Abstract
The cytotoxic activity of (-)-chlorizidine A, a marine alkaloid containing a unique fusion between a pyrroloisoindolone and dehydropyrrolizine, was explored using a combination of cellular and molecular methods. Our studies began by applying preliminary SAR evidence gathered from semisynthetic bioactivity evaluations, to prepare an active immunoaffinity fluorescent (IAF) probe. This probe was then used to identify two cytosolic proteins, GAPDH and hENO1, as the targets of (-)-chlorizidine A.
Keywords: natural products, mode of action, glycolysis, enolase, drug discovery
As part of an effort to identify bioactive natural products from marine microbes,[1-3] we recently described the isolation and structure elucidation of (-)-chlorizidine A (1, Scheme 1) a cytotoxic alkaloid from cultures of a Streptomyces sp., strain CNH-287, obtained from deep ocean sediments collected off the coast of San Clemente, California.[4] After initial optimization efforts, we were able to identify conditions to reproducibly produce (−)-chlorizidine A (1) from cultures of the strain on a 20 L scale (∼1 mg/L). The isolated natural product was then used to examine the structural requirements for activity.
Scheme 1.
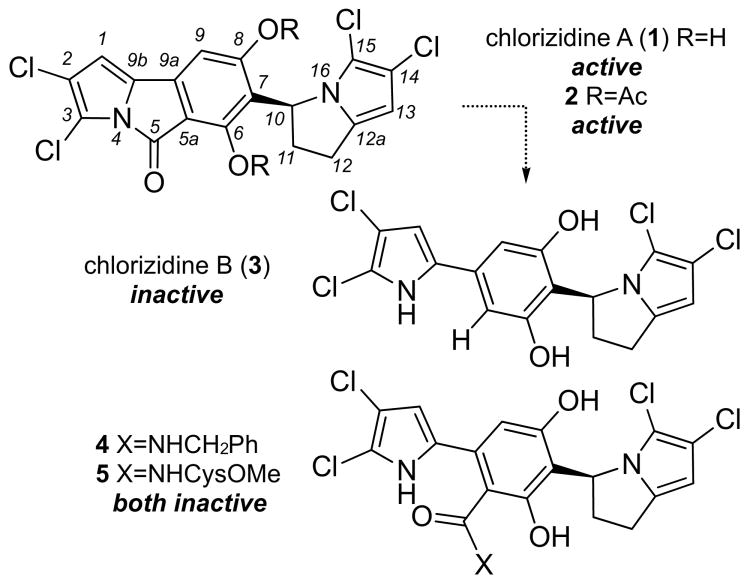
Structures of chlorizidine A (1), chlorizidine A bis-acetate (2), chlorizidine B (3) and adducts 4-5. Conversion of 1 to an inactive congener 3 arose by hydrolysis followed by decarboxylation. In addition, nucleophiles readily added to 1 resulting in adducts such as 4 or 5 that also displayed a loss of activity in HCT-116 cells.[4]
As described during the isolation efforts,[4] methylation of the phenols of 1 resulted in a bis-methyl ether, which lacked cytotoxicity in the in vitro HCT-116 colon carcinoma assay (IC50 value > 50 μM). Acetylation, on the other hand, provide the more stable bis-acetate 2 (Scheme 1) that offered an increase in activity (IC50 values were determined as 1.8±1.2 μM for 2 and 4.0±0.9 μM for 1, respectively),[4] suggesting that the low activity of 1 may arise due to instability in cells or a lack of cellular penetrance. This observation was further supported by the fact that we also obtained samples of a hydrolyzed and decarboxylated product (−)-chlorizidine B (3, Scheme 1) during the isolation effort.[4] Further studies indicated that the C5 carbonyl was indeed subject to nucleophilic attack as demonstrated by the addition of benzylamine and cysteine-O-methyl ester producing 4 and 5, respectively (Scheme 1).[4] Additional analyses also demonstrated that the congener (−)-chlorizidine B (3) was also inactive in HCT-116 bioassays indicating that the C5 amide was key to the activity observed in 1.[4]
