nyska A, Richards JH, et al. Dietary salt exacerbates isoproterenol-induced cardiomyopathy in rats. Toxicol Pathol. 2011a;39:925–937. doi: 10.1177/0192623311416373. [DOI] [PubMed] [Google Scholar]
- Carll AP, Willis MS, Lust RM, Costa DL, Farraj AK. Merits of Non-Invasive Rat Models of Left Ventricular Heart Failure. Cardiovasc Toxicol. 2011b doi: 10.1007/s12012-011-9103-5. [DOI] [PubMed] [Google Scholar]
- Carll AP, Hazari MS, Perez CM, Krantz QT, King CJ, Winsett DW, et al. Whole and particle-free diesel exhausts differentially affect cardiac electrophysiology, blood pressure, and autonomic balance in heart failure-prone rats. Toxicol Sci. 2012;128:490–499. doi: 10.1093/toxsci/kfs162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carll AP, Hazari MS, Perez CM, Krantz QT, King CJ, Haykal-Coates N, et al. An autonomic link between inhaled diesel exhaust and impaired cardiac performance: insight from treadmill and dobutamine challenges in heart failure-prone rats. Toxicol Sci. 2013a;135:425–436. doi: 10.1093/toxsci/kft155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carll AP, Lust RM, Hazari MS, Perez CM, Krantz QT, King CJ, et al. Diesel exhaust inhalation increases cardiac output, bradyarrhythmias, and parasympathetic tone in aged heart failure-prone rats. Toxicol Sci. 2013b;131:583–595. doi: 10.1093/toxsci/kfs295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang HC, Hsueh TW, Chang CC, Hwang JS, Chuang KJ, Yan YH, et al. Nickel-regulated heart rate variability: the roles of oxidative stress and inflammation. Toxicol Appl Pharmacol. 2013;266:298–306. doi: 10.1016/j.taap.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JCG. Reflexes Evoked from Tracheobronchial Tree and Lungs. In: Pollock DM, editor. Comprehensive Physiology. Wiley Online Library:The American Physiological Society; 2011. pp. 395–429. [Google Scholar]
- Costa DL, Dreher KL. Bioavailable transition metals in particulate matter mediate cardiopulmonary injury in healthy and compromised animal models. Environ Health Perspect. 1997;105(Suppl 5):1053–1060. doi: 10.1289/ehp.97105s51053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. Jama. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farraj AK, Haykal-Coates N, Winsett DW, Hazari MS, Carll AP, Rowan WH, et al. Increased non-conducted P-wave arrhythmias after a single oil fly ash inhalation exposure in hypertensive rats. Environ Health Perspect. 2009;117:709. doi: 10.1289/ehp.0800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farraj AK, Hazari MS, Haykal-Coates N, Lamb C, Winsett DW, Ge Y, et al. ST depression, arrhythmia, vagal dominance, and reduced cardiac micro-RNA in particulate-exposed rats. Am J Respir Cell Mol Biol. 2011;44:185–196. doi: 10.1165/rcmb.2009-0456OC. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Bailar JC, 3rd, Burnett RT, Brook JR, Tamblyn R, Bonvalot Y, et al. Identifying subgroups of the general population that may be susceptible to short-term increases in particulate air pollution: a time-series study in Montreal, Quebec. ResRepHealth EffInst. 2000;97:7. [PubMed] [Google Scholar]
- Gosse P, Coulon P, Papaioannou G, Litalien J, Lemetayer P. Atrioventricular conduction in the hypertensive patient: influence of aging, pulse pressure, and arterial stiffness. Rejuvenation Res. 2011;14:405–410. doi: 10.1089/rej.2010.1152. [DOI] [PubMed] [Google Scholar]
