Transplantation of progenitors from induced pluripotent stem cells reprogrammed by lentiviral vectors led to the formation of invasive teratocarcinoma-like tumors in more than 90% of immunodeficient mice. Combined transgene-free reprogramming and elimination of residual pluripotent cells by enzymatic dissociation ensured tumor-free transplantation, ultimately enabling regeneration of type 1 diabetes-specific human islet structures in vivo.
Keywords: Type 1 diabetes, Teratoma, iPS cells, Insertional mutagenesis, Integration
Abstract
Human induced pluripotent stem cells (iPSCs) and derived progeny provide invaluable regenerative platforms, yet their clinical translation has been compromised by their biosafety concern. Here, we assessed the safety of transplanting patient-derived iPSC-generated pancreatic endoderm/progenitor cells. Transplantation of progenitors from iPSCs reprogrammed by lentiviral vectors (LV-iPSCs) led to the formation of invasive teratocarcinoma-like tumors in more than 90% of immunodeficient mice. Moreover, removal of primary tumors from LV-iPSC progeny-transplanted hosts generated secondary and metastatic tumors. Combined transgene-free (TGF) reprogramming and elimination of residual pluripotent cells by enzymatic dissociation ensured tumor-free transplantation, ultimately enabling regeneration of type 1 diabetes-specific human islet structures in vivo. The incidence of tumor formation in TGF-iPSCs was titratable, depending on the oncogenic load, with reintegration of the cMYC expressing vector abolishing tumor-free transplantation. Thus, transgene-free cMYC-independent reprogramming and elimination of residual pluripotent cells are mandatory steps in achieving transplantation of iPSC progeny for customized and safe islet regeneration in vivo.
Significance
Pluripotent stem cell therapy for diabetes relies on the safety as well as the quality of derived insulin-producing cells. Data from this study highlight prominent tumorigenic risks of induced pluripotent stem cell (iPSC) products, especially when reprogrammed with integrating vectors. Two major underlying mechanisms in iPSC tumorigenicity are residual pluripotent cells and cMYC overload by vector integration. This study also demonstrated that combined transgene-free reprogramming and enzymatic dissociation allows teratoma-free transplantation of iPSC progeny in the mouse model in testing the tumorigenicity of iPSC products. Further safety assessment and improvement in iPSC specification into a mature β cell phenotype would lead to safe islet replacement therapy for diabetes.
Introduction
Ectopic expression of defined pluripotency-associated transcription factors achieves nuclear reprogramming of adult somatic cells [1]. As such, induced pluripotent stem cells (iPSCs) are generated in an embryo-independent manner and demonstrate unlimited self-renewal and pluripotent lineage differentiation capacity. In principle, iPSCs would offer a valuable regenerative platform for diagnostic and therapeutic applications [2, 3].
However, use of integrating reprogramming vectors—a first-generation reprogramming strategy—has been associated with genomic modifications of derived iPSCs. Moreover, the resulting variations in vector integration sites, residual expression/reactivation of integrated transgenes, or the degrees of induced reprogramming can all affect the redifferentiation capacity, leading to heterogeneity in the derivation of patient iPSCs [4, 5]. Integration of viral vectors also results in insertional mutagenesis due to undesired activation/suppression of essential host genes proximal to integration sites [6–9]. Stable introduction of an integrating vector encoding an oncogene, such as cMYC, can furthermore impose a tumorigenic load upon iPSC progeny [10]. Accordingly, newer reprogramming technologies have been introduced to facilitate generation of transgene-free iPSCs, devoid from genomic modification [11–16]. As a case in point, nonintegrating reprogramming circumvents most of the issues associated with integrating vectors. Nevertheless, the impact of reprogramming strategies on ensuing differentiation capacity and safety remain elusive, limiting translation of iPSC-based platforms.
Type 1 diabetes mellitus (T1D) is caused by autoimmune destruction of insulin-producing pancreatic β cells, resulting in severe insulin deficiency and a lifelong dependence on insulin-replacement therapies [17]. Various regenerative approaches have been explored as an alternative aimed at restoration of β-cell function in the setting of T1D. This includes potential use of embryonic stem cell (ESC)-derived insulin-producing cells [18]. Guided differentiation of ESCs—through stepwise specification into definitive endoderm, pancreatic endoderm, and endocrine progenitor cell stages—has successfully generated pancreatic multihormonal endocrine cells in vitro [19–21]. Furthermore, in vivo maturation of ESC-derived pancreatic progenitors has demonstrated regeneration of functional β cells, which are capable of restoring normal glucose metabolism in diabetic mice [20, 22]. Implementing an embryo-independent approach for pluripotent stem cell generation, iPSCs—bioengineered by integrating lentiviral reprogramming vectors—also demonstrate the feasibility of yielding autologous insulin-producing β-like cells from patients with diabetes in vitro [4, 23, 24]. However, limited information is available on the safety of transplanting diabetic patient-derived iPSC-generated pancreatic progenitors in vivo.
For therapeutic applications of iPSCs, the safety of iPSC products is critical. After the recent first-in-human iPSC treatment of age-related macular degeneration [25], other iPSC-based applications are being considered. Herein, we define the requirements necessary to achieve tumor-free transplantation of iPSC progeny and customize safe islet regeneration in vivo.
Materials and Methods
Written informed consent was obtained from a T1D patient for skin biopsy. De-identified surgical waste specimens were used to derive type 2 diabetes (T2D)-specific keratinocytes. All animal and human studies were approved by the Mayo Clinic Institutional Animal Care and Use Committee, Institutional Review Board, and Biospecimen Review Board. Information for cytogenetic analysis, spontaneous differentiation, and microarray are included as supplemental online data.
Cells
LV-iPSC clones from peripheral blood and hematopoietic progenitor cells (PBMC-LV#S1 and HPC-LV#A1) were generated as previously reported [26]. LV-iPSCs, derived from a nondiabetic donor (ND2-LV#N1) and patients with T2D (SW8-LV#20I and SW10-LV#5P) were established as previously described [23]. T1D-specific iPSCs (LV-iPSC and TGF-iPSC clones) generated from a 42-year-old male patient who has had T1D for 37 years and the TGF-iPSC clone from a 67-year-old patient with T2D were derived as previously described [27]. TGF-iPSC clone peripheral blood mononuclear cell (PBMC)-DSSV was derived from PBMCs as reported [26] by using Sendai viral reprogramming vectors [27]. All iPSC clones were maintained in Pluriton Reprogramming Medium (Stemgent, Cambridge, MA, https://www.stemgent.com) supplemented with 25% (vol/vol) mTeSR-1 maintenance medium (StemCell Technologies, Vancouver, BC, Canada, http://www.stemcell.com) on BD Matrigel-coated (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) cell culture plates.