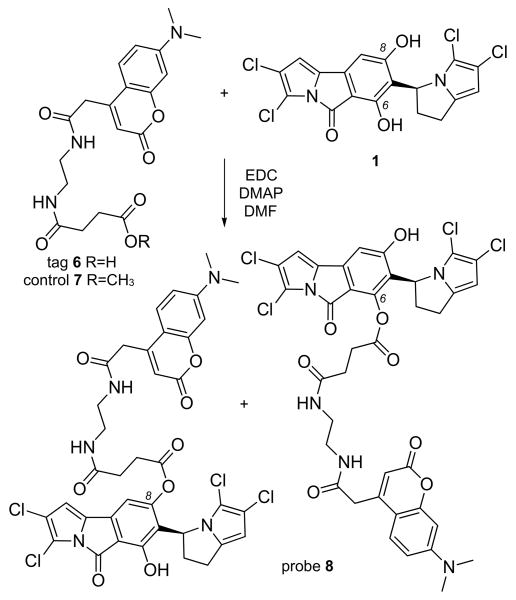
We then applied an immunoaffinity fluorescent (IAF) protocol developed in our laboratories to characterize the activity of 1 in tumor cells.[5-7] Merging our SAR data[4] with the functionally-diverse IAF tags,[8] we targeted the C6 and C8 phenols for installation of the acid-terminal immunoaffinity fluorescent (IAF) tag 6. Using a carbodiimide coupling (Scheme 2), a mono-labeled probe 8 was prepared by esterification of 1 with acid 6.[9] NMR analysis indicated that while probe 8 was pure (purity was also confirmed via LC/MS and HPLC analyses), it was likely a mixture of the C6 and C8 isomers (Supporting Information). We have previously observed a similar lack of selectivity in labeling polyphenolic natural products.[10] Biological analyses indicated that probe 8 retained activity in HCT-116 cells with an IC50 value of 2.2±0.8 μM, while compound 7, a control for the IAF tag, was inactive (IC50 value >50 μM in HCT-116 cells). Comparable results were also observed in other cell lines (Table S2, Supporting Information).
Scheme 2.
Synthesis of probe 8 by the esterification of 1 with IAF tag 6. Methyl ester 7 was prepared as a negative control for the IAF tag. NMR analyses suggested the presence of both C6 and C8 isomers as duplicate peaks were observed at proximal positions, H9/C9 and H10/C10, while single peaks at remote positions such as H1/C1 and H13/C13 (see Supporting Information).
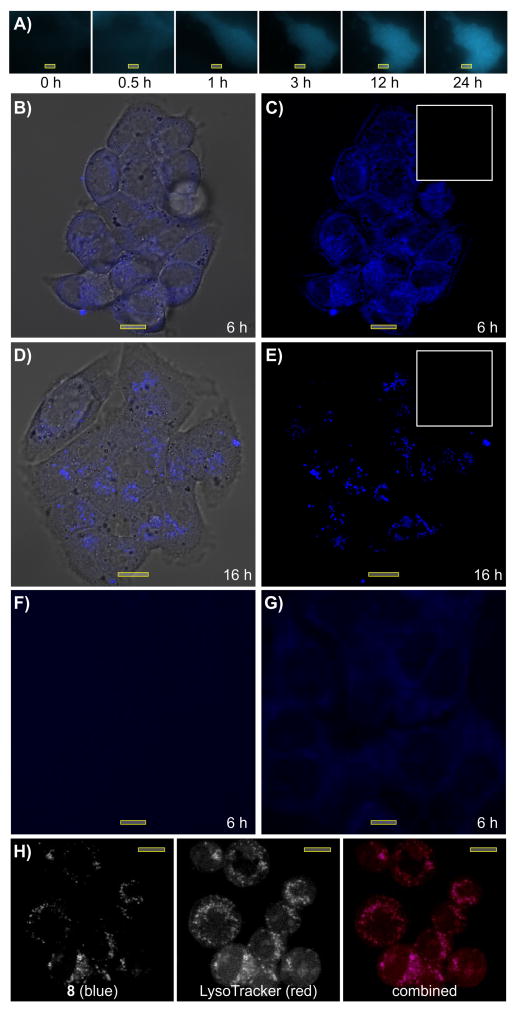
Our target evaluation studies began by examining the uptake and subcellular localization of 8 in HCT-116 cells. Using time course fluorescence microscopy (Fig. 1A), we found that probe 8 readily entered HCT-116 cells, appeared within the cytosol and remained there for the entire imaging time course. Confocal imaging provided further support, depicting probe 8 within the cytosol (Fig. 1B-1C) after 6 h even after washing with media. The uptake of 8 was reduced by either pretreatment with 1 (Fig. 1F) or by subsequent treatment with 1 (Fig. 1G) confirming that the localization of the blue fluorescence from 8 correlated with the activity of 1.
