Abstract
Background
Accurate quantification of malignant cells in the peripheral blood of patients with cutaneous T cell lymphoma (CTCL) is important for early detection, prognosis, and monitoring disease burden.
Objective
Determine the spectrum of current clinical practices; critically evaluate elements of current ISCL B1 and B2 staging criteria; and assess the potential role of TCR-Vβ analysis by flow cytometry.
Methods
We assessed current clinical practices by survey, and performed a retrospective analysis of 161 patients evaluated at Yale (2011-2014) to compare the sensitivity, specificity, PPV, and NPV of parameters for ISCL B2 staging.
Results
There was heterogeneity in clinical practices among institutions. ISCL B1 criteria did not capture five Yale cohort patients with immunophenotypic abnormalities who later progressed. TCR-Vβ testing was more specific than PCR and aided diagnosis in detecting clonality, but was of limited benefit in quantification of tumor burden.
Limitations
Because of limited follow-up involving a single center, further investigation will be necessary to conclude whether our proposed diagnostic algorithm is of general clinical benefit.
Conclusion
We propose further study of “modified B1 criteria”: CD4/CD8 ratio ≥5, %CD4+/CD26- ≥ 20%, %CD4+/CD7- ≥ 20%, with evidence of clonality. TCR-Vβ testing should be considered in future diagnostic and staging algorithms.
Keywords: Cutaneous T cell lymphoma, mycosis fungoides, Sézary syndrome, flow cytometry, TCR-Vβ, peripheral blood analysis
Introduction
Cutaneous T-cell lymphoma (CTCL) is a relatively rare and heterogeneous group of indolent skin-homing neoplasms of memory CD4+ T cells that can variably involve the lymph nodes and peripheral blood.1 Leukemic involvement has been found to be an independent prognostic factor in CTCL, and identification and quantification of blood involvement is important for early detection of disease as well as for monitoring tumor burden over time.2-4
In 2007, the ISCL/EORTC published revised guidelines for the assessment of peripheral blood involvement. B0 and B1 ratings are defined by Sézary counts (SCs), (B0 < 5% SCs; B1 > 5% SCs but either less than 1.0K/μl absolute SCs or absence of a clonal TCR rearrangement, or both) while the current B2 rating is defined by clonal rearrangement of the TCR in conjunction with any one of the following: 1) >1000 SCs/μl, 2) increased CD4+ or CD3+ population with CD4/CD8 >10, or 3) an increase in CD4+ cells with an abnormal phenotype (i.e. >40% CD4+/CD7-; >30% CD4+/CD26-).5
Current guidelines continue to utilize the identification and enumeration of the morphologically atypical Sézary cells. The sensitivity of SCs is limited by inter-observer subjectivity and the fact that many patients with blood involvement will not manifest SCs.5 SCs may also be found in patients with benign inflammatory conditions as well as in normal individuals, albeit usually in smaller quantities.6
As our understanding of cell surface markers and atypical phenotypes in CTCL has improved, flow cytometry has proven to be more robust than Sézary counts. However, there is no single marker that is both sensitive and specific for blood involvement. 7 An elevated CD4/CD8 ratio, and loss of CD7 and/or CD26 on CD3+CD4+ T cells, have commonly been cited as occurring in malignant T cells of CTCL but are also observed physiologically and in benign erythrodermas.8-10,11,12 Central to the diagnosis of peripheral blood involvement is the identification of T cell clonality by PCR. Yet despite its superior sensitivity, PCR in CTCL patients is relatively non-specific, with some studies even concluding that “clonality” demonstrated by PCR was more common in patients with benign conditions than in patients with SS.13-15
Flow cytometric analysis using antibodies directed against the T cell receptor (TCR)β chain has been shown to demonstrate clonality in between 79% to 100% of patients with blood involvement.16,17 However, TCR-Vβ has been found in previous studies to be less sensitive than PCR.14 Thus, to improve the sensitivity of TCR-Vβ analysis, some groups have begun to analyze Vβ expression on subset T cell populations after gating (i.e. first selecting cells for further flow cytometric analysis), such as on CD4+CD7- T cells or other identified phenotypic aberrancies.17
Commercially available Vβ antibodies are capable of recognizing only 70% of the TCR-Vβ repertoires in normal T-cells.18 A known clone by PCR may also demonstrate “non-reactivity,” where a non-detectable, dominant clone suppresses the usage of other Vβ families, leading to a restricted TCR repertoire. 18,19 Hence, several studies have suggested that failure of at least 70% of a gated T cell population to react to the panel of Vβ antibodies is indicative of clonality. 20
Despite the recommendations by the ISCL/EORTC, there is a paucity of data examining the performance parameters of individual criteria for detecting blood involvement and there are no guidelines for testing for clonality by TCR-Vβ flow cytometric analysis. Herein, we endeavored to determine the current spectrum of clinical practices and then aimed to (1) identify which, if any, of the markers most frequently used to identify abnormalities in the peripheral blood possess superior performance measures, (2) determine the utility of TCR-Vβ testing for detection of blood involvement in CTCL and situations in which this test should be used, and (3) suggest recommendations for B1 criteria that are not based on SC enumeration.
