Abstract
A major focus of cancer research for several decades has been understanding the ability of tumors to induce new blood vessel formation, a process known as angiogenesis. Unfortunately, only limited success has been achieved in the clinical application of angiogenesis inhibitors. We now know that lymphangiogenesis, the growth of lymphatic vessels, likely also plays a major role in tumor progression. Thus, therapeutic strategies targeting lymphangiogenesis or both lymphangiogenesis and angiogenesis may represent promising approaches for treating cancer and other diseases. Importantly, research progress toward understanding lymphangiogenesis is significantly behind that related to angiogenesis. A PubMed search of ‘angiogenesis’ returns nearly 80,000 articles, whereas a search of ‘lymphangiogenesis’ returns approximately 2,635 articles. This stark contrast can be explained by the lack of molecular markers for identifying the invisible lymphatic vasculature that persisted until less than two decades ago combined with the intensity of research interest in angiogenesis during the past half-century. Still, significant strides have been made in developing strategies to modulate lymphangiogenesis, largely using ocular disease models. Here, we review the current knowledge of lymphangiogenesis in the context of knockout models, ocular diseases, the biology of activators and inhibitors, and the potential for therapeutic interventions targeting this process.
Keywords: VEGF-C, VEGFR-3, LYVE-1, prox-1, podoplanin, dry eye disease, corneal transplant, herpetic stromal keratitis, glaucoma, ocular tumor
1. INTRODUCTION
The lymphatic vasculature is responsible for collecting excess fluid and macromolecules from capillary beds, returning these elements to the blood circulation, and capturing and delivering antigens to lymph nodes to induce immunological responses.1 Thus, lymphangiogenesis, the generation of new lymphatic vessels from pre-existing lymphatics, serves important functions during embryonic development and wound healing, but disruption of the fine balance between pro-lymphangiogenic and anti-lymphangiogenic factors can cause certain pathologies. For example, excess lymphangiogenesis can result in tumor metastasis2; 3 and deficient lymphangiogenesis can lead to lymphedema. In the eye specifically, transmission of immunogenic stimuli from a corneal graft through both lymphatic and blood vessels can lead to graft rejection, and the presence of lymphatic vessels in the host prior to corneal transplantation has been shown to be a key predictor of poor outcome, thus demonstrating the importance of lymphatics in transplantation.4; 5; 6; 7; 8 Because the identification and visualization of normally invisible lymphatic vessels was originally difficult, the contribution of these vessels in graft rejection was largely overlooked. However, the discovery of molecular markers for lymphatic vessels has helped advance lymphangiogenesis research in the past 20 years, including the recently discovery of a classical lymphatic drainage system in the central nervous system. 9
Normally, the cornea lacks lymphatic vessels and can tolerate foreign antigens without mounting a systemic immune response, a concept termed “immune privilege.”10; 11 This makes corneal transplant acceptance possible without human leukocyte antigen (HLA) matching.12 The eye has an anterior chamber-associated immune deviation (ACAID) through which inflammatory and immune cells are naturally suppressed when foreign antigens are introduced into the anterior chamber, preventing a systemic immune response.12 When a corneal graft is introduced, it forms the anterior surface of the anterior chamber, and through ACAID, rejection can be avoided.12 However, in the presence of corneal neovascularization, the integrity of the ACAID is lost, and the risk associated with graft rejection increases significantly because immune privilege is no longer applicable.13
The normal adult cornea is both avascular and alymphatic, and it is, therefore, an ideal model for easily assessing both forms of vessel formation.10; 11; 14 Lymphangiogenesis in the cornea occurs when lymphatic vessels either grow from pre-existing vessels in the limbus of the eye or form de novo.15 Under hypoxic and inflammatory conditions, various members of the vascular endothelial growth factor (VEGF) family are released by inflammatory cells to stimulate both angiogenesis and lymphangiogenesis.16; 17; 18 Elucidation of the mechanisms by which lymphangiogenesis occurs in the cornea can lead to the development of therapeutics targeted at reducing corneal neovascularization and may be extrapolated to the prevention of tumor cell metastasis in cancer patients.19; 20; 21
In this review, we first provide an overview of lymphatic markers and knockout models currently used to study lymphatic development and emphasize why the cornea serves as a great model for studying lymphangiogenesis. We also provide updated descriptions of the normal ocular surface anatomy, factors involved in regulating lymphangiogenesis, and ocular diseases associated with lymphangiogenesis. Finally, we discuss strategies for modulating lymphangiogenesis in the context of disease.
2. CORNEAL LYMPHANGIOGENESIS AND ANGIOGENESIS
The cornea is normally avascular and serves as an ideal model in which to study both angiogenesis and lymphangiogenesis.1; 4; 11; 15 However, in various ocular pathologies, 22 corneal angiogenesis and lymphangiogenesis are induced, and corneal transparency is lost. The presence of newly formed blood and lymphatic vessels induces leakage of proteins, lipids, and calcium within the cornea, which results in a reduction in visual acuity and increases the risk of graft rejection after corneal transplantation.4; 5; 6; 7; 8 Prior to the discovery of lymphatic markers, corneal neovascularization was believed to only involve blood vessels, since the lymphatic vessels are biomicroscopically undetectable.23 However, we now know that pathologic lymphangiogenesis is usually present with angiogenesis and occurs in the setting of an inflammatory insult directly to the cornea, overriding the angiogenic and lymphangiogenic privilege of the cornea.24 Members of the VEGF family (VEGF-A, -B, -C, and –D) are the primary mediators of both angiogenesis and lymphangiogenesis. Lymphangiogenesis is primarily mediated by VEGF-C and VEGF-D binding to vascular endothelial growth factor receptor 3 (VEGFR-3) on lymphatic endothelial cells (LECs).25; 26 Bone marrow-derived cells, such as macrophages, produce both VEGF-C and VEGF-D.11; 27; 28 If inflammation occurs after corneal transplantation, macrophages will enter via the blood vasculature in response to cytokines and other mediators of inflammation, whereas antigens are transported by antigen-presenting cells to regional lymph nodes via the lymphatic vasculature.28
3. LYMPHATIC MARKERS
A major breakthrough in the study of lymphangiogenesis occurred with the introduction of lymphatic-specific markers that made lymphatic vessel visualization more accessible and facilitated significant scientific advancements. The ideal characteristic of a lymphatic-specific marker is its exclusive expression on LECs.29; 30 However, this is rarely the case because many of the lymphatic-specific markers currently used are also expressed on certain nonendothelial cells. Thus, the most important feature is that these markers are not expressed on blood vessels and can be used to distinguish lymphatic vessels from blood vessels. A summary of lymphatic-specific markers is provided in Table 1.
Table 1.
Lymphatic markers and their presence on lymphatic and/or blood vessels
| Lymphatic Marker | Present on Lymphatic Vessels | Present on Blood Vessels | Reference(s) |
|---|---|---|---|
| LYVE-1 | ++ | +a | 31; 37 |
| VEGFR-3 | + | +b | 43; 44 |
| Prox-1 | ++ | − | 78 |
| Podoplanin | ++ | − | 61; 69 |
| Desmoplakin | + | − | 79 |
| CCL21 | + | − | 80 |
LYVE-1 is present on blood vessels in the liver.
VEGFR-3 is present on blood vessels in many organs
3.1. LYVE-1
Banerji et al. discovered lymphatic vessel endothelial hyaluronan (HA) receptor 1 (LYVE-1), a member of the Link superfamily of HA-binding proteins, by searching the expression sequence tag database for cDNAs homologous to CD44, the only other major member of the Link superfamily of HA receptors.31 Although both CD44 and LYVE-1 bind to HA, CD44 is not expressed on lymphatic vessels and is instead primarily located on blood vascular endothelial cells. In contrast, LYVE-1 is highly expressed on lymphatic vessels and serves as a lymphatic- specific marker.31 LYVE-1 binds to and internalizes HA31; 32 and is speculated to assist HA in its involvement with cell migration in the lymphatic system33 and to transport HA to the liver or regional lymph nodes for degradation.34 Platanova et al. reported that LYVE-1 and FGF2 have a functional relationship in which LYVE-1 and FGF2 interaction inhibits FGF2-induced lymphangiogenesis and also prevents TNF-β–dependent down-regulation of LYVE-1.35
LYVE-1 mRNA has been detected in a variety of organs where its cellular expression pattern was analyzed to determine its status as a lymphatic-specific marker. An abundant amount of LYVE-1 mRNA was detected in the human spleen sinusoidal endothelium, lymph nodes, heart, lung, and fetal liver.31; 36 LYVE-1 mRNA was also detected in smaller amounts in the human appendix, muscles, placental syncytiotrophoblasts, bone marrow, and adult liver sinusoidal endothelium.31; 36 In the liver, LYVE-1 is expressed in both lymphatic and blood endothelial cells and cannot be used to differentiate lymphatic vessels from blood vessels.37 Instead, prospero homeobox 1 (Prox-1) is present on liver lymphatic vessels, but not on liver blood vessels, and is a better lymphatic marker for the liver.37 When different diseased states of the liver were examined, it was found that blood vessels in cirrhotic livers express less LYVE-1 than blood vessels in healthy livers, whereas blood vessels of hepatocellular carcinomas express no LYVE-1.37 In both of these states, LYVE-1 expression in the liver lymphatic vessels remained the same.37 Aside from its expression in the liver, LYVE-1 is confined to LECs in adult humans and mice and can overall be used as a lymphatic-specific marker.31 LYVE-1 expression is most visible in the draining lymphatic vessels of the gastrointestinal system, the lacteals that drain intestinal villi, and the subdermal lymphatic vessels of the skin.31 On the lymphatic vessel, LYVE-1 has a bipolar distribution and is present on both the luminal and abluminal surfaces of the lymphatic endothelium.31 LYVE-1 and FGF2 have a bifunctional relationship in which FGF2 regulates LYVE-1 expression by reversing the TNF-β–dependent downregulation of LYVE-1 and increasing the overall expression of LYVE-1.35 In contrast, LYVE-1 acts on FGF2 and inhibits FGF2-dependent lymphangiogenesis.35 LYVE-1 expression in corneal lymphatics is variable, with an increase in the number of LYVE-1 absent gaps along corneal lymphatic vessels up until 8 weeks of age, when LYVE-1 expression begins to increase.38 Nakao et al. reported that the LYVE-1 absent regions potentially serve as microvalves that facilitate unidirectional lymphatic flow and as immunological hot spots for stromal macrophage re-entry into the lymphatic vessels.38
LYVE-1 expression in corneal lymphatics is variable, with an increase in the number of LYVE-1 absent gaps in the lymphatic vessels from birth until week 8.
Through the detection of LYVE-1 with anti-LYVE-1 antibodies, the presence of lymphangiogenesis in the cornea,15 inflammatory diseases,39 and the skin via VEGF-C induction40 has been established. The major advantages associated with using LYVE-1 as a lymphatic marker are its specificity and its use as a method to visualize lymphatic vessels in vivo through anti-LYVE-1 antibodies conjugated with fluorophores.41
3.2. VEGFR-3
VEGFR-3 interaction with VEGF-C and VEGF-D induces lymphatic vessel sprouting from the venous system, an early step in lymphangiogenesis.42 The complete absence of VEGFR-3 is embryonic lethal (for more information regarding VEGFR-3 knockout, refer to section 4.4.).42 VEGFR-3 is expressed on all blood vessels embryonically, but becomes predominantly expressed in LECs later in development.43; 44 Although VEGFR-3 is frequently used in research as a marker for lymphatic vessels, alone it is not sufficient because VEGFR-3 can be expressed on blood vessels.45 Specifically, studies have identified VEGFR-3 on the tip cells of the retina blood vessels,46, 47 in chronic inflammatory wounds,48; 49; 50; 51 in tumors during neovascularization,48; 49; 50; 51 and on fenestrated capillaries of normal tissues.46 VEGFR-3 loses its lymphatic specificity in tumors because its expression is upregulated on blood vessels.50 Previous studies using VEGFR-3 as a lymphatic marker have identified the presence of lymphatic vessels in cutaneous lymphangiomatosis,52 hemangiomas,52 and regions surrounding lymphomas53 and breast carcinomas.53; 54 Furthermore, the presence of VEGFR-3 has revealed the lymphatic endothelium origin of AIDS-associated Karposi’s sarcoma.53 These studies also used antibodies against vascular-specific markers (i.e., CD31, PAL-E, and von Willebrand factor) to ensure that the detected VEGFR-3 was indeed on lymphatic vessels and not on blood vessels.52; 53
3.3. Prox-1
The transcription factor Prox-1 is a lymphatic-specific marker and required for LEC commitment from blood endothelial cells (BECs).55 Prox-1 expression is restricted to lymphatic vessels and has no effect on the development or function of blood vessels.55 Thus, the embryonic lethality of Prox-1 knockout murine models is attributed to defects in the lymphatic system (for more information regarding Prox-1 knockout, refer to section 4.1.). Prox-1 is predominantly restricted to LECs of normal tissues and tumors.56 In addition to LECs, Prox-1 is also expressed in hepatocytes,37 some photoreceptors in the liver,37 cardiomyocytes,57; 58 and pancreatic epithelial cells.57; 58 Prox-1 is co-expressed with CD31/PECAM-1, a transmembrane protein present on both blood and lymphatic vessels, and staining for both Prox-1 and CD31 is the most reliable way to identify LECs given that Prox-1 can be present on non-endothelial cells.59; 60 One of the major advantages of using Prox-1 as a lymphatic marker is that it is absent on blood vessels, and unlike LYVE-1, Prox-1 can be used to identify lymphatic vessels in the liver.37 Wilting et al. compared Prox-1 to VEGFR-3 and concluded that it is a much better lymphatic marker due to its lymphatic specificity in all tissue types.59 Overall, Prox-1 is a reliable lymphatic marker in normal and pathological human tissue.59
3.4. Podoplanin
Podoplanin was first described by Wetterwald et al., who identified the protein on osteocytes and osteoblasts and called it E11 antigen.61 Soon after, Breiteneder-Geleff et al. discovered the same protein on rat podocytes and named it podoplanin.62 Alternate names for podoplanin include OTS-8,63 M2A antigen,64 T1α,65 Aggrus,66 and glycoprotein 36.67 In all organs examined, podoplanin is expressed on LECs and not BECs, but it is also expressed on many nonendothelial cell types.68 The adult lung is a major site for T1α/podoplanin expression,65 and other sites include osteocytes,61; 63 the choroid plexus,61 podocytes,62 skin endothelium,69 and central nervous system ependymal cells.68
Before Schacht et al. discovered that mouse monoclonal D2-40 antibody can recognize human podoplanin, it was very difficult to study human podoplanin expression due to a lack of protein detection methods.68 An obstacle in detecting lymphatic vessel through immunohistochemistry is the absence of a single exclusive lymphatic marker; thus, current guidelines recommend the use of at least two lymphatic-specific markers for identifying lymphatic vessels in the eye.70 Cursiefen et al. used both podoplanin and LYVE-1 to confirm that lymphangiogenesis occurs in the cornea.15 Currently, podoplanin is used to identify many different types of tumors: lymphangiomas,69; 71 Karposi’s sarcomas,69; 71; 72 angiosarcomas,69; 71; 73 and squamous cell carcinomas.74 Additionally, podoplanin has also been found to be a potential diagnostic marker for epitheliod mesothelioma73; 75; 76 and gonadal and extragonadal germ cell tumors.64; 68; 77
4. KNOCKOUT AND MUTANT MODELS FOR STUDYING LYMPHANGIOGENESIS
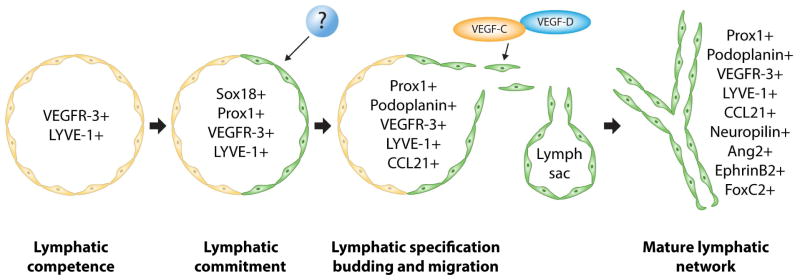
Many factors have been implicated in the process of lymphangiogenesis with varying degrees of importance. Knockout models allow researchers to gain insight into the specific functions these factors have in the development of the lymphatic system. Factors can be involved in the initial development of the lymphatic system or in downstream maturation events (Fig. 1). A clear understanding of the process of lymphatic system development and the unique roles each factor plays serves as an important clinical tool for developing treatments for diseases involving the lymphatic system. A summary of factors involved in lymphangiogenesis and their corresponding knockout models is provided in Table 2.
Fig. 1.
Diagram of proteins essential to specific steps in the development of the lymphatic system. (Adapted from Cueni and Detmar45, with permission from Nature Publishing group).
Table 2.