Figure 1.
Subcellular localization of 8 in HCT-116 cells. A) Time course imaging of HCT-116 cells treated with 10 μM 8. Confocal microscopic images depicting HCT-116 cells treated with B,C) 5 μM 8 for 6 h or D,E) 5 μM 8 for 16 h and washed with media lacking 8 prior to imaging. Images are provided (B,D) with and C,E) without overlaid bright field images. Insets in C) and E) depict the same experiments repeated with IAF control 7 showing complete removal after washing. F) A confocal microscopic image depicting HCT-116 cells treated 25 μM 1 for 1 h prior to staining with 5 μM 8 for 6 h. G) A confocal microscopic image depicting HCT-116 cells treated with 5 μM 8 for 6 h and then treated with 25 μM 1 for 1 h prior to imaging. H) A confocal microscopic image depicting HCT-116 cells treated with 5 μM 8 for 16 h and then co-stained with 2.5 μM LysoTracker red DND-99 for 15 min before imaging. The appearance of violet in the combined image indicates the co-localization of blue from 8 and red from the LysoTracker red DND-99. Bars denote 10 μm.
Over the following 10 h, probe 8 was transferred from cytosol into subcellular vesicles (Fig. 1D-1E), while the IAF control 7 was completely washed from the cells (inset, Fig. 1E). Using co-staining experiments, we were able to confirm that this subcellular localization was in the lysosomes. As shown in Fig. 1h, the blue fluorescence from 8 co-localized with the red fluorescence from LysoTracker red DND-99.
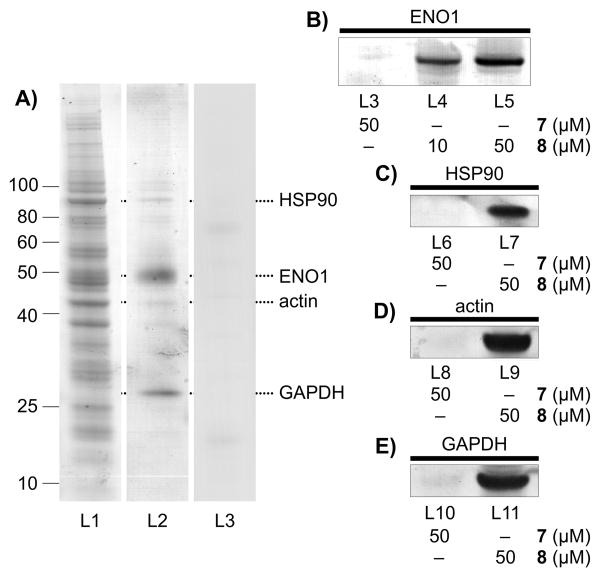
Next, we examined the affinity features of the IAF probe to screen for protein targets. We applied our immunoprecipitation (IP) strategy using Affigel 10 resin containing the covalently linked monoclonal antibody XRI-TF35, to identify proteins targeted by 8 in HCT-116 cell lysate (lane L1, Fig. 2A). Using conditions where treatment with the IAF control 7 returned no background (less than 1 ng total protein per IP),[7] we consistently obtained four bands (lane L2, Fig. 2A) with the major bands at 50 kDa (strongest) and 26 kDa and weaker bands at 90 and 45 kDa, respectively from lysates immunoprecipitated with 8. The amount of protein returned was dependent on the concentration of 8 and the ratio of bands remained consistent over multiple repetitions.