Immunostaining
For immunostaining of iPSCs and iPSC progeny, cells were cultured in Matrigel-coated Lab-Tek Chamber slides (Nunclone). For immunostaining of pancreatic or renal samples, tissues were embedded and frozen in OCT compound (Sakura, Torrance, CA, http://www.sakura-americas.com). Cells/tissues were then fixed in 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and blocked in PBS with 5% fetal bovine serum (FBS). Cells were then stained with specific antibodies, including insulin (catalog no. 12018, Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), C-peptide (catalog no. 4593; Cell Signaling Technology, Beverly, MA, http://www.cellsignal.com), TRA-1-60 (catalog no. SCR001; EMD Millipore, Billerica, MA, http://www.millipore.com), OCT4 (catalog no. 2750; Cell Signaling Technology), SOX2 (catalog no. 2748; Cell Signaling Technology), NANOG (catalog no. ab21624; Abcam, Cambridge, MA, http://www.abcam.com/), FOXA2 (catalog no. 07-633; Millipore), β-III tubulin (catalog no. 41489; Abcam), and CD31 (catalog no. SC1506; Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com). Nuclei were counterstained by 4′,6-diamidino-2-phenylindole (DAPI). Stained cells were observed through a Zeiss LSM 780 (Zeiss, Stuttgart, Germany, http://www.zeiss.com) confocal laser scanning microscope, and the images were analyzed by using the Zeiss imaging software.
Reverse-Transcription Polymerase Chain Reaction
Total cellular RNA was isolated by using the RNeasy Mini Kit (Qiagen, Hilden, Germany, http://www.qiagen.com). cDNA synthesis was performed with 1 µg of RNA by using RNA to cDNA EcoDry Premix (Clontech, Mountain View, CA, http://www.clontech.com/). Predesigned primers from Invitrogen were purchased to detect PDX1, NKX6.1, GCG, and INS transcripts.
Differentiation of iPS Cells Into Insulin-Producing Cells
iPSC clones were treated with 25 ng/ml Wnt3a (R&D Systems, Minneapolis, MN, https://www.rndsystems.com) and 100 ng/ml activin A (Peprotech, Rocky Hill, NJ, http://www.peprotech.com) in advanced RPMI (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) supplemented with Pen/Strep for a day, followed by 100 ng/ml activin A treatment in advanced RPMI supplemented with 0.2% fetal calf serum (FCS) (Invitrogen) for 2 days to generate definitive endoderm cells (day 3). Cells were then cultured in high-glucose DMEM (HG-DMEM; Invitrogen), supplemented with 20% (vol/vol) advanced RPMI medium containing 50 ng/ml FGF10 (R&D systems), 0.25 µM KAAD-cyclopamine (CYC), and 2% FCS for 2 days, followed by treatment with 50 ng/ml FGF10, 0.25 µM CYC, and 2 µM all-trans retinoic acid (Sigma-Aldrich) in HG-DMEM supplemented with 20% advanced RPMI, Pen/Strep, 1× B27 supplement (Invitrogen) for 4 days, resulting in primitive gut tube-like cells (day 9). Cells were further cultured in 50 ng/ml FGF10, 5 nM Indolactam V (ILV) (Axxora, San Diego, CA, http://www.axxora.com/), and 55 nM GLP-1 (Sigma) in HG-DMEM supplemented with 20% advanced RPMI and 1× B27 for 4 days to generate pancreatic endoderm cells (day 13). For generation of insulin-producing cells, cells were further cultured in the HG-DMEM medium supplemented with 55 nM GLP-1 and 1× B27 for 6 days, followed by incubation with 50 ng/ml hepatocyte growth factor (R&D Systems), 50 ng/ml insulin-like growth factor 1 (R&D Systems), and 55 nM GLP-1 in HG-DMEM with 1× B27 for 5 days (day 24).
Transplantation of iPSCs and iPSC Progeny
SCID-beige mice were anesthetized, the flank was incised and kidney exposed, a small incision was made in the kidney capsule, and a blunt needle was used to create a pocket under the kidney capsule. After transplantation of undifferentiated iPSCs or iPSC-derived pancreatic endoderm cells, the kidney was placed back into the abdomen, and the incision was closed. Mice were then maintained for 1–8 months and sacrificed for harvesting normal and iPS-transplanted kidneys. OCT tissue-fixative-embedded frozen tissues were cryosectioned for H&E and immunostaining.