Methods
Survey
A survey study of six multiple-choice questions (SurveyMonkey) was sent by email to members of the USCLC and the MDS to characterize clinical practices for analyzing blood in CTCL. Topics included indications for testing for blood involvement, stage(s) at which blood was tested, diagnostic tools, T cell markers tested during flow cytometric analysis, laboratories to which specimens were sent for analysis, and contexts in which Vβ analysis was performed.
Patient selection
A retrospective analysis was carried out including 161 patients evaluated at the Yale Cutaneous Lymphoma Center between 2011 and 2014 who were tested for TCR-Vβ restriction. All patients were retrospectively staged according to the current ISCL guidelines and tumor burden was assessed using the modified severity weight assessment tool.62 The diagnosis of CTCL was confirmed in 133 patients by biopsy, two patients were diagnosed based on blood involvement in constellation with clinical features, and all other patients were diagnosed based on clinical presentation. Yale University's IRB granted exempt status for this retrospective case series.
Flow cytometry
In the course of patients' treatment at the Yale Cutaneous Lymphoma Center, peripheral blood samples were obtained at various time points. Flow cytometry analysis was performed using fluorochrome-conjugated antibodies for the following antigens: CD2, CD3, CD4, CD5, CD7, CD8, CD25, CD26, CD45, CD45RA, and CD45RO. Blood was longitudinally assessed for CD4/CD8 ratio, CD4+CD7- and CD4+CD26- phenotype percentages, and CD3+CD4+ absolute number. Yale Laboratory Medicine does not perform Sézary counts due to the aforementioned limitations. If atypical lymphocytes were noted based on immunophenotypic alterations, this percentage was multiplied by the absolute lymphocyte count to approximate the number of “Sézary cells”, allowing for qualification for B2 or B1 criteria based on the absolute number of atypical circulating cells. Blood involvement was defined by the ISCL B2 and B1 ratings. 5
Polymerase chain reaction
Genomic DNA was extracted from peripheral blood samples and tested for TCR beta and gamma gene rearrangements. Regions of the gene were amplified enzymatically, and the amplified products were characterized for the presence of PCR products in the 75-110bp range on acrylamide gels. The primers V-γ-11, V-γ -101, J-γ-1, and Jp-γ-12 were used for TCR-γ gene rearrangement amplification.21 The primer set D2 and J2 was used for TCR-β gene rearrangement.22
TCR-Vβ testing
Testing for TCR-Vβ restriction was performed for all patients by ARUP Laboratories, (Salt Lake City, Utah). Briefly, evaluation of the TCR-Vβ expression was performed using 5-color immunophenotyping including primarily antibodies to CD3 (ECD, Coulter Immunotech), CD4 (PC-7, Coulter Immunotech), CD7 (PC-5, Coulter Immunotech) as well as fluorochrome (FITC and PE) labeled antibodies to the various TCR-Vβ's within the IOTest Beta Mark TCR-Vβ Repertoire kit (Beckman Coutler). The IOTest Beta Mark TCR-Vβ Repertoire kit evaluates 24 different TCR-Vβ utilizing 8 tubes. Each tube in conjunction with CD3, CD4, and CD7 evaluates 3 different TCR-Vβ's labeled with FITC, PE or FITC plus PE.