Figure 2.
Affinity analyses. A) Immunoprecipitation (IP) of HCT-116 cell lysate (1 mg/mL total protein) in PBS pH 7.2 containing 10 μM 8 returned four distinct protein bands after staining with GelCode Blue (Pierce). Lane L1-3 depicts HCT-116 cell lysate (L1), the IP fraction (L2) from probe 8, and the IP fraction (L2) from control 7. B) Western blot analysis depicting the level of hENO1 in the IP fraction of HCT-116 cells with IAF control 7 and probe 8. C) Western blot analysis depicting the validation of HSP90 in fraction of HCT-116 cell lysate immunoprecipitated with probe 8. D) Western blot analysis depicting the validation of actin in fraction of HCT-116 cell lysate immunoprecipitated with probe 8. E) Western blot analysis depicting the validation of GAPDH in fraction of HCT-116 cell lysate immunoprecipitated with probe 8. Ten fold more IP fraction was loaded on the gel for the experiments in C-E) as in B), as noted by the increased blot intensity.
Using trypsin-digest LC-MS/MS analyses, we examined bands from multiple repeats of the IP procedure. The major band at 48 kDa was identified as alpha-enolase, hENO1, with 12 matching peptides identified (Fig. 3A). The next band at 27 kDa, with ∼ 20% of the intensity of the 48 kDa band, was identified as D-glyceraldehyde-3-phosphate dehydrogenase, GAPDH (Fig. 3B). The two minor bands at 90 kDa and 45 kDa (lane L2, Fig. 2A) were also evaluated and identified as HSP90 (Fig. 3C) and actin (Fig. 3D), respectively.
Figure 3.
Protein ID analyses. A)-D) Protein sequences depicting the peptides (shaded) obtained for each of the protein bands immunoprecipitated in Fig. 2A. Dark shading denotes peptides observed and light shading highlights sites of cleavage.
We then turned to western blot analyses to validate the presence of the identified proteins within the fractions immunoprecipitated with probe 8. A dose dependent return of hENO1 was obtained as noted in lanes L4-L5 (Fig. 2B) treated with probe 8, but not in experiments conducted with control 7 (lane L3, Fig. 2B). Comparable evidence was also obtained for the bands corresponding to HSP90 (Fig. 2C), actin (Fig. 2D) and GAPDH (Fig. 2E) when immunoprecipitating with 8, therein supporting the protein ID analyses. The fact that each of the proteins was apparent in the fraction immunoprecipitated by 8 but not 7 provided strong support for their targeting by the active motif in 1.