Results
Pancreatic Progenitor Cells From Lentivirus-Reprogrammed iPSCs Form Invasive Teratocarcinoma-Like Tumors
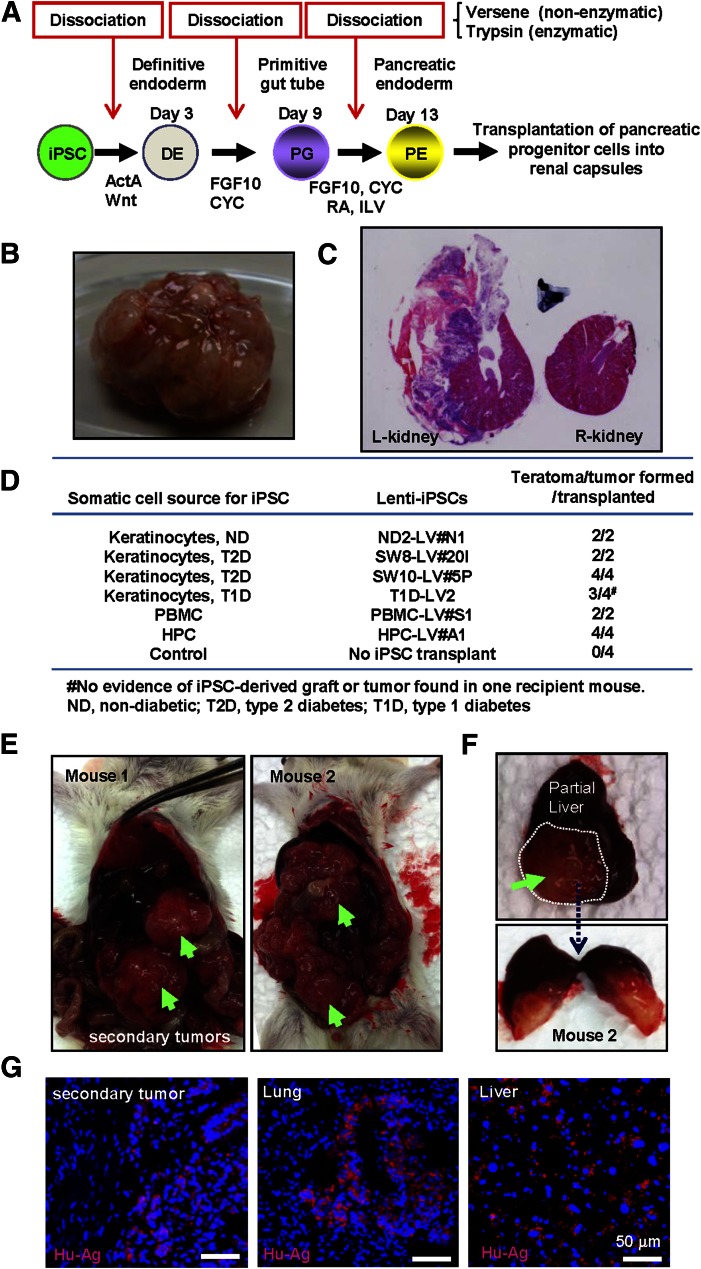
Stepwise differentiation facilitates generation of PDX1- and NKX6.1-expressing pancreatic endoderm cells from pluripotent stem cell sources [22, 28]. Previous studies have demonstrated successful human islet regeneration in vivo upon transplantation of ESC-derived pancreatic endoderm/progenitor cells in immunocompromised mice [22, 29]. To assess the efficiency of human islet regeneration and the risk of teratoma formation upon transplantation of lentivirus-reprogrammed iPSC (LV-iPSC)-derived pancreatic endoderm cells, previously characterized LV-iPSCs, made from nondiabetic healthy donors and verified for their pluripotency and differentiation propensities, were differentiated into pancreatic endoderm cells (Fig. 1A), and 1 million cells were transplanted under the kidney capsule of SCID-beige mice. Within 4–6 weeks, LV-iPSC grafts gave rise to ∼2-cm solid tumors (Fig. 1B). Histology of the cross-section of the grafts demonstrated diverse cell types within the complex architecture of the graft, including glandular epithelium, adipose, muscular, and poorly differentiated tissues. Notably, the LV-iPSC-derived tumors were found invading into the kidney structure (Fig. 1C). Consistently, transplantation of pancreatic progenitors from various LV-iPSC lines resulted in rapid tumor formation in immunocompromised mice (more than 90% of cases, i.e., 17 of 18 recipient mice) (Fig. 1D).
Figure 1.
Pancreatic progenitor cells from lentivirus-reprogrammed iPSCs (LV-iPSCs) form invasive teratocarcinoma-like tumors. (A): Stepwise differentiation protocol for generation of pancreatic endoderm cells from human iPS cells. Schematic diagram of the differentiation process is shown. (B): A representative image of left kidney, completely covered by an iPSC-derived tumor. (C): Cross-sections of the left kidney, transplanted with iPSC progeny, and the control right kidney from the same animal were imaged after H&E staining. Note the disruption of kidney structure by the iPSC-derived tumor. (D): Summary of the incidence of tumor formation upon transplantation of iPSC progeny. (E): Formation of secondary tumors upon removal of the primary tumor by unilateral nephrectomy. Secondary tumors are indicated by green arrows. (F): A metastatic tumor found on the liver surface (upper) and its cross-section (lower) are shown. Note the tumor invading deep inside the liver. (G): Immunostaining with anti-human antigen antibody was performed to detect human iPSC-derived cells in the tumors. The secondary tumors found in the peritoneal cavity, and metastatic tumors found in the lung and the liver, were shown to be human cells (shown with red signals). Nuclei were counterstained by DAPI. Abbreviations: ActA, Activin A; CYC, KAAD-cyclopamine; DAPI, 4′,6-diamidino-2-phenylindole; HPC, hematopoietic progenitor cells; ILV, indolactam V; iPSCs, induced pluripotent stem cells; ND, nondiabetic; PBMC, peripheral blood mononuclear cells; RA, all-trans retinoic acid; Wnt, Wnt 3a.
Naturally occurring teratomas are generally benign [30, 31]. We therefore performed unilateral nephrectomy to remove the iPSC-derived tumors. Unexpectedly, large secondary tumors developed within 3 weeks (four of four mice tested) (Fig. 1E), in some cases with additional metastatic tumors in the liver (Fig. 1F) or the lung (not shown). The metastatic tumor was found invading deep into tissue parenchyma (Fig. 1F). An antibody specific for human mitochondrial antigen (supplemental online Fig. 1) detected human cells within mouse tissues harvested from the recipient mice, including secondary tumors in the peritoneal cavity and metastatic tumors in the lung and liver (Fig. 1G), indicating that the tumors had originated from human iPSC grafts. These results revealed a high tumorigenicity of LV-iPSC-derived cells and highly aggressive cancerous properties of derived tumors in SCID-beige mice.
Combined Transgene-Free Reprogramming and Enzymatic Dissociation Allows Teratoma-Free Transplantation of iPSC Progeny and Regeneration of T1D-Specific Human Islets
To dissect tumorigenic risks of pluripotency from the use of integrating reprogramming vectors, we assessed the safety of pancreatic progenitors derived from transgene-free iPSCs (TGF-iPSCs) established by using nonintegrating Sendai viral reprogramming vectors. We used TGF-iPSC lines from patients with type 1 and 2 diabetes [27] and PBMCs (supplemental online Fig. 2). To assess the influence of sustained reprogramming vector integration on the ensuing redifferentiation process, we first tested the pancreatic differentiation propensities of LV- and TGF-iPSCs upon guided differentiation. All clones were differentiated into NKX6.1-positive pancreatic endoderm cells at day 13 (supplemental online Fig. 3A). Further directed differentiation resulted in induction of insulin at day 24 (supplemental online Fig. 3B). Flow cytometry analysis showed that approximately 80% and 10% of iPSC progeny expressed PDX1 and insulin at this stage (supplemental online Fig. 3C). Although some clonal variations were evident upon differentiation of five LV-iPSC and four TGF-iPSC lines, there was no notable difference in pancreatic differentiation capacities between TGF- and LV-iPSC clones (supplemental online Fig. 3D).