Murine knockout models and their lymphatic phenotypes reveal gene functions in lymphangiogenesis
| Murine Model | Lethality | Lymphatic Phenotype | Gene Function | Reference(s) |
|---|---|---|---|---|
| Prox-1 | ||||
| Prox1+/− | P2–P3 | Abdominal chylous ascites. Detectable but dysfunctional lymphatic vessels. | Prox-1 is required for the maintenance of lymphatic cell budding and sprouting and is critical in BEC commitment to LECs. | 55; 78; 8 |
| Prox1−/− | E14.5 | Arrested sprouting with no lymphatic vessels present. Venous ECs lack lymphatic markers LYVE-1 and SLC but contain blood markers CD34 and laminin. | ||
| SRY-related HMG-box 18 (Sox18) | ||||
| Sox18+/− | Normal | Fine, dense, and more branched lymphatic vessels. | Sox18 is a transcription factor that is responsible for inducing the expression of Prox-1 on lymphatic vasculature precursor cells. | 84; 85 |
| Sox18−/− | 14.5 d.p.c. | No lymphatic vessels present. No venous Prox-1 expression. | ||
| Vascular endothelial growth factors C and D (VEGF-C and VEGF-D) | ||||
| Vegfc+/− | Normal or perinatal | Abdominal chylous ascites. Cutaneous lymphatic hypoplasia with lymphedema in adults. | VEGF-C controls the process of Prox-1– positive LEC sprouting and migration from embryonic veins. VEGF-C is required for LEC proliferation and survival. VEGFD plays a similar but not as impactful role as VEGF-C. | 42; 87 |
| Vegfc−/− | E17–E19 | No detected lymphatic vessels. Severe embryonic lymphedema. | ||
| Vegfd−/− | Normal | Normal lymphatic vasculature. | ||
| Vegfc−/− x Vegfd−/− | E16.5 | Same phenotype as Vegfc−/−. | ||
| Vascular endothelial growth factor receptor 3 (VEGFR-3) | ||||
| Vegfr3+/neo | Normal | Leaky and dysfunctional lymphatic vessels. Transient abdominal chylous ascites after birth. | VEGFR-3 is regulated by VEGF-C and VEGF-D and is necessary for LEC proliferation and survival. VEGFR-3 is also necessary for angiogenesis. | 42; 148 |
| Vegfr3neo/neo | Perinatal | No detectable lymphatic vessels. Cardiovascular failure. | ||
| Vegfr3lz/lz | E10.5 | No detectable lymphatic vessels. | ||
| Podoplanin | ||||
| T1α/podoplanin+/− | Normal | Similar to wild-type mice. Some regions of dilated lymphatic vessels with incomplete lymphatic network formation. | Podoplanin plays a critical role in lymphatic patterning and network formation. Specifically, podoplanin promotes LEC migration, adhesion, and tubulogenesis. | 98; 100 |
| T1α/podoplanin−/− | Perinatal | Congenital lymph edema, impaired lymphatic transport, lymphangiectasia, undetectable small lymphatic capillaries, absent abdominal lacteals, and lack of anatamosing lymphatic vessels between superficial and subcutaneous lymphatic networks. Incomplete separation between blood vascular and lymphatic vascular systems. | ||
| Angiopoietin 2 (Ang2) | ||||
| Ang2+/− | Normal | Collecting lymphatics with valves are present. Needs additional investigation. | Ang2 is required for the remodeling and maturation of the primary lymphatic plexus. This includes the emergence of a secondary lymphatic plexus along with the development of collecting lymphatic vessels with valves. | 108; 110 |
| Ang2−/− | Perinatal or normal | Abdominal chylous ascites and chylothorax. Lymphedema present. Lack of collecting lymphatic vessels with valves. Primary and secondary lymphatic plexus hypoplasia. Impaired lymphatic maturation and remodeling. | ||
| EphrinB2 | ||||
| EphrinB2ΔV/ΔV | Perinatal | No lymphatic valves. Chylothorax. | EphrinB2 is essential in regulating valve formation and morphology in developing lymphatics. Data regarding whether ephrinB2 forward or reverse signaling is responsible for valve formation are conflicting. | 111; 114; 122 |
| EphrinB26YFΔV/6YFΔV | Perinatal | No lymphatic valves. Chylothorax. | ||
| EphrinB2lacZ/lacZ | Perinatal | Normal lymphatic valves detected at E18. | ||
| EphrinB26YFΔV/lacZ | Normal | Normal lymphatic development. | ||
| Forkhead box c2 (Foxc2) | ||||
| Foxc2+/− | Normal | Lymphatic hyperplasia and reflux. Distichiasis. | Foxc2 is necessary for the lymphatic vessel maturation and valve formation. | 125; 127; 130; 131; 132 |
| Foxc2−/− | E12.5 | Cardiovascular and skeletal defects. Impaired lymphatic maturation, remodeling, and valve formation. | ||
| Neuropilin-2 (NRP2) | ||||
| Nrp2+/− | Normal | Appears unaffected. | Nrp2 is necessary for new lymphatic vessels sprouting from pre-existing lymphatic vessels. Nrp2 inhibits tip cell retraction and thus promotes VEGF-C induced tip cell extension from the lymphatic sprout. | 94; 136; 138 |
| Nrp2+/−vegfr3+/− | Reduced Mendelian ratio | Lymphatic hypoplasia in the skin with lymphatic vessel enlargement and increased branching. Reduced number of tip cells with defective filopodia extension from existing tip cells. Enlarged lymph sac and severe edema present. | ||
| Nrp2−/− | Reduced Mendelian ratio | |||
| SLP-76 and Syk | ||||
| Slp-76−/− or Syk−/− | Perinatal | Nonseparation phenotype. Chylous ascites. Lymphatic vessels are filled with blood. | Slp-76 and Syk are hematopoietic intracellular signaling proteins required for the separation of the closed blood vascularsystem from the open lymphatic system. | 142; 143 |
| C-type lectin-like receptor 2 (CLEC-2) | ||||
| Clec-2+/− | Normal | Normal | CLEC-2 serves as the intermediate between podoplanin and SLP-76/Syk. LEC podoplanin binds to CLEC-2 to activate platelet cells and induce release of granules that inhibit lymphangiogenesis. | 100; 101; 145; 146 |
| Clec-2−/− | Perinatal | Nonseparation phenotype. | ||
| Elk3 (Net) | ||||
| Elk3−/− | Perinatal | Chylothorax and lymphangiectasis. | N/A | 147 |
| Integrin α9 | ||||
| α9−/− | Perinatal | Chylothorax and lymphangiectasis. | Integrin-α9 binds to VEGF-C and VEGF-D and also interacts with VEGFR-3. | 149; 150 |
4.1. Prox-1 knockout models
Prox1, prospero-related homeobox transcription factor gene, is expressed in the developing central nervous system, lens, pancreas, heart, and liver of mice.58 Wigle and Oliver found that Prox1 is present in a subset of BECs that eventually gives rise to the lymphatic system.55 Through two primary types of murine Prox1 knockout models, Prox1’s critical regulatory role in the development of the lymphatic system and the commitment of BECs into LECs was discovered.55
The Prox1 heterozygous knockout murine model, Prox1+/−, is viable at birth, but lethal 2–3 days later.55 Hemizygous Prox1+/− mice develop dysfunctional lymphatic vessels and a chylous ascites phenotype, which presents with chyle in the intestines, at the time of death.55; 78 This is in contrast to the nullizygous murine knockout model, Prox1−/−, which is lethal at E14.5 and has no lymphatic vasculature present.55; 78 At E14.5, both Prox1+/− and Prox1−/− mice present with severe edema, suggesting the haploinsufficiency of Prox1 in lymphatic development.55
A more detailed analysis of the embryonic development of Prox1+/− and Prox1−/− mice reveals Prox1’s specific effects on the development of the lymphatic system. Early lymphatic commitment and development involves two main steps: budding and sprouting. In budding, endothelial venous cells bud out to form lymphatic sacs. In sprouting, endothelial cells from the lymphatic sacs sprout to give rise to lymphatic vessels. At E10.5, Prox1+/− and Prox1−/− mice are developmentally similar and both exhibit normal budding.55 However, by E11.5, Prox1−/− mice have suppressed levels of endothelial budding with random migration due to defective polarization, and by E12, complete arrest of budding is observed.55; 81 These findings reveal that Prox1 is not required for the initial endothelial cell budding from the cardinal vein but is required for the maintenance of further endothelial cell budding and sprouting.78 At E13.5, Prox1+/− mice show normal endothelial sprouting, whereas Prox1−/− mice exhibit no sprouting and an absence of lymphatic vessels.81 During the process of budding and sprouting, Prox1 plays a role in the lymphatic commitment of budding endothelial cells by upregulating the lymphatic-specific markers VEGF-C, LYVE-1, and secondary lymphoid tissue chemokine (SLC) to help BECs commit to a lymphatic fate.81 Budding endothelial cells in Prox1+/− mice are similar to those in wild-type mice in that they begin to express these lymphatic-specific markers and suppress expression of their original blood vascular markers.81 Prox1−/− mice on the other hand do not express LYVE-1 or SLC and only express low levels of VEGFR-3, while also expressing high levels of the blood vascular markers CD34 and laminin, overall adopting a blood vascular phenotype.81
After the discovery that Prox-1 is necessary for lymphatic development from the embryonic venous blood, Hong et al. and Petrova et al. performed experiments using human dermal microvascular endothelial cells (HDMECs) and found that Prox-1 expression can reprogram BECs into LECs.82; 83 The introduction of Prox-1 to HDMECs successfully induced the expression of lymphatic-specific markers VEGFR-3 and podoplanin and suppressed the expression of some of the previously elevated blood vascular markers.82; 83
4.2. Sox18 knockout models
SRY-related HMG-box 18 (Sox18) is a transcription factor that is expressed on lymphatic vasculature precursor cells and is necessary for inducing Prox-1 expression.84 Mutations in Sox18 cause the autosomal recessive and dominant forms of hypotrichosis-lymphoedema-telangiectasia syndrome in humans.85
Heterozygous Sox18+/− mice survive to adulthood but have very dense and branched lymphatic vessels.84 Homozygous Sox18−/− mice die at 14 days post coitum (d.p.c.) and have no Prox-1 venous expression and subsequent lymphatic development.84 Thus, Sox18 is required only during initial lymphatic development, and its absence leads to a complete halt in further development.84
4.3. VEGF-C and VEGF-D knockout models
Vascular endothelial growth factors C and D (VEGF-C and VEGF-D) are the only known high affinity ligands for VEGFR-3 (flt-4), a receptor unique to adult lymphatic vessels.44 These ligands play a critical role in regulating lymphangiogenesis and are necessary for LEC migration and survival.42 Schoppmann et al. found that tumor-associated macrophages express high levels of VEGF-C and VEGF-D, which are necessary for the induction of peritumoral lymphangiogenesis and contribute to cancer metastasis.86 The development of VEGF-C and VEGF-D murine knockout models revealed the involvement of VEGF-C in LEC sprouting from embryonic veins during early lymphatic development and the comparatively trivial role that VEGF-D plays in lymphangiogenesis.42; 87
Hemizygous Vegfc+/− murine models presented with lymphatic vessel defects, emphasizing the necessity for two functional Vegfc alleles for normal lymphatic development.42 In contrast to nullizygous Vegfc−/− mice, a fraction of Vegfc haploinsufficient mice survive to adulthood, but many die perinatally.42 When examined at E13, Vegfc+/− mice have normal or only slightly reduced lymph sac formation, a process primarily guided by Prox1 and as a whole unaffected by VEGF-C levels.42 At the time of birth, Vegfc+/− mice present with lymphatic hypoplasia in all of the studied organs, including the skin.42 During the first few postnatal weeks, lymphatic capillaries slowly grow into most of these organs; however, this effort is not sufficient to completely revert the mice to a wild-type phenotype.42 The lymphatic defects persist in the skin, and the cutaneous lymphatic vessel hypoplasia does not improve with time, contrary to what is seen in the other organs.42 When the mice reach adulthood, lymphedema and abdominal chylous ascites are present as a result of the lymphatic hypoplasia.42 The lymphatic phenotype seen in Vegfc+/− mice can be rescued by VEGF-D overexpression as seen in Haiko et al’s K14-hVEGF-D;Vegfc+/− compound mice, indicating some overlap in the functions of VEGF-C and VEGF-D.87 Küchler et al. investigated the role of VEGF-C in zebrafish and found that lymphatic vessel development in zebrafish was also sensitive to levels of VEGF-C and VEGFR-3 signaling.88
Nullizygous Vegfc−/− mice all die embryonically, with most dying between E15.5 and E17.5.42 Defects presented as lymphedema are seen as early as E12.42 Without VEGF-C, lymph sacs and lymphatic vessels are completely absent, because Prox-1-positive venous endothelial cells cannot proliferate, survive, or sprout into lymphatic vessels and thus undergo apoptosis as evidenced histologically by macrophage infiltration.42 Similar results were seen in Xenopus laevis tadpole VEGF-C knockout models.89 Typically, VEGFR-3 is initially expressed on all blood and lymphatic vessels, but through the course of normal development, blood vessel VEGFR-3 is downregulated and the receptor can only be found on lymphatic vessels. In Vegfc−/− mice, the VEGFR-3 on blood vessels is never downregulated, but it is in Vegfc+/− mice, suggesting that VEGF-C participates in the downregulation of VEGFR-3.42, 26 Overall, the primary effect of complete VEGF-C knockout is the absence of lymphatic vessels, which has been proven to be embryonic lethal.
To examine the specific role of VEGF-D in lymphangiogenesis, Haiko et al. developed Vegfd−/− mice and Vegfc−/−;Vegfd−/− double knockout mice.87 Vegfd−/− mice show no lymphatic abnormalities, indicating that VEGF-D plays a secondary role to VEGF-C in regulating lymphangiogenesis.87 The Vegfc−/−;Vegfd−/− double knockout mice are phenotypically similar to the Vegfc−/− mice, with both models presenting no lymphatic vessels at the time of embryonic death.87
Through these various knockout models, the specific and necessary roles of VEGF-C in LEC proliferation, migration, and sprouting from the lymph sacs have been determined.42 Although VEFG-C is not required for the Prox-1 dominated BEC commitment to LECs, it is needed for the continued development of lymphatic vessels.42
4.4. VEGFR-3/flt-4 knockout models
VEGFR-3 (or flt-4) is initially present on all blood and lymphatic vessels during development. Eventually, VEGFR-3 is upregulated in Prox-1–expressing cells that sprout from the venous system to form the lymphatic system and is downregulated in blood vessels. By the time development is complete, VEGFR-3 is almost exclusively present in lymphatic vessels.43; 46 Upon VEGF-C and VEGF-D binding to VEGFR-3, downstream signaling leads to the proliferation, migration, and survival of cultured human LECs.90 In humans, VEGFR-3’s role in inducing lymphangiogenesis has been established, and a missense mutation in VEGFR-3, the cause of primary human lymphedema or Milroy’s disease, leads to lymphatic hypoplasia.91; 92; 93 Because VEGF-C and VEGF-D are the only known ligands of VEGFR-3, knockout models can be used to reveal potential functions of VEGFR-3 that are independent of VEGF-C and VEGF-D. VEGFR-3 knockout, conditional knockout, and missense mutation models are clinically important tools for investigating the role of VEGFR-3 in lymphangiogenesis and Milroy’s disease.
Chy mice have a missense mutation in one Vegfr3 allele and serve as a murine model for Milroy’s disease.93; 94 Both Chy mice and Vegfc+/− mice present with abdominal chylous ascites and a deficiency in subcutaneous lymphatic vessels.42; 94; 95 Vegfr3+/neo mice, a conditional heterozygous knockout model, are phenotypically similar to Vegfc+/− and Chy mice, with all three presenting with abdominal chylous ascites.42 The haploinsufficient nature of Vegfr3 can be seen when comparing Vegfr3+/neo and Vegfr3neo/neo conditional knockout models. Vegfr3neo/neo mice, similar to Vegfc−/− mice, are embryonic lethal, but Vegfr3+/neo mice can survive to adulthood.42 When examined at E17.5, Vegfr3neo/neo mice lack lymphatic vessels in their skin, whereas Vegfr3+/neo mice retain some lymphatic vessel remnants.42 The lymphatic phenotypes present in VEGFR-3 knockout mice are similar to those seen in VEGF-C knockout mice; however, Vegfr3−/− mice embryos were found to have cardiovascular failure, a phenotype not present in Vegfc−/− mice.42 This finding indicates that VEGFR-3 has a ligand other than VEGF-C and VEGF-D that plays a role in angiogenesis.
4.5. Podoplanin knockout models
Podoplanin is a transmembrane glycoprotein initially discovered for its ability to control human kidney podocyte shape.96 Podoplanin and VEGF-C both play integral roles in lymphatic vasculature development and are both regulated by the homeobox gene Prox1.82 After further investigation, it was found that podoplanin is primarily expressed on the lymphatic epithelium and is also one of the most highly expressed lymphatic-specific markers.69; 83 Schacht et al. produced hemizygous and nullizygous knockout models of murine podoplanin (found on alveolar type 1 cells of the lung) to examine the exact role that podoplanin plays in lymphangiogenesis.69; 97; 98
Unlike the previously discussed genes involved in lymphangiogenesis, podoplanin is not essential for life, and T1α/podoplanin+/− mice are healthy, fertile, and differ minimally from wild-type mice. Upon examining the intestinal and cutaneous lymphatic networks in T1α/podoplanin+/− mice, Schacht et al. saw that the lymphatic vessels are dense, well-organized, and have no deficiencies in lymphatic transportation.98 The only differences present in T1α/podoplanin+/− mice compared to their wild-type counterparts were the presence of some dilated lymphatic vessels and a few regions with incomplete network formation, which were both not significantly detrimental.98
Nullizygous T1α/podoplanin−/− mice die at birth due to respiratory failure from the loss of T1α in alveolar type 1 cells.99 Podoplanin plays a role in the later stages of lymphangiogenesis, and its absence does not prevent the lymphatic system from developing. Although a lymphatic system is detected at the time of death, it contains severe organizational and functional defects attributed to podoplanin’s involvement in lymphatic pattern formation.98 These defects include a diminished lymphatic transport capability, congenital lymphedema, lymphangectasia in abdominal and cutaneous lymphatic vessels, undetectable small lymphatic capillaries, and absent intestinal lymphatic lacteals for dietary lipid absorption.98 Many of these defects, including the lymphangiectasia, may potentially be caused by the lack of anastomosing lymphatic vessels typically present between superficial and subcutaneous lymphatic networks.98 In another study, Bertozzi et al. found that complete absence of podoplanin leads to an incomplete separation between blood and lymphatic vessels.100 This nonseparation phenotype is similar to that seen in slp-76−/− and Clec-2−/− mice (discussed later).100; 101 Podoplanin, C-type lectin-like receptor 2 (CLEC-2), SLP-76, and Syk are all components of a signaling pathway involved in the separation between blood vascular and lymphatic systems, and the complete absence of podoplanin leads to the presence of chyle and blood in mice mesenteric and intestinal lymphatic vessels.100
After observing the effects of podoplanin through knockout models, Schacht et al. confirmed their conclusions by observing the effects of podoplanin overexpression.98 When two different cell lines (human microvascular endothelial cells and murine hemangioendothelioma-derived EOMA cells) were overexpressed T1α/podoplanin, their lymphatic cells showed very long and thin cell extensions, increased migration and adhesion, and an increased propensity for tubule formation.98 Even though podoplanin is not involved in early lymphatic development, it plays a crucial and necessary role in the later stages of lymphatic patterning and networking.