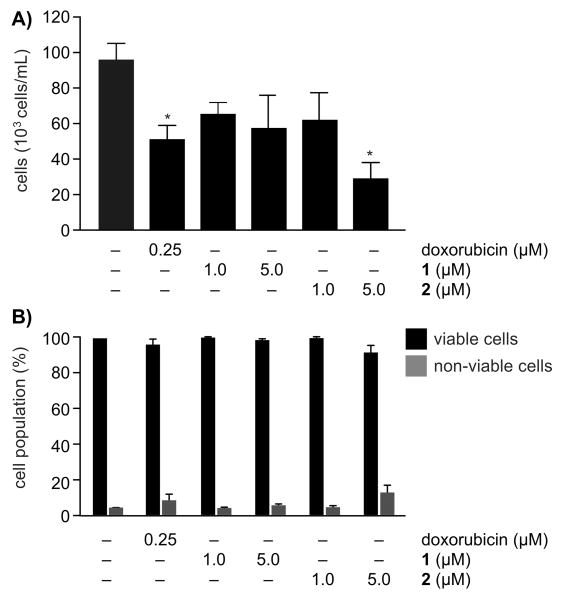
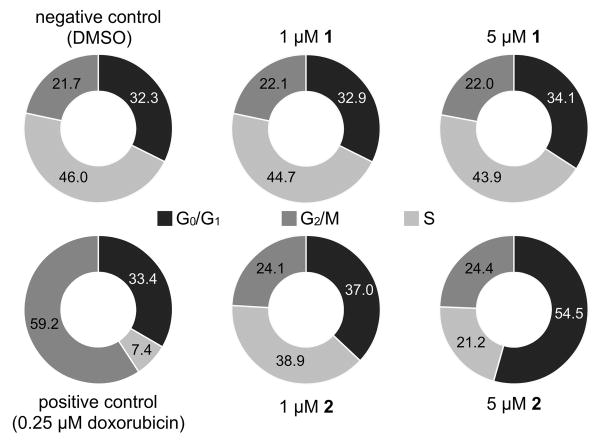
To further explore this activity, we further characterized the activities of 1 and its corresponding bis-acetate 2 in HCT-116 cells. As indicated in Fig. 4, treatment of HCT-116 cells with either 1 or 2 resulted in reduced concentrations of cells with a minor adjustment of the percentage of non-viable cells, indicating that treatment led to the inhibition of proliferation rather than cell death. We then evaluated the effects of 1 and 2 on the cell cycle. As shown in Fig. 5, compound 1 did not display statistically relevant effects on the population of cells. Treatment with the more active and stable bis-acetate 2 resulted in an increased population of cells in G1 phase with a corresponding loss of cells in S phase. This observation was consistent with the fact that inhibiting the glycolytic pathway results in halting the cell cycle at G1 phase in HCT-116 as well as HeLa cells.[11] Furthermore, these data are consistent with recent observations[12] that indicate that the levels of glycolysis are enhanced during G1 phase.
Figure 4.
Cell density and viability analyses. A) Cell density analyses with 1 and its bis-acetate 2. B) Cell viability analyses with 1 and bis-acetate 2. * p < 0.05, ANOVA followed by Dunnett's test.
Figure 5.
Cell cycle analyses. HCT-116 cells were treated with the ascribed compound for 24 h. The cells were then collected and evaluated by flow cytometry. Levels are given as indicated by G0/G1 (dark grey), G2/M (grey) and S (light grey). Histograms depicting the corresponding levels are provided in the Supporting Information.
Interested in further exploring this response, we conducted isothermal calorimetry (ITC) studies using recombinantly expressed hENO1.[13] Using nano-ITC, we obtained a Kd of 1.9 ±0.9 μM over multiple titrations of hENO1 with 1 (Supporting Figure S2). These studies also indicated that the binding occurred with a stoichiometry of 1.4-1.6 molecules of 1 per hENO1. Comparable analyses on heat-decomposed samples of 1 or bis-acetate 2 did not show binding under the same conditions, as it was indistinguishable from background, indicating that while 2 displays activity it arises only after hydrolysis to 1. Further analyses however did indicate that probe 8 retained binding to hENO1 with a Kd of 0.69±0.01 μM (Supporting Figure S2), suggesting that while full acetylation removed activity the mono-acetylated probe was active. Combined, these data validated the interaction between 1 and hENO1.