Selective gating using CD3, CD4 and CD7 allowed analysis of the total T-cell population (CD3+) as well as various CD3+ T-cell subset populations such as CD3+, CD4+, CD7-. A T-cell population was considered clonal if greater than 50% of the analyzed population expressed a single TCR-Vβ or if the analyzed population expressed less than a total of 20% of the evaluated TCR-Vβs. The latter would indicate that 80% or more of the analyzed population failed to react with any of the TCR-Vβs antibodies, presumptively due to expression of a TCR-Vβ not recognized by the antibody panel in the repertoire kit. We defined an “equivocal” test as one that showed 20-50% reactivity. The number of circulating atypical cells was calculated using TCR-Vβ by multiplying the percentage of atypical T cells by the TCR-Vβ expression in that population by the total number of T cells (absolute CD4+CD3+).
Statistical Analysis
The student t test was used to compare percentages of CD4+CD7-, CD4+CD26-, and CD4/CD8 ratios between B0/B1 and B2 groups. The number of patients in each group meeting each criterion for B2 involvement was compared using the Fischer exact test for comparison of proportions. A p value<0.05 was considered statistically significant.
Results
Current clinical practices
Of 33 institutions surveyed, there was substantial variation with regard to the CTCL stage at which blood involvement was assessed, tools utilized for assessment of blood involvement, and T cell receptor markers tested by flow cytometry (Table 1). 51.4% reported obtaining peripheral blood smears for SC counting and only 14.3% reported utilizing Vβ assays for detection of a clonal T cell population.
Table 1. Results of Survey Study.
| Question | Answer choice | Percentage respondents (%) |
|---|---|---|
| Stage(s) at which assessment of peripheral blood for malignant involvement is initiated. | Stage I | 60 |
| Stage II | 80 | |
| Stage III | 86 | |
| Stage IV | 74 | |
| Testing utilized to assess for peripheral blood involvement | PCR for TCR clonal gene rearrangement | 83 |
| TCR V-beta analysis | 14 | |
| Peripheral blood smear for SC | 51 | |
| None of the above | 14 | |
| Purpose of TCR-Vβ analysis | Assess clonal expansion | 100 |
| Assess tumor quantity | 0 | |
| To monitor tumor burden during treatment | 0 | |
| Laboratory to which blood specimens were sent for analysis. | Home institutional | 86 |
| Outside laboratory | 14 |
ISCL B1 staging
Twelve individuals satisfied ISCL B1 criteria by having greater than 5% circulating atypical cells, but <1k/μl (4 T4, 1 T3, 5 T2, 2 T1). Ten of these twelve had TCR gene arrangements by PCR of which six had TCR-Vβ restricted clones, and two had “equivocal” TCR-Vβ results.
Analysis of current B2 criteria
161 patients were identified who were tested for TCR-Vβ restriction between 2011 and 2014. Five patients had concurrent CLL, 3 patients had follicular mucinosis, 2 patients had PCSM-TCL, and 6 patients had folliculotropic MF.
Thirty-four patients met current B2 ISCL criteria. This group included patients with greater tumor burden as assessed by T stage and mSWAT at time of TCR-Vβ testing who required a greater number of systemic therapies (Table 2). Patients fulfilling B2 criteria had higher median values for %CD4+CD7-, %CD4+CD26-, and CD4/CD8 ratios than those fulfilling B0/B1 criteria (p<0.05 in all cases). The proportion of patients who satisfied individual components of the ISCL criteria for B2 blood rating was greater in those with blood involvement of B2 rating than in the those without B2 blood involvement (p<0.001). Additionally, the proportion of patients with a Vβ-detected clonal T cell population was greater in patients with blood involvement than in those without (p<0.0001) (Table 3).
Table 2. Comparison of clinical characteristics between ISCL B2 and non-B2 groups.