4.6. Ang2 knockout models
Angiopoietin 2 (Ang2) is a member of the vascular growth factor family. Ang2 has contradictory roles in angiogenesis and lymphangiogenesis depending on a number of factors. In angiogenesis, Ang2 promotes vessel regression in the absence of VEGF, but promotes vessel growth in the presence of VEGF.102; 103; 104 Ang2 carries out its effects through binding to Tie2, a tyrosine kinase receptor specific to BECs, and through unknown mechanisms, Ang2 can either activate or repress Tie2 signaling.105; 106; 107 Although its name implies a primary effect on vascularization, Ang2 knockout models reveal that Ang2 is integral in the fundamental processes of lymphatic maturation and remodeling.108; 109
Ang2’s involvement in lymphatic vasculature maturation and remodeling makes it an indispensible factor in normal lymphatic functioning as seen in Ang2−/− mice. Most Ang2−/− mice die within 2 weeks of birth, and the few that survive to adulthood exhibit severe lymphatic dysfunctions including chylous ascites, chylothorax, and lymphedema.108; 110 In wild-type mice, lymphatic remodeling occurs between P0–P5 and involves sprouting of the secondary lymphatic plexus from the primary plexus and the transformation of the remaining primary plexus into collecting lymphatic vessels with valves.109; 111 Dellinger et al. compared wild-type and Ang2−/− mice at three different postnatal time points and found that Ang2−/− mice have hypoplastic primary and secondary lymphatic networks and do not adopt the collecting lymphatic phenotype, which is characterized by elongated LECs and downregulated LYVE-1 levels.108 Overall, Ang2−/− mice display a disorganized lymphatic system with sparse lymphatic vessels that do not surround and follow major blood vessels.
4.7. EphrinB2 knockout models
EphrinB2, a transmembrane ligand that binds to the receptor tyrosine kinase EphB4, participates in a unique bidirectional signaling pathway. The forward signaling is EphB4-dependent and involves ephrinB2 binding to EphB4, inducing EphB4 autophosphorylation and activating downstream signaling pathways.112; 113 The reverse signaling is ephrinB2-dependent and involves the ephrinB2’s C-terminal PDZ-binding motif signaling back to its ephrinB2 host cell.114; 115 Thus, when a cell containing ephrinB2 encounters an EphB4-containing cell, the signal has the potential of propagating in both the forward and reverse directions. EphrinB2 is highly expressed in the LECs of collecting lymphatic vessel valves and plays an important role in regulating lymphatic valve formation and morphology along with lymphatic capillary sprouting.111; 116; 117; 118; 119 Complete and correct valve formation ensures the unidirectional flow of lymph and is imperative for a proper functioning lymphatic system, especially at the lymphatic venous junctions.
Bazigou et al. discovered that complete deletion of efnb2 in mature lymphatic vessels leads to a decreased number and deformed morphology of the lymphatic luminal valves, suggesting that a continuous presence of ephrinB2 is necessary for valve maintenance.120 The amount of ephrinB2 expression is also correlated with the amount and integrity of valves formed. In a mouse model with a heterozygous deletion of efnb2, fewer lymphatic valves are present in the cornea in comparison to the number in wild-type mice.121
Because ephrinB2 carries out both forward and reverse signaling, many researchers have aimed to determine the type of ephrinB2 signaling and the specific region of ephrinB2 responsible for lymphatic valve formation. Makinen et al. created ephrinB2ΔV/ΔV mice by removing the valine in the C-terminal PDZ-binding motif of ephrinB2 and found that this motif executes reverse signaling and is involved in lymphatic valve formation.111 The ephrinB2ΔV/ΔV mice die within 3 weeks after birth and present with chylothorax, absent luminal valves, lymphatic leakage and backflow, and incomplete separation between blood vessels and lymphatic vessels.111 Zhang et al. extended the ephrinB2ΔV/ΔV model by also replacing the six critical intracellular tyrosine residues with phenylalanine to prevent tyrosine phosphorylation in EphB4-dependent forward signaling.122 The impaired forward signaling in these ephrinB26YFΔV/6YFΔV mice was confirmed through observation of a decrease in the number of phosphorylated EphB4 receptors.122 These ephrinB26YFΔV/6YFΔV mice die perinatally, lack lymphatic valve development, and have an overall phenotype similar to that of ephrinB2ΔV/ΔV mice.122 Interestingly, the administration of an EphB4 agonistic antibody can save ephrinB26YFΔV/+ mice from lymphatic valve defects and suggests that forward signaling is also required for proper valve formation.122 Having already removed the critical signaling regions in ephrinB26YFΔV/6YFΔV mice, Zhang et al. replaced the entire intracellular region of ephrinB2 with β-gal to create ephrinB2lacZ/lacZ mice.122 Although ephrinB2lacZ/lacZ mice are perinatally lethal, they show normal lymphatic development with an abundance of lymphatic valves at E18.122 This suggests that the PDZ-binding motif and subsequent reverse ephrinB2 signaling is not required for lymphatic valve formation.122 To confirm this, Zhang et al. mated their previous two mutant models to form ephrinB2lacZ/6YFΔV mice, which survive to adulthood and exhibit a normal lymphatic phenotype, implying that ephrinB2lacZ can compensate for the ephrinB26YFΔV allele.122
4.8. FOXC2 knockout models
Forkhead Box C2 (FOXC2, also named mesenchymal fork head-1) is a transcription factor that is expressed in both developing embryos and adults.123; 124; 125; 126 FOXC2’s role in lymphangiogenesis is of particular importance because of the phenotypic similarities seen between Foxc2 heterozygous mutant mice and humans with lymphatic-distichiasis syndrome (LD), a rare autosomal dominant genetic disorder caused by mutations in FOXC2 and characterized by the presence of primary lymphedema and distichiasis.127; 128; 129 Foxc2 is required for lymphatic maturation, remodeling, and valve formation, and the consequences of its absence can be seen in hemizygous and nullizygous knockout models.
Heterozygous Foxc2+/− mice survive to adulthood and appear grossly normal.127 However, upon further examination, common lymphatic defects include lymphatic hyperplasia and dilation, lymphatic reflux from the cisterna chili into dilated lymphatic channels in the hepatic hilum, and increases in the number and size of lymph nodes.127 All of the hemizygous mice present with distichiasis, a condition in which an extra row of eyelashes is present.127 Overall, Foxc2+/− mice are a good model for the lymphatic and ocular phenotypes seen in LD.127
The absence of both Foxc2 alleles is embryonic lethal, with a majority of nullizygous Foxc2−/− mice dying by E12.5.130; 131 Severe cardiovascular and skeletal defects are present in all embryos, indicating that Foxc2 has an indispensible role in cardiovascular remodeling and skeletal formation.130; 131 Lymphatic development arrests at the primary plexus stage in Foxc2−/− mice, suggesting that Foxc2 is not required for initial lymphatic development; however, maturation of the primary plexus into collecting vessels and formation of luminal valves does not occur.125; 132 Typically, lymphatic vessels develop a collecting vessel phenotype through downregulation of Prox-1, LYVE-1, VEGFR-3, and SLC expression. However, these markers are not downregulated in Foxc2−/− mice, and their lymphatic vessels remain in an immature capillary-like state characterized by high expression of VEGFR-3.132
4.9. Nrp2 knockout models
Neuropilin-2 (Nrp2) was initially discovered as a transmembrane nervous system receptor that binds to class 3 semaphorins to guide the direction of axon development.133; 134; 135 Later, it was found that Nrp2 also binds to VEGF-C and plays a role in lymphangiogenesis.94 Nrp2 is initially expressed in the developing nervous, vascular, and lymphatic systems, but by E13, it is downregulated in the nervous and vascular systems and only highly expressed in the lymphatic system.136 Although the exact effects of Nrp2 on lymphangiogenesis are not known, the clinical significance of Nrp2 is highlighted by Caunt et al.’s finding that blocking the interaction between Nrp2 and VEGF-C decreases tumor lymphangiogenesis and metastasis.137 Through various mutant murine models, research groups have found that Nrp2 exerts its main lymphangiogenic effects by promoting lymphatic sprouting from pre-existing lymphatic vessels.136; 138
Three different murine nrp2 knockout models have been created, nrp2+/−, nrp2−/−, and nrp2+/;vegfr3+/−, to examine the effects of Nrp2 on lymphangiogenesis.136; 138 Of these models, nrp2+/− mice appear to be similar to wild-type mice and lack any detectable lymphatic defects.136 The nrp2−/− and nrp2+/−;vegfr3+/− double heterozygous mice show significant overlap and similarities in lymphatic defects.138 Although both of these mice are viable, they reproduce at a reduced Mendelian ratio, indicating embryonic death.136; 138 Because the superficial dermal lymphatic network is formed through sprouting from deeper major lymphatic vessels, the dermis is an ideal region to observe the effects of Nrp2.136 The skin lymphatic vessels in both nrp2−/− and nrp2+/−;vegfr3+/− mice are characterized by a reduced area of lymphatic coverage with enlarged and poorly branched lymphatic vessels.138 Furthermore, nrp2−/− mice have skin lymphatic vessels located in atypical regions of the dermis.136 Nrp2 guides the process of lymphatic sprouting from pre-existing lymphatic vessels by modulating tip cell stability in sprouting.136; 138 In nrp2−/− and nrp2+/−;vegfr3+/− mice, the number of tip cells is reduced with a majority of the tip cells failing to extend their filopodia in sprouting.136; 138 With impaired lymphangiogenesis, the lymph sac becomes enlarged in these mice and severe edema is detected by E15.5.136; 138
Studies so far indicate that Nrp2 is not required for the initial lymphatic sprouting from the venous system but rather for the further growth of the lymphatic network from pre-existing lymphatic vessels.136 Nrp2 modulates this sprouting by directly inhibiting tip cell retraction and stalling to increase its overall stability and thus indirectly promoting tip cell extension in response to VEGF-C.136 Because both VEGFR-3 and Nrp2 are co-expressed on lymphatic vessels and both bind to VEGF-C, it is speculated that Nrp2 is a VEGFR-3 co-receptor that mediates VEGF-C–induced lymphangiogenesis without activating downstream signaling pathways directly.94; 136; 139; 140
4.10. SLP-76 and Syk knockout models
Adaptor protein SH2 domain-containing leukocyte protein of 76 kDa (SLP-76) and spleen tyrosine kinase (Syk) are intracellular signaling proteins on hematopoietic cells that are involved in the separation of blood vessels from lymphatic vessels.141; 142 Both Slp-76−/− and Syk−/− knockout mice display a similar phenotype in which blood vessels and lymphatic vessels are not completely separated in the developing mice. This nonseparation phenotype is also seen in podoplanin and CLEC-2 knockout mice.100; 101 Specifically, Slp-76−/− and Syk−/− mice lack arterio-venous-lymphatic shunts and have a direct vascular and lymphatic connection that leads to blood-filled lymphatic vessels.142 SLP-76 and Syk in hematopoietic cells prevent LECs from connecting to pre-existing blood vessels and are essential in forming the separation between the closed blood vascular system and the open lymphatic system.142; 143
4.11. CLEC-2 knockout models
CLEC-2 is a receptor present on platelet cells that binds to its endogenous ligand, podoplanin, present on LECs.144 CLEC-2 is the mediator between podoplanin and the SLP-76 and Syk pathway and an important component in inhibiting developing lymphatic vessels from fusing with blood vessels.145; 146 Clec-2+/− mice are healthy, fertile, and do not present with lymphatic defects.100 Clec-2−/− mice, on the other hand, are embryonic lethal or die within the first few weeks after birth due to respiratory failure.100; 101 The nonseparation phenotype of Clec-2−/− mice can be explained by CLEC-2’s interactions with podoplanin, SLP-76, and Syk. During the course of lymphatic development at the venous lymphatic junction, podoplanin present on LECs binds to the CLEC-2 on platelet cells to activate the platelet cells.101 Once activated, the platelets release granules with contents that inhibit lymphangiogenesis in vivo.101 The platelet releasates involved in platelet-mediated inhibition of lymphangiogenesis include bone morphogenetic protein-9 (BMP-9), transforming growth factor-β (TGF-β), platelet factor 4, angiostatin, and endostatin.101 Of these releasates, BMP-9 is thought to be the most important and potent because it is the only one that inhibits tube formation by LECs.101
4.12. Elk3 (Net) knockout model
Elk3 (or Net) is a member of the Ets-domain transcription factor and ternary complex factor families.147 The exact mechanism of Elk3’s involvement in lymphangiogenesis is unknown, but it co-localizes with VEGFR-3 in the thoracic duct and the intestinal and cutaneous lymphatic vessels.147 Elk3−/− mice die shortly after birth and present with chylothorax and lymphangiectasia.147
5. LYMPHATIC STRUCTURE IN THE LIMBUS
The limbus is the border between the opaque sclera and transparent cornea and contains blood vessels that arise primarily from the anterior ciliary arteries and lymphatic vessels that connect to the conjunctival lymphatic network.151 Despite the small size of the limbus, it has several vital ocular functions, which include maintaining nourishment for the cornea and containing the pathways for outflow from the aqueous humor.151 The lymphatic and blood vasculature within the limbus are not evenly distributed and may be heterogeneic in patients.152; 153 Understanding the molecular mechanisms driving lymphatic heterogeneity and the lymphangiogenic or angiogenic response during ocular pathologies and transplant rejection may provide insight into the etiology of some of these diseases and strategies for certain procedures and treatments.153 Ecoiffier et al. found that lymphatic vessels were nasally dominated in the limbus and in the cornea under inflammatory conditions.152 Because the lymphatic vessels are more prone to develop on the nasal side, treatments using subconjunctival injection of anti-lymphangiogenic reagents or surgical procedures such as corneal and limbal transplantation may be more effectively approached via the nasal side.152 Age may also factor into the distribution of limbal lymphatic vessels.154 Hos et al. found that older mice have fewer resting limbal lymphatic vessel sprouts than young mice, and that younger mice show greater lymphatic corneal neovascularization in response to an inflammatory stimulus.154
The limbus is believed to serve a crucial role in preventing corneal neovascularization and maintaining corneal avascularity.155 Significant increases in inflammation and corneal neovascularization have been demonstrated in experimental limbal damage or pathological limbal stem cell deficiency, suggesting that the limbus serves as a physical barrier to angiogenesis and lymphangiogenesis.156; 157; 158 Collin et al. found that corneal lymphangiogenesis arises from limbal lymphatics in the vascularized rabbit cornea.159 Lymphatic vessel formation from the limbus is primarily mediated by VEGFR-3 binding to its ligands VEGF-C and VEGF-D.2; 3; 160; 161; 162; 163; 164; 165; 166 The presence of other mechanisms for corneal lymphangiogenesis has not been established. Our research group has questioned the role of the limbus as merely a physical barrier and found that removal of half of the limbus results in corneal neovascularization from the opposite side.167 Further research is needed to understand the molecular pathways by which the limbus maintains corneal avascularity.
6. DISEASES ASSOCIATED WITH CORNEAL LYMPHANGIOGENESIS
6.1. Dry eye disease
Dry eye disease (DED) is an immune-inflammation mediated disorder that affects the ocular surface resulting in abnormal tear composition.168 More than 10 million Americans 50 years and older have this disease.169 DED severely affects patients’ vision-related quality of life and may lead to psychological and physically debilitating symptoms.168 Cyclosporine is the only treatment for DED currently approved by the U.S. Food and Drug Administration (FDA), but its use is primarily palliative.170 Inflammation of the ocular surface is maintained by the entry of primarily CD4+ T cells.171 In DED, lymphangiogenesis in the cornea occurs without hemangiogenesis, and the lymphatics grow from the limbal areas to the central cornea.172 Because DED occurs without hemangiogenesis, inflammation leads to selective upregulation of VEGF-C, VEGF-D, and VEGFR-3.172 Proinflammatory cytokines, such as interleukin (IL)-17 and IL-1β, are present at increased levels in corneas of patients with DED and are essential for regulating the gene expression of VEGFR-3 and VEGF ligands.173; 174; 175
Goyal et al. administered systemic anti-VEGF-C treatment in a murine model before inducing DED and found significant reductions in lymphatic area and caliber compared to the untreated group.176 VEGF-A binding to VEGFR-2 can also induce lymphangiogenesis, but these lymphatic vessels have a poorly functional phenotype, exhibiting dilated and leaky vessels.177 When VEGF-C is interrupted with anti-VEGF-C treatment, the residual lymphatics are likely less effective at carrying antigen-presenting cells.176 Mice that are treated with anti-VEGF-C also show decreased expression of interferon gamma (IFNγ) and IL-17, which are potent inflammatory attractants.176 By disrupting the VEGF-C/VEGFR-3 axis, the afferent arm of the immune cycle is interrupted. The anti-VEGF-C treatment also suppresses epithelial disease associated with DED, likely by reducing the expression of proinflammatory cytokines.176 Lee et al. also found therapeutic efficacy of epigallocatechin gallate (EGCG), a principal extract of green tea, in murine dry eyes by suppressing expression of inflammatory cytokines (IL-1β and CCL2), VEGF-A and VEGF-D, and CD11b+ cells.178 Thrombospondin-1 (TSP-1) has recently been identified as a key endogenous inhibitor for corneal lymphangiogenesis related to DED and may have potential as a therapeutic target for DED.179 TSP-1 promotes the cleavage of TGF-β into the active form, which has anti-inflammatory roles and induces regulatory T cells.179 TSP-1 inhibits lymphangiogenesis by binding to CD36 present on macrophages and downregulating their production of VEGF-C.180 Without TSP-1’s anti-lymphangiogenic effects, TSP-1–deficient mice have spontaneous isolated lymphatic outgrowths.180 TSP-1 null mice also have a lacrimal gland and corneal inflammation and are used as mouse models of Sjögren syndrome.181 Cho et al. recently found that surgical insults such as corneal suture or incision aggravate preoperative DED in a murine model.182 Thus, dry eye may be part of a pre-lymphangiogenic milieu that is amplified upon corneal injury.182 Surgeons may need to use more aggressive anti-inflammatory treatment in patients with pre-existing DED.