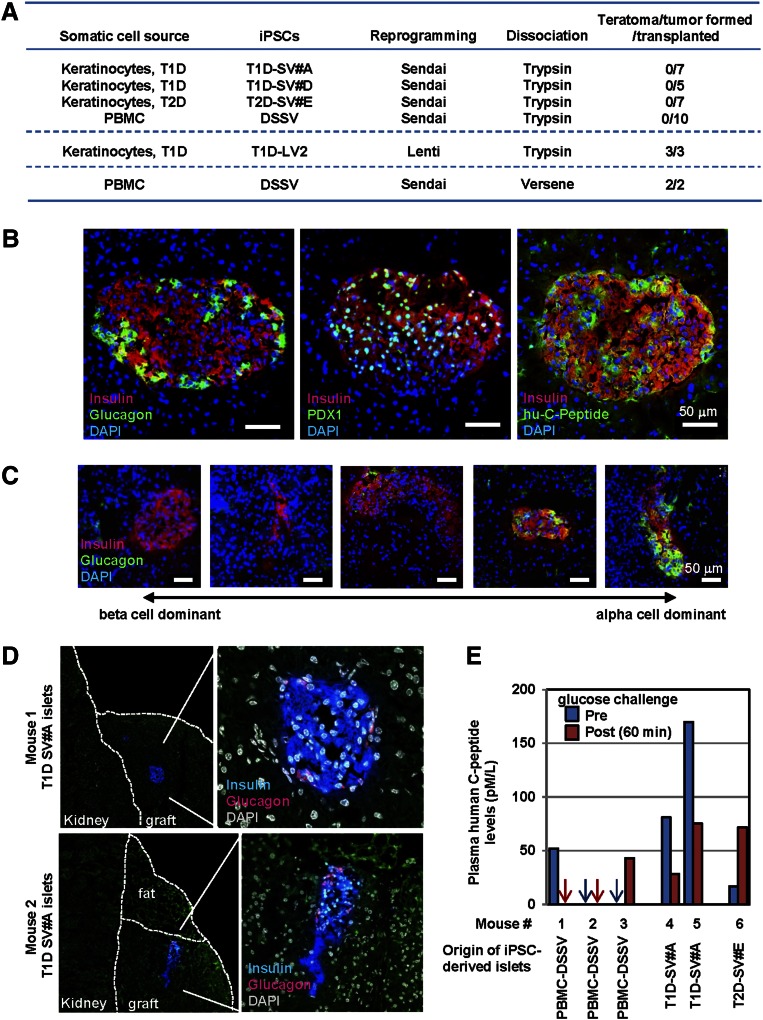
When TGF-iPSC clones were differentiated into pancreatic progenitor cells, by using the same differentiation protocol with nonenzymatic dissociation steps (Fig. 1A) and transplanted beneath the kidney capsule, TGF-iPSC grafts developed teratoma-like tumors (Fig. 2A). Because human pluripotent stem cells are sensitive to enzymatic single cell dissociation [32, 33], we used trypsinization-mediated cell dissociation to eliminate contamination by pluripotent cells of the iPSC-derived progeny population. Transplantation of the TGF-iPSC progeny, differentiated and enzymatically dissociated, resulted in no tumor formation at 3 and 8 months after transplantation (0 of 29 and 0 of 3 recipient mice with tumor) (Fig. 2A). In sharp contrast, enzymatic dissociation did not prevent tumor formation of LV-iPSC-derived pancreatic endoderm cells (Fig. 2A; 3 of 3 recipient mice transplanted with T1D-specific LV-iPSC progeny).
Figure 2.
Combined transgene-free reprogramming and enzymatic dissociation allows teratoma-free transplantation of iPSC progeny and regeneration of human islets from T1D patients. (A): Summary of the incidence of tumor formation upon transplantation of pancreatic endoderm cells from TGF- and LV-iPSCs, made by Sendai viral and lentiviral reprogramming vectors, respectively. Mice were observed for 3 months for the TGF-iPSC progeny, differentiated with the enzymatic dissociation protocol. Mice having received pancreatic endoderm cells from LV-iPSCs or TGF-iPSCs, underwent differentiation with the enzymatic or nonenzymatic dissociation protocols, respectively. Recipients, consequently, were sacrificed at 3-5 weeks post transplantation due to enlarged iPSC-derived tumors. (B): Sections of recovered TGF-iPSC-derived grafts in the kidney capsules were assessed for formation of human islet-like structures by immunostaining with specific antibodies against insulin, glucagon, PDX1 and human C-peptide. Nuclei were counterstained by DAPI (blue). (C): Representative immunostaining images of TGF-iPSC-derived islets with heterogeneity in the predominance of β- and α-cell contents from a single recipient mouse are shown. (D): Regeneration of human islets from a patient with T1D. SCID beige mice received pancreatic progenitor cells derived from TGF-iPSCs originating from a skin biopsy. Recovered grafts were assessed for islet regeneration by insulin and glucagon antibodies. Nuclei were counter stained by DAPI (white). (E): Circulating levels of human C-peptide was measured at 2 months after transplantation of iPSC-derived pancreatic progenitor cells. Plasma samples were harvested at fasting (pre) and 60 min after glucose challenge (post) conditions to measure human C-peptide levels by ELISA, an assay without cross-reactivity toward mouse C-peptide. No statistical significant glucose-responsive insulin secretion was observed. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; ELISA, enzyme-linked immunosorbent assay; iPSC, induced pluripotent stem cell; LV-iPSC, lentivirus-reprogrammed induced pluripotent stem cell; PBMC, peripheral blood mononuclear cell; TGF-iPSC, transgene-free induced pluripotent stem cell; T1D, type 1 diabetes mellitus.