| ISCL | B2+ | B2- | p values |
|---|---|---|---|
| No. | 34 | 127 | |
| No. systemic therapies | 2 (0-7) | 0 (0-6) | <0.0001* |
| mSWAT | 40 (4-100) | 15 (0-180) | <0.0001* |
| T1 (patches/plaques <10% BSA) | 4/34 (11.8%) | 55/120 (45.8%) | 0.0002** |
| T2 (patches/plaques ≥10% BSA) | 14/34 (41.2%) | 45/120 (35%) | 0.6948** |
| T3 (tumor(s) present) | 1/34 (2.9%) | 7/120 (5.8%) | 1.000** |
| T4 (erythroderma) | 13/34 (38.2%) | 10/120 (8.3%) | 0.0002** |
| No. with circulating atypical cells | 27/34 (79%) | 12/127 (9.4%) | 0.0001** |
Table 2 footnote: BSA, body surface area; B1 and B2 were defined by ISLC criteria (if atypical lymphocytes were noted based on immunophenotypic alterations only, this percentage was multiplied by the absolute lymphocyte count to approximate the number of “Sézary cells” where no such counts were performed/available)
Table 3. Comparison of flow cytometric and molecular data between ISCL B2 and non-B2 groups.
| ISCL | B2+ | B2- | p values |
|---|---|---|---|
| No. | 34 | 127 | |
| CD4/CD8 ratio | 5.4 (1.3-67) | 2.7 (0.6-24.3) | <0.0001* |
| Abs CD4 (K) | 1.17 (0.06-7.8) | 0.87 (0.21-2.5) | <0.0001* |
| CD45RO+ | 43.6 (12.9 -83.1) | 30.3 (9.4-77.7) | <0.0001* |
| % CD4+/CD7- | 22.3 (4.1-90) | 8.8 (1.8-44.3) | <0.0001* |
| % CD4+/CD26- | 35.4 (11.9-71.6) | 10.7 (2-38.8) | <0.0001* |
| Abs lymphocyte | 1.9 (0.13-10.4) | 1.6 (0.5-3.9) | <0.0001* |
| CD4/CD8 ≥ 10 | 9/34 (26%) | 2/127 (1.6%) | <0.0001** |
| CD4+/CD7-≥ 40% | 8/33 (24%) | 1/125 (0.8%) | <0.0001** |
| CD4+/CD26- ≥ 30% | 25/30 (83%) | 3/126 (2.4%) | <0.0001** |
| PCR + | 34/34(100%) | 46/126 (37%) | <0.0001** |
| TCR-VB + | 25/34 (74%) 5 by non-reactivity | 11/127 (10.2%) 4 by non-reactivity | <0.0001** |
| TCR- VB+ (including equivocal) | 29/34 (85%) | 26/128 (20%) | <0.0001** |
student t-test
Fischer exact test p-value
Table 3 footnote: B1 and B2 were defined by ISLC criteria (if atypical lymphocytes were noted based on immunophenotypic alterations only, this percentage was multiplied by the absolute lymphocyte count to approximate the number of “Sézary cells” where no such counts were performed/available)
The sensitivity, specificity, PPV, and NPV of individual components of the ISCL criteria for blood involvement and for Vβ analysis were calculated and compared (CD4/CD8 ratio ≥ 10, CD4+/CD7- ≥ 40%, CD4+/CD26- ≥ 30%, positive PCR for clonality) (Table 4). CD4+/CD7- ≥ 40% possessed the highest specificity for blood involvement while %CD4+/CD26- ≥ 30% possessed the highest sensitivity and NPV. Both had comparable PPVs. Consistent with other studies, patients with blood involvement demonstrated a stable phenotype over time.
Table 4. Comparison of individual ISCL performance parameters.
| Parameter | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| CD4/CD8 | 26.5 | 98.4 | 81.8 | 83.3 |
| CD4+CD7- ≥ 40% | 23.5 | 99.2 | 88.9 | 83.2 |
| CD4+CD26-≥ 30% | 83.3 | 97.6 | 89.2 | 96.0 |
| CD4+CD26-≥ 25% | 93.3 | 90.5 | 70 | 98.2 |
| CD4+CD26-≥ 40% | 50 | 100 | 100 | 89.4 |
| PCR+ | 100 | 63.4 | 42.5 | 100 |
| TCR-Vβ > 50% or total reactivity <20% | 73.5 | 90.6 | 67.6 | 92.8 |
TCR-Vβ analysis
When gated on an atypical population (defined by abnormalities in CD3, CD4, and/or CD7) TCR-Vβ testing revealed clonal expansions in 36 individuals. TCR-Vβ clonal restriction was identified in 25 of the 34 B2 patients (73.5%) and allowed for the identification of clonal expansion in patients with as low as 1% circulating atypical cells. Five of these 25 patients (20.0%) met TCR-Vβ criteria of clonality through non-reactivity (<70% reactivity). TCR-Vβ testing demonstrated lower sensitivity (73.5%) compared to PCR (by definition 100%) but was significantly more specific for blood involvement (90.6% vs. 64.0%). Twelve individuals had TCR-Vβ restricted clones in the non-B2 population (9.4%). In contrast, when gated on all CD3+ cells, TCR-Vβ testing only revealed clonal populations in thirteen patients (12 B2 patients, 1 non-B2).