6.2. Corneal transplant rejection
Corneal transplantation is one of the most prevalent and successful ocular procedures. Because the cornea is avascular and immunologically privileged, corneal transplantation does not require HLA matching or the use of high-dose immunosuppressants.183 However, complications can arise if the recipient has severely inflamed and prevascularized beds prior to transplantation, providing a mechanism by which antigen-loaded dendritic cells from the graft gain immediate access to the lymph nodes where allosensitization can occur.5; 184 The rejection rates in high-risk patients can exceed 70%.185; 186; 187 Effector cells reject the graft by entering through the vascularized cornea, and thus, the requirements of both hemangiogenesis and lymphangiogenesis for graft rejection are met. Recent research has focused on modulating the afferent arm of the immune system to prevent graft rejection. The risk of human corneal graft rejection is correlated with lymphangiogenesis, and the presence of lymphangiogenesis is a signal for poor prognosis of the allograft.188 Using a suture-induced corneal neovascularization assay, selective lymphangiogenesis inhibitors have been evaluated.8 Grafts placed into a hemvascularized recipient bed show similar survival rates as grafts placed into a completely avascular recipient bed.8 However, if the recipient had lymphatic vessels within the bed, graft survival is significantly lower, suggesting that lymphatic vessels are more important for graft rejection than blood vessels.8 Patients that carry a certain combination of single nucleotide polymorphisms in IL-17F, VEGF-A, and tumor necrosis factor (TNF)-α of the ACGTCT haplotype may be at increased risk of developing corneal transplant rejection, which may require the surgeon to adjust the therapeutic approach according to a patient’s genetics.189 The immune response against allogeneic corneal tissue is also intensified in the presence of allergic conjunctivitis, which has been shown to have corneal lymphangiogenesis involvement.190; 191
Corticosteroid therapy remains the mainstay for preventing corneal transplant rejection, and the initiation time point for treatment is crucial for graft survival.192 Specifically, presurgical corticosteroid treatment improves graft survival in high-risk patients than extended treatment.192 The anti-lymphangiogenic and anti-angiogenic potency of corticosteroids depends on the specific corticosteroid used. Corticosteroids with stronger anti-inflammatory activity, such as dexamethasone, exert stronger in vivo anti-lymphangiogenic and anti-angiogenic effects in comparison to prednisone and fluoromethalone, corticosteroids with weaker anti-inflammatory properties.193 The strength of corticosteroid used and its adverse drug reactions should both be considered when administering steroids for promoting graft survival. Cho et al. reported that combining steroid treatment with VEGFR-1 morpholino, a synthetically produced molecule that binds mRNA to inhibit translation or alternative splicing, decreases both angiogenesis and lymphangiogenesis and improves corneal graft survival in a high-risk corneal transplantation murine model.194 In addition, a combination of Flt23k nanoparticles that deliver plasmids expressing anti-VEGF intrareceptor Flt23k and steroid treatment significantly reduces lymphangiogenesis and improves graft survival in a mouse model of corneal transplantation.195
Initial approaches to inhibiting lymphangiogenesis begin by targeting members of the lymphangiogenesis signaling pathway. Studies show that inhibiting VEGF-A in the postoperative corneal transplant reduces both angiogenesis and lymphangiogenesis, improving the overall graft survival.5; 6; 7;8 Additionally, targeting VEGFR-3 has also been shown to potentiate anti-lymphangiogenic effects beneficial in prolonging corneal graft survival.4
By administering a soluble form of VEGFR-2 (sVEGFR-2) that specifically traps VEGF-C, Albuquerque et al. observed a significant and selective reduction in lymphangiogenesis without affecting hemangiogenesis.196 Thus, treatment with sVEGFR-2 has the therapeutic potential to improve corneal allograft survival. Emami-Naeini et al. used a soluble form of VEGFR-3 (sVEGFR-3), which consisted of the ligand-binding domain of VEGFR-3 fused to the Fc domain of immunoglobulin, and evaluated its therapeutic effects on corneal transplant survival.197 Treatment of mice after corneal allogeneic transplantation and intrastromal suture placement with sVEGFR-3 inhibited lymphangiogenesis, improved graft survival, reduced the frequency of allosensitized T cells, and decreased the frequency of IFN-γ–secreting T cells compared to a control group.197 Zhang et al. showed that the combined blockade of VEGFR-3 and very late antigen-1, a mediator of corneal inflammatory lymphangiogenesis in vivo, using neutralizing antibodies against the two receptors increases high-risk transplant survival.198
Maruyama et al. administered podoplanin-neutralizing antibody in vivo to understand the role of podoplanin in corneal transplant rejection.199 They found that neutralization of podoplanin reduced lymphatic vessel growth and the presence of macrophages, suggesting the potential role of podoplanin as a therapeutic target.199 Bucher et al. used photodynamic therapy to regress mature lymphatic vessels prior to corneal transplantation in a murine model.200 Photodynamic therapy followed by corneal instrastromal photosensitizer verteporfin injection resulted in selective regression of lymphatic vessels.200 This may prove to be an effective strategy to reduce the risk of graft rejection in high-risk patients prior to surgery.200
Hua et al. showed the therapeutic efficacy of resolvin D1 analogue (RvD1a), a potent lipid mediator of anti-inflammatory effects, in a murine model.201 Mice treated with RvD1a had decreased T-cell infiltration into the corneal graft and a reduced frequency of IFN-γ–secreting T cells in draining lymph nodes.201 RvD1a inhibits the maturation of dendritic cells as well as alloimmune sensitization after corneal transplantation.201 Recently, Dohlman et al. compared the efficacies of VEGF trap, anti-VEGF-C, and sVEGFR-3 in vivo.202 They found that the VEGF trap aflibercept, which primarily neutralizes VEGF-A, is significantly more effective than current therapies at reducing the rate of corneal transplant rejection, suggesting that the efferent arm of the immune system via hemangiogenesis plays a more important role than lymphangiogenesis in corneal transplant rejection.
Tang et al. reported that knockdown of neuropilin-2, a co-receptor for VEGF-C, selectively inhibits lymphangiogenesis in vivo prior to corneal transplantation.203 By employing an artificial microRNA to knockdown neuropilin-2 in a mouse model of high-risk corneal transplantation, they determined that inhibiting neuropilin-2 may selectively inhibit lymphangiogenesis to improve graft survival rate.203
Hos et al. reported that blockade of IRS-1, a pro-lymphangiogenic factor, results in in vivo inhibition of lymphangiogenesis.204 GS-101 antisense oligonucleotide against IRS-1, under the trade name Aganirsen, is currently in phase III clinical trials for the treatment of corneal graft rejection. Cursiefen et al. reported that GS-101 eye drops administered at a daily dose of 86 μg/day effectively inhibit active corneal angiogenesis and lead to its regression.205; 206
6.3. Herpetic stromal keratitis
The most severe form of corneal herpes simplex virus-1 (HSV-1) infection, herpetic stromal keratitis (HSK), is the leading cause of blindness in developing countries.207 HSV-1 is a very infectious human pathogen with a seroconversion rate between 50–90%.208 In HSK, lymphangiogenesis is induced in the alymphatic cornea and immune privilege is lost. During the initial stage of HSK development, inflammatory lymphangiogenesis is induced exclusively through VEGF-A and VEGFR-2 signaling pathways, and the lymphatic vessels persist even after the infection has subsided.209 This differs from inflammatory lymphangiogenesis that occurs during bacterial infections or wound healing, which involves recruitment of macrophages and VEGF-C and VEGF-D activity.209 The reported upregulation of VEGF-A is attributed to VEGF-A production by infected corneal epithelial cells and is an atypical host response to a viral infection, making it rather a unique characteristic of HSV-1.209 The lymphatic vessels produced from VEGF-A and VEGFR-2 interaction are more dilated and leaky than the typical VEGF-C–induced lymphatic vessels.210; 211; 212 Although the integrity of the VEGF-A–induced lymphatic vessels is inferior, they are still capable of transporting antigens to draining lymph nodes and impeding immune privilege.209 Infiltrating neutrophils213; 214 and CD4+ T cells215; 216 in later stages of HSK development are responsible for most of the damage seen in HSK.
Current standard treatments for HSK, including topical antivirals to antagonize viral replication and corticosteroids to limit the immune response, do not address the presence of corneal lymphatic vessels. Corneal lymphangiogenesis in HSK leads to stromal opacity, and corneal transplantation may be required to restore vision.209 However, transplant rejection rates are higher in HSK patients.217 Because VEGF-A–dependent lymphangiogenesis occurs in HSK, VEGF-A serves as a promising therapeutic target, and drugs targeting VEGF-A such as bevacizumab,218; 219; 220; 221 ranibizumab,193 and VEGF trap5; 222 have shown positive results as potential anti-lymphangiogenic treatments for HSK. Although the exact role of insulin receptor substrate-1 in lymphangiogenesis remains unknown, anti-sense oligonucleotides against IRS-1 (insulin receptor substrate-1) inhibit lymphangiogenesis in both mouse models204 and human HSK patients205; 206 and are potential therapeutic targets for the suppression of lymphangiogenesis.
GS-101 antisense oligonucleotide blockade of IRS-1 has also been shown to inhibit lymphangiogenesis and angiogenesis as a treatment for corneal keratitis patients and is currently in clinical trials as the first topical anti-angiogenic agent for the cornea.206
6.4. Glaucoma
Glaucoma, a neurodegenerative disease that leads to optic nerve neuropathy, is caused by impaired aqueous humor drainage, which subsequently leads to an increased intraocular pressure (IOP). The two passageways for draining the aqueous humor are the conventional or trabecular outflow pathway223 and the unconventional or uveoscleral outflow pathway involving the ciliary body224; 225. The presence or absence of “true” lymphatic vessels in the human eye is still a controversial topic. Heindl et al. pointed out that there is no sufficient evidence that ciliary body lymphatic vessels are true lymphatic vessels without extraocular extension226 and that the presence of intraocular lymphangiogenesis in ciliary body melanomas with extraocular extensions is associated with a poorer prognosis227. In addition, immunohistochemical staining for LYVE-1 on postmortem human adult eyes reveals the absence of lymphatic vessels.228 New guidelines for the identification of lymphatic vessels in the eye, including using at least two different immunohistochemical stains, aim to clarify the identification of lymphatic vessels.70
Some studies show that compared to the rest of the eye, the ciliary body has the most lymphatic vessels.229 Current glaucoma treatments target the trabecular and uveoscleral outflow pathways to lower the IOP, but if these medications do not work, surgical treatment with the risk of vision loss may be required. Latanoprost, a prostaglandin F2 analog, is a commonly prescribed topical antiglaucoma drug that acts on the uveoscleral outflow pathway.230; 231 Recently, Tam et al. reported that latanoprost increases lymphatic drainage via the uveoscleral pathway by 400%, suggesting that the efficacy of the treatment lies in its effects on the lymphatic system.230 However, Hos et al. found that tafluprost, a prostaglandin F2 analog, has no effect on murine corneal angiogenesis or lymphangiogenesis and can be used to treat inflammation without affecting the vascular and lymphatic profile of the cornea.232 With this knowledge, a better understanding of the lymphatics of the eye and their role in glaucoma can lead to the development of better therapeutic interventions for glaucoma and other ocular pathologies involving an increased IOP.
6.5. Ocular tumors
Many types of cancers can occur in the eye, with lymphangiogenesis involvement depending on the cell type and location. Heindl et al. reported that the transformation of a conjunctival intraepithelial neoplasia into an invasive squamous cell carcinoma (SCC) of the conjunctiva involves conjunctival lymphangiogenesis.233 Furthermore, the risk of SCC recurrence is associated with the amount of lymphangiogenesis present.233 In another study, Heindl et al. reported that intraocular lymphangiogenesis increases tumor size and metastasis, and is thus correlated with mortality in patients with malignant melanoma and extraocular extensions.227 Recently, Hos et al. described the role of tumor-associated lymphangiogenesis in ocular malignancies and its potential role in future therapeutic development.20
7. MODULATION OF LYMPHANGIOGENESIS
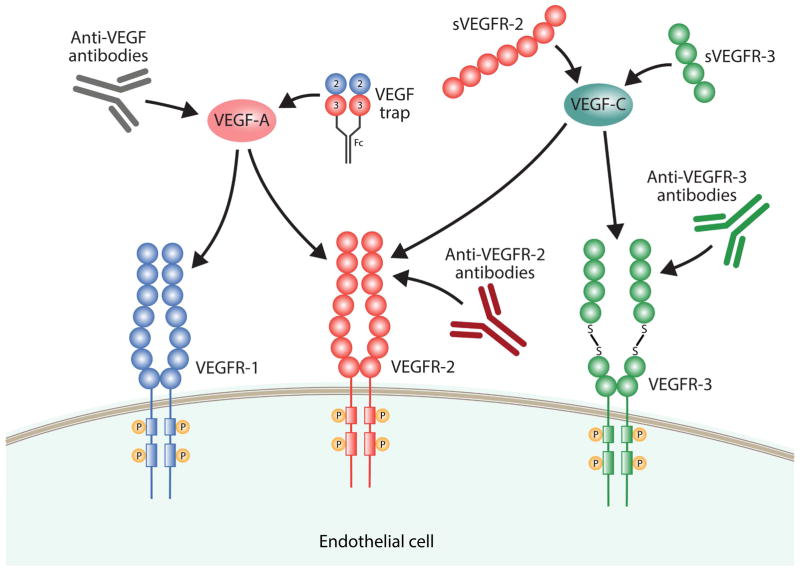
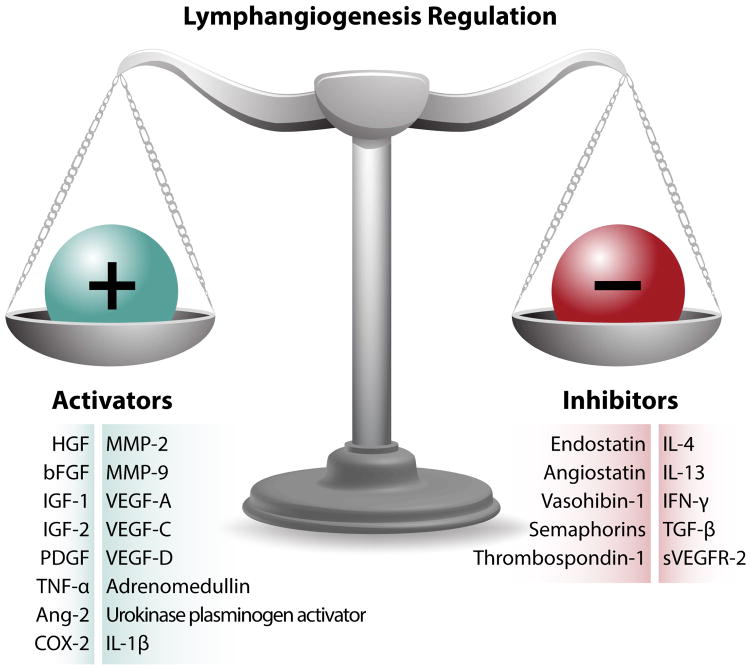
Many endogenous and pharmaceutical factors have been found to modulate lymphangiogenesis, either by activating or inhibiting it. Because fewer anti-lymphangiogenic factors have been identified in the literature compared to pro-lymphangiogenic factors, we focus here on inhibitors of lymphangiogenesis. Many molecules inhibit lymphangiogenesis by modulating members of the VEGF and VEGFR family (Fig. 2). Table 3 and Fig. 3 summarize both endogenous and pharmaceutical activators and inhibitors of lymphangiogenesis, and a more detailed explanation of pro-lymphangiogenic factors can be found in Zheng et al.234.
Fig. 2.
Table 3.
Endogenous and pharmaceutical compounds that modulate lymphangiogenesis.
| Mechanism of Action | Reference(s) | |
|---|---|---|
| Lymphangiogenesis Activators | ||
| VEGF-A | Directly binds to VEGFR-2 on pre-existing lymphatic cells. Indirectly recruits inflammatory cells that produce VEGF-C and VEGF-D. | 221; 238; 242 |
| VEGF-C and VEGF-D | Directly binds to VEGFR-3 on lymphatic cells. Indirectly stimulates lymphangiogenesis. | 295 |
| PDGFa | A direct lymphangiogenic factor that binds to tyrosine kinase receptors on LECs and induces a signaling pathway similar to VEGF-C binding to VEGFR-3. | 296 |
| bFGFb | bFGF or FGF-2, binds to VEGFR-3 and indirectly stimulates lymphangiogenesis. LYVE-1 can bind to bFGF and inhibit bFGF-dependent lymphangiogenesis. | 35; 256 |
| IGF-1 and IGF- 2c | IGF induces phosphorylation of intracellular signaling factors and extracellular kinases in LECs | 297 |
| HGFd | Mechanism is unknown, but it is believed that HGF can bind to VEGFR-3 and induce lymphangiogenesis. | 298 |
| Ang | Mechanism is unknown, but it is believed that Ang-2 and Ang- 2 induces lymphangiogenesis through binding to its Tie2 receptor. | 299; 300; 301; 302 |
| IRS-1 | Mechanism is unknown, but it is believed that IRS-1 promotes macrophage infiltration, which plays a role in lymphangiogenesis. Inhibition of IRS-1 by GS-101 (Aganirsen) suppresses lymphangiogenesis. | 204; 205; 206 |
| Lymphangiogenesis Inhibitors | ||
| sVEGFR-2 | Binds to VEGF-C and prevents it from binding to VEGFR-3 and inducing lymphangiogenesis. | 196 |
| Class 3 Semaphorins | Binds to Nrp2 on lymphatic cells. Believed to inhibit VEGF-C phosphorylation of VEGFR-3. | 136; 303 |
| Endostatin | Competes with VEGF-C for binding to VEGFR-3. | 291 |
| Vasohibin-1 | Believed to inhibit the pro-lymphangiogenic effects of VEGF- A. | 289; 304 |
| Anti-VEGFR-2 | A neutralizing antibody that directly binds to VEGFR-2 and prevents it from interacting with VEGF-C. | 248; 249 |
| Anti-VEGFR-3 | A neutralizing antibody that binds VEGFR-3 and prevents VEGF-C binding to VEGFR-3. | 254 |
| VEGF trap | A soluble decoy receptor that binds and traps VEGF, preventing it from binding to VEGFR-1 and VEGFR-2 and inducing lymphangiogenesis. | 259; 5; 238; 261 |
| sVEGFR-3 | A soluble receptor that binds to and traps VEGF-C, preventing it from binding VEGFR-3 and inducing lymphangiogenesis. | 266 |
| Canstatin | Inhibits Ang-1–induced lymphangiogenesis. | 271 |
| Lamstatin | Believed to stimulate apoptosis. | 272 |
| 16K N terminal human prolactin | Induces LEC apoptosis. | 273 |
| Small peptide derived from somatotrophin-contained domain | Induces LEC apoptosis. | 274 |
| MMPI270 | Inhibits the actions of MMP2 and MMP9, two pro- lymphangiogenic members of the MMP family. | 276 |
| COX-2 inhibitors | Inhibits the action of COX2 enzymes implicated in lymphangiogenesis. | 280; 281; 46 |
| siRNA | Downregulates VEGF-C expression by gene silencing. | 282 |
| Curcurmin | Believed to inhibit tubule formation through Akt and MMP2 pathways. | 283 |
Note. – PDGF = platelet-derived growth factor; bFGF = basic fibroblast growth factor; IGF = insulin-like growth factor; HGF = hepatocyte growth factor.