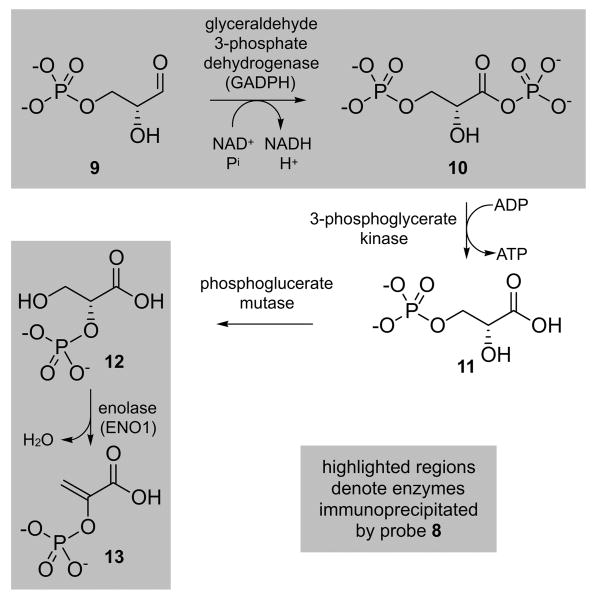
Interestingly, the two most abundant immunoprecipitated proteins hENO1 and GAPDH share a close proximity in the glycolytic pathway (Scheme 3) with GAPDH acting on glyceraldehyde-3-phospate (9) and hENO1 on 3-phospho-D-glycerate (12). It is quite remarkable that the top proteins isolated by IP studies with 8 are two proteins steps apart within the same pathway. This evidence clearly suggests that (-)-chlorizidine A (1) may play a role in signaling processes regulated by GAPDH or hENO1.
Scheme 3.
Steps 6-9 of the glycolysis of D-glucose, denoting the roles of GAPDH (step 6) and hENO1 (step 9), the most abundant immunoprecipitated proteins found in HCT-116 cell lysates when using probe 8.
In addition, this discovery suggests that 1 or stabilized derivatives such as 2 may serve as tools to further study the role of hENO1 and GAPDH in tumor cell growth and progression. This suggestion is further supported by the immunoprecipitation of hENO1 and GAPDH by probe 8, as well as by the fact that probe 8, hENO1[14] and GAPDH[15] localized within the cytosol. The identification of HSP90 (Fig. 2C) and actin (Fig. 2D), observed in lesser quantities during the target identification process, could suggest further interactivity between hENO1 or GAPDH and cytoskeletal[16] or heat shock[17] proteins. Alternatively, and most likely, the isolation of HSP90 and actin, two abundant proteins in mammalian cells, could have resulted from non-specific binding events based upon the nucleophilic reactivity of the C5 carbonyl in probe 8.[18]
The glycolytic pathway is well recognized as a primary target for therapeutic intervention, as most invasive cancers display specific modulations of cellular energy metabolism.[19] Many of the enzymes within this pathway have subsequently been identified as therapeutic targets. For instance, GAPDH has an established role in cell death and carcinogenesis.[20] Recently, it has been shown to induce cancer cell senescence.[21] This, combined with the fact that hENO1 has been linked with tumor progression in several cases of breast and lung cancer,[22] as well as serving as an auto antigen associated with Hashimoto's encephalopathy[23] and severe asthma,[24] suggests possible biomedical utility for (-)-chlorizidine A (1) or a suitable, more stable analogue. Initial evidence suggests that 1, 2 and 8 display selectivity to tumor cell lines as indicated by the enhanced activity in PC-3M (prostate carcinoma) and HCT-116 (colon carcinoma) cells as compared to normal MRC-5 cells (Table S1 in the Supporting Information).