Discussion
Assessment of blood involvement in CTCL offers prognostic significance yet guidelines for blood analysis are sparse, and Sézary cell enumeration is subjective yet remains a component of ISCL B2 and B1 criteria. We conducted this study with the goal of exploring a new approach for the assessment of blood involvement in CTCL.
Current Practices
We found significant heterogeneity among institutions in the assessment of peripheral blood involvement in CTCL. Over half of respondents indicated use of peripheral smears for SC quantification despite the impracticality of the tool and evidence of drawbacks, including subjectivity of results and nonspecific nature of morphologic assessment. 5-7 While nearly 90% of respondents checked for CD7 expression, only about 40% checked for loss of CD26. Analysis of TCR-Vβ was also infrequently utilized and institutions that indicated performing TCR-Vβ analysis used the test solely to demonstrate clonality in the blood.
Analysis of ISCL B2 criteria
We next aimed to work towards a new approach for the assessment of blood involvement. We first analyzed each individual component of the current ISCL B2 criteria. We found significant differences in all components of the ISCL criteria between ISCL B2 and non-B2 groups. Loss of CD26 was the most sensitive marker for blood involvement and only five individuals did not meet the CD4+/CD26-≥30% criterion. With a goal to improve the sensitivity of current B2 criteria, we assessed the utility of the parameter %CD4+/CD26- ≥ 25%. This change increased the parameter's sensitivity without a great cost to its specificity (Table 4). In contrast to other studies which found loss of CD26 to be more specific in CTCL than loss of CD7, our study found the criteria for CD4+CD7->40% to be the most specific of the ISCL parameters.23
Modified B1 criteria
To help formulate B1 criteria that are based on immunophenotypic data instead of morphology, we applied the following conditions to our current group: CD4/CD8 ratio ≥5, %CD4+/CD26- ≥ 20%, %CD4+/CD7- ≥ 20%. We identified eighteen individuals with evidence of clonality, all with TCR gene rearrangements, four with TCR-Vβ restriction, and four with an “equivocal” TCR-Vβ result. Four patients had T4 disease and required systemic therapy; five had T2 disease with progression of blood involvement necessitating systemic therapy; and one had T3 disease and developed metastatic lesions. Sixteen patients were observed longitudinally – twelve progressed (75.0%) and four (3 T1, 1 T2) improved and eventually did not meet the revised B1 criteria.
Our modified B1 criteria identified 10 individuals not captured by current ISCL criteria. Five had advanced stage disease (oneT4, one T3, and 3 T2 disease) that required systemic therapy. Importantly, the three ISCL B1 individuals who did not meet our modified criteria had either indolent disease (T1, not requiring systemic therapy) or met B2 criteria using TCR-Vβ restriction as evidence for clonality; thus, implementation of these criteria would not omit clinically significant patients.
Role of TCR-Vβ testing
We next attempted to clarify the role of TCR-Vβ testing in assessing blood involvement in CTCL. In line with other investigators, we found that TCR-Vβ testing on gated atypical populations was more specific yet less sensitive than PCR. In our group, testing for clonality using TCR-Vβ would allow one patient to meet criteria for B2 blood involvement. Consistent with other studies, in patients where a TCR-Vβ clone is detectable, it may be useful to continue to monitor tumor burden by TCR-Vβ expression. 20,24,25
Regarding the gating on a small atypical population, it has been postulated the test may be overly sensitive and lead to false positives. In our study, eleven “false positives” were identified (TCR-Vβ restricted in non-B2 group). However, nine patients had some evidence of blood involvement with PCR testing; six had other flow cytometric abnormalities (CD4/CD8>5, %CD4+/CD26-≥ 20, %CD4+/CD7- ≥ 20). Of the two patients who had TCR-Vβ restricted clones without concordant PCR positivity, one was lost to follow up and one developed PCR positivity, qualifying her for B2 blood involvement and later requiring systemic therapy.