Fig. 3.
Lymphangiogenesis is highly regulated via a balance between various activators and inhibitors. Lymphangiogenesis occurs when either the levels of activators are increased or inhibitors are decreased, whereas lymphedema may develop if either the activators are downregulated or the inhibitors are upregulated. (Adapted from Bruce R. Zetter235, with permission from Nature Publishing group).
7.1 Pharmacological modulation
7.1.1 Anti-VEGF-A
Although VEGF-A is primarily associated with angiogenesis in solid tumors, several studies have demonstrated that VEGF-A also regulates lymphangiogenesis.5; 236; 237 VEGF-A has been shown to induce lymphatic vessel formation in both mouse5 and rat238 models of corneal injury. Inhibition of VEGF-A in these studies inhibited the growth of lymphatic vessels, suggesting that VEGF-A activates both angiogenesis and lymphangiogenesis through multiple mechanisms.239 The current belief is that VEGF-A directs lymphangiogenesis via indirect and direct mechanisms. Through its indirect mechanism, VEGF-A recruits inflammatory cells that supply lymphangiogenic factors, VEGF-C and VEGF-D.238 The direct mechanism involves VEGF-A directly binding to VEGFR-2 on pre-existing lymphatic vessels.29; 240; 241 Whitehurst et al. showed that anti-VEGF-A neutralizing antibody 2C3 suppresses tumor lymphangiogenesis and metastasis in an orthotopic breast tumor model, suggesting that VEGF-A neutralizing therapeutics may prove to be clinically useful for preventing metastasis in breast cancer patients.242 Bevacizumab is a recombinant, humanized, monoclonal antibody that targets VEGF-A and is approved by the FDA for various metastatic carcinomas. Bock et al. found that bevacizumab inhibits not only corneal angiogenesis but also lymphangiogenesis.221
Serum eye drops are prescribed for ocular surface disorders associated with reduced trophic factors.243 Because these eye drops contain growth factors, they often induce lymphangiogenesis and angiogenesis.244 Thus, serum eye drops have antagonistic effects relative to bevacizumab both in vitro and in vivo, suggesting that the opposing effects of serum eye drops and bevacizumab must be taken into account when considering combinatory therapies.245 Li et al. have shown that by blocking insulin-like growth factor-I receptor and administering bevacizumab in a human gastric cancer cell line, both angiogenesis and lymphangiogenesis are greatly reduced; thus, this combination may be a potential therapeutic approach for gastrointestinal cancers.246 The combination of fotemustine and bevacizumab has been shown to be clinically efficacious in untreated metastatic melanoma patients during a phase II clinical trial in Italy.247
7.1.2 Anti-VEGFR-2
Because anti-VEGFR-2 antibodies have been found to be effective at inhibiting angiogenesis in vitro248 and in vivo,249 their effects on lymphangiogenesis have also been evaluated. Kodera et al. found that anti-VEGFR-2 treatment results in only partial reduction in LEC growth and function.250 Using an orthotopic spontaneous breast cancer metastasis model, Roberts et al. observed that anti-VEGFR-2 antibodies suppress tumor lymphangiogenesis, and the combination of anti-VEGFR-2 and anti-VEGFR-3 antibodies is more potent at decreasing lung and lymph node metastases than either antibody alone.251 Other research groups also have found that anti-VEGFR-2 antibodies inhibit the growth of lymphatic vessels, but their efficacy is limited compared to other pharmacological modulators of lymphangiogenesis.252; 253
7.1.3. Anti-VEGFR-3
VEGFR-3 interaction with VEGF-C and VEGF-D is essential in normal lymphatic development. Using a mouse monoclonal anti-VEGFR-3 antibody that antagonizes binding of VEGFR-3 to VEGF-C, Pytowski et al. demonstrated that the neutralizing antibody can block normal and VEGF-C-enhanced lymphangiogenesis in wild-type and tumor murine models without affecting the integrity of previously formed lymphatic vessels.254 Direct targeting of VEGFR-3 has been shown to almost completely inhibit ingrowth of lymphatic vessels without affecting angiogenesis.255 The use of VEGFR-3 neutralizing antibodies also has been implicated in reducing corneal lymphangiogenesis,256 lymph node metastasis and lymphatic vessel density in primary tumors,256 and lymphangiogenesis in gastric cancer.257 The major disadvantage of anti-VEGFR-3 therapy as an anti-tumor treatment is the timing of tumor detection, because anti-VEGFR-3 therapy does not affect previously formed lymphatic vessels and will only prevent future lymphangiogenic events.254 Still, the anti-lymphangiogenic effects of anti-VEGFR-3 therapy show clinical potential for a variety of diseases.
7.1.4 VEGF Trap
VEGF trap is a soluble chimera of the binding domains of VEGFR-1 and VEGFR-2 coupled to a human Fc fragment.258 VEGF trap has been shown to have similar pharmacokinetics as monoclonal anti-VEGF antibodies but has a higher affinity for VEGF.258 By suppressing lymphangiogenesis and angiogenesis, VEGF trap suppresses the inflammatory response in murine corneal suture models238 and improves graft survival in mice after normal-risk corneal transplation.5 Fukasawa and Kore reported that VEGF trap also suppresses pancreatic ductal adenocarcinoma lymph node enlargement, growth, and metastasis.259 Two side effects of systemic administration of VEGF trap are hypertension and proteinuria, and thus, caution should be used when considering VEGF trap treatment in patients with a history of hypertension or impaired renal function.260
Intravitreal aflibercept, also known as VEGF trap-eye, is a form of VEGF trap that is purified and formulated for intraocular injection.261 Heier et al. reported that aflibercept injections elicit similar results as ranibizumab for the treatment of age-related macular degeneration (AMD), but to achieve the same results, ranibizumab injections must be administered twice as frequently as aflibercept injections.261 However, a recent study comparing aflibercept and ranibizumab treatment patterns for AMD showed that the number of injections patients receive is comparable in the first year of treatment with aflibercept or ranibizumab; this may render the previously reported aflibercept-associated benefits of a decreased treatment and compliance burden and injection-related risks inapplicable.262 In another study, aflibercept was found to have greater efficacy for improving visual acuity in patients with diabetic macular edema in comparison to bevacizumab and ranibizumab, while also allowing for a greater interval of time between injections.263 The FDA has approved aflibercept under the trade name Eylea for the treatment of wet AMD and diabetic retinopathy in patients with diabetic macular edema.
Topical application of ranibizumab has been shown to directly inhibit corneal lymphangiogenesis and angiogenesis in vitro and in vivo, and thus, ranibizumab is a potential therapeutic agent for conditions with limbal stem cell deficiency, such as chemical burns.264 The FDA approved pegaptanib, an antibody against the human isoform of VEGF-A, for the treatment of AMD. Unlike the other therapies discussed above, pegaptanib has anti-angiogenic effects, but no effects on lymphangiogenesis.265
7.1.5. sVEGFR-3
sVEGFR-3 is composed of the soluble extracellular ligand-binding domain of VEGFR-3. sVEGFR-3 inhibits lymphangiogenesis and causes regression of previously formed lymphatic vessels by binding to and trapping VEGF-C.266 The anti-lymphangiogenic effects of sVEGFR-3 make it a potential therapeutic agent for the treatment of various cancers. In a murine orthopic urinary bladder cancer model, sVEGFR-3 gene therapy was found to suppress lymphangiogenesis and metastasis.267 Additionally, sVEGFR-3 gene therapy in an endometrial cancer model reduces lymph node and lung metastasis, two important prognostic factors in endometrial cancer.197 When administered in conjunction with chemotherapy, sVEGFR-1, -2, and -3 gene therapy reduces lymphangiogenesis and tumor growth in ovarian cancer models and is more efficacious than treatment with sVEGFR-1, -2, or -3 alone.268; 269 sVEGFR-3 also has been shown to increase corneal allograft survival through the suppression of lymphangiogenesis, T cell allosensitization, and corneal allograft rejection.197; 270
7.1.6. Other
Additional molecules reported to exhibit anti-lymphangiogenic properties include modified forms of endogenous molecules, synthetic drugs, and herbal components. Many endogenous molecules have anti-lymphangiogenic properties, but obstacles such as a short half-life and insufficient therapeutic concentrations prevent these molecules from suppressing lymphangiogenesis without modification. Recombinant forms of canstatin271 and lamstatin272, fragments found at the C-terminal of type IV collagen’s α2 and α6 chains, respectively, reduce LEC migration and exhibit anti-lymphangiogenic properties. When administered in melanoma mice models, the 16K N-terminal region of human prolactin has been reported to decrease the number of lymphatic vessels in both the primary tumor and sentinel lymph nodes through the inhibition of LEC proliferation, migration, and tube formation.273 Small peptides derived from somatotropin-containing domains have also been shown to exhibit anti-angiogenic properties.274
Another approach to inhibiting lymphangiogenesis is to pharmacologically target endogenously produced pro-lymphangiogenic molecules. Members of the matrix metalloproteinase (MMP) family, MMP-2 and MMP-9, exhibit pro-lymphangiogenic properties275 and are inhibited by the synthetic MMP inhibitor MMI270.276 Cyclooxygenase-2 (COX-2) expression is implicated in many different cancers,277; 278; 279 and COX-2 inhibitors celecoxib,280; 281 under the trade name Celebrex, and rofecoxib,46 under the trade name Vioxx, have been reported to inhibit lymphangiogenesis. Another pharmaceutical method for inhibiting lymphangiogenesis involves the use of siRNA gene silencing to downregulate the expression of VEGF-C, a potent stimulator of lymphangiogenesis.282
Curcurmin, a natural component of the plant turmeric, has also been reported to show anti-lymphangiogenic potential.283
7.2. Endogenous modulation
Endogenous anti-lymphangiogenic molecules play important regulatory roles in preventing excess lymphatic vessel development. During tumor development and metastasis, the balance between anti-lymphangiogenic and pro-lymphangiogenic factors is offset, with lymphangiogenesis activators overpowering inhibitors. The current literature related to endogenous anti-lymphangiogenic molecules is limited, and thus, these factors represent an avenue for further research.
sVEGFR-2 is a monomer composed of the soluble extracellular domain of membrane-bound VEGFR-2 and created through alternative splicing. sVEGFR-2 functions as an anti-lymphangiogenic factor by antagonizing VEGF-C.196 sVEGFR-2 is produced by the corneal epithelium and plays an integral role in maintaining the alymphatic nature of the cornea.196 Administration of exogenous sVEGFR-2 can inhibit cornea suture-induced and corneal transplant-induced lymphangiogenesis to increase corneal allograft survival.196 Furthermore, a reduction in sVEGFR-2 levels leads to lymphatic invasion of the cornea and skin and is implicated in the progression of neuroblastoma.196; 284 The primary advantage of administering sVEGFR-2 to inhibit lymphangiogenesis is its specificity in targeting the lymphatic system and its lack of influence on the vascular system.196
Sempahorins were initially discovered for their ability to guide axon growth cones upon binding to Nrp2 during neuronal development. Members of class 3 sempahorins are believed to inhibit lymphangiogenesis through their interactions with Nrp2.136 Moreover, class 3 sempahorins exhibit anti-tumorigenic activity in certain cancer cells by inducing apoptosis.285; 286; 287; 288
Vasohibin-1 is an anti-lymphangiogenic protein that is endogenously produced by BECs and has been reported to inhibit tumor lymphangiogenesis, lymph node metastasis, and corneal lymphangiogenesis.289
Endostatin is the C-terminal fragment of type XVIII collagen that exhibits both anti-angiogenic and anti-lymphangiogenic properties. 290 Specifically, endostatin inhibits VEGF-stimulated LEC proliferation and migration.291
TSP-1, an ECM protein, endogenously inhibits lymphangiogenesis in the cornea and other tissues by binding to CD36 on macrophages and downregulating their production of VEGF-C.180
TRAIL is a type II transmembrane protein member of the TNF family expressed in epithelial and endothelial layers of the cornea.292 Corneal expression of TRAIL has been shown to inhibit lymphangiogenesis.293; 294
CONCLUSION
Lymphangiogenesis has now been implicated in many diseases for which treatments with angiogenic inhibitors have failed, and several research groups are on the brink of uncovering pathophysiological mechanisms involving lymphangiogenesis. Recent and ongoing research continues to show us that lymphangiogenesis may be as important as angiogenesis in regulating physiological processes and in the development of certain pathologies. Thus, a thorough understanding of lymphangiogenesis in the contexts of knockout models, the cornea, and ocular diseases has the potential to support the development of therapeutics for various diseases in which lymphangiogenesis is upregulated or downregulated, such as cancer, lymphedema, and some ocular diseases.
Acknowledgments
This study was supported by grants from the National Institutes of Health [EY10101 (D.T.A.), R01EY018594 (M.I.R), EY023691, EY021886, I01 BX002386 (J.H.C), EY01792, and UL1TR000050] and an unrestricted grant from Research to Prevent Blindness, New York, NY.