Recovered TGF-iPSC-derived grafts were assessed for formation of human islet-like structures. Regeneration of human islets was found in recipient mice transplanted with PBMC-DSSV progeny (Fig. 2B). Unlike mouse islets, which have β cells predominantly in the core of the cluster and scarce α cells in the periphery, TGF-iPSC-derived islets displayed insulin-positive β cells and glucagon-positive α cells throughout islet clusters, reminiscent of authentic human islets (Fig. 2B). iPSC-derived insulin-positive cells were also positive for the mature β-cell marker PDX1 and the proinsulin byproduct human C-peptide (Fig. 2B). Heterogeneity was observed in the predominance of β and α cells in regenerated islets (Fig. 2C). In fact, transplantation of pancreatic progenitors from T1D-specific TGF-iPSCs (SV#A) facilitated regeneration of human islets from one T1D patient (Fig. 2D), yet absence of insulin-positive cells was observed in grafts derived from another T1D (SV#D). Use of pancreatic progenitor cells from T2D-specific TGF-iPSCs (SV#E) led to regeneration of few islet-like structures (not shown). Glucose challenge did not increase circulating levels of human C-peptide (Fig. 2E).
Integrating cMYC Vector Imposes Tumorigenic Load in iPSCs
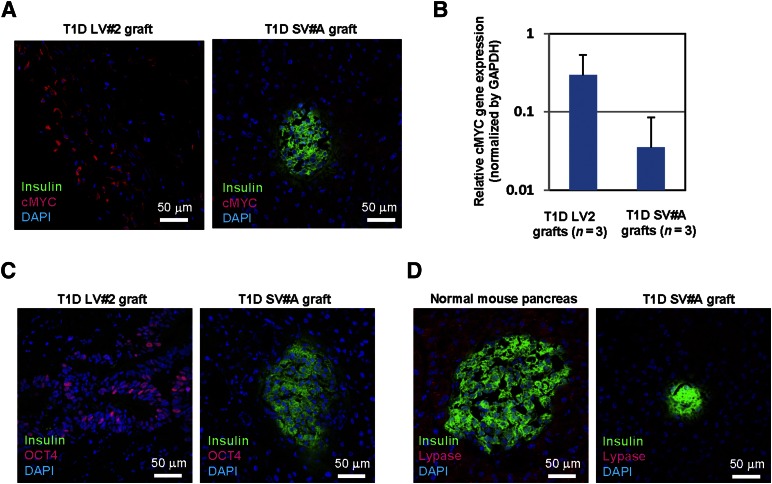
To assess the underlying mechanism of notable tumorigenicity of LV-iPSC-derived pancreatic endoderm cells, we first analyzed the representative iPSC-derived grafts for expression of cMYC, OCT4, and pancreatic acinar cell marker lypase. cMYC-positive cells were frequently found in LV-iPSC-derived tumors, whereas no notable cMYC signal was found in TGF-iPSC-derived grafts (Fig. 3A). We also found a trend of increased cMYC expression in LV-iPSC-derived grafts (Fig. 3B). Conversely, OCT4-positive cells were rare. Only one graft from LV-iPSCs showed notable OCT4 expression in ductal cells (Fig. 3C). Although we found good lipase expression in normal mouse pancreas, none of the iPSC-derived grafts showed notable lipase expression (Fig. 3D).
Figure 3.
Detection of pluripotency-associated factors in LV-iPSC-derived grafts. (A): Sections of recovered LV-iPSC- and TGF-iPSC-derived grafts in the kidney capsules were assessed for cMYC expression by immunostaining with specific antibodies against cMYC (red) and insulin (green). Nuclei were counter-stained by DAPI (blue). (B): The levels of cMYC transcripts in the grafts were determined by real-time reverse-transcription polymerase chain reaction. RNA samples from T1D LV2- and T1D SV#A-derived grafts (n = 3 each) were used for the assay. (C): Sections of recovered LV-iPSC- and TGF-iPSC-derived grafts in the kidney capsules were assessed for OCT4 expression by immunostaining with specific antibodies against OCT4 (red) and insulin (green). Nuclei were counterstained by DAPI (blue). (D): Sections of recovered LV-iPSC- and TGF-iPSC-derived grafts, as well as normal mouse pancreas, were assessed for pancreatic lipase expression by immunostaining with specific antibodies against lypase (red) and insulin (green). Nuclei were counterstained by DAPI (blue). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; iPSC, induced pluripotent stem cell; LV-iPSC, lentivirus-reprogrammed iPSC; TGF-iPSC, transgene-free iPSC; T1D, type 1 diabetes mellitus.
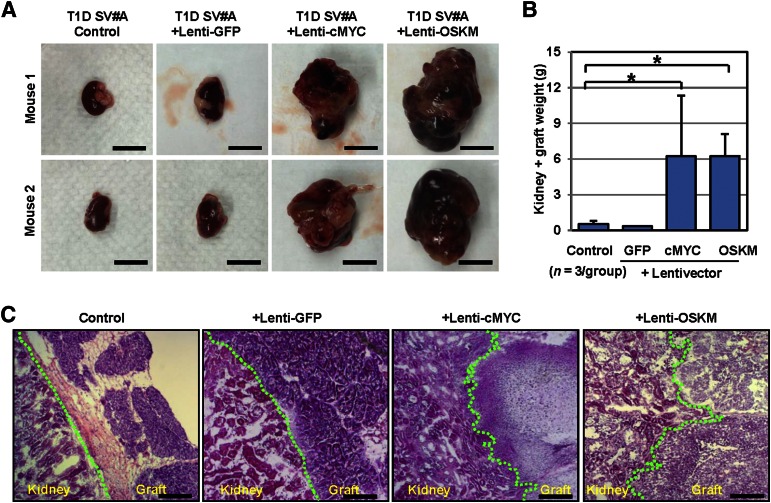
To verify the role of integrated reprogramming factors in the tumorigenicity of LV-iPSC progeny, we modified nontumorigenic T1D-speicific TGF-iPSCs by a series of lentiviral vectors and assessed the influence of lentiviral content on the incidence of tumor formation. We tested four TGF-iPSC-derived lines, including (a) unmodified control, (b) lentivector integration control at multiplicity of infection (MOI) titers of 4 (+ Lenti-GFP), (c) cMYC lentivector integration (+ Lenti-cMYC, at MOI = 4), and (d) all four transcription factor lentivector integration (+ Lenti-OCT4, SOX2, KLF4, and c-MYC [OSKM]; Lenti-OCT4, Lenti-SOX2, Lenti-KLF4, and Lenti-cMYC, at MOI = 4 each). Upon transplantation of iPSC-derived pancreatic progenitors from Lenti-cMYC and Lenti-OSKM iPSCs, recipient mice developed large solid tumors (Fig. 4A, 4B). No notable tumor formation was observed in mice transplanted with control or Lenti-GFP-modified iPSC progeny. Of note, histological analysis of the grafts demonstrated that lentiviral introduction of cMYC led to invasion of the graft/tumor into the kidney cortex structure, although unmodified or Lenti-GFP-modified iPSC grafts showed no sign of invasion (Fig. 4C).