Conversely, there were nine “false negatives,” patients who had blood involvement by the ISCL criteria but nonetheless failed to demonstrated TCR Vβ-restriction by flow cytometry. Four patients demonstrated equivocal TCR-Vβ restriction (30-47%), and the other five patients had atypical CD4+CD26- populations. Thus, it is possible that if gated on CD4+CD26-, these patients too would have TCR-Vβ restricted populations. Currently, ARUP Laboratories does not use loss of CD26 to gate on atypical populations. We would propose gating on CD4+CD26- populations in order to increase the sensitivity of TCR-Vβ testing.
“Equivocal” TCR-Vβ testing
Four B2 patients had TCR-Vβ “equivocal” testing (greater than 20% and less than 50%). Three of these four were followed on average for 7.6 months and all required systemic therapy, suggesting the utility of TCR-Vβ testing to upstage higher risk individuals. In one patient, an equivocal test occurred during a transition between two malignant clonal populations. In contrast, equivocal TCR-Vβ testing in ISCL B1-B0 patients (n=15) was not clinically significant and no patients had disease progression.
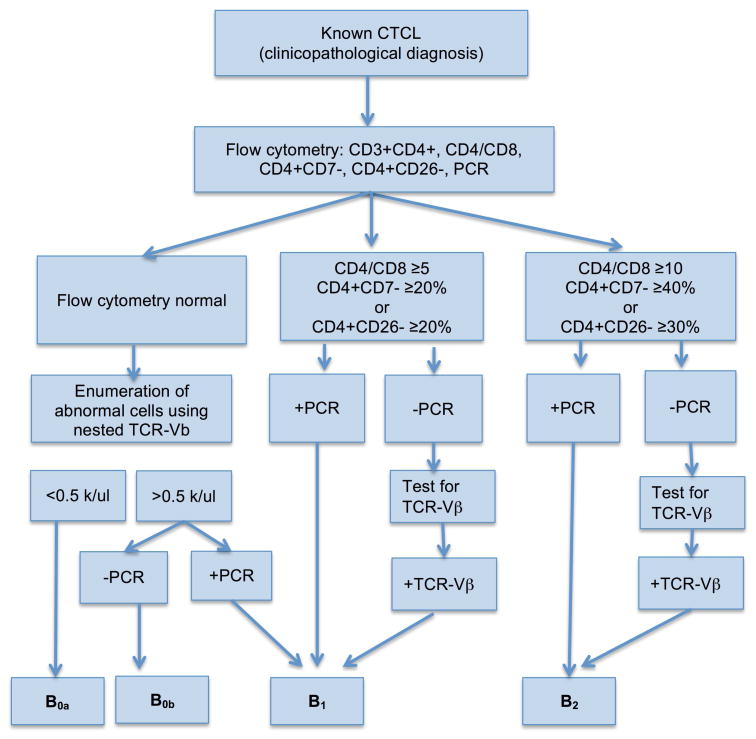
Figure 1. Proposed algorithm for assessment of blood involvement in CTCL.
This algorithm may aid in further research investigating the prognostic significance of our proposed modified B1 criteria and “equivocal” TCR-Vβ tests. As loss of CD26 was the most sensitive parameter of the current B2 criteria, we would suggest investigating the potential for TCR-Vβ testing in CD4+CD26- populations. B1 and B2 were defined by ISLC criteria (if atypical lymphocytes were noted based on immunophenotypic alterations only, this percentage was multiplied by the absolute lymphocyte count to approximate the number of “Sézary cells” where no such counts were performed/available).
Capsule summary.
Accurate assessment of blood involvement in cutaneous T cell lymphoma (CTCL) is important for disease detection and monitoring.
We examine the potential of use of TCR-Vβ analysis to inform B1 staging criteria.
We provide a novel algorithm to aid the diagnosis and monitoring of blood involvement in CTCL.