Recent evidence clearly suggests a role for anticancer agents that target proteins associated with the glycolytic pathway.[24] The fact that an active IAF probe has been developed herein also suggests that it may be adapted as a probe for confocal microscopy. This is supported by the fact that while probe 8 initially localized within the cytosol, overtime it localized into lysosome or autophagosomal compartments, as illustrated by comparing the localization of 8 after 6 h (Fig. 1B-1C) versus that at 16 h (Fig. 1D-1E). Efforts are now underway to develop a detailed understanding of the regulatory effects of 1, including determining if 1, and associated analogues such as 2, act by blocking the activity of hENO1 and GAPDH or by other mechanisms such as regulating protein interactivity associated with these proteins.[26-28] Further efforts will be focused on determining the structural details of these binding events and elucidating the pathways that connect the targeting of hENO1 and/or GAPDH to the observed cell death phenomenon.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Health, National Cancer Institute under grant R37-CA044848 (to W.F.) and Brazilian National Council for Scientific and Technological Development CNPq (to L.V.C.L). We thank Dr. Chambers Hughes for assistance with preparation of the IAF probes described herein. Special acknowledgement goes out to Prof. Gabriela Chiosis (Memorial Sloan Kettering Cancer Center) and Dr. Hardik J. Patel (Memorial Sloan Kettering Cancer Center) for conducting the fluorescence polarization binding analyses with Hsp90a, Grp94 and TRAP-1. We also thank our UC San Diego colleagues Dr. Majid Ghassemian, Dr. Yongxuan Su, Dr. Anthony Mrse and Dr. Kersi Pestonjamasp for assistance with protein mass spectroscopy, small molecule mass spectroscopy, NMR spectroscopy, and confocal microscopy, respectively. Confocal microscopy was conducted at the Universidade Federal do Ceará and the UC San Diego Microscopy Shared Resource Center, with the latter being funded through NIH grant P30 NS047101.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/###.
References
- 1.Fenical W, Jensen PR. Nat Chem Biol. 2006;2:666–673. doi: 10.1038/nchembio841. [DOI] [PubMed] [Google Scholar]
- 2.Hughes CC, Fenical W. Chemistry. 2010;16:12512–12525. doi: 10.1002/chem.201001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maldonado LW, Fenical W, Jensen PR, Kauffman CA, Mincer TJ, Ward AC, Bull AT, Goodfellow M. Int J Syst Evol Microbiol. 2005;55:1759–1766. doi: 10.1099/ijs.0.63625-0. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Mico X, Jensen PR, Fenical W, Hughes CC. Org Lett. 2013;15:988–991. doi: 10.1021/ol303374e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes CC, MacMillan JB, Gaudêncio SP, Jensen PR, Fenical W. Angew Chem Int Ed. 2008;121:742–746. [Google Scholar]; Angew Chem. 2008;48:725–727. [Google Scholar]
- 6.Hughes CC, Yang YL, Liu WT, Dorrestein PC, La Clair JJ, Fenical W. J Am Chem Soc. 2008;131:12094–12096. doi: 10.1021/ja903149u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu WL, Guizzunti G, Foley TL, Burkart MD, La Clair JJ. J Nat Prod. 2010;73:1659–1666. doi: 10.1021/np100371k. [DOI] [PubMed] [Google Scholar]
- 8.Alexander MD, Burkart MD, Leonard MS, Portonovo P, Liang B, Ding X, Joullié MM, Gulledge BM, Aggen JB, Chamberlin AR, Sandler J, Fenical W, Cui J, Gharpure SJ, Polosukhin A, Zhang HR, Evans PA, Richardson AD, Harper MK, Ireland CM, Vong BG, Brady TP, Theodorakis EA, La Clair JJ. Chembiochem. 2006;7:409–416. doi: 10.1002/cbic.200500466. [DOI] [PubMed] [Google Scholar]