Abbreviations
- HLA
human leukocyte antigen
- ACAID
anterior chamber-associated immune deviation
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- LEC
lymphatic endothelial cell
- LYVE-1
lymphatic vessel endothelial hyaluronan receptor 1
- TNF
tumor necrosis factor
- HA
hyaluronan
- Prox-1
prospero homeobox 1
- BEC
blood endothelial cell
- SLC
secondary lymphoid tissue chemokine
- HDMEC
human dermal microvascular endothelial cell
- Sox18
SRY-related HMG-box 18
- CLEC-2
C-type lectin-like receptor 2
- Ang
angiopoietin
- Foxc2
forkhead box c2
- LD
lymphatic-distichiasis syndrome
- Nrp2
neuropilin 2
- Syk
spleen tyrosine kinase
- BMP
bone morphogenetic protein
- DED
dry eye disease
- IL
interleukin
- EGCG
epigallocatechin gallate
- TSP-1
thrombospondin-1
- IFNγ
interferonγ
- RvD1a
resolving D1 analogue
- HSK
herpetic stromal keratitis
- HSV-1
herpes simplex virus-1
- IOP
intraocular pressure
- SCC
squamous cell carcinoma
- AMD
age-related macular degeneration
- IRS-1
insulin receptor substrate-1
Footnotes
CONFLICT OF INTEREST
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES***
- 1.Collin HB. Lymphatic drainage of 131-I-albumin from the vascularized cornea. Invest Ophthalmol. 1970;9:146–155. [PubMed] [Google Scholar]
- 2.He Y, Kozaki K, Karpanen T, et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- 3.Skobe M, Hawighorst T, Jackson DG, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–815. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 5.Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45:2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 6.Bachmann BO, Bock F, Wiegand SJ, et al. Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation. Arch Ophthalmol. 2008;126:71–77. doi: 10.1001/archopht.126.1.71. [DOI] [PubMed] [Google Scholar]
- 7.Bachmann BO, Luetjen-Drecoll E, Bock F, et al. Transient postoperative vascular endothelial growth factor (VEGF)-neutralisation improves graft survival in corneas with partly regressed inflammatory neovascularisation. Br J Ophthalmol. 2009;93:1075–1080. doi: 10.1136/bjo.2008.145128. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich T, Bock F, Yuen D, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184:535–539. doi: 10.4049/jimmunol.0903180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cursiefen C. Immune privilege and angiogenic privilege of the cornea. Chem Immunol Allergy. 2007;92:50–57. doi: 10.1159/000099253. [DOI] [PubMed] [Google Scholar]
- 11.Cursiefen C, Chen L, Saint-Geniez M, et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc Natl Acad Sci U S A. 2006;103:11405–11410. doi: 10.1073/pnas.0506112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein-Streilein J, Streilein JW. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. Int Rev Immunol. 2002;21:123–152. doi: 10.1080/08830180212066. [DOI] [PubMed] [Google Scholar]
- 13.Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Invest Ophthalmol Vis Sci. 1996;37:2485–2494. [PubMed] [Google Scholar]
- 14.Qazi Y, Wong G, Monson B, et al. Corneal transparency: genesis, maintenance and dysfunction. Brain Res Bull. 2010;81:198–210. doi: 10.1016/j.brainresbull.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cursiefen C, Schlotzer-Schrehardt U, Kuchle M, et al. Lymphatic vessels in vascularized human corneas: immunohistochemical investigation using LYVE-1 and podoplanin. Invest Ophthalmol Vis Sci. 2002;43:2127–2135. [PubMed] [Google Scholar]
- 16.Cao Y, Linden P, Shima D, et al. In vivo angiogenic activity and hypoxia induction of heterodimers of placenta growth factor/vascular endothelial growth factor. J Clin Invest. 1996;98:2507–2511. doi: 10.1172/JCI119069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 18.Shima DT, Adamis AP, Ferrara N, et al. Hypoxic induction of endothelial cell growth factors in retinal cells: identification and characterization of vascular endothelial growth factor (VEGF) as the mitogen. Mol Med. 1995;1:182–193. [PMC free article] [PubMed] [Google Scholar]
- 19.Chauhan SK, Dohlman TH, Dana R. Corneal Lymphatics: Role in Ocular Inflammation as Inducer and Responder of Adaptive Immunity. J Clin Cell Immunol. 2014;5 doi: 10.4172/2155-9899.1000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hos D, Schlereth SL, Bock F, et al. Antilymphangiogenic therapy to promote transplant survival and to reduce cancer metastasis: what can we learn from the eye? Semin Cell Dev Biol. 2015;38:117–130. doi: 10.1016/j.semcdb.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Bock F, Maruyama K, Regenfuss B, et al. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog Retin Eye Res. 2013;34:89–124. doi: 10.1016/j.preteyeres.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Tshionyi M, Shay E, Lunde E, et al. Hemangiogenesis and lymphangiogenesis in corneal pathology. Cornea. 2012;31:74–80. doi: 10.1097/ICO.0b013e31821dd986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22:273–281. doi: 10.1097/00003226-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Cursiefen C, Maruyama K, Jackson DG, et al. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006;25:443–447. doi: 10.1097/01.ico.0000183485.85636.ff. [DOI] [PubMed] [Google Scholar]
- 25.Mäkinen T, Veikkola T, Mustjoki S, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. 2001 doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han KY, Chang JH, Dugas-Ford J, et al. Involvement of lysosomal degradation in VEGF-C-induced down-regulation of VEGFR-3. FEBS Lett. 2014;588:4357–4363. doi: 10.1016/j.febslet.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoppmann SF, Birner P, Stöckl J, et al. Tumor-Associated Macrophages Express Lymphatic Endothelial Growth Factors and Are Related to Peritumoral Lymphangiogenesis. The American Journal of Pathology. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maruyama K, Ii M, Cursiefen C, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirakawa S, Hong YK, Harvey N, et al. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162:575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kriehuber E, Breiteneder-Geleff S, Groeger M, et al. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J Exp Med. 2001;194:797–808. doi: 10.1084/jem.194.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prevo R, Banerji S, Ferguson DJ, et al. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem. 2001;276:19420–19430. doi: 10.1074/jbc.M011004200. [DOI] [PubMed] [Google Scholar]
- 33.Jackson DG, Prevo R, Clasper S, Banerji S. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001;22:317–321. doi: 10.1016/s1471-4906(01)01936-6. [DOI] [PubMed] [Google Scholar]
- 34.Sleeman JP, Krishnan J, Kirkin V, Baumann P. Markers for the lymphatic endothelium: in search of the holy grail? Microsc Res Tech. 2001;55:61–69. doi: 10.1002/jemt.1157. [DOI] [PubMed] [Google Scholar]
- 35.Platonova N, Miquel G, Regenfuss B, et al. Evidence for the interaction of fibroblast growth factor-2 with the lymphatic endothelial cell marker LYVE-1. Blood. 2013;121:1229–1237. doi: 10.1182/blood-2012-08-450502. [DOI] [PubMed] [Google Scholar]
- 36.Jackson DG. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS. 2004;112:526–538. doi: 10.1111/j.1600-0463.2004.apm11207-0811.x. [DOI] [PubMed] [Google Scholar]
- 37.Mouta Carreira C, Nasser SM, di Tomaso E, et al. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61:8079–8084. [PubMed] [Google Scholar]
- 38.Nakao S, Zandi S, Faez S, et al. Discontinuous LYVE-1 expression in corneal limbal lymphatics: dual function as microvalves and immunological hot spots. FASEB J. 2012;26:808–817. doi: 10.1096/fj.11-183897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grant AJ, Goddard S, Ahmed-Choudhury J, et al. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am J Pathol. 2002;160:1445–1455. doi: 10.1016/S0002-9440(10)62570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enholm B, Karpanen T, Jeltsch M, et al. Adenoviral expression of vascular endothelial growth factor-C induces lymphangiogenesis in the skin. Circ Res. 2001;88:623–629. doi: 10.1161/01.res.88.6.623. [DOI] [PubMed] [Google Scholar]
- 41.McElroy M, Hayashi K, Garmy-Susini B, et al. Fluorescent LYVE-1 antibody to image dynamically lymphatic trafficking of cancer cells in vivo. J Surg Res. 2009;151:68–73. doi: 10.1016/j.jss.2007.12.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karkkainen MJ, Haiko P, Sainio K, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 43.Kaipainen A, Korhonen J, Mustonen T, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kukk E, Lymboussaki A, Taira S, et al. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122:3829–3837. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- 45.Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126:2167–2177. doi: 10.1038/sj.jid.5700464. [DOI] [PubMed] [Google Scholar]
- 46.Partanen TA, Arola J, Saaristo A, et al. VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J. 2000;14:2087–2096. doi: 10.1096/fj.99-1049com. [DOI] [PubMed] [Google Scholar]
- 47.Tammela T, Zarkada G, Nurmi H, et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol. 2011;13:1202–1213. doi: 10.1038/ncb2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kubo H, Fujiwara T, Jussila L, et al. Involvement of vascular endothelial growth factor receptor-3 in maintenance of integrity of endothelial cell lining during tumor angiogenesis. Blood. 2000;96:546–553. [PubMed] [Google Scholar]
- 49.Paavonen K, Puolakkainen P, Jussila L, et al. Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am J Pathol. 2000;156:1499–1504. doi: 10.1016/S0002-9440(10)65021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Partanen TA, Alitalo K, Miettinen M. Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor 3 in 185 vascular tumors. Cancer. 1999;86:2406–2412. [PubMed] [Google Scholar]
- 51.Valtola R, Salven P, Heikkila P, et al. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am J Pathol. 1999;154:1381–1390. doi: 10.1016/S0002-9440(10)65392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lymboussaki A, Partanen TA, Olofsson B, et al. Expression of the vascular endothelial growth factor C receptor VEGFR-3 in lymphatic endothelium of the skin and in vascular tumors. Am J Pathol. 1998;153:395–403. doi: 10.1016/S0002-9440(10)65583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jussila L, Valtola R, Partanen TA, et al. Lymphatic endothelium and Kaposi’s sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res. 1998;58:1599–1604. [PubMed] [Google Scholar]
- 54.Longatto Filho A, Martins A, Costa SM, Schmitt FC. VEGFR-3 expression in breast cancer tissue is not restricted to lymphatic vessels. Pathol Res Pract. 2005;201:93–99. doi: 10.1016/j.prp.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 55.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 56.Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16:773–783. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez-Niedenfuhr M, Papoutsi M, Christ B, et al. Prox1 is a marker of ectodermal placodes, endodermal compartments, lymphatic endothelium and lymphangioblasts. Anat Embryol (Berl) 2001;204:399–406. doi: 10.1007/s00429-001-0214-9. [DOI] [PubMed] [Google Scholar]
- 58.Oliver G, Sosa-Pineda B, Geisendorf S, et al. Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech Dev. 1993;44:3–16. doi: 10.1016/0925-4773(93)90012-m. [DOI] [PubMed] [Google Scholar]
- 59.Wilting J, Papoutsi M, Christ B, et al. The transcription factor Prox1 is a marker for lymphatic endothelial cells in normal and diseased human tissues. FASEB J. 2002;16:1271–1273. doi: 10.1096/fj.01-1010fje. [DOI] [PubMed] [Google Scholar]
- 60.Simmons DL, Walker C, Power C, Pigott R. Molecular cloning of CD31, a putative intercellular adhesion molecule closely related to carcinoembryonic antigen. J Exp Med. 1990;171:2147–2152. doi: 10.1084/jem.171.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wetterwald A, Hoffstetter W, Cecchini MG, et al. Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone. 1996;18:125–132. doi: 10.1016/8756-3282(95)00457-2. [DOI] [PubMed] [Google Scholar]
- 62.Breiteneder-Geleff S, Matsui K, Soleiman A, et al. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol. 1997;151:1141–1152. [PMC free article] [PubMed] [Google Scholar]
- 63.Nose K, Saito H, Kuroki T. Isolation of a gene sequence induced later by tumor-promoting 12-O-tetradecanoylphorbol-13-acetate in mouse osteoblastic cells (MC3T3-E1) and expressed constitutively in ras-transformed cells. Cell Growth Differ. 1990;1:511–518. [PubMed] [Google Scholar]
- 64.Marks A, Sutherland DR, Bailey D, et al. Characterization and distribution of an oncofetal antigen (M2A antigen) expressed on testicular germ cell tumours. Br J Cancer. 1999;80:569–578. doi: 10.1038/sj.bjc.6690393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rishi AK, Joyce-Brady M, Fisher J, et al. Cloning, characterization, and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev Biol. 1995;167:294–306. doi: 10.1006/dbio.1995.1024. [DOI] [PubMed] [Google Scholar]
- 66.Kato Y, Fujita N, Kunita A, et al. Molecular identification of Aggrus/T1alpha as a platelet aggregation-inducing factor expressed in colorectal tumors. J Biol Chem. 2003;278:51599–51605. doi: 10.1074/jbc.M309935200. [DOI] [PubMed] [Google Scholar]
- 67.Zimmer G, Oeffner F, Von Messling V, et al. Cloning and characterization of gp36, a human mucin-type glycoprotein preferentially expressed in vascular endothelium. Biochem J. 1999;341(Pt 2):277–284. [PMC free article] [PubMed] [Google Scholar]
- 68.Schacht V, Dadras SS, Johnson LA, et al. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913–921. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Breiteneder-Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schroedl F, Kaser-Eichberger A, Schlereth SL, et al. Consensus statement on the immunohistochemical detection of ocular lymphatic vessels. Invest Ophthalmol Vis Sci. 2014;55:6440–6442. doi: 10.1167/iovs.14-15638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2–40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434–440. doi: 10.1038/modpathol.3880543. [DOI] [PubMed] [Google Scholar]
- 72.Weninger W, Partanen TA, Breiteneder-Geleff S, et al. Expression of vascular endothelial growth factor receptor-3 and podoplanin suggests a lymphatic endothelial cell origin of Kaposi’s sarcoma tumor cells. Lab Invest. 1999;79:243–251. [PubMed] [Google Scholar]
- 73.Ordonez NG. D2–40 and podoplanin are highly specific and sensitive immunohistochemical markers of epithelioid malignant mesothelioma. Hum Pathol. 2005;36:372–380. doi: 10.1016/j.humpath.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 74.Kyzas PA, Geleff S, Batistatou A, et al. Evidence for lymphangiogenesis and its prognostic implications in head and neck squamous cell carcinoma. J Pathol. 2005;206:170–177. doi: 10.1002/path.1776. [DOI] [PubMed] [Google Scholar]
- 75.Chu AY, Litzky LA, Pasha TL, et al. Utility of D2–40, a novel mesothelial marker, in the diagnosis of malignant mesothelioma. Mod Pathol. 2005;18:105–110. doi: 10.1038/modpathol.3800259. [DOI] [PubMed] [Google Scholar]
- 76.Kimura N, Kimura I. Podoplanin as a marker for mesothelioma. Pathol Int. 2005;55:83–86. doi: 10.1111/j.1440-1827.2005.01791.x. [DOI] [PubMed] [Google Scholar]
- 77.Kato Y, Sasagawa I, Kaneko M, et al. Aggrus: a diagnostic marker that distinguishes seminoma from embryonal carcinoma in testicular germ cell tumors. Oncogene. 2004;23:8552–8556. doi: 10.1038/sj.onc.1207869. [DOI] [PubMed] [Google Scholar]
- 78.Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- 79.Ebata N, Nodasaka Y, Sawa Y, et al. Desmoplakin as a specific marker of lymphatic vessels. Microvasc Res. 2001;61:40–48. doi: 10.1006/mvre.2000.2280. [DOI] [PubMed] [Google Scholar]
- 80.Gunn MD, Tangemann K, Tam C, et al. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wigle JT, Harvey N, Detmar M, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hong YK, Harvey N, Noh YH, et al. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- 83.Petrova TV, Makinen T, Makela TP, et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Francois M, Caprini A, Hosking B, et al. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 85.Irrthum A, Devriendt K, Chitayat D, et al. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am J Hum Genet. 2003;72:1470–1478. doi: 10.1086/375614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schoppmann SF, Birner P, Stockl J, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haiko P, Makinen T, Keskitalo S, et al. Deletion of vascular endothelial growth factor C (VEGF-C) and VEGF-D is not equivalent to VEGF receptor 3 deletion in mouse embryos. Mol Cell Biol. 2008;28:4843–4850. doi: 10.1128/MCB.02214-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuchler AM, Gjini E, Peterson-Maduro J, et al. Development of the zebrafish lymphatic system requires VEGFC signaling. Curr Biol. 2006;16:1244–1248. doi: 10.1016/j.cub.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 89.Ny A, Koch M, Schneider M, et al. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat Med. 2005;11:998–1004. doi: 10.1038/nm1285. [DOI] [PubMed] [Google Scholar]
- 90.Makinen T, Veikkola T, Mustjoki S, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Veikkola T, Jussila L, Makinen T, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20:1223–1231. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jeltsch M, Kaipainen A, Joukov V, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 93.Karkkainen MJ, Ferrell RE, Lawrence EC, et al. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat Genet. 2000;25:153–159. doi: 10.1038/75997. [DOI] [PubMed] [Google Scholar]
- 94.Karkkainen MJ, Saaristo A, Jussila L, et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci U S A. 2001;98:12677–12682. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lyon MF, Glenister PH, Loutit JF, et al. A presumed deletion covering the W and Ph loci of the mouse. Genet Res. 1984;44:161–168. doi: 10.1017/s0016672300026367. [DOI] [PubMed] [Google Scholar]
- 96.Matsui K, Breitender-Geleff S, Soleiman A, et al. Podoplanin, a novel 43-kDa membrane protein, controls the shape of podocytes. Nephrol Dial Transplant. 1999;14(Suppl 1):9–11. doi: 10.1093/ndt/14.suppl_1.9. [DOI] [PubMed] [Google Scholar]
- 97.Dobbs LG, Williams MC, Gonzalez R. Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim Biophys Acta. 1988;970:146–156. doi: 10.1016/0167-4889(88)90173-5. [DOI] [PubMed] [Google Scholar]
- 98.Schacht V, Ramirez MI, Hong YK, et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramirez MI, Millien G, Hinds A, et al. T1alpha, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev Biol. 2003;256:61–72. doi: 10.1016/s0012-1606(02)00098-2. [DOI] [PubMed] [Google Scholar]
- 100.Bertozzi CC, Schmaier AA, Mericko P, et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116:661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Osada M, Inoue O, Ding G, et al. Platelet activation receptor CLEC-2 regulates blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of lymphatic endothelial cells. J Biol Chem. 2012;287:22241–22252. doi: 10.1074/jbc.M111.329987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goede V, Schmidt T, Kimmina S, et al. Analysis of blood vessel maturation processes during cyclic ovarian angiogenesis. Lab Invest. 1998;78:1385–1394. [PubMed] [Google Scholar]
- 103.Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 104.Zagzag D, Hooper A, Friedlander DR, et al. In situ expression of angiopoietins in astrocytomas identifies angiopoietin-2 as an early marker of tumor angiogenesis. Exp Neurol. 1999;159:391–400. doi: 10.1006/exnr.1999.7162. [DOI] [PubMed] [Google Scholar]
- 105.Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 106.Mochizuki Y, Nakamura T, Kanetake H, Kanda S. Angiopoietin 2 stimulates migration and tube-like structure formation of murine brain capillary endothelial cells through c-Fes and c-Fyn. J Cell Sci. 2002;115:175–183. doi: 10.1242/jcs.115.1.175. [DOI] [PubMed] [Google Scholar]