Figure 4.
Integrating cMYC vector imposes tumorigenic load in iPSCs. (A): Four groups of SCID-beige mice (n = 3) received pancreatic progenitor cells from control, Lenti-GFP-, Lenti-cMYC- and Lenti-OSKM-superinfected TGF-iPSCs through transplantation into renal capsules of recipient SCID-beige mice. Three weeks after transplantation, left kidneys with iPSC grafts were recovered. Representative images of left kidneys with iPSC grafts are shown. Scale bars indicate 1 cm. (B): The averages of total kidney and graft weights were determined and compared among control, Lenti-GFP-, Lenti-cMYC- and Lenti-OSKM groups. *, p < .05 versus control by t test. Data represent the mean ± SEM. (C): Cross-sections of the junctions between the kidney and iPSC-derived grafts were visualized upon H&E staining. The junctions are indicated by a green line. Scale bars = 200 µm. Abbreviations: GFP, green fluorescent protein; iPSCs, induced pluripotent stem cells; OSKM, OCT4, SOX2, KLF4, and cMYC; TGF-iPSCs, transgene-free induced pluripotent stem cells.
Discussion
Here, we demonstrate tumor-free regeneration of human islets from iPSCs, including those from a T1D patient. Regeneration of iPSC-derived islets carrying patient-specific genetic traits would enable a personalized characterization of patient-matched β-cell properties. Moreover, the clinical use of autologous iPSC-derived islets would allow the mapping of patient-specific autoimmune responses against β cells assisting in the comprehension of pathogenic events triggering development of T1D. Safe insulin-producing surrogate cells from TGF-iPSCs would ultimately provide a foundation for individualized cell therapy in the setting of T1D, independent of allogeneic postmortem pancreatic tissue or stem cell sources.
A critical limitation toward translation is the risk of uncontrolled proliferation and differentiation inherent to pluripotent stem cells [34–36]. Characterization of human ESC-derived teratomas has revealed their close resemblance to spontaneous benign teratomas [37]. Because of potential contamination of residual pluripotent cells, transplantation of ESC progeny cells harbors a risk of developing teratoma-like tumors, and elimination of residual pluripotent cells facilitates tumor-free transplantation of ESC-derived therapeutics [34, 38]. Here, our primary focus was on the safety, in particular, tumorigenicity, of iPSC-derived pancreatic progenitor cells. Successful regeneration of mature β cells has been reported after long-term in vivo maturation of ESC-derived pancreatic endoderm cells, but not ESC-derived β cells [22]. Therefore, we transplanted iPSC-derived pancreatic endoderm cells at day 13. We found that close to 100% of immunocompromised recipient mice developed teratoma-like tumors when pancreatic progenitor cells derived from LV- and TGF-iPSCs were transplanted into kidney capsules of SCID-beige mice, indicating that lineage commitment through our original stepwise differentiation protocol may not be sufficient to ensure safe transplantation in the setting of iPSC-based therapy. Previously, purging undifferentiated pluripotent stem cells in ESC and iPSC products has been achieved through genetic selection, cell sorting of fully differentiated cell populations, or pluripotency-targeted drug treatments [34, 38–41]. Because human pluripotent stem cells, but not murine PSCs, are highly sensitive to enzymatic dissociation, and single-cell dissociation immediately kills human PSCs [32, 33], here we used simple enzymatic dissociation steps to eliminate contaminated pluripotent cells from the iPSC-derived pancreatic progenitor population. Although β cells are sensitive to trypsin-induced dispersion, iPSC-derived pancreatic endoderm cells were more resistant to brief enzymatic dissociation. The modified protocol achieved complete blockade of tumor formation upon transplantation of TGF-iPSC-derived progeny, providing a reliable solution for eliminating contaminated pluripotent cells and ensuring safe transplantation of TGF-iPSC progeny. This observation also indicates residual undifferentiated cells as the primary cause of tumorigenicity of TGF-iPSC products.
The tumors originating from LV-iPSCs were frequently found to be invasive and metastatic. Trypsinization failed to prevent tumor formation upon transplantation of pancreatic progenitor cells from LV-iPSCs, but not TGF-iPSCs, suggesting the existence of additional mechanisms leading to increased tumorigenicity of LV-iPSC-derived cells. Our data with lentivirally modified TGF-iPSC lines clearly demonstrate that integration of the cMYC-expressing lentiviral vector plays a critical role in imposing an extra tumorigenic load upon iPSCs. Although spontaneous tumor formation through reactivation of the cMyc transgene has been reported in germline-competent iPSC-derived mice [10], our results are the first to convincingly demonstrate the increased tumorigenic risks of human iPSCs with integrated cMYC vectors. Our observation is in line with the necessity of cMYC for the invasion and metastasis of cancer cells in experimental xenografts, independent of its effects on proliferation and survival [42].
Cell doses are known to affect the tumorigenicity of pluripotent stem cells [43, 44]. Site-dependent tumorigenicity of human ESCs has also been reported: 25%–100% through subcutaneous, 12.5% through intramuscular, and 100% through kidney capsule transplantations [45]. Kanemura et al. has verified the safety of iPSC products through transplantation of up to 1.5 × 104 retinal pigment epithelium cells, derived from TGF-iPSCs reprogrammed by a cMYC-free episomal vector, into the subretinal spaces in nude rats [46], which have no functional T cells but retain intact B and natural killer cell functions. We transplanted 106 pancreatic endoderm cells from iPSCs, made with lentiviral or Sendai viral vectors, into the kidney capsules of SCID-beige mice that have defective B, T, and natural killer cell functions. Because our study used a highly sensitive mouse model for teratoma formation, it is possible that the use of 106 iPSC derivatives in the kidney capsule of severely immunodefective mice may have overestimated the tumorigenic risk of LV-iPSC products. Nevertheless, use of the most rigorous mouse model in testing iPSC tumorigenicity is critical to ensuring patient safety. Variable study designs and outcomes on the safety of iPSCs and iPSC products also highlight the challenges to evaluate the tumorigenic potential of human iPSC products in xenograft models. In this context, it is notable that undifferentiated autologous rhesus monkey iPSCs, made with an excisable reprogramming vector, form mature teratomas in a dose-dependent manner, whereas iPSC-derived mesodermal stromal cells differentiate into bone without any evidence of tumor formation in autologous recipients [47]. Takahashi and colleagues have demonstrated no evidence of tumor formation in a rhesus monkey recipient upon transplantation of autologous iPSC-derived retinal epithelium cells [48]. These studies suggest low tumorigenic risks of TGF-iPSC products in autologous, immunocompetent recipients.