- Hatch GE, Boykin E, Graham JA, Lewtas J, Pott F, Loud K, et al. Inhalable particles and pulmonary host defense: in vivo and in vitro effects of ambient air and combustion particles. Environ Res. 1985;36:67–80. doi: 10.1016/0013-9351(85)90008-8. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, McCrimmon DR. Respiratory neurons mediating the Breuer-Hering reflex prolongation of expiration in rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:6526–6536. doi: 10.1523/JNEUROSCI.16-20-06526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazari MS, Haykal-Coates N, Winsett DW, Costa DL, Farraj AK. A single exposure to particulate or gaseous air pollution increases the risk of aconitine-induced cardiac arrhythmia in hypertensive rats. Toxicol Sci. 2009;112:532–542. doi: 10.1093/toxsci/kfp214. [DOI] [PubMed] [Google Scholar]
- Hazari MS, Callaway J, Winsett DW, Lamb C, Haykal-Coates N, Krantz QT, et al. Dobutamine “stress” test and latent cardiac susceptibility to inhaled diesel exhaust in normal and hypertensive rats. Environ Health Perspect. 2012;120:1088–1093. doi: 10.1289/ehp.1104684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TN, Urthaler F, Hageman GR. Reflex heart block. Baroreflex, chemoreflex and bronchopulmonary reflex causes. Am J Cardiol. 1980;45:1182–1188. doi: 10.1016/0002-9149(80)90476-2. [DOI] [PubMed] [Google Scholar]
- Jaskot R, Charlet EG, Grose EC, Grady MA, Roycroft JH. An automated analysis of glutathione peroxidase, S-transferase, and reductase activity in animal tissues. J Analyt Toxicol. 1983;7:86. doi: 10.1093/jat/7.2.86. [DOI] [PubMed] [Google Scholar]
- Johnson B. NASA Releases Images of Beijing Air Pollution. [10 Nov. 2014];CBS News. 2013 15 Jan. 2013. http://www.cbsnews.com/news/nasa-releases-images-of-beijing-air-pollution/
- Kodavanti UP, Schladweiler MC, Ledbetter AD, Hauser R, Christiani DC, McGee J, et al. Temporal association between pulmonary and systemic effects of particulate matter in healthy and cardiovascular compromised rats. J Toxicol Environ Health A. 2002;65:1545–1569. doi: 10.1080/00984100290071667. [DOI] [PubMed] [Google Scholar]
- Lamb CM, Hazari MS, Haykal-Coates N, Carll AP, Krantz QT, King C, et al. Divergent electrocardiographic responses to whole and particle-free diesel exhaust inhalation in spontaneously hypertensive rats. Toxicol Sci. 2012;125:558–568. doi: 10.1093/toxsci/kfr296. [DOI] [PubMed] [Google Scholar]
- Ledbetter AD, Killough PM, Hudson GF. A low-sample-consumption dry-particulate aerosol generator for use in nose-only inhalation exposures. Inhal Toxicol. 1998;10:239. [Google Scholar]
- Marek W, Muckenhoff K, Prabhakar NR. Significance of pulmonary vagal afferents for respiratory muscle activity in the cat. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society 59 Suppl. 2008;6:407–420. [PubMed] [Google Scholar]
- Mills NL, Finlayson AE, Gonzalez MC, Tornqvist H, Barath S, Vink E, et al. Diesel exhaust inhalation does not affect heart rhythm or heart rate variability. Heart. 2011;97:544–550. doi: 10.1136/hrt.2010.199042. [DOI] [PubMed] [Google Scholar]
- Muggenburg BA, Barr EB, Cheng YS, Seagrave JC, Tilley LP, Mauderley JL. Effect of inhaled residual oil fly ash on the electrocardiogram of dogs. Inhal Toxicol. 2000;12(Suppl 4):189–208. doi: 10.1080/08958370050165049. [DOI] [PubMed] [Google Scholar]
- Nadziejko C, Chen LC, Nadas A, Hwang JS. The ‘Fishing License’ method for analysing the time course of effects in repeated measurements. Stat Med. 2004;23:1399. doi: 10.1002/sim.1727. [DOI] [PubMed] [Google Scholar]
- Nanoff C, Freissmuth M, Tuisl E, Schutz W. A different desensitization pattern of cardiac beta-adrenoceptor subtypes by prolonged in vivo infusion of isoprenaline. J Cardiovasc Pharmacol. 1989;13:198–203. doi: 10.1097/00005344-198902000-00004. [DOI] [PubMed] [Google Scholar]
- NASA. Smog Shuts Down Harbin. [10 Nov. 2014];NASA Earth Observatory. 2013 23 Oct. 2013. http://earthobservatory.nasa.gov/IOTD/view.php?id=82220&src=eoa-iotd.
- Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain, behavior, and immunity. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Pham H, Bonham AC, Pinkerton KE, Chen CY. Central neuroplasticity and decreased heart rate variability after particulate matter exposure in mice. Environ Health Perspect. 2009;117:1448–1453. doi: 10.1289/ehp.0900674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, 3rd, Verrier RL, Lovett EG, Larson AC, Raizenne ME, Kanner RE, et al. Heart rate variability associated with particulate air pollution. Am Heart J. 1999;138:890. doi: 10.1016/s0002-8703(99)70014-1. [DOI] [PubMed] [Google Scholar]
- Quinlan T, Spivack S, Mossman BT. Regulation of antioxidant enzymes in lung after oxidant injury. Environ Health Perspect. 1994;102(Suppl 2):79–87. doi: 10.1289/ehp.9410279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riediker M, Cascio WE, Griggs TR, Herbst MC, Bromberg PA, Neas L, et al. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med. 2004a;169:934–940. doi: 10.1164/rccm.200310-1463OC. [DOI] [PubMed] [Google Scholar]
- Riediker M, Devlin RB, Griggs TR, Herbst MC, Bromberg PA, Williams RW, et al. Cardiovascular effects in patrol officers are associated with fine particulate matter from brake wear and engine emissions. Part Fibre Toxicol. 2004b;1:2. doi: 10.1186/1743-8977-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riediker M. Cardiovascular effects of fine particulate matter components in highway patrol officers. Inhal Toxicol. 2007;19(Suppl 1):99–105. doi: 10.1080/08958370701495238. [DOI] [PubMed] [Google Scholar]
- Rowan WH, Campen MJ, Wichers LB, Watkinson WP. Heart rate variability in rodents: uses and caveats in toxicological studies. Cardiovasc Toxicol. 2007;7:28. doi: 10.1007/s12012-007-0004-6. [DOI] [PubMed] [Google Scholar]
- Savelieva I, Yap YG, Yi G, Guo X, Camm AJ, Malik M. Comparative reproducibility of QT, QT peak, and T peak-T end intervals and dispersion in normal subjects, patients with myocardial infarction, and patients with hypertrophic cardiomyopathy. Pacing Clin Electrophysiol. 1998;21:2376–2381. doi: 10.1111/j.1540-8159.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- Schulz H, Harder V, Ibald-Mulli A, Khandoga A, Koenig W, Krombach F, et al. Cardiovascular Effects of Fine and Ultrafine Particles. J Aerosol Med. 2005;18:1. doi: 10.1089/jam.2005.18.1. [DOI] [PubMed] [Google Scholar]
- Shah AS, Langrish JP, Nair H, McAllister DA, Hunter AL, Donaldson K, et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet. 2013;382:1039–1048. doi: 10.1016/S0140-6736(13)60898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M, Ino H, Okeie K, Yamaguchi M, Nagata M, Hayashi K, et al. T-peak to T-end interval may be a better predictor of high-risk patients with hypertrophic cardiomyopathy associated with a cardiac troponin I mutation than QT dispersion. Clinical cardiology. 2002;25:335–339. doi: 10.1002/clc.4950250706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlo K, Wita K, Trusz-Gluza M, Tabor Z. Late phase of repolarization (TpeakTend) as a prognostic marker of left ventricle remodeling in patients with anterior myocardial infarction treated with primary coronary intervention. Cardiology journal. 2010;17:244–248. [PubMed] [Google Scholar]
- Takeshita D, Shimizu J, Kitagawa Y, Yamashita D, Tohne K, Nakajima-Takenaka C, et al. Isoproterenol-induced hypertrophied rat hearts: does short-term treatment correspond to long-term treatment? J Physiol Sci. 2008;58:179. doi: 10.2170/physiolsci.RP004508. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Campen M, Bierman A, Flanders SE, Broman KW, Rabold R. Particle effects on heart-rate regulation in senescent mice. Inhal Toxicol. 2004;16:381. doi: 10.1080/08958370490439551. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Champion HC, Takimoto E, Gabrielson K, Bedja D, Misra V, et al. Exposure to inhaled particulate matter impairs cardiac function in senescent mice. Am J Physiol - Reg Integ Compar Physiol. 2008;295:R252. doi: 10.1152/ajpregu.00697.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF. Vagal tone and the inflammatory reflex. Cleveland Clinic journal of medicine. 2009;76(Suppl 2):S23–26. doi: 10.3949/ccjm.76.s2.05. [DOI] [PubMed] [Google Scholar]
- Wellenius G, Saldiva P, Batalha J, Murthy G, Coull B, Verrier R, et al. Electrocardiographic changes during exposure to residual oil fly ash (ROFA) particles in a rate model of myocardial infarction. Toxicol Sci. 2002;66:327–335. doi: 10.1093/toxsci/66.2.327. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Bateson TF, Mittleman MA, Schwartz J. Particulate Air Pollution and the Rate of Hospitalization for Congestive Heart Failure among Medicare Beneficiaries in Pittsburgh, PA. American Journal of Epidemiology. 2005;161:1030. doi: 10.1093/aje/kwi135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe J, Lee LY. Airway reflexes, autonomic function, and cardiovascular responses. Environ Health Perspect. 2001;109(Suppl 4):579–584. doi: 10.1289/ehp.01109s4579. [DOI] [PMC free article] [PubMed] [Google Scholar]