Acknowledgments
Yale SPORE in Skin Cancer (grant from NIH/NCI)
Abbreviations and acronyms
- CTCL
Cutaneous T cell lymphoma
- MF
mycosis fungoides
- SS
Sézary syndrome
- SC
Sézary counts
- TCR-Vβ
T-cell receptor Vβ
- ISCL
International Society for Cutaneous Lymphomas
- EORTC
European Organisation for Research and Treatment of Cancer
- USCLC
United States Cutaneous Lymphoma Consortium
- MDS
Medical Dermatology Society
- IRB
Institutional Review Board
- PPV
positive predictive value
- NPV
and negative predictive value
- PCSM-TCL
Primary cutaneous CD4 positive small/medium T cell lymphoma
- CLL
chronic lymphocytic leukemia
- mSWAT
Modified Skin Weighted Assessment Tool
Footnotes
Conflicts of interest: Dr. Hussong is the Director of Laboratory for ARUP Laboratories, Salt Lake City, UT; is on the Executive Committee for ARUP Laboratories, Salt Lake City, UT. Mr. Mohl is a technical supervisor for ARUP Laboratories, Salt Lake City, UT. Ms. Hill is a hematologic flow cytometry specialist for ARUP Laboratories, Salt Lake City, UT.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005 Apr;115(4):798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003 Jul;139(7):857–866. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- 3.Scarisbrick JJ, Whittaker S, Evans AV, et al. Prognostic significance of tumor burden in the blood of patients with erythrodermic primary cutaneous T-cell lymphoma. Blood. 2001 Feb 1;97(3):624–630. doi: 10.1182/blood.v97.3.624. [DOI] [PubMed] [Google Scholar]
- 4.Schechter GP, Bunn PA, Fischmann AB, et al. Blood and lymph node T lymphocytes in cutaneous T cell lymphoma: evaluation by light microscopy. Cancer treatment reports. 1979 Apr;63(4):571–574. [PubMed] [Google Scholar]
- 5.Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007 Sep 15;110(6):1713–1722. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 6.Klemke CD, Brade J, Weckesser S, et al. The diagnosis of Sézary syndrome on peripheral blood by flow cytometry requires the use of multiple markers. The British journal of dermatology. 2008 Sep;159(4):871–880. doi: 10.1111/j.1365-2133.2008.08739.x. [DOI] [PubMed] [Google Scholar]
- 7.Heald P, Yan SL, Edelson R. Profound deficiency in normal circulating T cells in erythrodermic cutaneous T-cell lymphoma. Arch Dermatol. 1994 Feb;130(2):198–203. [PubMed] [Google Scholar]
- 8.Harmon CB, Witzig TE, Katzmann JA, Pittelkow MR. Detection of circulating T cells with CD4+CD7- immunophenotype in patients with benign and malignant lymphoproliferative dermatoses. J Am Acad Dermatol. 1996 Sep;35(3 Pt 1):404–410. doi: 10.1016/s0190-9622(96)90605-2. [DOI] [PubMed] [Google Scholar]
- 9.Jung LK, Fu SM, Hara T, Kapoor N, Good RA. Defective expression of T cell-associated glycoprotein in severe combined immunodeficiency. J Clin Invest. 1986 Mar;77(3):940–946. doi: 10.1172/JCI112393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinhold U, Abken H, Kukel S, et al. CD7- T cells represent a subset of normal human blood lymphocytes. J Immunol. 1993 Mar 1;150(5):2081–2089. [PubMed] [Google Scholar]
- 11.Papadavid E, Economidou J, Psarra A, et al. The relevance of peripheral blood T-helper 1 and 2 cytokine pattern in the evaluation of patients with mycosis fungoides and Sézary syndrome. Br J Dermatol. 2003 Apr;148(4):709–718. doi: 10.1046/j.1365-2133.2003.05224.x. [DOI] [PubMed] [Google Scholar]
- 12.Vonderheid EC, Bernengo MG. The Sézary syndrome: hematologic criteria. Hematol Oncol Clin North Am. 2003 Dec;17(6):1367–1389. doi: 10.1016/s0889-8588(03)00120-5. , viii. [DOI] [PubMed] [Google Scholar]