- 107.Teichert-Kuliszewska K, Maisonpierre PC, Jones N, et al. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc Res. 2001;49:659–670. doi: 10.1016/s0008-6363(00)00231-5. [DOI] [PubMed] [Google Scholar]
- 108.Dellinger M, Hunter R, Bernas M, et al. Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin-2 deficient mice. Dev Biol. 2008;319:309–320. doi: 10.1016/j.ydbio.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sabin FR. On the development of the superficial lymphatics in the skin of the pig. American Journal of Anatomy. 1904;3:183–195. [Google Scholar]
- 110.Gale NW, Thurston G, Hackett SF, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 111.Makinen T, Adams RH, Bailey J, et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pitulescu ME, Adams RH. Eph/ephrin molecules--a hub for signaling and endocytosis. Genes Dev. 2010;24:2480–2492. doi: 10.1101/gad.1973910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kuijper S, Turner CJ, Adams RH. Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc Med. 2007;17:145–151. doi: 10.1016/j.tcm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 114.Wang Y, Nakayama M, Pitulescu ME, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 115.Sawamiphak S, Seidel S, Essmann CL, et al. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465:487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 116.Bazigou E, Makinen T. Flow control in our vessels: vascular valves make sure there is no way back. Cell Mol Life Sci. 2013;70:1055–1066. doi: 10.1007/s00018-012-1110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 118.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 119.Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol. 2011;193:607–618. doi: 10.1083/jcb.201012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bazigou E, Lyons OT, Smith A, et al. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J Clin Invest. 2011;121:2984–2992. doi: 10.1172/JCI58050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Katsuta H, Fukushima Y, Maruyama K, et al. EphrinB2-EphB4 signals regulate formation and maintenance of funnel-shaped valves in corneal lymphatic capillaries. Invest Ophthalmol Vis Sci. 2013;54:4102–4108. doi: 10.1167/iovs.12-11436. [DOI] [PubMed] [Google Scholar]
- 122.Zhang G, Brady J, Liang WC, et al. EphB4 forward signalling regulates lymphatic valve development. Nat Commun. 2015;6:6625. doi: 10.1038/ncomms7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- 124.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- 125.Dagenais SL, Hartsough RL, Erickson RP, et al. Foxc2 is expressed in developing lymphatic vessels and other tissues associated with lymphedema-distichiasis syndrome. Gene Expr Patterns. 2004;4:611–619. doi: 10.1016/j.modgep.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 126.Petrova TV, Karpanen T, Norrmen C, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 127.Kriederman BM, Myloyde TL, Witte MH, et al. FOXC2 haploinsufficient mice are a model for human autosomal dominant lymphedema-distichiasis syndrome. Hum Mol Genet. 2003;12:1179–1185. doi: 10.1093/hmg/ddg123. [DOI] [PubMed] [Google Scholar]
- 128.Fang J, Dagenais SL, Erickson RP, et al. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am J Hum Genet. 2000;67:1382–1388. doi: 10.1086/316915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Erickson RP, Dagenais SL, Caulder MS, et al. Clinical heterogeneity in lymphoedema-distichiasis with FOXC2 truncating mutations. J Med Genet. 2001;38:761–766. doi: 10.1136/jmg.38.11.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Iida K, Koseki H, Kakinuma H, et al. Essential roles of the winged helix transcription factor MFH-1 in aortic arch patterning and skeletogenesis. Development. 1997;124:4627–4638. doi: 10.1242/dev.124.22.4627. [DOI] [PubMed] [Google Scholar]
- 131.Winnier GE, Hargett L, Hogan BL. The winged helix transcription factor MFH1 is required for proliferation and patterning of paraxial mesoderm in the mouse embryo. Genes Dev. 1997;11:926–940. doi: 10.1101/gad.11.7.926. [DOI] [PubMed] [Google Scholar]
- 132.Norrmen C, Ivanov KI, Cheng J, et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J Cell Biol. 2009;185:439–457. doi: 10.1083/jcb.200901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen H, Chedotal A, He Z, et al. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–559. doi: 10.1016/s0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 134.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 135.Kolodkin AL, Levengood DV, Rowe EG, et al. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 136.Yuan L, Moyon D, Pardanaud L, et al. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- 137.Caunt M, Mak J, Liang WC, et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell. 2008;13:331–342. doi: 10.1016/j.ccr.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 138.Xu Y, Yuan L, Mak J, et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J Cell Biol. 2010;188:115–130. doi: 10.1083/jcb.200903137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Favier B, Alam A, Barron P, et al. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108:1243–1250. doi: 10.1182/blood-2005-11-4447. [DOI] [PubMed] [Google Scholar]
- 140.Karpanen T, Heckman CA, Keskitalo S, et al. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J. 2006;20:1462–1472. doi: 10.1096/fj.05-5646com. [DOI] [PubMed] [Google Scholar]
- 141.Rafii S, Skobe M. Splitting vessels: keeping lymph apart from blood. Nat Med. 2003;9:166–168. doi: 10.1038/nm0203-166. [DOI] [PubMed] [Google Scholar]
- 142.Abtahian F, Guerriero A, Sebzda E, et al. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299:247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sebzda E, Hibbard C, Sweeney S, et al. Syk and Slp-76 mutant mice reveal a cell-autonomous hematopoietic cell contribution to vascular development. Dev Cell. 2006;11:349–361. doi: 10.1016/j.devcel.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 144.Suzuki-Inoue K, Kato Y, Inoue O, et al. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993–26001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 145.Fuller GL, Williams JA, Tomlinson MG, et al. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem. 2007;282:12397–12409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suzuki-Inoue K, Fuller GL, Garcia A, et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 147.Ayadi A, Zheng H, Sobieszczuk P, et al. Net-targeted mutant mice develop a vascular phenotype and up-regulate egr-1. EMBO J. 2001;20:5139–5152. doi: 10.1093/emboj/20.18.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dumont DJ, Jussila L, Taipale J, et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 149.Huang XZ, Wu JF, Ferrando R, et al. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J Biol Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Van Buskirk EM. The anatomy of the limbus. Eye (Lond) 1989;3(Pt 2):101–108. doi: 10.1038/eye.1989.16. [DOI] [PubMed] [Google Scholar]
- 152.Ecoiffier T, Yuen D, Chen L. Differential distribution of blood and lymphatic vessels in the murine cornea. Invest Ophthalmol Vis Sci. 2010;51:2436–2440. doi: 10.1167/iovs.09-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nakao S, Maruyama K, Zandi S, et al. Lymphangiogenesis and angiogenesis: concurrence and/or dependence? Studies in inbred mouse strains. FASEB J. 2010;24:504–513. doi: 10.1096/fj.09-134056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hos D, Bachmann B, Bock F, et al. Age-related changes in murine limbal lymphatic vessels and corneal lymphangiogenesis. Exp Eye Res. 2008;87:427–432. doi: 10.1016/j.exer.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 155.Ma DH, Chen JK, Zhang F, et al. Regulation of corneal angiogenesis in limbal stem cell deficiency. Prog Retin Eye Res. 2006;25:563–590. doi: 10.1016/j.preteyeres.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 156.Espana EM, Grueterich M, Romano AC, et al. Idiopathic limbal stem cell deficiency. Ophthalmology. 2002;109:2004–2010. doi: 10.1016/s0161-6420(02)01250-2. [DOI] [PubMed] [Google Scholar]
- 157.Espana EM, Ti SE, Grueterich M, et al. Corneal stromal changes following reconstruction by ex vivo expanded limbal epithelial cells in rabbits with total limbal stem cell deficiency. The British Journal of Ophthalmology. 2003;87:1509–1514. doi: 10.1136/bjo.87.12.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Grueterich M, Espana EM, Tseng SC. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003;48:631–646. doi: 10.1016/j.survophthal.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 159.Collin HB. Endothelial cell lined lymphatics in the vascularized rabbit cornea. Investigative Ophthalmology & Visual Science. 1966;5:337–354. [PubMed] [Google Scholar]
- 160.Kopfstein L, Veikkola T, Djonov VG, et al. Distinct roles of vascular endothelial growth factor-D in lymphangiogenesis and metastasis. Am J Pathol. 2007;170:1348–1361. doi: 10.2353/ajpath.2007.060835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Krishnan J, Kirkin V, Steffen A, et al. Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res. 2003;63:713–722. [PubMed] [Google Scholar]
- 162.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 163.Karpanen T, Egeblad M, Karkkainen MJ, et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–1790. [PubMed] [Google Scholar]
- 164.Mandriota SJ, Jussila L, Jeltsch M, et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stacker SA, Caesar C, Baldwin ME, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 166.Veikkola T, Jussila L, Makinen T, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. 2001 doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ellenberg D, Azar DT, Hallak JA, et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010;29:208–248. doi: 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 169.Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clinical Ophthalmology (Auckland, NZ) 2009;3:405–412. doi: 10.2147/opth.s5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Perry HD, Solomon R, Donnenfeld ED, et al. EValuation of topical cyclosporine for the treatment of dry eye disease. Archives of Ophthalmology. 2008;126:1046–1050. doi: 10.1001/archopht.126.8.1046. [DOI] [PubMed] [Google Scholar]
- 171.Annan JE, Chauhan SK, Ecoiffier T, et al. Characterization of Effector T cells in Dry Eye Disease. Investigative ophthalmology & visual science. 2009;50:3802–3807. doi: 10.1167/iovs.08-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Goyal S, Chauhan SK, El Annan J, et al. Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity? Arch Ophthalmol. 2010;128:819–824. doi: 10.1001/archophthalmol.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Numasaki M, Fukushi J, Ono M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 174.Carmi Y, Voronov E, Dotan S, et al. The role of macrophage-derived IL-1 in induction and maintenance of angiogenesis. J Immunol. 2009;183:4705–4714. doi: 10.4049/jimmunol.0901511. [DOI] [PubMed] [Google Scholar]
- 175.Watari K, Nakao S, Fotovati A, et al. Role of macrophages in inflammatory lymphangiogenesis: Enhanced production of vascular endothelial growth factor C and D through NF-κB activation. Biochemical and Biophysical Research Communications. 2008;377:826–831. doi: 10.1016/j.bbrc.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 176.Goyal S, Chauhan SK, Dana R. Blockade of prolymphangiogenic vascular endothelial growth factor C in dry eye disease. Arch Ophthalmol. 2012;130:84–89. doi: 10.1001/archophthalmol.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. Journal of Clinical Investigation. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee HS, Chauhan SK, Okanobo A, et al. Therapeutic efficacy of topical epigallocatechin gallate in murine dry eye. Cornea. 2011;30:1465–1472. doi: 10.1097/ICO.0b013e31821c9b5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schollhorn L, Bock F, Cursiefen C. Thrombospondin-1 as a Regulator of Corneal Inflammation and Lymphangiogenesis: Effects on Dry Eye Disease and Corneal Graft Immunology. J Ocul Pharmacol Ther. 2015 doi: 10.1089/jop.2015.0020. [DOI] [PubMed] [Google Scholar]
- 180.Cursiefen C, Maruyama K, Bock F, et al. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J Exp Med. 2011;208:1083–1092. doi: 10.1084/jem.20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Contreras-Ruiz L, Regenfuss B, Mir FA, et al. Conjunctival inflammation in thrombospondin-1 deficient mouse model of Sjogren’s syndrome. PLoS One. 2013;8:e75937. doi: 10.1371/journal.pone.0075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cho YK, Archer B, Ambati BK. Dry eye predisposes to corneal neovascularization and lymphangiogenesis after corneal injury in a murine model. Cornea. 2014;33:621–627. doi: 10.1097/ICO.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 183.Group CCTSR. The Collaborative Corneal Transplantation Studies (CCTS) Effectiveness of Histocompatibility Matching in High-Risk Corneal Transplantation. Archives of Ophthalmology. 1992;110:1392. [PubMed] [Google Scholar]
- 184.Niederkorn JY. The immune privilege of corneal grafts. J Leukoc Biol. 2003;74:167–171. doi: 10.1189/jlb.1102543. [DOI] [PubMed] [Google Scholar]
- 185.Niederkorn JY. High-risk corneal allografts and why they lose their immune privilege. Curr Opin Allergy Clin Immunol. 2010;10:493–497. doi: 10.1097/ACI.0b013e32833dfa11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Weisbrod DJ, Sit M, Naor J, Slomovic AR. Outcomes of repeat penetrating keratoplasty and risk factors for graft failure. Cornea. 2003;22:429–434. doi: 10.1097/00003226-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 187.Bachmann B, Taylor RS, Cursiefen C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: an evidence-based meta-analysis. Ophthalmology. 2010;117:1300–1305. e1307. doi: 10.1016/j.ophtha.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 188.Zheng Y, Lin H, Ling S. Clinicopathological correlation analysis of (lymph) angiogenesis and corneal graft rejection. Mol Vis. 2011;17:1694–1700. [PMC free article] [PubMed] [Google Scholar]
- 189.Winton HL, Bidwell JL, Armitage WJ. Haplotype analysis on chromosome 6p of tumor necrosis factor alpha, vascular endothelial growth factor A, and interleukin-17F alleles associated with corneal transplant rejection. Transplant Proc. 2014;46:1540–1547. doi: 10.1016/j.transproceed.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 190.Flynn TH, Ohbayashi M, Dawson M, et al. The effect of perioperative allergic conjunctivitis on corneal lymphangiogenesis after corneal transplantation. Br J Ophthalmol. 2011;95:1451–1456. doi: 10.1136/bjo.2010.201939. [DOI] [PubMed] [Google Scholar]
- 191.Lee HS, Hos D, Blanco T, et al. Involvement of corneal lymphangiogenesis in a mouse model of allergic eye disease. Invest Ophthalmol Vis Sci. 2015;56:3140–3148. doi: 10.1167/iovs.14-16186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim HK, Choi JA, Uehara H, et al. Presurgical corticosteroid treatment improves corneal transplant survival in mice. Cornea. 2013;32:1591–1598. doi: 10.1097/ICO.0b013e31829ebb0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hos D, Saban DR, Bock F, et al. Suppression of inflammatory corneal lymphangiogenesis by application of topical corticosteroids. Arch Ophthalmol. 2011;129:445–452. doi: 10.1001/archophthalmol.2011.42. [DOI] [PubMed] [Google Scholar]
- 194.Cho YK, Zhang X, Uehara H, et al. Vascular Endothelial Growth Factor Receptor 1 morpholino increases graft survival in a murine penetrating keratoplasty model. Invest Ophthalmol Vis Sci. 2012;53:8458–8471. doi: 10.1167/iovs.12-10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cho YK, Uehara H, Young JR, et al. Flt23k nanoparticles offer additive benefit in graft survival and anti-angiogenic effects when combined with triamcinolone. Invest Ophthalmol Vis Sci. 2012;53:2328–2336. doi: 10.1167/iovs.11-8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Albuquerque RJ, Hayashi T, Cho WG, et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med. 2009;15:1023–1030. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Emami-Naeini P, Dohlman TH, Omoto M, et al. Soluble vascular endothelial growth factor receptor-3 suppresses allosensitization and promotes corneal allograft survival. Graefes Arch Clin Exp Ophthalmol. 2014;252:1755–1762. doi: 10.1007/s00417-014-2749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang H, Grimaldo S, Yuen D, Chen L. Combined blockade of VEGFR-3 and VLA-1 markedly promotes high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2011;52:6529–6535. doi: 10.1167/iovs.11-7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Maruyama Y, Maruyama K, Kato Y, et al. The effect of podoplanin inhibition on lymphangiogenesis under pathological conditions. Invest Ophthalmol Vis Sci. 2014;55:4813–4822. doi: 10.1167/iovs.13-13711. [DOI] [PubMed] [Google Scholar]
- 200.Bucher F, Bi Y, Gehlsen U, et al. Regression of mature lymphatic vessels in the cornea by photodynamic therapy. Br J Ophthalmol. 2014;98:391–395. doi: 10.1136/bjophthalmol-2013-303887. [DOI] [PubMed] [Google Scholar]
- 201.Hua J, Jin Y, Chen Y, et al. The resolvin D1 analogue controls maturation of dendritic cells and suppresses alloimmunity in corneal transplantation. Invest Ophthalmol Vis Sci. 2014;55:5944–5951. doi: 10.1167/iovs.14-14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dohlman TH, Omoto M, Hua J, et al. VEGF-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation. 2015;99:678–686. doi: 10.1097/TP.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 203.Tang XL, Sun JF, Wang XY, et al. Blocking neuropilin-2 enhances corneal allograft survival by selectively inhibiting lymphangiogenesis on vascularized beds. Mol Vis. 2010;16:2354–2361. [PMC free article] [PubMed] [Google Scholar]
- 204.Hos D, Regenfuss B, Bock F, et al. Blockade of insulin receptor substrate-1 inhibits corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2011;52:5778–5785. doi: 10.1167/iovs.10-6816. [DOI] [PubMed] [Google Scholar]
- 205.Cursiefen C, Bock F, Horn FK, et al. GS-101 antisense oligonucleotide eye drops inhibit corneal neovascularization: interim results of a randomized phase II trial. Ophthalmology. 2009;116:1630–1637. doi: 10.1016/j.ophtha.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 206.Cursiefen C, Viaud E, Bock F, et al. Aganirsen antisense oligonucleotide eye drops inhibit keratitis-induced corneal neovascularization and reduce need for transplantation: the I-CAN study. Ophthalmology. 2014;121:1683–1692. doi: 10.1016/j.ophtha.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 207.Liesegang TJ, Melton LJ, 3rd, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1155–1159. doi: 10.1001/archopht.1989.01070020221029. [DOI] [PubMed] [Google Scholar]
- 208.Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186(Suppl 1):S3–28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 209.Wuest TR, Carr DJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med. 2010;207:101–115. doi: 10.1084/jem.20091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nagy JA, Vasile E, Feng D, et al. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002;196:1497–1506. doi: 10.1084/jem.20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kajiya K, Hirakawa S, Detmar M. Vascular endothelial growth factor-A mediates ultraviolet B-induced impairment of lymphatic vessel function. Am J Pathol. 2006;169:1496–1503. doi: 10.2353/ajpath.2006.060197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hong YK, Shin JW, Detmar M. Development of the lymphatic vascular system: a mystery unravels. Dev Dyn. 2004;231:462–473. doi: 10.1002/dvdy.20179. [DOI] [PubMed] [Google Scholar]