We acknowledge that the number of transplanted cells was relatively high (106 cells) in our study. It would be informative to establish the lower limiting cell number that establishes tumorigenicity. Nevertheless, considering that we need to transplant 200 islets (∼2 × 105 cells) to reverse diabetes in mice and 5,000 islets (∼5 × 106 cells) per kilogram for humans, assessing the tumorigenicity of high numbers of iPSC progeny is critical. For future clinical applications of iPSC-derived pancreatic progeny, we need to establish no tumorigenic risk after transplantation of more than 10 million iPSC-derived pancreatic progeny.
Pluripotent stem cell therapy for diabetes relies on the safety as well as the quality of derived insulin-producing cells. Previous studies have demonstrated frequent generation of human β cells with functional characteristics of immature β cells [49], with striking clonal/intrapatient variations in the propensity for pancreatic differentiation [4, 24]. Although TGF-iPSC derivatives offered safe transplantation, transgene-free reprogramming did not substantially improve pancreatic differentiation propensities in vitro, when compared with those of LV-iPSCs. Additionally, regenerated human islets in vivo through transplantation of iPSC-derived pancreatic endoderm cells did not show proper glucose responsive C-peptide secretion at 3 months after transplantation. Observed low β-cell differentiation yield in vitro was likely because of our relatively short differentiation protocol (up to 24 days). In addition, our protocol did not have several recently identified small molecules and growth factors, which can promote β-cell regeneration/maturation, such as ALK2/3 inhibitor LDN193189, thyroid hormone T3, or betacellulin [22, 29]. We are currently in the process of optimizing our differentiation protocol with such small molecules and growth factors.
The gene expression levels shown in supplemental online Fig. 3C appear to suggest low β-cell differentiation yield in vitro [arbitrary gene expression levels 103- to 105-fold less than those of glyceraldehyde-3-phosphate dehydrogenase (GAPDH)]. This is partly because we determined the relative transcript levels, but not absolute transcript copy numbers, among iPSC lines. The gene detection sensitivity simply depends on the level of cycle threshold lines and the primers/probes used for the assay, and our data do not necessarily mean that the levels of insulin or PDX1 transcript levels were 105-fold lower than those of GAPDH.
The observed low circulating levels of C-peptide after transplantation of iPSC-derived pancreatic endoderm cells were likely due to suboptimal in vitro specification and in vivo maturation. Long-term in vitro differentiation and prolonged in vivo maturation have been reported to improve glucose responsiveness of islet like cells from selected ESC lines [22, 29]. Thus, it is plausible that extended in vivo maturation steps may improve the glucose responsiveness of iPSC-derived islets. Alternatively, selection of TGF-iPSC clones with better pancreatic differentiation propensities, and the use of recently reported differentiation protocols with better specification for mature β-cell phenotype [20, 21], could improve the quality of TGF-iPSC-derived islets.
Conclusion
In summary, our data highlight prominent tumorigenic risks of iPSC products, especially when reprogrammed with integrating vectors. Two major underlying mechanisms in iPSC tumorigenicity are residual pluripotent cells and cMYC overload by vector integration. We also demonstrate that combined TGF reprogramming and enzymatic dissociation allow teratoma-free transplantation of iPSC progeny in the most rigorous mouse model in testing the tumorigenicity of iPSC products. Further long-term safety assessment and improvement in TGF-iPSC specification into a mature β-cell phenotype would lead to customized safe islet replacement therapy for T1D.
Supplementary Material
Acknowledgments
We thank Jim E. Tarara (Mayo Clinic) for technical supports and helpful suggestions. This work was supported by the Sheikh Khalifa Bin Zayed Al Nahyan Regenerative Diabetes Research Program to Cure Diabetes Using Stem Cells (Y.I., D.A.W., and Y.C.K.), George M. Eisenberg Foundation for Charities Stem Cell Trust, NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK085516 (to Y.C.K.), NIH GM Grant R43 GM112316 (to Y.I.), Urdang Fund for Cellular Therapy for Type 1 Diabetes (to Y.I. and Y.C.K.), Vann Family Fund in Diabetes Research (to Y.I.), J.W. Kieckhefer Fund in Regenerative Medicine to Support Research in Type 1 Diabetes (to Y.I.), Paul A. and Ruth M. Schilling Medical Research Endowment Fund (to Y.I.), Marriott family, and Mayo Clinic Center for Regenerative Medicine (Y.I. and A.T.). A.T. is recognized through the Michael S. and Mary Sue Shannon Family Directorship, the Mayo Clinic Center for Regenerative Medicine, and the Marriott Family Professorship of Cardiovascular Diseases Research.