- 13.Delfau-Larue MH, Laroche L, Wechsler J, et al. Diagnostic value of dominant T-cell clones in peripheral blood in 363 patients presenting consecutively with a clinical suspicion of cutaneous lymphoma. Blood. 2000 Nov 1;96(9):2987–2992. [PubMed] [Google Scholar]
- 14.Muche JM, Lukowsky A, Asadullah K, Gellrich S, Sterry W. Demonstration of frequent occurrence of clonal T cells in the peripheral blood of patients with primary cutaneous T-cell lymphoma. Blood. 1997 Aug 15;90(4):1636–1642. [PubMed] [Google Scholar]
- 15.Vonderheid EC. On the diagnosis of erythrodermic cutaneous T-cell lymphoma. J Cutan Pathol. 2006 Feb;33(Suppl 1):27–42. doi: 10.1111/j.0303-6987.2006.00541.x. [DOI] [PubMed] [Google Scholar]
- 16.Morice WG, Kimlinger T, Katzmann JA, et al. Flow cytometric assessment of TCR-Vbeta expression in the evaluation of peripheral blood involvement by T-cell lymphoproliferative disorders: a comparison with conventional T-cell immunophenotyping and molecular genetic techniques. Am J Clin Pathol. 2004 Mar;121(3):373–383. doi: 10.1309/3A32-DTVM-H640-M2QA. [DOI] [PubMed] [Google Scholar]
- 17.Feng B, Jorgensen JL, Jones D, et al. Flow cytometric detection of peripheral blood involvement by mycosis fungoides and Sézary syndrome using T-cell receptor Vbeta chain antibodies and its application in blood staging. Mod Pathol. 2010 Feb;23(2):284–295. doi: 10.1038/modpathol.2009.175. [DOI] [PubMed] [Google Scholar]
- 18.Bigler RD, Boselli CM, Foley B, Vonderheid EC. Failure of anti-T-cell receptor V beta antibodies to consistently identify a malignant T-cell clone in Sézary syndrome. Am J Pathol. 1996 Nov;149(5):1477–1483. [PMC free article] [PubMed] [Google Scholar]
- 19.Vonderheid EC, Boselli CM, Conroy M, et al. Evidence for restricted Vbeta usage in the leukemic phase of cutaneous T cell lymphoma. J Invest Dermatol. 2005 Mar;124(3):651–661. doi: 10.1111/j.0022-202X.2004.23586.x. [DOI] [PubMed] [Google Scholar]
- 20.Morice WG, Katzmann JA, Pittelkow MR, el-Azhary RA, Gibson LE, Hanson CA. A comparison of morphologic features, flow cytometry, TCR-Vbeta analysis, and TCR-PCR in qualitative and quantitative assessment of peripheral blood involvement by Sézary syndrome. Am J Clin Pathol. 2006 Mar;125(3):364–374. [PubMed] [Google Scholar]
- 21.McCarthy KP, Sloane JP, Kabarowski JH, Matutes E, Wiedemann LM. A simplified method of detection of clonal rearrangements of the T-cell receptor-gamma chain gene. Diagn Mol Pathol. 1992 Sep;1(3):173–179. [PubMed] [Google Scholar]
- 22.McCarthy KP, Sloane JP, Kabarowski JH, Matutes E, Wiedemann LM. The rapid detection of clonal T-cell proliferations in patients with lymphoid disorders. Am J Pathol. 1991 Apr;138(4):821–828. [PMC free article] [PubMed] [Google Scholar]
- 23.Bernengo MG, Novelli M, Quaglino P, et al. The relevance of the CD4+ CD26-subset in the identification of circulating Sézary cells. Br J Dermatol. 2001 Jan;144(1):125–135. doi: 10.1046/j.1365-2133.2001.04014.x. [DOI] [PubMed] [Google Scholar]
- 24.Ferenczi K, Yawalkar N, Jones D, Kupper TS. Monitoring the decrease of circulating malignant T cells in cutaneous T-cell lymphoma during photopheresis and interferon therapy. Arch Dermatol. 2003 Jul;139(7):909–913. doi: 10.1001/archderm.139.7.909. [DOI] [PubMed] [Google Scholar]
- 25.Schwab C, Willers J, Niederer E, et al. The use of anti-T-cell receptor-Vbeta antibodies for the estimation of treatment success and phenotypic characterization of clonal T-cell populations in cutaneous T-cell lymphomas. Br J Haematol. 2002 Sep;118(4):1019–1026. doi: 10.1046/j.1365-2141.2002.03726.x. [DOI] [PubMed] [Google Scholar]