- 213.Duan R, Remeijer L, van Dun JM, et al. Granulocyte macrophage colony-stimulating factor expression in human herpetic stromal keratitis: implications for the role of neutrophils in HSK. Invest Ophthalmol Vis Sci. 2007;48:277–284. doi: 10.1167/iovs.06-0053. [DOI] [PubMed] [Google Scholar]
- 214.Yan XT, Tumpey TM, Kunkel SL, et al. Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Invest Ophthalmol Vis Sci. 1998;39:1854–1862. [PubMed] [Google Scholar]
- 215.Thomas J, Rouse BT. Immunopathogenesis of herpetic ocular disease. Immunol Res. 1997;16:375–386. doi: 10.1007/BF02786400. [DOI] [PubMed] [Google Scholar]
- 216.Newell CK, Martin S, Sendele D, et al. Herpes simplex virus-induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J Virol. 1989;63:769–775. doi: 10.1128/jvi.63.2.769-775.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yamagami S, Dana MR. The critical role of lymph nodes in corneal alloimmunization and graft rejection. Invest Ophthalmol Vis Sci. 2001;42:1293–1298. [PubMed] [Google Scholar]
- 218.Hosseini H, Khalili MR. Therapeutic potential of bevacizumab (Avastin) in herpetic stromal keratitis (HSK) Med Hypotheses. 2007;69:568–570. doi: 10.1016/j.mehy.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 219.Stevenson W, Cheng SF, Dastjerdi MH, et al. Corneal neovascularization and the utility of topical VEGF inhibition: ranibizumab (Lucentis) vs bevacizumab (Avastin) Ocul Surf. 2012;10:67–83. doi: 10.1016/j.jtos.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Saravia M, Zapata G, Ferraiolo P, et al. Anti-VEGF monoclonal antibody-induced regression of corneal neovascularization and inflammation in a rabbit model of herpetic stromal keratitis. Graefes Arch Clin Exp Ophthalmol. 2009;247:1409–1416. doi: 10.1007/s00417-009-1101-y. [DOI] [PubMed] [Google Scholar]
- 221.Bock F, Onderka J, Dietrich T, et al. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2545–2552. doi: 10.1167/iovs.06-0570. [DOI] [PubMed] [Google Scholar]
- 222.Chang JH, Garg NK, Lunde E, et al. Corneal neovascularization: an anti-VEGF therapy review. Surv Ophthalmol. 2012;57:415–429. doi: 10.1016/j.survophthal.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24:612–637. doi: 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 224.Weinreb RN. Uveoscleral outflow: the other outflow pathway. J Glaucoma. 2000;9:343–345. doi: 10.1097/00061198-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 225.Toris CB, Pederson JE. Aqueous humor dynamics in experimental iridocyclitis. Invest Ophthalmol Vis Sci. 1987;28:477–481. [PubMed] [Google Scholar]
- 226.Heindl LM, Schrodl F, Lutjen-Drecoll E, Cursiefen C. Ciliary body lymphangiogenesis. Ophthalmology. 2013;120:e41–42. doi: 10.1016/j.ophtha.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 227.Heindl LM, Hofmann TN, Adler W, et al. Intraocular tumor-associated lymphangiogenesis a novel prognostic factor for ciliary body melanomas with extraocular extension? Ophthalmology. 2010;117:334–342. doi: 10.1016/j.ophtha.2009.06.057. [DOI] [PubMed] [Google Scholar]
- 228.Schroedl F, Brehmer A, Neuhuber WL, et al. The normal human choroid is endowed with a significant number of lymphatic vessel endothelial hyaluronate receptor 1 (LYVE-1)-positive macrophages. Invest Ophthalmol Vis Sci. 2008;49:5222–5229. doi: 10.1167/iovs.08-1721. [DOI] [PubMed] [Google Scholar]
- 229.Yucel YH, Johnston MG, Ly T, et al. Identification of lymphatics in the ciliary body of the human eye: a novel “uveolymphatic” outflow pathway. Exp Eye Res. 2009;89:810–819. doi: 10.1016/j.exer.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 230.Tam AL, Gupta N, Zhang Z, Yucel YH. Latanoprost Stimulates Ocular Lymphatic Drainage: An In Vivo Nanotracer Study. Transl Vis Sci Technol. 2013;2:3. doi: 10.1167/tvst.2.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nomura S, Hashimoto M. Pharmacological profiles of latanoprost (Xalatan), a novel anti-glaucoma drug. Nihon Yakurigaku Zasshi. 2000;115:280–286. doi: 10.1254/fpj.115.280. [DOI] [PubMed] [Google Scholar]
- 232.Hos D, Koch KR, Bock F, et al. Short- and long-term corneal vascular effects of tafluprost eye drops. Graefes Arch Clin Exp Ophthalmol. 2013;251:1919–1927. doi: 10.1007/s00417-013-2345-0. [DOI] [PubMed] [Google Scholar]
- 233.Heindl LM, Hofmann-Rummelt C, Adler W, et al. Tumor-associated lymphangiogenesis in the development of conjunctival squamous cell carcinoma. Ophthalmology. 2010;117:649–658. doi: 10.1016/j.ophtha.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 234.Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest. 2014;124:878–887. doi: 10.1172/JCI71603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zetter BR. The scientific contributions of M. Judah Folkman to cancer research. Nat Rev Cancer. 2008;8:647–654. doi: 10.1038/nrc2458. [DOI] [PubMed] [Google Scholar]
- 236.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69(Suppl 3):11–16. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 237.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 238.Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Nakao S, Zandi S, Hata Y, et al. Blood vessel endothelial VEGFR-2 delays lymphangiogenesis: an endogenous trapping mechanism links lymph- and angiogenesis. Blood. 2011;117:1081–1090. doi: 10.1182/blood-2010-02-267427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hirakawa S, Kodama S, Kunstfeld R, et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Roberts N, Kloos B, Cassella M, et al. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 2006;66:2650–2657. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- 242.Whitehurst B, Flister MJ, Bagaitkar J, et al. Anti-VEGF-A therapy reduces lymphatic vessel density and expression of VEGFR-3 in an orthotopic breast tumor model. Int J Cancer. 2007;121:2181–2191. doi: 10.1002/ijc.22937. [DOI] [PubMed] [Google Scholar]
- 243.Geerling G, MacLennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. The British Journal of Ophthalmology. 2004;88:1467–1474. doi: 10.1136/bjo.2004.044347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hajrasouliha AR, Sadrai Z, Chauhan SK, Dana R. b-FGF induces corneal blood and lymphatic vessel growth in a spatially distinct pattern. Cornea. 2012;31:804–809. doi: 10.1097/ICO.0b013e31823f8b5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hos D, Koch KR, Bucher F, et al. Serum eyedrops antagonize the anti(lymph)angiogenic effects of bevacizumab in vitro and in vivo. Invest Ophthalmol Vis Sci. 2013;54:6133–6142. doi: 10.1167/iovs.13-12460. [DOI] [PubMed] [Google Scholar]
- 246.Li H, Adachi Y, Yamamoto H, et al. Insulin-like growth factor-I receptor blockade reduces tumor angiogenesis and enhances the effects of bevacizumab for a human gastric cancer cell line, MKN45. Cancer. 2011;117:3135–3147. doi: 10.1002/cncr.25893. [DOI] [PubMed] [Google Scholar]
- 247.Del Vecchio M, Mortarini R, Canova S, et al. Bevacizumab plus fotemustine as first-line treatment in metastatic melanoma patients: clinical activity and modulation of angiogenesis and lymphangiogenesis factors. Clin Cancer Res. 2010;16:5862–5872. doi: 10.1158/1078-0432.CCR-10-2363. [DOI] [PubMed] [Google Scholar]
- 248.Tille J-C, Wang X, Lipson KE, et al. Vascular endothelial growth factor (VEGF) receptor-2 signaling mediates VEGF-CΔNΔC- and VEGF-A-induced angiogenesis in vitro. Experimental Cell Research. 2003;285:286–298. doi: 10.1016/s0014-4827(03)00053-3. [DOI] [PubMed] [Google Scholar]
- 249.Huang Y, Chen X, Dikov MM, et al. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. 2007 doi: 10.1182/blood-2007-01-065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kodera Y, Katanasaka Y, Kitamura Y, et al. Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3. Breast Cancer Research : BCR. 2011;13:R66–R66. doi: 10.1186/bcr2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Roberts N, Kloos B, Cassella M, et al. Inhibition of VEGFR-3 Activation with the Antagonistic Antibody More Potently Suppresses Lymph Node and Distant Metastases than Inactivation of VEGFR-2. Cancer Research. 2006;66:2650–2657. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- 252.Hong YK, Lange-Asschenfeldt B, Velasco P, et al. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 2004;18:1111–1113. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- 253.Burton JB, Priceman SJ, Sung JL, et al. Suppression of prostate cancer nodal and systemic metastasis by blockade of the lymphangiogenic axis. Cancer Res. 2008;68:7828–7837. doi: 10.1158/0008-5472.CAN-08-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Pytowski B, Goldman J, Persaud K, et al. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97:14–21. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- 255.Bock F, Onderka J, Dietrich T, et al. Blockade of VEGFR3-signalling specifically inhibits lymphangiogenesis in inflammatory corneal neovascularisation. Graefes Arch Clin Exp Ophthalmol. 2008;246:115–119. doi: 10.1007/s00417-007-0683-5. [DOI] [PubMed] [Google Scholar]
- 256.Kubo H, Cao R, Brakenhielm E, et al. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci U S A. 2002;99:8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Shimizu K, Kubo H, Yamaguchi K, et al. Suppression of VEGFR-3 signaling inhibits lymph node metastasis in gastric cancer. Cancer Sci. 2004;95:328–333. doi: 10.1111/j.1349-7006.2004.tb03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Konner J, Dupont J. Use of soluble recombinant decoy receptor vascular endothelial growth factor trap (VEGF Trap) to inhibit vascular endothelial growth factor activity. Clin Colorectal Cancer. 2004;4(Suppl 2):S81–85. doi: 10.3816/ccc.2004.s.013. [DOI] [PubMed] [Google Scholar]
- 259.Fukasawa M, Korc M. Vascular endothelial growth factor-trap suppresses tumorigenicity of multiple pancreatic cancer cell lines. Clin Cancer Res. 2004;10:3327–3332. doi: 10.1158/1078-0432.CCR-03-0820. [DOI] [PubMed] [Google Scholar]
- 260.Fedecostante M, Giulietti F, Spannella F, et al. PP.21.20: HYPERTENSIVE URGENCY AND PROTEINURIA WITH ACUTE DETERIORATION OF RENAL FUNCTION FOLLOWING INTRAVITREAL INJECTION OF AFLIBERCEPT. Journal of Hypertension. 2015;33:e324. [Google Scholar]
- 261.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 262.Ferreira A, Sagkriotis A, Olson M, et al. Treatment Frequency and Dosing Interval of Ranibizumab and Aflibercept for Neovascular Age-Related Macular Degeneration in Routine Clinical Practice in the USA. PLoS One. 2015;10:e0133968. doi: 10.1371/journal.pone.0133968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results From the VISTA and VIVID Studies. Ophthalmology. 2015 doi: 10.1016/j.ophtha.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 264.Bucher F, Parthasarathy A, Bergua A, et al. Topical Ranibizumab inhibits inflammatory corneal hem- and lymphangiogenesis. Acta Ophthalmol. 2014;92:143–148. doi: 10.1111/j.1755-3768.2012.02525.x. [DOI] [PubMed] [Google Scholar]
- 265.Lipp M, Bucher F, Parthasarathy A, et al. Blockade of the VEGF isoforms in inflammatory corneal hemangiogenesis and lymphangiogenesis. Graefes Arch Clin Exp Ophthalmol. 2014;252:943–949. doi: 10.1007/s00417-014-2626-2. [DOI] [PubMed] [Google Scholar]
- 266.Makinen T, Jussila L, Veikkola T, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 267.Yang H, Kim C, Kim MJ, et al. Soluble vascular endothelial growth factor receptor-3 suppresses lymphangiogenesis and lymphatic metastasis in bladder cancer. Mol Cancer. 2011;10:36. doi: 10.1186/1476-4598-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sallinen H, Anttila M, Narvainen J, et al. Antiangiogenic gene therapy with soluble VEGFR-1, -2, and -3 reduces the growth of solid human ovarian carcinoma in mice. Mol Ther. 2009;17:278–284. doi: 10.1038/mt.2008.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sopo M, Anttila M, Sallinen H, et al. Antiangiogenic gene therapy with soluble VEGF-receptors -1, -2 and -3 together with paclitaxel prolongs survival of mice with human ovarian carcinoma. Int J Cancer. 2012;131:2394–2401. doi: 10.1002/ijc.27495. [DOI] [PubMed] [Google Scholar]
- 270.Singh N, Tiem M, Watkins R, et al. Soluble vascular endothelial growth factor receptor 3 is essential for corneal alymphaticity. Blood. 2013;121:4242–4249. doi: 10.1182/blood-2012-08-453043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hwang-Bo J, Yoo KH, Park JH, et al. Recombinant canstatin inhibits angiopoietin-1-induced angiogenesis and lymphangiogenesis. Int J Cancer. 2012;131:298–309. doi: 10.1002/ijc.26353. [DOI] [PubMed] [Google Scholar]
- 272.Weckmann M, Moir LM, Heckman CA, et al. Lamstatin--a novel inhibitor of lymphangiogenesis derived from collagen IV. J Cell Mol Med. 2012;16:3062–3073. doi: 10.1111/j.1582-4934.2012.01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kinet V, Castermans K, Herkenne S, et al. The angiostatic protein 16K human prolactin significantly prevents tumor-induced lymphangiogenesis by affecting lymphatic endothelial cells. Endocrinology. 2011;152:4062–4071. doi: 10.1210/en.2011-1081. [DOI] [PubMed] [Google Scholar]
- 274.Lee E, Rosca EV, Pandey NB, Popel AS. Small peptides derived from somatotropin domain-containing proteins inhibit blood and lymphatic endothelial cell proliferation, migration, adhesion and tube formation. Int J Biochem Cell Biol. 2011;43:1812–1821. doi: 10.1016/j.biocel.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wong TS, Kwong DL, Sham JS, et al. Clinicopathologic significance of plasma matrix metalloproteinase-2 and -9 levels in patients with undifferentiated nasopharyngeal carcinoma. Eur J Surg Oncol. 2004;30:560–564. doi: 10.1016/j.ejso.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 276.Nakamura ES, Koizumi K, Kobayashi M, Saiki I. Inhibition of lymphangiogenesis-related properties of murine lymphatic endothelial cells and lymph node metastasis of lung cancer by the matrix metalloproteinase inhibitor MMI270. Cancer Sci. 2004;95:25–31. doi: 10.1111/j.1349-7006.2004.tb03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Timoshenko AV, Chakraborty C, Wagner GF, Lala PK. COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer. Br J Cancer. 2006;94:1154–1163. doi: 10.1038/sj.bjc.6603067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kyzas PA, Stefanou D, Agnantis NJ. COX-2 expression correlates with VEGF-C and lymph node metastases in patients with head and neck squamous cell carcinoma. Mod Pathol. 2005;18:153–160. doi: 10.1038/modpathol.3800244. [DOI] [PubMed] [Google Scholar]
- 279.Soumaoro LT, Uetake H, Takagi Y, et al. Coexpression of VEGF-C and Cox-2 in human colorectal cancer and its association with lymph node metastasis. Dis Colon Rectum. 2006;49:392–398. doi: 10.1007/s10350-005-0247-x. [DOI] [PubMed] [Google Scholar]
- 280.Barnes NL, Warnberg F, Farnie G, et al. Cyclooxygenase-2 inhibition: effects on tumour growth, cell cycling and lymphangiogenesis in a xenograft model of breast cancer. Br J Cancer. 2007;96:575–582. doi: 10.1038/sj.bjc.6603593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Alitalo K, Mohla S, Ruoslahti E. Lymphangiogenesis and cancer: meeting report. Cancer Res. 2004;64:9225–9229. doi: 10.1158/0008-5472.CAN-04-2475. [DOI] [PubMed] [Google Scholar]
- 282.Chen Z, Varney ML, Backora MW, et al. Down-regulation of vascular endothelial cell growth factor-C expression using small interfering RNA vectors in mammary tumors inhibits tumor lymphangiogenesis and spontaneous metastasis and enhances survival. Cancer Res. 2005;65:9004–9011. doi: 10.1158/0008-5472.CAN-05-0885. [DOI] [PubMed] [Google Scholar]
- 283.Matsuo M, Sakurai H, Koizumi K, Saiki I. Curcumin inhibits the formation of capillary-like tubes by rat lymphatic endothelial cells. Cancer Lett. 2007;251:288–295. doi: 10.1016/j.canlet.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 284.Becker J, Pavlakovic H, Ludewig F, et al. Neuroblastoma progression correlates with downregulation of the lymphangiogenesis inhibitor sVEGFR-2. Clin Cancer Res. 2010;16:1431–1441. doi: 10.1158/1078-0432.CCR-09-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Castro-Rivera E, Ran S, Thorpe P, Minna JD. Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effect. Proc Natl Acad Sci U S A. 2004;101:11432–11437. doi: 10.1073/pnas.0403969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Xiang R, Davalos AR, Hensel CH, et al. Semaphorin 3F gene from human 3p21.3 suppresses tumor formation in nude mice. Cancer Res. 2002;62:2637–2643. [PubMed] [Google Scholar]
- 287.Kessler O, Shraga-Heled N, Lange T, et al. Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res. 2004;64:1008–1015. doi: 10.1158/0008-5472.can-03-3090. [DOI] [PubMed] [Google Scholar]
- 288.Nasarre P, Constantin B, Rouhaud L, et al. Semaphorin SEMA3F and VEGF have opposing effects on cell attachment and spreading. Neoplasia. 2003;5:83–92. doi: 10.1016/s1476-5586(03)80020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Heishi T, Hosaka T, Suzuki Y, et al. Endogenous angiogenesis inhibitor vasohibin1 exhibits broad-spectrum antilymphangiogenic activity and suppresses lymph node metastasis. Am J Pathol. 2010;176:1950–1958. doi: 10.2353/ajpath.2010.090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Chang JH, Javier JA, Chang GY, et al. Functional characterization of neostatins, the MMP-derived, enzymatic cleavage products of type XVIII collagen. FEBS Lett. 2005;579:3601–3606. doi: 10.1016/j.febslet.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 291.Han KY, Azar DT, Sabri A, et al. Characterization of the interaction between endostatin short peptide and VEGF receptor 3. Protein Pept Lett. 2012;19:969–974. doi: 10.2174/092986612802084465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 293.Regenfuss B, Hos D, Bock F, et al. TRAIL Influences Human Lymphatic Microvascular Endothelial Cell (HMVECs) Proliferation and Lymphangiogenesis. Investigative Ophthalmology & Visual Science. 2012;53:2398–2398. [Google Scholar]
- 294.Regenfuss B, Dreisow ML, Hos D, et al. The Naive Murine Cornea as a Model System to Identify Novel Endogenous Regulators of Lymphangiogenesis: TRAIL and rtPA. Lymphat Res Biol. 2015;13:76–84. doi: 10.1089/lrb.2015.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Oh SJ, Jeltsch MM, Birkenhager R, et al. VEGF and VEGF-C. specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev Biol. 1997;188:96–109. doi: 10.1006/dbio.1997.8639. [DOI] [PubMed] [Google Scholar]
- 296.Cao R, Bjorndahl MA, Religa P, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 297.Bjorndahl M, Cao R, Nissen LJ, et al. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci U S A. 2005;102:15593–15598. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Cao R, Bjorndahl MA, Gallego MI, et al. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood. 2006;107:3531–3536. doi: 10.1182/blood-2005-06-2538. [DOI] [PubMed] [Google Scholar]
- 299.Thurston G. Role of Angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell Tissue Res. 2003;314:61–68. doi: 10.1007/s00441-003-0749-6. [DOI] [PubMed] [Google Scholar]
- 300.Veikkola T, Alitalo K. Dual Role of Ang2 in Postnatal Angiogenesis and Lymphangiogenesis. Developmental Cell. 2002;3:302–304. doi: 10.1016/s1534-5807(02)00231-9. [DOI] [PubMed] [Google Scholar]
- 301.Tammela T, Saaristo A, Lohela M, et al. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–4648. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- 302.Morisada T, Oike Y, Yamada Y, et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- 303.Mumblat Y, Kessler O, Ilan N, Neufeld G. Full-Length Semaphorin-3C Is an Inhibitor of Tumor Lymphangiogenesis and Metastasis. Cancer Res. 2015;75:2177–2186. doi: 10.1158/0008-5472.CAN-14-2464. [DOI] [PubMed] [Google Scholar]
- 304.Shirasuna K, Kobayashi A, Nitta A, et al. Possible action of vasohibin-1 as an inhibitor in the regulation of vascularization of the bovine corpus luteum. Reproduction. 2012;143:491–500. doi: 10.1530/REP-11-0465. [DOI] [PubMed] [Google Scholar]