- 9.Stout EP, Cervantes S, Prudhomme J, France S, La Clair JJ, Le Roch K, Kubanek J. ChemMedChem. 2011;6:1572–1577. doi: 10.1002/cmdc.201100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farnaes L, La Clair JJ, Fenical W. Org Biomol Chem. 2014;12:418–423. doi: 10.1039/c3ob41355a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shim H, Chun YS, Lewis BC, Dang CV. Proc Natl Acad Sci USA. 1998;95:1511–1516. doi: 10.1073/pnas.95.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao Y, Mukai K, Hishiki T, Kubo A, Ohmura M, Sugiura Y, Matsuura T, Nagahata Y, Hayakawa N, Yamamoto T, Fukuda R, Saya H, Suematsu M, Minamishima YA. Mol Cancer Res. 2013;11:973–985. doi: 10.1158/1541-7786.MCR-12-0669-T. [DOI] [PubMed] [Google Scholar]
- 13.Lee H, Guo Y, Ohta M, Xiong L, Stevenson B, Zhu JK. EMBO J. 2002;21:2692–2702. doi: 10.1093/emboj/21.11.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukano K, Kimura K. Methods Enzymol. 2014;542:115–124. doi: 10.1016/B978-0-12-416618-9.00006-6. [DOI] [PubMed] [Google Scholar]
- 15.Vöpel T, Makhatadze G. PLoS One. 2012;7:e39418. doi: 10.1371/journal.pone.0039418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lew CW, Tolan DR. J Biol Chem. 2012;287:42554–42564. doi: 10.1074/jbc.M112.405969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao X, Bloomston M, Zhang T, Frankel WL, Jia G, Wang B, Hall NC, Koch RM, Cheng H, Knopp MV, Sun D. Clin Cancer Res. 2008;14:1831–1839. doi: 10.1158/1078-0432.CCR-07-1607. [DOI] [PubMed] [Google Scholar]
- 18.Early evidence suggests that that this is the case at least for Hsp90. Fluorescent polarization analyses indicated that clorizidine A (1) has no significant binding affinity for Hsp90a, Grp94 or TRAP-1 proteins up to the concentration of 50 μM using the methods reported in. Taldone T, Patel PD, Patel M, Patel HJ, Evans CE, Rodina A, Ochiana S, Shah SK, Uddin M, Gewirth D, Chiosis G. J Med Chem. 2013;56:6803–6818. doi: 10.1021/jm400619b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granchi C, Fancelli D, Minutolo F. Bioorg Med Chem Lett. 2014;24:4915–4925. doi: 10.1016/j.bmcl.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 20.Colell A, Green DR, Ricci JE. Cell Death Differ. 2009;16:1573–1581. doi: 10.1038/cdd.2009.137. [DOI] [PubMed] [Google Scholar]
- 21.Nicholls C, Pinto AR, Li H, Li L, Wang L, Simpson R, Liu JP. Proc Natl Acad Sci U S A. 2012;109:13308–13313. doi: 10.1073/pnas.1206672109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Díaz-Ramos A, Roig-Borrellas A, García-Melero A, López-Alemany R. J Biomed Biotechnol. 2012;2012:156795. doi: 10.1155/2012/156795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoneda M, Fujii A, Ito A, Yokoyama Y, Nakagawa H, Kuriyama M. J Neuroimmunol. 2007;185:195–200. doi: 10.1016/j.jneuroim.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Nahm DH, Lee KH, Shin JY, Ye YM, Kang Y, Park HS. J Allergy Clin Immunol. 2006;118:376–381. doi: 10.1016/j.jaci.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Granchi C, Minutolo F, F ChemMedChem. 2012;7:1318–1350. doi: 10.1002/cmdc.201200176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu QF, Liu Y, Fan Y, Hua SN, Qu HY, Dong SW, Li RL, Zhao MY, Zhen Y, Yu XL, Chen YY, Luo RC, Li R, Li LB, Deng XJ, Fang WY, Liu Z, Song X. J Hematol Oncol. 2015;8:22. doi: 10.1186/s13045-015-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seidler NW. Adv Exp Med Biol. 2013;985:179–206. doi: 10.1007/978-94-007-4716-6_6. [DOI] [PubMed] [Google Scholar]
- 28.Nicholls C, Li H, Liu JP. Clin Exp Pharmacol Physiol. 2012;39:674–679. doi: 10.1111/j.1440-1681.2011.05599.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.