Author Contributions
M.M.E.K., S.O., and E.J.J.: conception and design, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; S.J.H.: collection and/or assembly of data, final approval of manuscript; J.M.T., S.G.M., and C.A.S.: collection and/or assembly of data; K.U. and N.F.: conception and design, final approval of manuscript; D.A.W., A.T., Y.C.K., and Y.I.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Inoue H, Nagata N, Kurokawa H, et al. iPS cells: A game changer for future medicine. EMBO J. 2014;33:409–417. doi: 10.1002/embj.201387098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherry AB, Daley GQ. Reprogrammed cells for disease modeling and regenerative medicine. Annu Rev Med. 2013;64:277–290. doi: 10.1146/annurev-med-050311-163324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thatava T, Kudva YC, Edukulla R, et al. Intrapatient variations in type 1 diabetes-specific iPS cell differentiation into insulin-producing cells. Mol Ther. 2013;21:228–239. doi: 10.1038/mt.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu BY, Weick JP, Yu J, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci USA. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moiani A, Paleari Y, Sartori D, et al. Lentiviral vector integration in the human genome induces alternative splicing and generates aberrant transcripts. J Clin Invest. 2012;122:1653–1666. doi: 10.1172/JCI61852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bokhoven M, Stephen SL, Knight S, et al. Insertional gene activation by lentiviral and gammaretroviral vectors. J Virol. 2009;83:283–294. doi: 10.1128/JVI.01865-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howe SJ, Mansour MR, Schwarzwaelder K, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 10.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 11.Kaji K, Norrby K, Paca A, et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanyam D, Lamouille S, Judson RL, et al. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat Biotechnol. 2011;29:443–448. doi: 10.1038/nbt.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anokye-Danso F, Trivedi CM, Juhr D, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seki T, Yuasa S, Oda M, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7:11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Hou P, Li Y, Zhang X, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 17.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holditch SJ, Terzic A, Ikeda Y. Concise review: Pluripotent stem cell-based regenerative applications for failing β-cell function. Stem Cells Translational Medicine. 2014;3:653–661. doi: 10.5966/sctm.2013-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 20.Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 21.Pagliuca FW, Millman JR, Gürtler M, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 23.Ohmine S, Squillace KA, Hartjes KA, et al. Reprogrammed keratinocytes from elderly type 2 diabetes patients suppress senescence genes to acquire induced pluripotency. Aging (Albany, NY) 2012;4:60–73. doi: 10.18632/aging.100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maehr R, Chen S, Snitow M, et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA. 2009;106:15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reardon S, Cyranoski D. Japan stem-cell trial stirs envy. Nature. 2014;513:287–288. doi: 10.1038/513287a. [DOI] [PubMed] [Google Scholar]
- 26.Ohmine S, Dietz AB, Deeds MC, et al. Induced pluripotent stem cells from GMP-grade hematopoietic progenitor cells and mononuclear myeloid cells. Stem Cell Res Ther. 2011;2:46. doi: 10.1186/scrt87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudva YC, Ohmine S, Greder LV, et al. Transgene-free disease-specific induced pluripotent stem cells from patients with type 1 and type 2 diabetes. Stem Cells Translational Medicine. 2012;1:451–461. doi: 10.5966/sctm.2011-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thatava T, Nelson TJ, Edukulla R, et al. Indolactam V/GLP-1-mediated differentiation of human iPS cells into glucose-responsive insulin-secreting progeny. Gene Ther. 2011;18:283–293. doi: 10.1038/gt.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rezania A, Bruin JE, Riedel MJ, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61:2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mann JR, Gray ES, Thornton C, et al. Mature and immature extracranial teratomas in children: The UK Children’s Cancer Study Group Experience. J Clin Oncol. 2008;26:3590–3597. doi: 10.1200/JCO.2008.16.0622. [DOI] [PubMed] [Google Scholar]
- 31.Lo Curto M, D’Angelo P, Cecchetto G, et al. Mature and immature teratomas: Results of the first paediatric Italian study. Pediatr Surg Int. 2007;23:315–322. doi: 10.1007/s00383-007-1890-1. [DOI] [PubMed] [Google Scholar]
- 32.Ohgushi M, Matsumura M, Eiraku M, et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010;7:225–239. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 34.Darabi R, Gehlbach K, Bachoo RM, et al. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat Med. 2008;14:134–143. doi: 10.1038/nm1705. [DOI] [PubMed] [Google Scholar]
- 35.Miura K, Okada Y, Aoi T, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 36.Nelson TJ, Martinez-Fernandez A, Yamada S, et al. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunningham JJ, Ulbright TM, Pera MF, et al. Lessons from human teratomas to guide development of safe stem cell therapies. Nat Biotechnol. 2012;30:849–857. doi: 10.1038/nbt.2329. [DOI] [PubMed] [Google Scholar]
- 38.Chung S, Shin BS, Hedlund E, et al. Genetic selection of sox1GFP-expressing neural precursors removes residual tumorigenic pluripotent stem cells and attenuates tumor formation after transplantation. J Neurochem. 2006;97:1467–1480. doi: 10.1111/j.1471-4159.2006.03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyles SP, Yamada S, Oommen S, et al. Inhibition of DNA topoisomerase II selectively reduces the threat of tumorigenicity following induced pluripotent stem cell-based myocardial therapy. Stem Cells Dev. 2014;23:2274–2282. doi: 10.1089/scd.2014.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong B, Watts KL, Gori JL, et al. Safeguarding nonhuman primate iPS cells with suicide genes. Mol Ther. 2011;19:1667–1675. doi: 10.1038/mt.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hattori F, Chen H, Yamashita H, et al. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Methods. 2010;7:61–66. doi: 10.1038/nmeth.1403. [DOI] [PubMed] [Google Scholar]
- 42.Wolfer A, Wittner BS, Irimia D, et al. MYC regulation of a “poor-prognosis” metastatic cancer cell state. Proc Natl Acad Sci USA. 2010;107:3698–3703. doi: 10.1073/pnas.0914203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behfar A, Perez-Terzic C, Faustino RS, et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J Exp Med. 2007;204:405–420. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamada S, Nelson TJ, Crespo-Diaz RJ, et al. Embryonic stem cell therapy of heart failure in genetic cardiomyopathy. Stem Cells. 2008;26:2644–2653. doi: 10.1634/stemcells.2008-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prokhorova TA, Harkness LM, Frandsen U, et al. Teratoma formation by human embryonic stem cells is site dependent and enhanced by the presence of Matrigel. Stem Cells Dev. 2009;18:47–54. doi: 10.1089/scd.2007.0266. [DOI] [PubMed] [Google Scholar]
- 46.Kanemura H, Go MJ, Shikamura M, et al. Tumorigenicity studies of induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration. PLoS One. 2014;9:e85336. doi: 10.1371/journal.pone.0085336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong SG, Winkler T, Wu C, et al. Path to the clinic: Assessment of iPSC-based cell therapies in vivo in a nonhuman primate model. Cell Reports. 2014;7:1298–1309. doi: 10.1016/j.celrep.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamao H, Mandai M, Okamoto S, et al. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014;2:205–218. doi: 10.1016/j.stemcr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hrvatin S, O’Donnell CW, Deng F, et al. Differentiated human stem cells resemble fetal, not adult, β cells. Proc Natl Acad Sci USA. 2014;111:3038–3043. doi: 10.1073/pnas.1400709111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.